Abstract
Triphenyl phosphate (TPHP) is primarily used as either a flame retardant or plasticizer, and is listed as an ingredient in nail polishes. However, the concentration of TPHP in nail polish and the extent of human exposure following applications have not been previously studied. We measured TPHP in ten different nail polish samples purchased from department stores and pharmacies in 2013–2014. Concentrations up to 1.68% TPHP by weight were detected in eight samples, including two that did not list TPHP as an ingredient. Two cohorts (n=26 participants) were recruited to assess fingernail painting as a pathway of TPHP exposure. Participants provided urine samples before and after applying one brand of polish containing 0.97% TPHP by weight. Diphenyl phosphate (DPHP), a TPHP metabolite, was then measured in urine samples (n=411) and found to increase nearly seven-fold 10–14 hours after fingernail painting (p<0.001). To determine relative contributions of inhalation and dermal exposure, ten participants also painted their nails and painted synthetic nails adhered to gloves on two separate occasions, and collected urine for 24 hours following applications. Urinary DPHP was significantly diminished when wearing gloves, suggesting that the primary exposure route is dermal. Our results indicate that nail polish may be a significant source of short-term TPHP exposure and a source of chronic exposure for frequent users or those occupationally exposed.
INTRODUCTION
Chemical additives are often incorporated into plastics and resins to alter the properties of the material or to protect from weathering or fire. For example, organophosphorus (OP) compounds are commonly added to polyurethane foam in furniture and baby products to purportedly slow the spread of fire.1, 2 In addition to being used as flame retardants, OP compounds are used in a variety of other applications. Triphenyl phosphate (TPHP), an aryl phosphate, is used both as a flame retardant in polyurethane foam and as a plasticizer in hydraulic fluids, lacquers, and varnishes.3, 4
Data on the toxicity of TPHP in humans is scarce; however, an emerging toxicological literature indicates that exposure is associated with endocrine impacts, reproductive and developmental toxicity, and genotoxicity.5–11 Short-term (21 day) exposure to TPHP in zebra fish, for example, has been associated with altered sex hormone balance and disruptions of reproductive performance.5 Additionally, TPHP exposure has been associated with peroxisome proliferator-activated receptor gamma agonism, which may impact metabolic function and weight gain.10, 11 Although limited to two studies, data in humans also suggest that TPHP alters endocrine function and impacts reproduction; altered thyroid hormone levels and decreased semen quality were both observed with increasing TPHP exposure.12, 13
Much of the general population is likely exposed to TPHP. Once absorbed, TPHP is rapidly metabolized to diphenyl phosphate (DPHP) and several other metabolites, and excreted in urine (Figure 1).14, 15 In our previous work we found detectable levels of DPHP, in greater than 90 percent of a convenience sample of adults living in North Carolina in the United States [n=53];16 several other studies from within and outside the U.S. have also reported near ubiquitous detection of DPHP.17–23 We also reported that DPHP levels were approximately twice as high in women compared to men, which may suggest differences in exposure patterns by sex.16 Similar patterns have been observed for some phthalate metabolites (e.g. monobenzyl phthalate and monoethyl phthalate), a finding which has been attributed in part to differences in the use of personal care products between males and females.24 TPHP is commonly listed as an ingredient in nail polishes; according to the Environmental Working Group’s Skin Deep® cosmetic database, TPHP is on the label of about half of polishes available for sale between 2012 and 2015,25 likely added to increase flexibility and durability.26 Recent media focus on working conditions faced by nail salon employees has drawn attention to occupational exposures to potentially hazardous chemicals in nail polish (e.g. toluene, formaldehyde, and dibutyl phthalate).27 However, human exposure to TPHP in nail polish and the potential resulting occupational and consumer health risks have not yet been characterized.
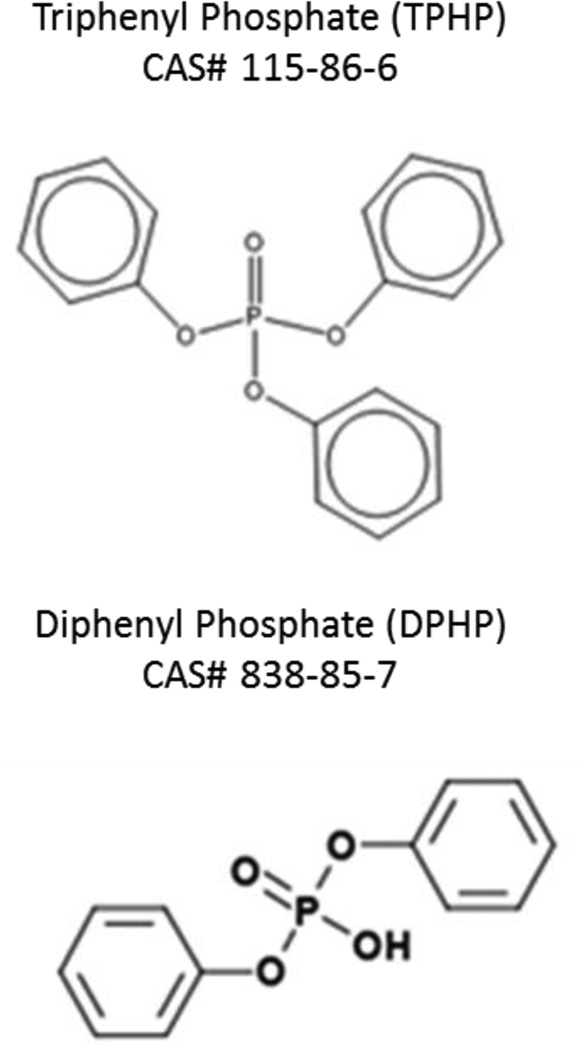
Figure 1.
Chemical structures of triphenyl phosphate and diphenyl phosphate.
The purpose of this study was to evaluate nail polish applications as a potential pathway of exposure to TPHP. We investigated the levels of TPHP in ten different nail polish samples and assessed urinary DPHP levels in two cohorts of study participants. In the first cohort (n=16), urine samples were collected before and after application of one TPHP-containing polish. In the second cohort (n=10), background exposures and the total estimated amount of TPHP absorbed via inhalation and dermal routes were assessed to better characterize the primary exposure pathway. This work provides useful information for understanding pathways of exposure to TPHP among the general population and for characterizing risks from TPHP use in nail polish.
MATERIALS AND METHODS
Characterization of Nail Polish TPHP
Nail polish samples were purchased in Durham and Wake counties in North Carolina from common department stores and pharmacies. This study was designed to measure the amount of TPHP in selected polishes, not as a random sample of nail polish. Therefore, product labels were assessed in order to purchase polishes that listed TPHP as an ingredient (n=7), as well as polishes that did not include TPHP as an ingredient (n=3). Three replicates bottles of three different polishes were also purchased to assess variability between replicate bottles. These bottles were purchased at different stores and over the course of several months.
To measure TPHP, a drop of each brand of nail polish was weighed, dissolved with acetone, and then diluted to 100 mL with dichloromethane (DCM). A 1 mL aliquot of this diluted solution was transferred to a glass amber autosampler vial, spiked with deuterated TPHP (d15TPHP), and analyzed using GC/MS using established methods.28 The mass of a 100 µL aliquot of each nail polish was recorded to calculate the TPHP density. Triplicate sub-analyses were conducted for seven different polishes to assess homogeneity within each bottle.
Study Participants (Cohorts 1 and 2)
Cohort 1 consisted of 16 female Duke University (Durham, North Carolina) undergraduate students recruited in the spring of 2014. Cohort 2 comprised ten female Duke University graduate students and employees recruited in the spring of 2015. The Duke University Institutional Review Board approved study protocols and participants gave informed consent. Participants were each provided with a new bottle of clear nail polish and a urine collection kit with instructions for collecting urine. All participants were given the same nail polish, a brand and type that was part of the nail polish TPHP assessment (see above) and contained 0.97% TPHP by weight. Although individual bottles provided to participants were not analyzed for TPHP, results of the nail polish TPHP assessment indicated that there was minimal variability in TPHP content among bottles of the same brand and type (see below). Participants from cohorts 1 and 2 also provided demographic information and completed a questionnaire about potential modifiers of exposure, including nail biting and the frequency of hand washing. Although our questionnaire included several categories for frequency of hand washing, we dichotomized the categories based on the median response. Similarly, nail biting was dichotomized as either none or any biting.
Cohort 1: Urine Collection
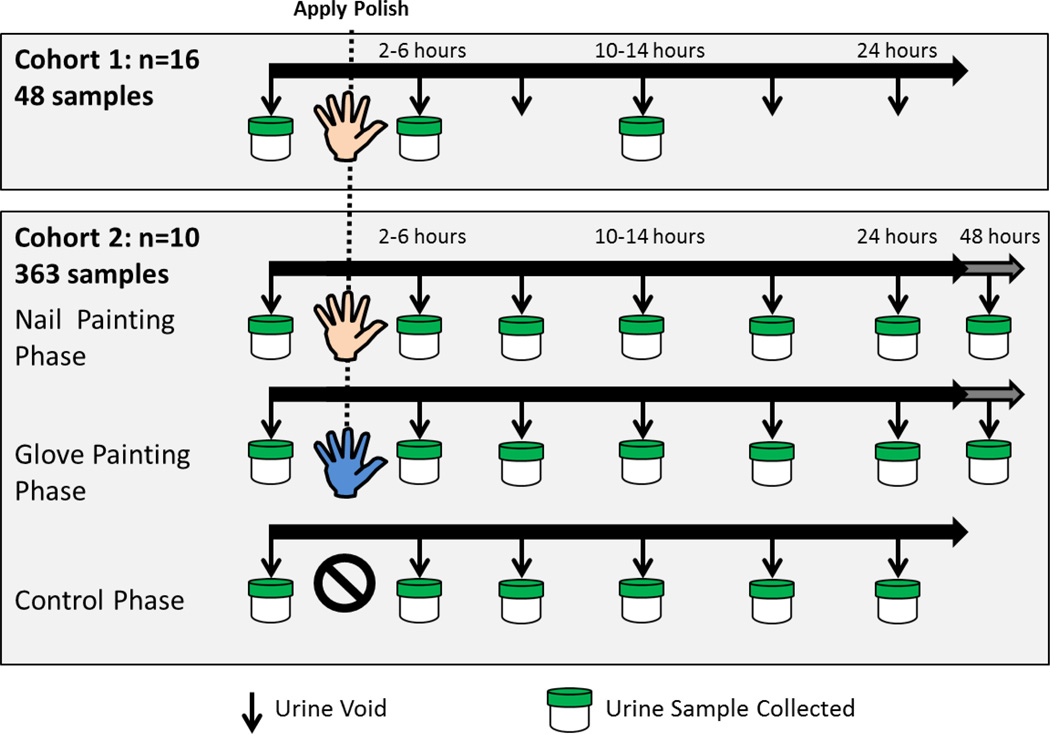
Participants were asked to remove existing nail polish from fingernails and toenails and then wait 72 hours before beginning the study. Participants then collected urine samples (~50 mL) at three time points (Figure 2). An initial urine sample was collected at a time of the participant’s choosing (T1). Approximately 24 hours later, participants painted their fingernails with two coats of the provided polish. Spot urine samples were then collected approximately 2–6 hours after nail polish application (T2) and 10–14 hours after application (T3). All urine samples were collected in standard polypropylene specimen containers and frozen until analysis.
Figure 2.
Sample collection by cohort and phase. For cohort 1, spot urine samples were collected before application of nail polish and then 2–6 hours and 10–14 hours later. For cohort 2, complete urine samples were collected over 24 or 48 hours.
Cohort 2: Urine Collection
As in cohort 1, participants were asked to remove nail polish from previous applications 72 hours before beginning the study and to not apply nail polish throughout the study except for the purpose of the study. Urine collection took place over three phases (control, nail painting, and glove painting; Figure 2). During each phase, participants collected all urine over either 24 or 48 hours (four participants collected urine for 48 hours) using a separate container for each void. Participants were asked to collect the entire volume of each void using 500mL polypropylene specimen containers and immediately freeze the sample. Participants first completed the control phase, in which they did not apply nail polish and collected all urine for 24 hours. During the nail painting phase, participants collected urine upon waking in the morning, and then proceeded to paint their fingernails with two coats of the provided nail polish and collect all urine for the following 24 or 48 hours. Nail polish was removed upon completion of urine collection. The glove painting phase mirrored the nail painting phase, but participants wore latex gloves and applied polish to synthetic nails attached to gloves. Participants were asked to keep the gloves on or near their hands for one hour post nail polish application to allow polish to completely dry before discarding the gloves. Both the synthetic nails and adhesive were tested for TPHP and were found to be below the method limit of detection (results not shown). Half the participants completed the nail painting phase before the glove painting phase, and half completed the glove painting phase before the nail painting phase. Participants waited a minimum of seven days between these two phases to ensure complete urinary excretion of DPHP from the previous phase.
Urine Processing
Extraction and measurement of DPHP in urine was performed as previously described.19, 29 Briefly, DPHP was measured using mixed-mode anion exchange solid phase extraction and a mass-labeled internal standard (d10-DPHP) with analysis by negative electrospray ionization liquid chromatography tandem mass spectrometry.29 d10-DPHP was used as an internal standard and spiked into all urine and lab blank samples prior to extraction. 13C-DPHP was spiked immediately prior to analysis to quantify d10-DPHP recovery. Average recovery for d10-DPHP was 115±22%. DPHP levels were also evaluated in laboratory blanks (n=5; purified water) for quality assurance purposes and were very low (mean = 0.11±0.07 ng). Therefore, the method detection limit (MDL) was calculated using three times the standard deviation of the blanks normalized to the urine volume extracted (MDL = 0.10 ng/mL). The total volume of each urine sample was measured for Cohort 2.
To account for urine dilution, specific gravity (SG) was also measured in each urine sample prior to analysis using a digital handheld refractometer (Atago, Bellevue, WA, USA). The average SG among samples was 1.013. Presented concentration data and results are based on SG corrected DPHP measurements;30 however, analyses with uncorrected measurements produced very similar results (results not shown).
Statistical Analyses
All statistical analyses were performed in the R Package 2.12.02 (Vienna, Austria). Descriptive statistics were calculated for TPHP in nail polish samples and for urinary DPHP concentrations. Values below the method detection limit (MDL) were replaced with MDL divided by the square root of 2.
Preliminary analyses indicated that urinary DPHP concentrations were log-normally distributed and therefore data were log10 transformed for further analyses. To evaluate the relationship between nail painting and urinary SG-corrected DPHP concentrations over time, we used a linear mixed effects regression model, which accommodates correlation in the data caused by multiple measurements per participant.31, 32 To aid in the interpretation of results, we exponentiated beta coefficients (10β) for each time point. Exponentiated beta coefficients represent the multiplicative change in DPHP levels compared to the pre-application sample. Urinary DPHP concentration data were combined from cohort 1 and cohort 2 (nail painting phase only) to match the time points of cohort 1 (T1 = pre-application; T2 = 2–6 hours post application; T3 =10–14 hours post application). Urinary DPHP concentrations for each individual over time were also plotted to visually examine trends and deviations from patterns observed for the cohorts as a whole.
To determine whether personal behavior modified exposure to TPHP via nail polish, we evaluated associations between nail biting and hand-washing frequency, and the change in urinary DPHP concentrations from T1 to T3, i.e. the change in DPHP between the pre-application and 10–14 hour post-application samples. Beta coefficients (10β) were exponentiated for each predictor, producing the multiplicative impact of each factor.
Using only data from cohort 2, we compared the total mass of DPHP excreted in each phase using a linear mixed effect model (accounting for residual correlation within individuals). We calculated the total mass of DPHP excreted per individual, i.e. multiplied the DPHP concentration (ng/mL) and sample volume (mL) from each void and then summed the DPHP mass to estimate total DPHP mass excreted over 24 and 48 hours (depending on individual) for each participant in each phase (control phase, nail painting phase, and glove painting phase). As with concentration values, DPHP mass data were log-normally distributed and were log10 transformed for analysis.
We estimated total TPHP mass in a nail polish application using the measured concentration of TPHP by weight in the nail polish provided to participants and assuming the average mass of nail polish applied to be 0.3 g, as previously estimated.33, 34 To estimate absorbed TPHP dose, we compared the estimated moles of TPHP in a nail polish application to moles of urinary DPHP excreted over 24 or 48 hours (depending on individual) in the nail painting phase, accounting for background levels by subtracting out the moles of DPHP excreted by each participant during the control phase (for individuals who provided 48 hour samples during the nail painting phase, we subtracted twice the individual’s 24 hour control phase DPHP).
RESULTS AND DISCUSSION
Nail Polish TPHP
The majority of the nail polish samples (8 of 10) contained measurable levels of TPHP; concentrations ranged from non-detectable (ND) to 16.8 mg/g (1.68% by sample weight) with a median of 8.9 mg/g TPHP (0.89% by weight; Table 1). Of the three polishes that did not list TPHP as an ingredient (despite listing other ingredients), two contained TPHP (0.49 and 0.61% by sample weight). These findings suggest that consumers may not be able to reliably avoid polishes containing TPHP based on product labels. Levels of TPHP were very similar in bottles of polish for which duplicates were purchased, despite being purchased over the course of several months and at different stores. These data suggest that there is little variability in TPHP content between batches. Similarly, replicate measurements made from the same bottle were quite similar, indicating nail polish is a homogeneous mixture with respect to TPHP. Our data also suggest that the levels of TPHP may be higher in clear polishes than colored polishes (median clear 1.16% and median colored 0.49%). As clear nail polishes are often used as a base coat or top coat, some individuals may be simultaneously exposed to TPHP in both colored polish and clear polish. However, due to our small sample size we caution against the over interpretation of this finding.
Table 1.
Characteristics of 10 nail polish samples purchased in 2013 and 2014. Polish #5 was used for both cohort 1 and 2 nail polish experiments.
| Polish Number | Brand | TPHP Listed as Ingredient |
Polish Type | THPH % by weight mean ± std |
|---|---|---|---|---|
| 1 | a | yes | Clear | 0.61 ± 0.03* |
| 2 | a | no | Clear | 1.16 ± 0.06* |
| 3 | b | yes | Clear | 1.68 ± 0.07* |
| 4 | c | yes | Clear | 1.51 ± 0.21# |
| 5 | d | yes | Clear | 0.97 ± 0.04# |
| 6 | e | yes | Colored | ND*,† |
| 7 | a | yes | Colored | 0.81 ± 0.03* |
| 8 | f | no | Colored | ND#,† |
| 9 | d | yes | Colored | 1.63 ± 0.03* |
| 10 | d | no | Colored | 0.49 ± 0.05* |
summary statistics based on replicates from the same bottle (n=3)
summary statistics based on samples from different bottles (n=3)
all samples below the limit of detection
We are unable to assess the average frequency of TPHP use in nail polishes because we specifically chose polishes that contain TPHP. According to the EWG’s Skin Deep® cosmetics database, TPHP is used in about half of nail polishes available for sale between 2012–2015;25 however, our findings indicating TPHP is unlabeled in some polishes suggests that the percentage of polishes containing TPHP may be higher. Levels of TPHP observed in samples were similar to those previously reported for other ingredients of concern in nail polish (e.g. dibutyl phthalate, which has generally been detected at <5% by weight in nail polish35, 36).
Background Urinary DPHP Concentrations
A total of 101 urine samples were collected for measurements of background DPHP (control samples), of which 87 contained detectable levels of TPHP. From cohort 1, a pre-application sample was collected from each participant (n=16). From cohort 2, 65 background samples were collected during the control phase, and additional control samples were collected from each participant (n=10) at the start of the nail painting and glove phases. The geometric mean (GM) for all control samples (combining both cohorts) was 0.96 ng/mL (range = < MDL – 14.74 ng/mL) and was very similar to urinary DPHP concentrations reported in other U.S. cohorts.12, 16–19
Urinary DPHP Concentrations After Nail Polish Application
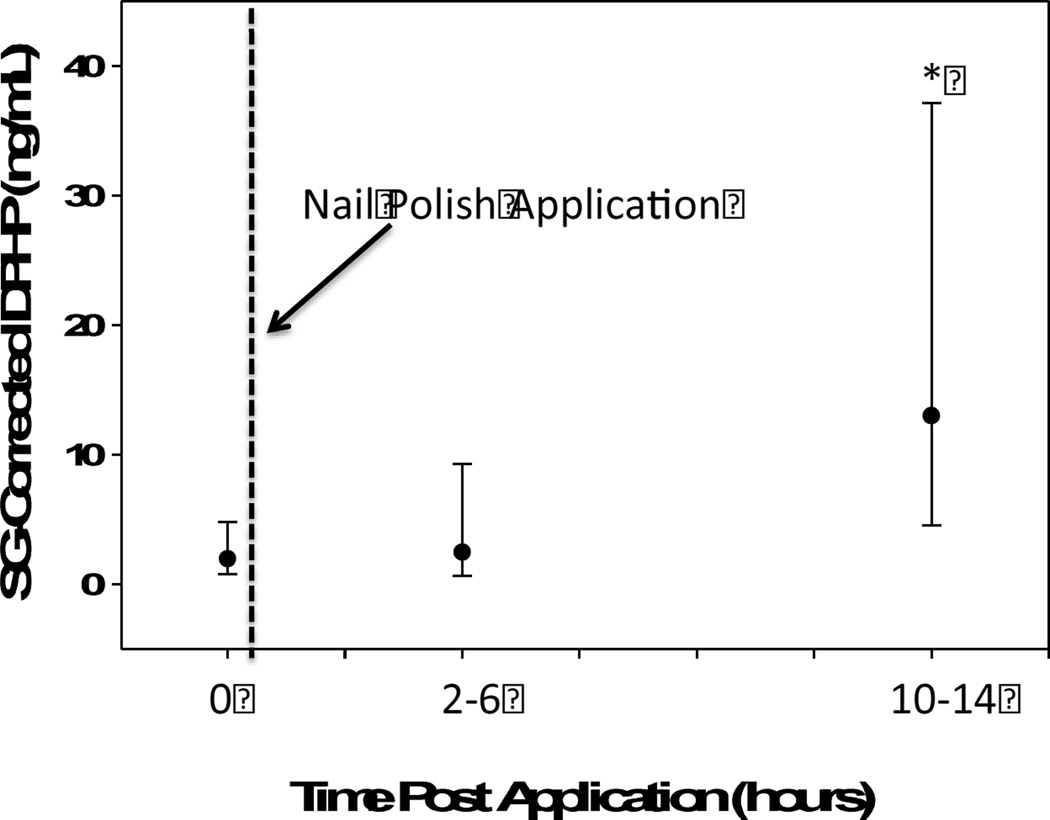
DPHP was detected in 24 out of 26 urine samples collected 2–6 hours following application of nail polish to fingernails (T2) (GM = 2.48 ng/mL; range = <MDL – 23.92 ng/mL; Figure 3) and all samples had detectable levels of DPHP 10–14 hours post-nail polish application (T3) (GM = 13.02 ng/mL; range = 1.15 – 168.29 ng/mL). Compared to T1, DPHP levels were higher at T2, but not significantly so (10β=1.26; 95% CI: 0.75, 2.10; p=0.38). However, a significant increase was observed at T3, with urinary DPHP levels 6.59 times those collected at T1 (95% CI: 3.95, 11.00; p<0.0001). These results suggest that nail polish use is likely a significant source of short-term TPHP exposure. Neither nail biting nor hand washing frequency was associated with the magnitude of change in urinary DPHP concentrations from T1 to T3, indicating that nail biting and hand washing frequency may not significantly modify exposure levels associated with nail painting (results not shown).
Figure 3.
Geometric mean and standard deviation of urinary DPHP levels measured at 3 time different points before and after applying nail polish. (N=26 participants). *Indicates statistically significant difference from pre-application urine sample (p<0.001).
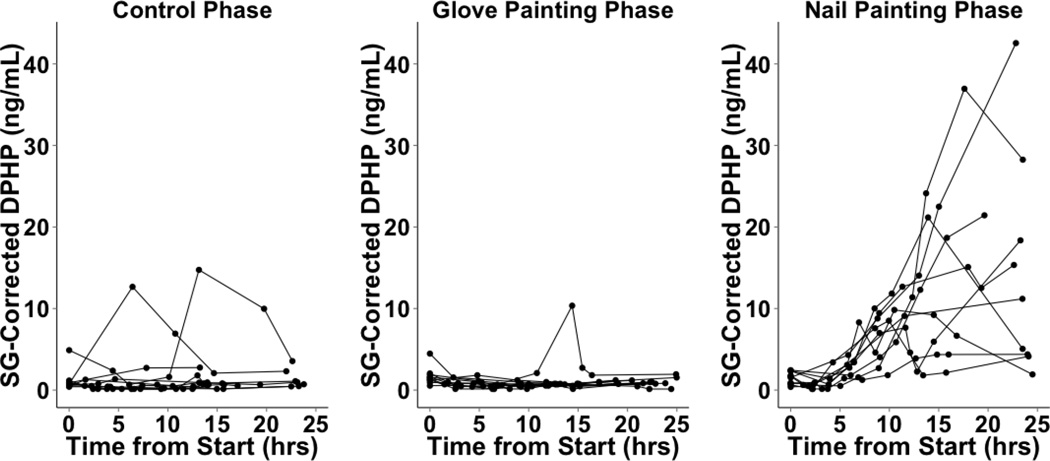
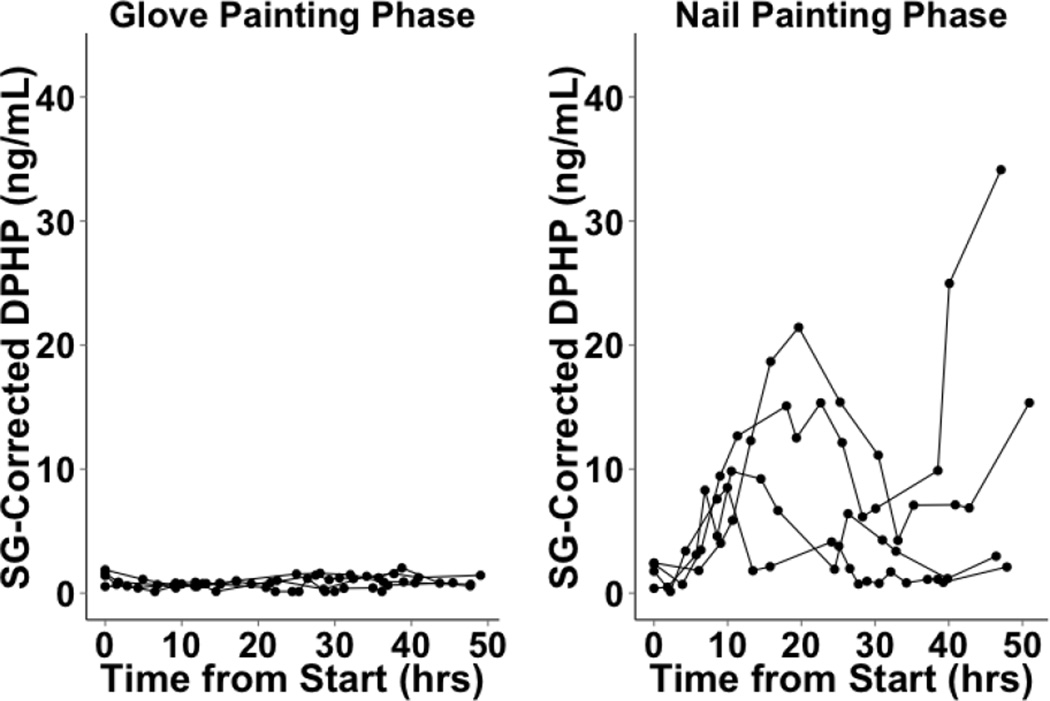
We cannot determine when DPHP concentrations peaked in cohort 1 because urine samples were collected at only two time points after polish application. In cohort 2, of the six participants who collected only 24 hours of urine samples following nail polish application to fingernails, two exhibited peaks within the time frame, while four exhibited maximum urinary DPHP in their final samples, suggesting that DPHP concentrations may have peaked after completion of urine collection for these individuals (Figure 4). Three of the four participants who collected 48 hours of urine samples exhibited peaks in urinary DPHP concentrations between 10 to 20 hours post nail polish application (Figure 5). The maximum urinary DPHP concentration of the fourth participant who provided 48 hours of samples occurred in their final sample. In the control and glove painting phases, urinary DPHP concentrations over 24 and 48 hours were lower and showed less variability compared to the nail painting phase for the majority of participants, although some aberrations were observed (Figures 4 and 5). Differences between individuals in rates of uptake, metabolism, excretion or residual confounding by other exposures to TPHP may have contributed to the variability observed in urinary DPHP concentrations over time. Unfortunately we did not collect information on personal characteristics that may be related to these factors such as race and ethnicity, body mass index or other environmental exposures (e.g. smoking and tobacco use).
Figure 4.
Cohort 2 SG-Corrected DPHP concentrations over 24 hours (n=10). In the control phase, nail polish was not applied. In the glove phase, participants wore latex gloves and applied the provided nail polish to synthetic nails attached to the gloves. In the nail painting (no-glove) phase, participants applied the provided nail polish directly to their nails.
Figure 5.
Cohort 2 SG-Corrected DPHP concentrations over 48 hours (n=4). In the control phase, nail polish was not applied. In the glove phase, participants wore latex gloves and applied the provided nail polish to synthetic nails attached to the gloves. In the nail painting (no-glove) phase, participants applied the provided nail polish directly to their nails.
Although our current work is focused on TPHP exposure, our urine extraction and analysis methods can also be used to measure biomarkers of exposure to other organophosphorus compounds, including tris (1,3-dichloro-2-propyl) phosphate (TDCIPP).29 We found no significant difference from T1 to T2, or T3 in the geometric mean concentrations of bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), a metabolite of the organophosphate TDCIPP, which is also detected frequently, and at similar levels, as DPHP metabolite.16–23 This result was expected as TDCIPP is not, to our knowledge, used as an ingredient in nail polish. Geometric mean concentration of BDCIPP at T1, T2, and T3 were 1.51 ng/mL, 1.56 ng/mL and 1.55 ng/mL, respectively.
Excreted Urinary DPHP Mass
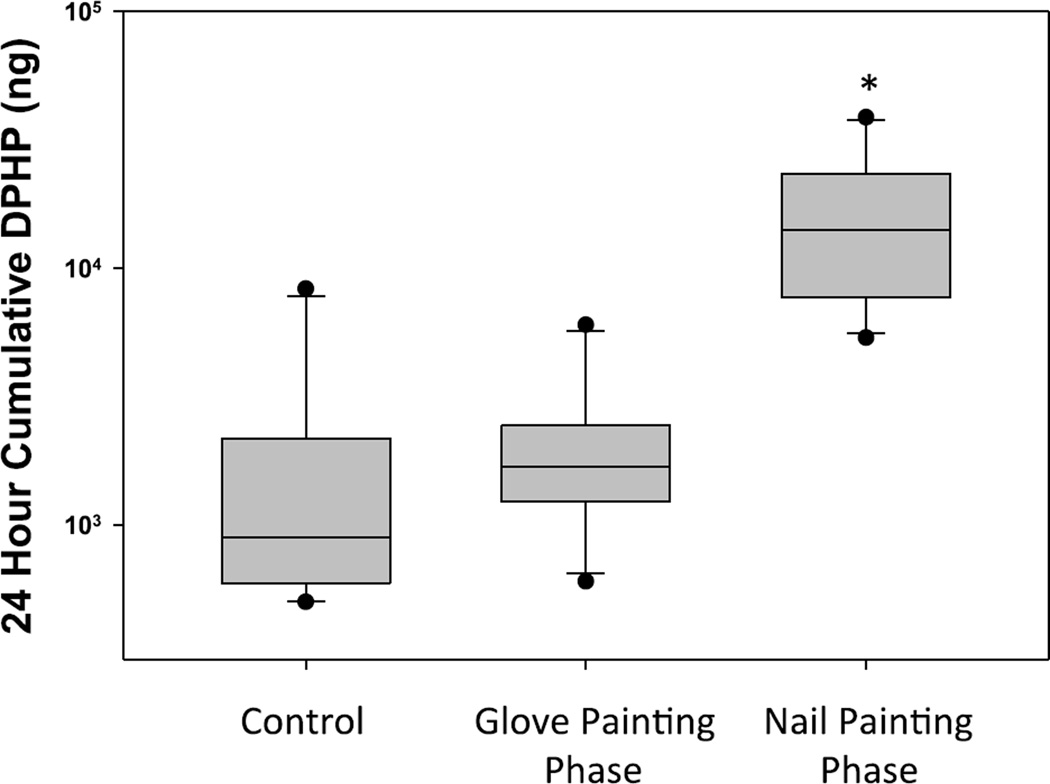
The geometric mean urinary DPHP mass excreted over 24 hours in the control phase of cohort 2 was 1.2 µg (range = 0.5 – 8.3 µg; Figure 6). The urinary DPHP mass excreted over 24 hours in the nail painting phase was 11.2 times that of the control phase (95% CI: 6.10, 20.61; p<0.0001), with a geometric mean of 13.7 µg (range = 5.4 – 38.8 µg). Urinary DPHP mass excreted over 24 hours in the glove-painting phase did not significantly differ from the control phase (10β =1.43; 95% CI: 0.78, 2.64; p = 0.23), with a geometric mean of 1.8 µg (range = 0.6 – 6.0 µg). These results increase the evidence that nail polish is a source of exposure to TPHP, as confounding by other exposures was shown to be minimal during the control phase. In addition, because the painting of gloves prevented dermal exposure, these results indicate that dermal absorption is likely the primary route of exposure to TPHP from nail polish use. The small, not statistically significant increase when wearing gloves may suggest a small amount of inhalation exposure; further experimentation with greater experimental power would be needed to test this hypothesis. These results also suggest that people in close proximity to nail polish applications may not have significant exposure to TPHP. However, additional research is needed to understand exposure routes in nail salons, as it is possible that multiple concurrent manicures in an enclosed space may contribute to a non-negligible amount of TPHP inhalation.
Figure 6.
Box whisker plots showing cumulative DPHP mass excreted over a 24 hours period for 10 participants in Cohort 2. In the control phase, nail polish was not applied. In the glove phase, participants wore latex gloves and applied the provided nail polish to synthetic nails attached to the gloves. In the nail painting (no-glove) phase, participants applied the provided nail polish directly to their nails. *Indicates statistically significant difference from pre-application urine sample (p<0.001).
We estimated that application of nail polish containing 0.97% TPHP by weight to fingernails is approximately 2.9 mg, or 8.9 × 10−6 mols, TPHP (molecular weight = 3.26 × 105 mg/mol). To estimate the proportion of TPHP from a nail polish application that is absorbed and metabolized to DPHP by the user, we took the ratio of moles of urinary DPHP (molecular weight = 2.50 × 105 mg/mol) excreted by each participant during the nail phase (with the number of moles from each participant’s control phase subtracted to account for background DPHP) to the moles of TPHP we estimated to be in a nail polish application. Based on these calculations, we found a geometric mean of 0.5% TPHP absorbed and metabolized to DPHP over 24 hours following a nail polish application (range = 0.2 – 1.7%). Over 48 hours, we also found a geometric mean of 0.5% TPHP absorbed and metabolized to DPHP (range = 0.1 – 2.2%), which suggests that the majority of TPHP applied may be absorbed, metabolized, and excreted during the first 24 hours following nail polish application. However, as there were only four participants who provided 48 hours of samples, we cannot conclusively determine the amount of time it takes for all absorbed TPHP to be metabolized to DPHP and excreted in urine. We also cannot rule out the possibility that dermal absorption of TPHP continues after 24 (48) hours of wearing nail polish, or that TPHP may partition to different compartments in the body (e.g. serum, lipids), where it may be stored beyond 24 (48) hours. In vitro work indicates that TPHP is metabolized to compounds other than DPHP (e.g. hydroxylated TPHP [OH-TPHP]).15 Our methods were not designed to measure other metabolites; however, a recent human study did not find OH-TPHP in urine samples, suggesting it may not be a relevant metabolite in vivo.20 Nonetheless, we cannot exclude the possibility that TPHP may be metabolized to other compounds, suggesting that our estimates of the percent of TPHP absorbed are likely underestimates.
From our current work, we cannot conclusively determine whether TPHP is absorbed through the nail plate or through the cuticles and surrounding skin. The nail plate, composed primarily of keratin, is generally characterized by low permeability to most molecules; however, nail polishes may contain other compounds that enhance absorption across the nail (e.g. solvents).37, 38 Tissues surrounding the nail also contain an extensive vascular network, which may readily facilitate chemical absorption through the skin.37 It is also possible that hand-to-mouth contact may result in incidental ingestion of TPHP following nail polish application, particularly for individuals biting their nails. Although we did not see associations between nail biting and DPHP levels, more refined measures of hand-to-mouth contacts may provide additional insights.
More research is needed to determine whether our reported levels of exposure to TPHP from nail polish use pose a risk to human health. TPHP and flame retardant mixtures containing TPHP (e.g. Firemaster® 550 [FM550]) have been associated with adverse outcomes in a growing number of experimental toxicology studies.5–11, 39 For example, in vitro TPHP exposure was found to divert mouse bone marrow cells away from osteogenesis toward adipogenesis, suggesting that TPHP may potentially affect bone health and weight gain.11 In vitro TPHP exposure has also been shown to impact estrogenic activity.40 In rats, perinatal exposure to FM550 has been associated with early puberty and obesity,39 which data suggest may be driven by TPHP (~10–20% of FM550 by weight).10, 11 In zebrafish, cardiotoxic effects including severe pericardial edema and blocked cardiac looping were induced by TPHP exposure during embryogenesis.41 Data in humans remain limited. Meeker et al., however, recently reported endocrine and reproductive impacts in adult men associated with levels of DPHP lower than those observed in our cohort prior to nail painting.18
Our results should be interpreted in the context of several strengths and limitations. In particular, cohort 2 collected the entire volume of each urine void over 24 or 48 hours. We designed the cohort 2 experiment using identical phases (control, nail painting, glove painting) to reduce confounding, for example by other exposures. In the glove-painting phase, we prevented dermal exposure, allowing us to determine the importance of this route of exposure. However, our sample size may have limited our ability to detect meaningful behavioral associations and to adjust for multiple covariates in regression analyses. Additionally, our sample was comprised of a highly homogeneous group of university students and employees. While this may limit the generalizability of our results to other populations, we do not anticipate that it would impact the validity of our results.
Despite these limitations, our data indicate that nail polish is a likely source of exposure to TPHP and that use may result in exposures substantially greater than background levels. As it has been estimated that adult women paint their nails approximately once per week on average, nail polish use may be a chronic source of exposure for many women.34, 42 Additional data are needed to determine exposure routes for individuals who are occupationally exposed in nail salons and to understand inter-individual variability in the quantity and timing of DPHP excretion following TPHP exposure.
HIGHLIGHTS.
Some nail polishes contain the plasticizer triphenyl phosphate (TPHP)
Urinary metabolites of TPHP increased 7-fold following nail polish application
TPHP exposure from nail polish appears to occur via dermal exposure
TPHP may be a replacement for phthalates in nail polish
ACKNOWLEDGEMENTS
The authors would like to thank all of our participants in this research study. Support for this project was provided in part by the Environmental Working Group and a donation from Fred and Alice Stanback. Partial support for effort was also provided by grants from the National Institute of Environmental Health Sciences, R01ES016099 (HMS) and R01ES015829 (TFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Consumer Product Safety Commission (CPSC) Nomination of FR Chemicals for NTP Testing. 2005 [Google Scholar]
- 4.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio) Aquat Toxicol. 2013;134–135:104–111. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012;114–115:173–181. doi: 10.1016/j.aquatox.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Kojima H, Takeuchi S, Itoh T, Iida M, Kobayashi S, Yoshida T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology. 2013;314(1):76–83. doi: 10.1016/j.tox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante J. Toxicity review of aromatic phosphate plasticizers. Bethesda, MD 20814: U.S. Consumer Product Safety Commission; 1999. [Google Scholar]
- 9.Babich MA. Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam. Bethesda, MD 20814: U.S. Consumer Product Safety Commission; 2006. [Google Scholar]
- 10.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett. 2014;228(2):93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillai H, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand Binding and Activation of PPARγ by Firemaster® 550: Effects on Adipogenesis and Osteogenesis in Vitro. Environ Health Perspect. 2014;122(11):1225–1232. doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocrine disruptors. 2013;1(1):e26306. doi: 10.4161/endo.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118(3):318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki K, Suzuki T, Takeda M, Uchiyama M. Metabolism of phosphoric acid triesters by rat liver homogenate. Bulletin of environmental contamination and toxicology. 1984;33(3):281–288. doi: 10.1007/BF01625544. [DOI] [PubMed] [Google Scholar]
- 15.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett. 2013;223(1):9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring Indoor Exposure to Organophosphate Flame Retardants: Hand Wipes and House Dust. Environ Health Perspect. 2015;123(2):160–165. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–172. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect. 2013;121(5):580–585. doi: 10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of Organophosphate Flame Retardants and 2-Ethylhexyl Tetrabromobenzoate (EH-TBB) in Urine from Paired Mothers and Toddlers. Environ Sci Technol. 2014;48(17) doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 20.Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, Covaci A. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int. 2015;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int. 2015;75:159–165. doi: 10.1016/j.envint.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol. 2014;48(23):13625–13633. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. Journal of chromatography. A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Environmental Working Group EWG's Skin Deep® Cosmetic Database. Available: http://www.ewg.org/skindeep/ [Google Scholar]
- 26.Personal Care Products Council Cosmetics Info: The Science & Safety Behind Your Favorite Products. available: http://www.cosmeticsinfo.org/ [Google Scholar]
- 27.Nir S. Perfect Nails, Poisoned Workers. The New York Times. 2015 [Google Scholar]
- 28.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper EM, Covaci A, van Nuijs AL, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(7):2123–2132. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association journal. 1993;54(10):615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro J, Bates D. Linear and Nonlinear Mixed Effects Models -"nlme' version 3.1-120. 2015 https://stat.ethz.ch/R-manual/R-devel/library/nlme/html/lme.html. [Google Scholar]
- 32.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New Jersey: John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 33.Biesterbos JW, Dudzina T, Delmaar CJ, Bakker MI, Russel FG, von Goetz N, Scheepers PT, Roeleveld N. Usage patterns of personal care products: important factors for exposure assessment. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;55:8–17. doi: 10.1016/j.fct.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Ficheux AS, Morisset T, Chevillotte G, Postic C, Roudot AC. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2014;66:36–43. doi: 10.1016/j.fct.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Hubinger JC, Havery DC. Analysis of consumer cosmetic products for phthalate esters. Journal of cosmetic science. 2006;57(2):127–137. [PubMed] [Google Scholar]
- 36.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environmental research. 2011;111(3):329–336. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Gupchup GV, Zatz JL. Structural characteristics and permeability properties of the human nail: A review. J. Cosmet. Sci. 1999;50:363–385. [Google Scholar]
- 38.Walters KA, Flynn GL. Permeability characteristics of the human nail plate. International journal of cosmetic science. 1983;5(6):231–246. doi: 10.1111/j.1467-2494.1983.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 39.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture FiremasteRR) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bittner GD, Denison MS, Yang CZ, Stoner MA, He G. Chemicals having estrogenic activity can be released from some bisphenol A-free, hard and clear, thermoplastic resins. Environmental health : a global access science source. 2015;14:103. doi: 10.1186/1476-069X-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci. 2013;133(1):144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 42.United States Environmetal Protection Agency. Exposure Factors Handbook: 2011 Edition. 2011 [Google Scholar]