Abstract
Objective
To assess the effects of fetal-neonatal iron deficiency on recognition memory in early infancy. Perinatal iron deficiency delays or disrupts hippocampal development in animal models and thus may impair related neural functions in human infants, such as recognition memory.
Study design
Event-related potentials were used in an auditory recognition memory task to compare 2-month-old Chinese infants with iron sufficiency or deficiency at birth. Fetal- neonatal iron deficiency was defined two ways: high zinc protoporphyrin/heme ratio (ZPP/H > 118 μmol/mol) or low serum ferritin (< 75 μg/l) in cord blood. Late slow wave (LSW) was used to measure infant recognition of mother’s voice.
Results
ERP patterns differed significantly for fetal-neonatal iron deficiency as defined by high cord ZPP/H but not low ferritin. Comparing 35 infants with iron deficiency (ZPP/H > 118 μmol/mol) to 92 with lower ZPP/H (iron-sufficient), only infants with iron sufficiency showed larger LSW amplitude for stranger’s voice than mother’s voice in frontal-central and parietal-occipital locations, indicating the recognition of mother’s voice.
Conclusions
Infants with iron sufficiency showed electrophysiological evidence of recognizing their mother’s voice, whereas infants with fetal-neonatal iron deficiency did not. Their poorer auditory recognition memory at two months of age is consistent with effects of fetal-neonatal iron deficiency on the developing hippocampus.
Keywords: iron deficiency, infant, ERP, recognition memory
Globally, 38% of pregnant women are anemic, mostly due to iron deficiency, potentially affecting ~32 million infants/year.(1) Despite routine prenatal iron supplementation in the US, 34% of pregnant US women are anemic (2) and 30% are iron deficiency in the 3rd trimester.(3) Although it was once thought that the fetus was protected from maternal iron deficiency, it appears that this is not completely true. Several cohort studies report associations between maternal and neonatal iron status, especially if mothers are markedly anemic or have very low iron stores.(4) Non-dietary factors also increase fetal iron deficiency risk through placental dysfunction in common conditions such as gestational diabetes and intrauterine growth restriction, which is mostly related to maternal hypertension.(5) Thus, in the US and elsewhere, the iron available to many fetuses may be inadequate to meet the needs of the developing brain.
Rodent models of fetal-neonatal iron deficiency show that the hippocampus – a neural structure important for memory and learning, among many other functions – is exquisitely sensitive to lack of iron during early development.(6) Research on fetal-neonatal iron deficiency in humans has been hampered not only by previous thinking that the fetus was protected but also by the challenge of assessing brain functioning in very young infants. Studies have used event related potentials (ERPs) to assess hippocampus-based auditory recognition memory in infants of diabetic mothers. (7) ERP is a form of neuroimaging that relies on non-invasive recording of the brain’s electrical activity in response to stimuli. Infants with iron deficiency of mothers with diabetes showed ERP evidence of impaired recognition memory, whereas those who were not iron deficiency at birth were similar to healthy controls. (8) These findings suggest that the observed impairment in recognition memory was related to fetal-neonatal iron deficiency. However, fetal exposure to metabolic abnormalities of the diabetic pregnancy may have also contributed.
A small study reported delayed development of the normal electrophysiological pattern of visual recognition memory in 9-month-old infants with iron-deficiency anemia (IDA).(9) The ERP findings were supported by behavioral testing in the same study.(10) Another study found evidence of impaired verbal recognition memory in 10-year-old Chilean children who had IDA as infants (at 6–18 months), compared with those who had not.(11) However, neither of these studies assessed iron status at birth, making it impossible to know what role fetal-neonatal iron deficiency may have played.
The primary aim of the present study was to determine the effects of fetal-neonatal iron deficiency on recognition memory using ERP in infants born of uncomplicated pregnancies. A secondary aim was to consider alternatives in defining fetal-neonatal iron deficiency, because there is no standard definition. We predicted that infants with fetal-neonatal iron deficiency would show poorer recognition memory at 2 months of age.
Methods
The ERP assessment of recognition memory was one component of a larger study on the neurodevelopmental effects of iron deficiency in early life. Study enrollment occurred between December 2008 and November 2011 in Fuyang County, a relatively prosperous rural area in Zhejiang Province, China. Parents provided signed informed consent. Pregnant women with normal uncomplicated pregnancies were invited to participate when they were randomly screened at a routine visit at 36–37 weeks gestation. Healthy full-term infants were eligible for neurodevelopmental testing. Specific entrance criteria included singleton birth with gestational age ≥ 37 weeks, birth weight ≥ 2,500 g, 5-minute Apgar score ≥7, no use of antibiotics (an indirect measure of confirmed or suspected infection), no placental abruption or uterine rupture, no birth injury or congenital deficits, no hemolytic or metabolic diseases, and normal hearing.(12) The majority of infants lived in 2-parent households. Most parents had completed high school; 32.5% of the infants had at least one parent who completed college. The study was approved by the Institutional Review Boards of the University of Michigan and the Children’s Hospital of Zhejiang University.
Cord blood was analyzed to identify infants with fetal-neonatal iron deficiency. In the few previous studies of fetal-neonatal iron deficiency and neurodevelopment,(9, 13, 14) cutoffs for low cord-blood ferritin have differed, and other iron status measures have not been used. We therefore measured cord zinc protoporphrin/heme (ZPP/H) ratio as well as cord ferritin, because ZPP/H may be a more sensitive indicator of iron deficiency.(15–17) Serum ferritin (SF) was assayed by chemiluminescent immunoassay (IMMULITE, Diagnostic Products),(18) and ZPP/H by ZP Hematofluorometer (Model 206D, AVIV Associates, NJ). We considered SF < 75 μg/l as the cutoff for fetal-neonatal iron deficiency, as in some previous studies.(17, 19) For ZPP/H, we used a cutoff of ZPP/H > 118 μmol/mol, corresponding to the 90th percentile in US studies (Pamela Kling, personal communication). (20)
At 2 months of age, 254 infants who were full-term without perinatal problems or acute or chronic illness received ERP assessment at the Children’s Hospital of Zhejiang University, Hangzhou, China. After initial ERP data processing, 92 infants were excluded, because less than 30% of their electrophysiology data were valid, ERP testing was incomplete, or there were technical problems. Of the 162 infants with usable ERP data, 28 had fetal-neonatal iron deficiency as defined by SF < 75 and 133 infants had iron sufficiency; 1 was excluded due to missing data on cord ferritin. For analyses using ZPP/H, 35 infants could not be included, as they did not have cord-blood ZPP/H data due to a delay in obtaining customs clearance for the hemtafluorometer. Of the 127 infants with ZPP/H data, 35 infants had iron deficiency as defined by ZPP ≥ 118 μmol/mol and 92 had iron sufficiency. No infant had both iron deficiency and anemia at birth.
Stimuli and Procedure
Trained experimenters (medical doctors with extra training in ERP in infants) performed the ERP recordings in the auditory recognition memory task in an electronically shielded room. Data were collected from infants who were quiet and alert while seated on the mother’s lap. The stimuli presented to infants consisted of 100 trials of mother’s voices and 100 trials of stranger’s voices. The stranger’s voice was the voice of the previously tested mother and thus varied for each infant. The stimuli were randomly presented to infants and the same voice was never presented consecutively more than twice. This testing lasted about 12 minutes. More details of this task can be found in our previous publications (12, 21).
ERP Recording and Preprocessing
The electroencephalogram (EEG) data were recorded with a 64-channel Hydrocel Geodesic Sensor net, NetAmps hardware, and Net Station data acquisition software (Electrical Geodesics Inc., Eugene, OR). Preprocessing and data reduction were carried out in Net Station 4.4 (Eugene, OR) including filtering, segmentation, artifacts detection, data rejection, interpolation, and re-reference. More information on ERP recording measurements and preprocessing steps were included in our previous publications (12, 21).
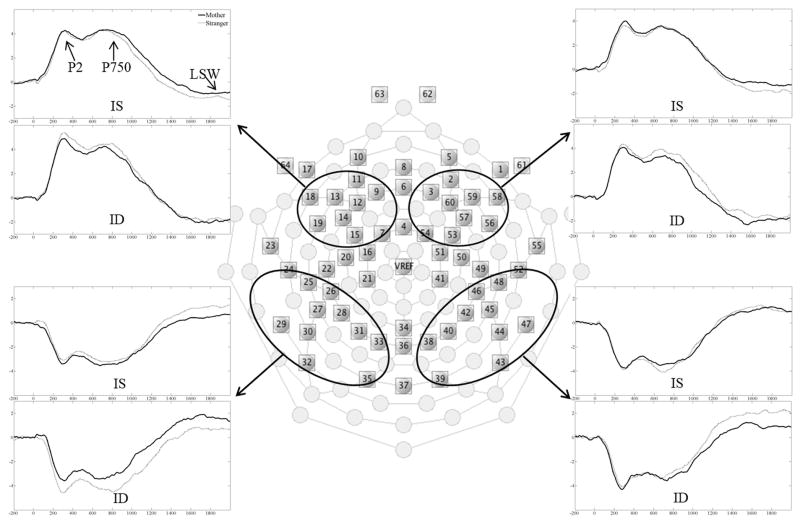
After preprocessing, we measured three ERP components: P2, P750, and a late slow wave (LSW).(21) LSW was the most important index in this study because it had been one of most widely used ERP component to measure memory updating and novelty detection. Additionally, P2 was used to measure voice discrimination or attention modulation. P750 was thought to represent processing of the second syllable in a 2-syllable word. Four regions of interests (ROI) were defined to analyze these three components (Figure 1): left frontal-central, right frontal-central, left parietal-occipital, and right parietal-occipital. The average of all channels in each region was computed to quantify ERPs. Consistent with previous studies, the P2 and P750 were defined as positive peaks in the front-central regions separately occurring about 200 ms and 750 ms after stimuli onset, respectively. Due to polarity inversion, they were defined as negative peaks in the parietal-occipital regions. Late slow wave (LSW) was identified as a negative-going slow wave in frontal-central regions and a positive-going slow wave in parietal-occipital regions occurring about 1500 ms after stimulus onset.
Figure 1.
Layout of the EGI 64-channel sensor net and the four regions of interest. The merged ERP waveforms are displayed for each region of interest and group. ID, iron deficiency; IS, iron sufficiency.
Statistical Analyses
The amplitude and latency of P2 and the amplitude of P750 and LSW were entered in mixed-design ANOVA models. The data from frontal-central and parietal-occipital regions established the within-subject factor of front-back. The data from left and right hemisphere established the within-subject factor of hemisphere. Condition (mother, stranger) and iron group were also included as within-subject and between-subject factors respectively. Potential confounding variables were added as covariates if there was a group difference or if previous studies indicated they were related to independent or dependent variables. Greenhouse-Geisser correction was used if the assumption of sphericity was violated. Further ANOVA analyses were carried out to understand significant interactions.
Results
There were statistically significant effects of fetal-neonatal iron deficiency on ERP outcomes when iron deficiency was defined as high ZPP/H but only one significant difference when low SF was the criterion. We therefore emphasize results for ZPP/H but also mention that SF finding (details of other SF analyses available on request).
Sample characteristics are shown by iron status group as defined by ZPP/H in the Table. All participants were from the same ethnic group (Han). No mother reported being a smoker or drug user. There were no statistically significant group differences in background characteristics except for more boys with iron sufficiency than those with iron deficiency (χ2 (1, N = 127) = 5.34, p < .05). Sex, therefore, was included as a covariate in further analyses.
Table 1.
Sample characteristics by iron status group*
| Iron group† | IS | ID |
|---|---|---|
| n | 92 | 35 |
| Infant characteristics | ||
| Sex, % male (n)‡ | 54.3 (50) | 31.4 (11) |
| Delivery type, % vaginal (n) | 26.1 (24) | 20.0 (7) |
| Gestation age, wk | 39.6 (0.9) | 39.5 (0.9) |
| Birth weight, kg | 3.39 (0.38) | 3.34 (0.49) |
| Age at ERP testing, d | 43.3 (2.0) | 44.0 (2.3) |
| 6-week weight-for-age z-score | 0.51 (0.79) | 0.41 (0.72) |
| Pb in cord blood, μg/dL § | 3.1 (1.3) | 3.2 (1.1) |
| ZPP, μmol/mol §,|| | 88.2 (16.5) | 145.5 (30.6) |
| STfR, mg/L § | 4.31 (2.4) | 4.01 (3.3) |
| Ferritin, μg/L | 156.7 (68.5) | 138.0 (77.9) |
| Hemoglobin, g/L | 143.5 (17.4) | 145.9 (18.2) |
| Family background | ||
| Mother’s age, y | 26.5 (3.3) | 26.8 (3.8) |
| Number of family members | 5.2 (1.2) | 5.1 (1.2) |
| First born, % yes (n) | 78.3 (72) | 82.9 (29) |
| Father in home, % yes (n) | 92.4 (85) | 91.4 (32) |
| Grandparents in home, % yes (n) | 83.7 (77) | 85.7 (30) |
| Parental education, % ≥ high school (n) | 69.2 (63) | 65.7 (23) |
| Father smokes, % yes (n) | 57.8 (48) | 62.5 (20) |
| Net income above poverty, % yes (n)¶ | 64.2 (58) | 63.6 (22) |
Values are means (standard deviation) for continuous variables and % (n) for categorical ones. Ns vary slightly due to missing data.
Iron status group based on ZPP/H; IS = iron sufficient, ID = iron deficient.
There were more boys in the IS than the ID group (χ2 (1, N = 127) = 5.34, p < .05).
Values are geometric means for these variables, which were log-transformed for analysis.
By definition, ZPP/H was higher in the ID than the IS group (F (1, 127) = 186.71, p < .001).
The local poverty line (<50,000 yuan per year) was based on Fuyang County Housing Assistance Policy for Low Income Families, 2012.
Regarding blood test results, the groups differed in ZPP/H by definition (F (1, 127) = 186.71, p < .001). There were no group differences in hemoglobin or serum transferrin receptor. The groups were also similar with respect to cord-blood lead (Pb) concentration; the means were low, and the highest Pb in a study infant was 8.10 μg/dL. There were only low-order correlations between ZPP/H and SF (r = −0.15, p = 0.10) or Pb (r = −0.17, p = 0.07). However, in light of iron-Pb physiologic interactions and important effects of Pb on neurodevelopment, including memory,(12, 22, 23) Pb was included as an additional covariate.
There was no condition difference in the number of good-quality segments, but infants with iron deficiency had more valid segments than infants with iron sufficiency, statistically significant for the mother condition (t (125) = 2.07, p < .05) and suggestive for the stranger condition (t (125) = 1.27, p = .09). Thus, the number of valid segments was also included as a covariate.
There was a main effect of front-back for P2, P750, and LSW, indicating the expected polarity inversion across the scalp (F (1, 119) = 41.10, p < .0001, ηp2 = 0.26; F (1, 119) = 251.65, p < .0001, ηp2 = 0.68; F (1, 119) = 389.93, p < .0001, ηp2 = 0.77). We report other results for the primary and secondary outcomes.
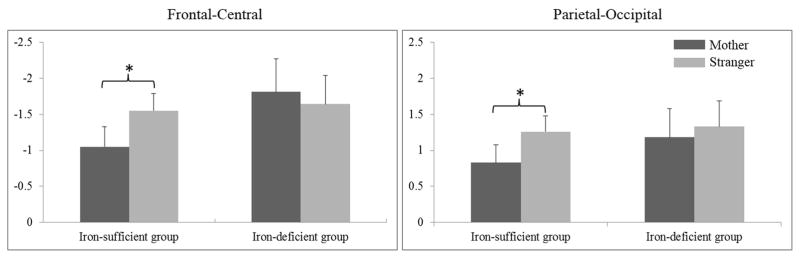
Primary outcome: LSW
For infants with iron sufficiency, LSW amplitude was more negative in the frontal-central location but more positive in the parietal-occipital location in the stranger than the mother condition, Figure 2. In contrast, for infants with iron deficiency there was no condition difference in LSW amplitude in any region of interest. However, there was a significant condition × front-back × hemisphere interaction (F (1, 30) = 4.41, p < .05, ηp2 = 0.13). Further analysis revealed that the interaction between condition and hemisphere was in the parietal-occipital location (F (1, 30) = 4.98, p < .05, ηp2 = 0.14). Infants with iron deficiency showed more positive LSW amplitude in the right than left hemisphere (mean = 2.02 μV, SE = 0.49 vs. mean = 0.63 μV, SE = 0.43) in the parietal-occipital location in the stranger condition (F (1, 30) = 5.57, p < .05, ηp2 = 0.16).
Figure 2.
Mean LSW amplitude (±SE) in frontal-central and parietal-occipital locations by condition and iron group (* p < .05). There were two interactions involving iron group: condition × hemisphere × group (F (1, 119) = 5.17, p < .05, ηp2 = 0.04) and condition × front-back × hemisphere × group (F (1, 119) = 6.02, p < .05, ηp2 = 0.05). For infants with sufficiency, there was a significant condition × front-back interaction (F (1, 85) = 5.77, p < .05, ηp2 = 0.06). Follow-up analyses indicated LSW amplitude in the frontal-central location was more negative in the stranger vs. mother condition (F (1, 85) = 5.56, p < .05, ηp2 = 0.06), whereas LSW amplitude in the parietal-occipital location was more positive in the stranger vs. mother condition (F (1, 85) = 4.91, p < .05, ηp2 = 0.06). For infants with iron deficiency, the condition effect was not significant in any region of interest.
Secondary outcomes: P2 and P750
P2 amplitude was larger over the left vs. right hemisphere (F (1, 119) = 7.99, p < .01, ηp2 = 0.06) and also larger in the mother vs. stranger condition (F (1, 119) = 4.24, p < .05, ηp2 = 0.03). There were two interactions involving iron group: front-back × hemisphere × group (F (1, 119) = 3.97, p < .05, ηp2 = 0.04) and condition × front-back × hemisphere × group (F (1, 119) = 4.25, p < .05, ηp2 = 0.03). Further analyses indicated that the interaction between condition, hemisphere, and group was significant only in the parietal-occipital location (F (1, 119) = 5.46, p < .05, ηp2 = 0.04). The interaction between hemisphere and group was significant only in the stranger condition (F (1, 120) = 5.21, p < .05, ηp2 = 0.04). The P2 amplitude in the left parietal-occipital location was more negative for infants with iron deficiency compared with infants with iron sufficiency in the stranger condition (mean = −3.56 μV, SE = 0.38 vs. mean = −2.47 μV, SE = 0.24, F (1, 120) = 6.18, p < .05, ηp2 = 0.05).
P2 latency was longer in the frontal-central vs. parietal-occipital location (F (1, 119) = 33.72, p < .0001, ηp2 = 0.22) and also in the left vs. right hemisphere (F (1, 119) = 14.72, p < .001, ηp2 = 0.11). There was a 3-way front-back × hemisphere × group interaction (F (1, 119) = 6.93, p < .05, ηp2 = 0.06). The interaction between front-back and hemisphere was observed only in the group with iron sufficiency (F (1, 85) = 8.91, p < .01, ηp2 = 0.10). Further analyses indicated that infants with iron sufficiency showed longer P2 latency in the left than right hemisphere only in the frontal-central location (mean = 308.82 ms, SE = 3.50 vs. mean = 292.03 ms, SE = 4.21, F (1, 85) = 21.78, p < .01, ηp2 = 0.20).
There were two interactions for P750 amplitude: front-back × hemisphere (F (1, 119) = 15.11, p < .001, ηp2 = 0.11) and condition × front-back × hemisphere × group (F (1, 119) = 3.97, p < .05, ηp2 = 0.03). Further analyses indicated the significant interaction between condition, hemisphere, and group only in the parietal-occipital location (F (1, 119) = 5.92, p < .05, ηp2 = 0.05). The interaction between condition and hemisphere was observed only in the group with iron deficiency (F (1, 30) = 5.39, p < .05, ηp2 = 0.15). Infants with iron deficiency showed more negative P750 amplitude in the stranger condition than the mother condition only in the left parietal-occipital location (mean = −3.98, SE = 0.40 vs. mean = −2.99, SE = 0.39, F (1, 30) = 4.47, p < .05, ηp2 = 0.13). For iron deficiency defined by SF, there was a three-way interaction: condition × hemisphere × group in P750 amplitude (F (1, 154) = 4.21, p < .05, ηp2 = 0.03). Further analysis revealed the interaction between hemisphere and group in stranger condition (F (1, 154) = 7.38, p < .01, ηp2 = 0.05). Infants with iron sufficiency showed higher amplitude in the left hemisphere than the right hemisphere (F (1, 128) = 10.90, p < .01, ηp2 = 0.08).
Discussion
Previous studies show that newborns and even fetuses are able to recognize the voices of their parents.(24, 25) Such recognition memory in infancy can predict cognitive functioning and intelligence in childhood.(26, 27) Thus, this cognitive ability might be a good indicator of developmental deficits or delay in early life. The few available previous ERP and behavioral studies report associations between iron deficiency in infancy and recognition memory.(8–11) The functional importance of recognition memory and its relationship with iron deficiency motivated the current study. We found that fetal-neonatal iron deficiency adversely affected recognition memory as measured by LSW, an ERP component that is widely used as an indicator of novelty detecting and memory updating. Infants with iron sufficiency showed more negative LSW in the stranger than the mother condition in the frontal-central location. A condition difference in negative LSW in this region is considered to indicate detecting the novelty of stranger’s voice. Moreover, the stranger’s voice elicited more positive LSW amplitude than the mother’s voice in the parietal-occipital location. A condition difference in positive LSW in this location is thought to reflect updating the memories of stranger’s voice. Thus, infants with iron sufficiency showed the normal pattern expected at this age. In contrast, infants with iron deficiency did not show condition differences in LSW amplitude. These results are consistent with previous studies that suggested weaker or no electrophysiologic evidence of recognition memory in infants with iron deficiency.(8, 9, 11) However, as noted in the introduction, prenatal iron status was unknown for two of the previous studies, and infants in the other study were born of diabetic mothers and thus exposed to other metabolic abnormalities. These findings taken together suggest that both prenatal and postnatal iron deficiency might affect the maturation of hippocampus and thus delay or impair the development of recognition memory. The studies also raise the question of the role of the timing of iron deficiency in cognitive development.
Recognition memory is thought to involve two processes: recollection and familiarity.(28) Recollection refers to retrieving specific information associated with the prior occurrence of a stimulus, whereas familiarity is a fast process that allows judging if the stimulus was previously presented, without retrieving any contextual detail. Little or no condition difference in LSW amplitude has been interpreted as difficulty in recognizing the mother’s voice. Our finding that infants with iron deficiency did not show a condition difference in LSW amplitude might mean they were not able to link the voices of their mothers with their prior experience. It could also mean that these infants were not sufficiently familiar with their mothers’ voices to activate the fast recognition process. Another potential explanation for the absence of condition difference in LSW amplitude is that, compared with infants with iron sufficiency, infants with iron deficiency had trouble encoding the stranger’s voice and thus were unable to update their memories.(21) Additionally, less arousal or engagement, as often observed in infants with iron deficiency, (10, 29) might contribute to their lower level of mother-stranger discrimination. Less arousal or engagement might also lead to less movement, which might help explain our observation of more valid segments in the group with iron deficiency, because movement artifact often makes segments invalid.
The hippocampus is an important neural structure for recognition memory. The hippocampus rapidly develops during late fetal and early neonatal periods and is one of the major brain structures impaired by early iron deficiency. In rodent models, early iron deficiency alters neurometabolism, dentritogenesis and gene expression in the hippocampus and decreases its growth factor expression, long-term potentiation (LTP), and energy availability.(30) These alterations were accompanied by impaired hippocampus-based learning and memory in preclinical models.(30, 31) Our LSW results show the pattern that would be expected if hippocampal impairment induced by fetal-neonatal iron deficiency adversely affected the recollection and/or familiarity processes of recognition memory in human infants. In addition to LSW, we found group differences in earlier ERP components, specifically, P2 and P750. P2 amplitude in the left parietal-occipital location was larger (more negative) for infants with deficiency than those with iron sufficiency in the stranger condition. P750 amplitude at the left parietal-occipital electrodes was more negative for the stranger than the mother condition only for infants with iron deficiency. Only one previous study reported an effect of iron deficiency on P2 latency; it involved infants of mothers with diabetes.(8) Previous studies did not address the effect of prenatal-neonatal iron deficiency on P750 in young infants. Therefore, our results warrant replication and confirmation before speculating on their interpretation.
This study reported several results related to hemisphere differences. Although it is hard to interpret these results due to the low spatial resolution of ERP methodology, MRI studies have revealed that human infants show hemisphere differences in processing speech in the first months of life.(32, 33) For example, 3-month-old infants showed left-lateralized brain activation when processing normal and reversed speech. Additionally, 1- to 4-month-old infants had hemisphere asymmetries in white matter tracts related to speech perception-production network.(34) Therefore, the hemisphere differences we observed might reflect the development of functional lateralization of the networks related to speech processing.
It should also be noted that we considered either ZPP/H or SF to define fetal-neonatal iron deficiency but found effects of iron deficiency on auditory recognition memory only for ZPP/H. High cord ZPP/H as the indicator of poor iron status at birth was associated with functional effects (poorer recognition memory), whereas low cord ferritin was not. ZPP/H captures aspects of iron’s role in hemoglobin synthesis, while ferritin reflects iron storage. Our findings thus support other research suggesting that that ZPP/H is a more sensitive measure for assessing functional fetal-neonatal iron status than SF.(13–15)
One important limitation of our study is that, like other observational studies, it cannot support causal inferences about relations between fetal-neonatal iron deficiency and recognition memory. Additionally, limited sample size did not allow us to analyze iron deficiency and Pb effects separately. Instead, we covaried Pb levels, which were very low. However, future research should consider how iron deficiency and Pb exposure in early life affect cognitive development separately and interactively.
In sum, our study showed that fetal-neonatal iron deficiency, as defined by high ZPP/H, was associated with poorer electrophysiological evidence of auditory recognition memory in 2-month-old infants. Because the hippocampus is important for recognition memory, the results are consistent with the findings in animal models that fetal iron deficiency in late gestation adversely affects the developing hippocampus.
Acknowledgments
Supported the US National Institutes of Health (NIH; P01 HD039386 [PI: B.L.]) and the National Natural Science Foundation of China (NNSFC) (81273085 [PI: J.S.]). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or NNSFC.
We thank Dr Yaping Shi and her colleagues for their assistance with participant enrollment and cord blood collection. We also thank Liqin Chen for the iron status measures, Zheng Shen for Pb analysis, Mingyan Li, Chai Ji, Zhiwei Zhu, and all team members for their assistance with data collection. Project staff received training in the auditory recognition memory paradigm in the laboratory of Dr Raye-Ann deRegnier (Northwestern University) and in ERP testing in infants more generally in the laboratory of Dr Charles A. Nelson (Harvard University).
ABBREVIATIONS
- ZPP/H
zinc protoporphyrin/heme ratio
- ERP
event-related potentials
- IDA
iron-deficiency anemia
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalenius K, Brindley P, Smith B, Reinold C, Grummer-Strawn L. Pregnancy nutrition surveillance 2010 Report. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 3.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93:1312–20. doi: 10.3945/ajcn.110.007195. [DOI] [PubMed] [Google Scholar]
- 4.Cao C, Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013;71:35–51. doi: 10.1111/j.1753-4887.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- 5.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozoff B, Georgieff MK. Iron Deficiency and Brain Development. Semin Pediatr Neurol. 2006;13:158–65. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.deRegnier R-A, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137:777–84. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 8.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier R-A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 9.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, et al. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e45. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126:e427–e34. doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160:1027–33. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng F, Mai X, Zhan J, Xu L, Shao J, Meeker J, et al. Low-level prenatal lead exposure alters auditory recognition memory in 2-month-old infants: an event-related potentials (ERPs) study. Dev Neuropsychol. 2014;39:516–28. doi: 10.1080/87565641.2014.959172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labbé RF, Dewanji A. Iron assessment tests: transferrin receptor vis-à-vis zinc protoporphyrin. Clin Biochem. 2004;37:165–74. doi: 10.1016/j.clinbiochem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Yu KH. Effectiveness of zinc protoporphyrin/heme ratio for screening iron deficiency in preschool-aged children. Nutr Res Pract. 2011;5:40–5. doi: 10.4162/nrp.2011.5.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magge H, Sprinz P, Adams WG, Drainoni M, Meyers A. Zinc protoporphyrin and iron deficiency screening: trends and therapeutic response in an urban pediatric center. JAMA Pediatr. 2013;167:361–7. doi: 10.1001/jamapediatrics.2013.751. [DOI] [PubMed] [Google Scholar]
- 16.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and c-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–32. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 17.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156:377–81. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, et al. Maternal serum ferritin concentration Is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142(11):2004–9. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 20.McLimore HM, Phillips AK, Blohowiak S, Pham DQD, Coe CL, Fischer BA, et al. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediat Hematol Onc. 2013;35:473–7. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai X, Xu L, Li M, Shao J, Zhao Z, deRegnier R-A, et al. Auditory recognition memory in 2-month-old infants as assessed by event-related potentials. Dev Neuropsychol. 2012;37:400–14. doi: 10.1080/87565641.2011.650807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright RO, Tsaih S-W, Schwartz J, Wright RJ, Hu H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J Pediatr. 2003;142:9–14. doi: 10.1067/mpd.2003.mpd0344. [DOI] [PubMed] [Google Scholar]
- 23.Bradman A, Eskenazi B, Sutton P, Athanasoulis M, Goldman LR. Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ Health Persp. 2001;109:1079–84. doi: 10.1289/ehp.011091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehler J, Bertoncini J, BarriŠre M, Jassik-Gerschenfeld D. Infant recognition of mother’s voice. Perception. 1978;7:491–7. doi: 10.1068/p070491. [DOI] [PubMed] [Google Scholar]
- 25.Kisilevsky BS, Hains SMJ, Lee K, Xie X, Huang H, Ye HH, et al. Effects of experience on fetal voice recognition. Psychol Sci. 2003;14:220–4. doi: 10.1111/1467-9280.02435. [DOI] [PubMed] [Google Scholar]
- 26.Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev Psychol. 1995;31:685–96. [Google Scholar]
- 27.McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 1993;64:57–79. [PubMed] [Google Scholar]
- 28.Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114:152–76. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. e3. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69:S43–S8. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubach GR, Coe CL. Selective impairment of cognitive performance in the young monkey following recovery from iron deficiency. J Dev Behav Pediatr. 2008;29:11–7. doi: 10.1097/DBP.0b013e31815f24a9. [DOI] [PubMed] [Google Scholar]
- 32.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional Neuroimaging of Speech Perception in Infants. Science. 2002;298:2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 33.Peña M, Maki A, Kovačić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci. 2003;100:11702–5. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19:414–23. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]