Abstract
Background
The hornworm Manduca sexta exhibits a defensive strike to noxious assaults, a response that is robust and is easily observed by experimenters. Von Frey filaments and methods typical for studying nociception in other animals were used to assess the strike response in M. sexta.
New Methods
A series of von Frey filaments was applied to the body wall in ascending order and the data generated were used to determine the strike threshold by (i) the up-and-down method, (ii) the first response method, and (iii) the simplified up-and-down order method (SUDO). The effect of a noxious pinch on strike threshold was assessed.
Comparison with existing methods
To our knowledge none of these methods has been used on M. sexta previously, making the use of the up-and-down and SUDO methods the first in an invertebrate. The use of the first response method has been used in other invertebrates, and the method appears equally suited to M. sexta.
Results
All three methods were successful in monitoring the threshold sensitivity to touch, which was lowered (sensitized) by tissue damage induced with a pinch. Sensitization lasted 19 h.
Conclusions
All three methods of assessing nociception were successfully applied to quantify the defensive strike response in M. sexta, although the SUDO method required empirical assessment of which filament to start the test sequence with. The results revealed both short- and long-term sensitization. These methods should prove to be useful for quantifying sensitization in M. sexta.
Keywords: defensive strike response, larva, von Frey filaments, SUDO method, up-and-down method, insect
1. INTRODUCTION
Nociception, which includes the perception and processing of noxious stimuli, is a response to potential or actual tissue damage. The nervous system may then be sensitized in response to noxious stimuli, as a form of non-associative learning that may last days to months (e.g. Castellucci et al., 1986; Woolf and Walters, 1991). This presumably serves to heighten the ability of individuals to respond to and protect themselves from potentially harmful events and protect injured areas during healing. In humans, such noxious stimuli manifest in the subjective experience of pain (reviewed by Walters, 1994; Rahn et al., 2013), and the result of abnormally persistent nociceptive sensitization includes conditions such as allodynia (such as that experienced on the surface of a scar long after an operation or wound), where normally innocuous stimuli are interpreted as noxious or painful), or hyperalgesia, where painful stimuli are perceived as more painful than normal. These neuropathic conditions can occur following a single insult or after repeated injury, chronic inflammation and in some genetic conditions (Binder et al., 2011).
In humans, the severity of pain a person is experiencing is typically ascertained through verbal or other subjective ratings of discomfort, although quantitative sensory testing (QST) may become the standard clinically and in clinical research (reviewed by Backonja et al., 2009; Cruz-Almeida and Fillingim, 2014). In this case, von Frey filaments, which provide a calibrated force to a portion of the subject’s body, are used to test the mechanical sensitivity. In fact, the use of these filaments has been exploited for the precise measurement of mechanosensitivity in most vertebrates in pain research. In models such as rats, defensive behaviors are monitored by a number of different assays. For instance, the tail flick, hind paw withdrawal and jump-flinch are all responses to noxious stimuli that may be sensitized after formalin injection, high-intensity light beams, hot plates or after spinal nerve ligation surgery (e.g. Juszkiewicz-Donsbach and Levy, 1962; Bonnet and Peterson, 1975; Pitcher et al., 1999; Kim et al., 2014; reviewed by Bannon, 2001; Bannon and Malmberg, 2007; Gregory et al., 2013). These responses are quantified by assessing either the latency to respond to the noxious stimulus or the intensity of the stimulus causing a response (the threshold response).
For years, most threshold response measurements using von Frey filaments recorded the “first response” to stimulation, with an ascending series of increasingly stiff filaments (e.g. Illich and Walters, 1997; Alupay et al., 2014). The “up-and-down” method, that was originally designed to estimate the stability of explosives, was first used in animal models to estimate lethal doses of drugs (Dixon and Mood, 1948; Dixon, 1965) and has since become the standard for assessing vertebrate nociception (Crocker and Russell, 1984; Chaplan et al., 1994). This method starts with a mid-range filament, and increases or decreases linearly, depending on the response of the animal. A defensive response results in a lower filament force being used next, while lack of a response dictates that a higher strength filament be used for the next test. This is repeated a number of times, and a table of probability thresholds was developed in rodents, depending on the sequence of responses and a maximum likelihood model (Dixon and Mood, 1948; Dixon, 1965; Chaplan et al., 1994). More recently, a simplified up-down order (“SUDO”) method was developed, where only five stimuli are required to determine nociceptive threshold, without the need for a look-up table. In this method, each animal receives the same number of touches from von Frey filaments. If the last touch by the filament yields a response, a set value is added to the filament force, whereas if no response is noted, a set value is subtracted from that filament force (Bonin et al., 2014; see also methods section 2.5). This method, however, requires empirical determination of a suitable starting force.
A number of invertebrate nociceptive models exist, the most well studied being Aplysia californica (reviewed by Walters and Moroz, 2009). The breadth of invertebrate studies has provided evidence of the relationship of invertebrate sensitization mechanisms to mammalian pain and memory mechanisms (e.g. Walters and Moroz, 2009; Crook and Walters, 2011, Crook et al., 2013). In many of these studies, the “first response” method of assessment was used to show nociceptive sensitization of behavior (e.g. Lewin, 2003; Alupay et al., 2014) and of mechanosensory neurons (Illich and Walters, 1997). Short and long-term sensitization of the siphon gill and tail withdrawal in A. californica paired with electrophysiology led to a fundamental understanding of the enhancement of synaptic transmission between sensory and motor neurons, which were important building blocks in the evolution of learning and memory (e.g. Carew et al., 1983; Walters, 1987; Schacher et al., 1988; Lewin and Walters, 1999). In Caenorhabditis elegans, thermal avoidance behavior was characterized through video monitoring of whole worm movements (e.g. Wittenburg and Baumeister, 1999). In insects, Drosophila was monitored visually for thermal avoidance behaviors after a variety of genetic manipulations (Tracey et al., 2003; Neely et al., 2010; Babcock et al., 2011; reviewed by Kim et al., 2011).
The hornworm, Manduca sexta, is another possible model organism for such research. It has conspicuous and reproducible defensive behaviors that include proleg withdrawal (Wiel and Weeks, 1996) and defensive strike and thrashing responses (Walters et al., 2001) to predators such as birds. The change in the number of defensive strikes for a set stimulus was the original method used for assessing nociceptive sensitization (Walters et al., 2001). In this case, the behavioral change associated with sensitization was recorded for less than an hour, whereas sensitization has been noted for much longer periods in many other invertebrates (e.g. Gasull et al., 2005). Moreover, this assay depended on an all-or-none response, and subtle changes in nociceptive sensitivity were not noted. Finally, a sequence of 16 applications of the von Frey filaments was required, leaving the possibility of habituation as a confounding factor.
Developing an invertebrate model using the first response, up-and-down and SUDO methods should prove important in understanding the conservation of pain signaling in animals, but may also alleviate ethical issues associated with testing vertebrates. The conserved nature of pain receptors such as the TRP receptors (e.g. Kim et al., 2012; Saito et al., 2012; Sardar et al., 2012; Palovcak et al., 2015), and associated signal transduction machinery (e.g. Lin et al., 1997; Lewin and Walters, 1999; Nikitin and Kozyrev, 2000) suggest that such a model will be invaluable in this field of research. Using an organism such as M. sexta is cheap and currently unregulated, allowing rapid and large scale testing of signaling mechanisms and pharmaceuticals. The goal of our research was to adapt and test the first response, up-and-down and SUDO methods to quantify the defensive strike response in M. sexta. To our knowledge, the use of the up-and-down and SUDO methods has not yet been incorporated into the quantification of any invertebrate defensive responses. M. sexta may thus provide a valuable tool for studying the signal transduction mechanisms inducing sensitivity after injuries leading to hyperalgesia or allodynia as well as the role of long-term memory in providing nocifensive behaviors in animals.
2. MATERIALS AND METHODS
2.1. Animals
Manduca sexta L. were raised under a 17:7h photoperiod with a thermoperiod of 27°C:25°C as described previously (Fuse and Truman, 2002). Free feeding was allowed on an artificial diet (MP Biomedical Irvine CA USA: based on Bell and Joachim, 1976). Animals were individually housed in small plastic cups with lids. Pharate fifth instar larvae were staged using external morphological markers (Truman et al., 1980; Copenhaver and Truman, 1982). Each animal was observed and timed for ecdysis and allowed to feed ad libitum and rest in the incubator for 24 h. Larvae were tested and provided stimuli 24 h after ecdysis to the fifth larval stage (L5) and then retested 1 and 19 h after the presentation of the noxious stimulus.
2.2. Strike response and its measurement by von Frey filaments
The behavior being monitored was called a “defensive strike response” whereby an animal rapidly bent, bringing its head and mouth close to the site of insult as a defensive response (Walters et al., 2001; van Griethuijsen et al., 2013). Although other responses have been observed in M. sexta, such as thrashing (Walters et al., 2001) or proleg withdrawal (Wiel and Weeks, 1996), the strike response is optimum for behavioral testing due to its robust and objectively distinct nature. The defensive strike response by M. sexta was videotaped using a digital camcorder (Cannon ZR-40 and Sony DCR-TRV250) and was also assessed subjectively in real time for the duration of the experiment (Merchasin, 2009). All video images were imported onto a Macintosh G4 PowerBook or a MacBook Pro using the application iMovie. The number of strikes elicited was assessed from the video footage in a blind manner. Subjective scoring (no video) and blind scoring (video) were assessed statistically to determine if a difference in scoring technique (ability to recognize a defensive strike) existed. Since no significant differences were noted (p>0.5; data not shown) all studies were conducted with subjective assessment of the defensive strike response.
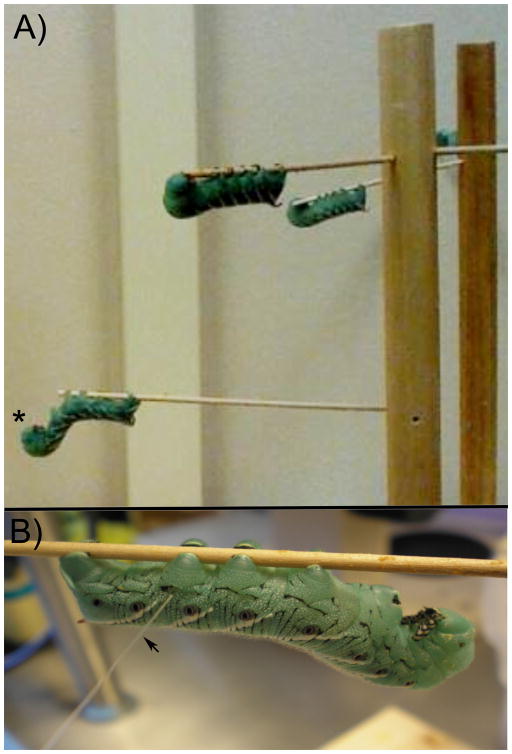
Animals were removed from their housing and allowed to acclimate to the wooden tree apparatus and settle for at least 15 min. Animals settled on a “branch” of the tree apparatus and assumed the sphinx position (Figure 1a). Animals that did not settle were excluded from the study. In this resting position the abdominal prolegs were clearly visible and accessible for testing. Abdominal proleg 6 was typically chosen for testing because the more anterior prolegs were often involved in the defensive responses, while proleg 6 stayed firmly gripped to the horizontal wooden rod that the caterpillar attached to. However, other prolegs could be tested to yield similar results. The proleg contralateral to the site of noxious stimulation was assessed for nociceptive sensitivity unless otherwise noted (Fig. 1b).
Figure 1. Manduca sexta resting tree apparatus.
A) A tree apparatus for testing multiple larvae is shown. The center dowel has numerous holes for placement of thin wooden rods. These rods provide a base for M. sexta to rest, with a grip surface for the prolegs. When the larvae assume the upside down “sphinx” position (*), they are ready to be tested. B) Manduca being tested at proleg 6 with a von Frey monofilament (arrow). If the pinch occurs on the same proleg, the site of testing is ipsilateral to noxious stimulation. If the pinch occurs on the opposite proleg, the site of testing is contralateral to noxious stimulation.
Following a baseline test of sensitivity, each animal of the experimental group was provided a noxious pinch as described in section 2.3. Each animal was tested again anywhere from 15 min to 1 h after the noxious stimulus, to observe any changes in sensitivity. After testing at the acute time point the animals were weighed and then returned to their home containers in temperature and light-regulated incubators (see section 2.1). They were again allowed to feed ad libitum. Before testing a second time 19 h after the noxious stimulus, larvae were again placed on the wooden tree apparatus and allowed to settle for 15 min. The animals were tested to assess the long-term change in sensitivity following the noxious insult. If larvae did not settle on the apparatus this second time, they were still excluded from the data.
2.3. Application of a noxious pinch
Blunt forceps were used to pinch an abdominal proleg for 2 sec. The proleg contralateral to the site of nociceptive monitoring was used for pinching. This was typically proleg 6, since larvae rarely lost their grip with this proleg. Cuticle was not ruptured by the pinch, as assessed by a lack of hemolymph leakage. During the pinch the animal could be seen rapidly bending to strike precisely at the stimulus (see also Figure 2 of Walters et al., 2001). On some occasions opposite striking and back and forth thrashing were also noted. For controls, the proleg was touched gently by the forceps for 2 sec rather than pinched.
2.4. Threshold estimation by “up-and-down” Method
The animal was first tested for sensitivity to mechanical stimulation using a series of von Frey filaments (Stoelting, Wood Dale, Illinois, USA), which are soft nylon filaments calibrated to known forces, providing discrete units of pressure (Table 1). They are used to measure the threshold of sensitivity at which an animal finds a stimulus noxious and withdraws its body part (Chaplan et al., 1994). The smaller filament sizes are not noxious to M. sexta but at larger filament diameters the stiffness (bending force) of the filament increases and becomes noxious to the insect (personal observation).
Table 1.
Properties of von Frey filaments used to test withdrawal reflex. Forces based on www.stoeltingco.com.
| Filament # | Fiber label | Bending force (mN) |
|---|---|---|
| 1 | 1.65 | 0.078 |
| 2 | 2.36 | 0.196 |
| 3 | 2.44 | 0.392 |
| 4 | 2.83 | 0.686 |
| 5 | 3.22 | 1.569 |
| 6 | 3.61 | 3.922 |
| 7* | 3.84 | 5.882 |
| 8 | 4.08 | 9.804 |
| 9* | 4.17 | 13.725 |
| 10 | 4.31 | 19.608 |
| 11 | 4.56 | 39.216 |
| 12* | 4.74 | 58.824 |
| 13* | 4.93 | 78.431 |
| 14 | 5.07 | 98.039 |
indicates filaments omitted with the up-and-down method
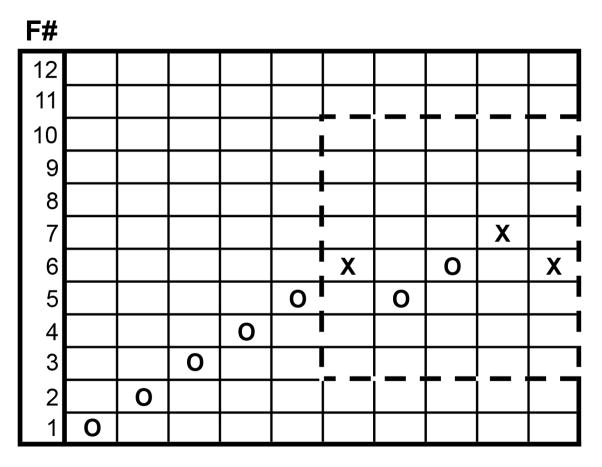
Each filament was applied by approaching the body wall very slowly, placing the filament in the center of the proleg and then pressing at a 90° angle until it bent. Thus a known amount of force was applied for each filament. Further bending would not apply additional force. Subtle changes in positioning or velocity of positioning of the filament did not appear to alter responses significantly in M. sexta (Walters et al., 2001). Each filament was applied for 2 sec and then withdrawn. The response was recorded as an “X” for a defensive strike or an “O” for no strike. For each test fiber, if the response was a strike, the force of the fiber was lowered by one increment for the next test, whereas if there was no strike, the force was increased by one increment. This process was repeated until the first transition from striking to not striking - or vice versa - was reached, after which force was applied to the animal an additional four times following the up and down paradigm (see Fig. 2).
Figure 2. Test sequence template.
A test sequence shows responses starting at filament #1 (F#1). When there is no response “O” is inserted and the next higher filament is used. When there is a response “X” is inserted and the next lower filament is used. After the first positive response (X) 5 more responses are recorded. The dashed box indicates the data used to test the SUDO method starting at filament #6 (range 3–10).
This up and down pattern is what gives this assay its name. It allows the experimenter to narrow in on the exact nociceptive sensitivity of each animal as defined by the force required to elicit a behavioral response. The pattern elicited by touches determined the final sensitivity of the animal based on the patterns previously described (Dixon and Mood, 1948; Dixon, 1965; Chaplan et al., 1994). To modify this method for M. sexta we used 10 von Frey filaments that applied 0.078 mN, 0.196 mN, 0.392 mN, 0.686 mN, 1.569 mN, 3.922 mN, 9.804 mN, 19.608 mN, 39.216 mN, and 98.039 mN (identified as filaments #1, 2, 3, 4, 5, 6, 8, 10, 11 and 14; see Table 1). These filaments were chosen such that the base ten logarithm of the force values are nearly equally spaced with an average increase of 0.344 in log units. In other words, the millinewton forces increase geometrically with a common ratio roughly equal to 2.208 (100.344). This was done to satisfy the equal spacing condition set forth by Dixon and Mood (1948) and Dixon (1965) and is the value of constant “d” following notation in his and other publications. The initial force to begin the sequences was chosen to be 0.392 mN (filament #3). This force was chosen as it was near to the predicted force that has a 50% probability of eliciting a strike, another condition imposed in the original up-down method (Dixon and Mood, 1948; Dixon, 1965). This value was based on empirical observation in our lab. The log of the force of the last filament tested was also recorded as “Xf”. The entire “XO” sequence was then used to find the value “k” in the lookup table by (Dixon and Mood, 1948; Dixon, 1965). However if the sequence exceeded nine tests, the maximum allowable by the table, the responses at the beginning of the sequence were omitted until the length of the sequence was nine tests long. It has been stated that these initial responses are of minimal consequence as they are at intensities far from the threshold of interest (Dixon and Mood, 1948; Dixon, 1965; Chaplan et al., 1994). The recorded values were then entered into the equation F50 = 10Xf + kd, where F50 was the threshold force for 50% chance to strike. If the animal did not respond to the highest filament (98mN; #14) before the sequence could be completed then the animal was assigned an F50 value of 98mN, a conservative estimate. Likewise, if the animal did respond to the lowest filament (0.078mN; #1) an F50 value of 0.078mN was given.
2.5. Threshold estimation by simplified up-and-down order (SUDO) Method
Following Bonin et al. (2014), filament numbers were assigned to the lower 14 fibers of a 20 von Frey filaments set that ranged from 0.078mN to 2940mN, where filament #1 was 0.078 mN and filament #14 was 98.039 mN (Table 1). The highest 6 fibers were not used. The simplified up-and-down (SUDO) method requires an empirically determined filament force range and starts at the mid-range filament for five consecutive touches (Bonin et al., 2014). If a strike is observed on the first touch then the next lowest filament is chosen as described for the up-and-down method above (Section 2.4 and also see boxed region of Fig. 2). If no strike is noted then the next higher filament is used. This continues for a total of five rounds. If the final filament causes a strike, the final filament value is that filament number minus 0.5 stimulus intervals. If no strike occurs the final filament number has 0.5 stimulus intervals added to it and is designated as SUDO result. Then given the logarithmic nature of the filament number relative to the force applied (Stoelting operating manual; www.stoeltingco.com) the force can be determined from the newly designated SUDO result (see equation 1). This equation is based on two correlation equations. Filament number was converted to manufacturer filament label fit using a binomial regression (Eqn. 1a), and manufacturer label was converted to force by a logarithmic regression (Eqn. 1b).
| Equation 1 |
| Equation 1a |
| Equation 1b |
In the current study we determined a mid-range force for 24 h 5th stage (L5) larvae empirically by using the data obtained from the up-and-down method, starting at filaments #3 4 5 6 or 7. An example of the data used when starting at filament #6 (3.61 mN; Table 1) is seen in Fig. 2. In this case the final filament size was determined to be filament #5.5 yielding a force of 2.41 mN.
2.6. Threshold estimation by “first response” method
To evaluate the threshold response by the first response method (“1st X”), we analyzed the data used to calculate SUDO values (see Section 2.5) and identified the force required to elicit the first strike before and after noxious stimulation. Briefly, beginning with the lowest force von Frey filament, animals were tested for a response. If the animal did not respond then the force was increased by using the next fiber. The force at which animals first responded was recorded as the threshold force. Thus not all SUDO data was used, if no strike was ever elicited at the highest filament.
2.7. Statistical Analysis
Statistical analyses were conducted with Sigmastat or GraphPad Prism 6 and are described in the figure legends for each experiment. Data are presented as averages with error bars representing the standard errors of the mean (SEM) unless stated otherwise.
3. RESULTS
3.1. Up-and-down method reveals short-terms sensitization after a pinch
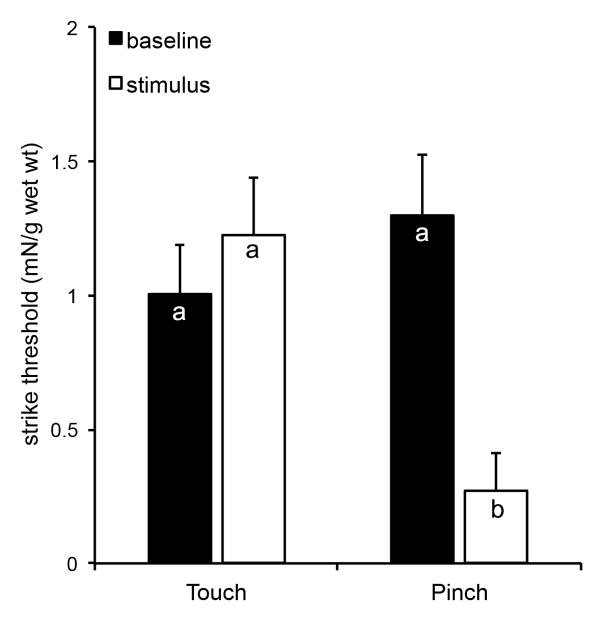
The up-and-down method revealed that on average, larvae initially responded to touch with approximately 1 mN of force (Fig. 3) and became sensitized to a pinch within 1 h, responding to a force of approximately 0.25 mN. This was not the case after a light touch, suggesting that the noxious stimulus elicited the change in threshold sensitivity. That is, all baseline and touch data were not significantly different from one another (P>0.1) while the pinched group was significantly lower than all other groups, one hour later (P<0.005). Use of the up-and-down method resulted in as few as 3 touches with von Frey filaments (typically occurring after sensitization) and as many as 12 touches (typically occurring prior to sensitization). Therefore, since baseline responses generally required more touches, there could have been some level of habituation. However, this would have yielded values closer to the sensitized values, and since there were significant differences, the up-and-down method appeared robust enough to withstand potential habituation effects.
Figure 3. The up-and-down method to assess the defensive strike response.
The up-and-down method was used to assess the sensitivity to touch after a noxious pinch to contralateral proleg A6. The test was conducted at baseline (black bars) and 1 h after the pinch and compared to animals only receiving a light touch, as controls (white bars). Bars are average forces required to elicit a strike per g wet weight of each animal (mN/g wet wt). The error bars represent the standard errors of the mean. The sample size is 16 for the touch group and 18 for the pinch group. Letters indicate similarities between groups (p>0.1), based on Two-Way Repeated Measures ANOVA with the Holm-Sidak Pairwise Multiple Comparison Method. The pinch results in a significantly lower strike threshold (p<0.005).
3.2. Comparison of first response and SUDO methods to estimate strike threshold before and after noxious pinch
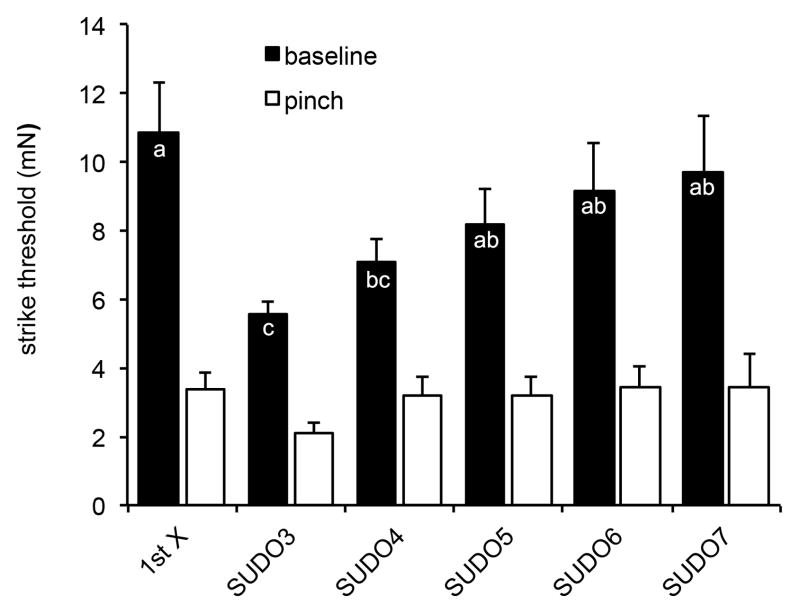
Bonin et al. (2014) have suggested that 5 touches with increasing and/or decreasing filaments is sufficient to replicate data from the up-and-down method. We looked at similar effects on the sensitivity before and after a pinch in M. sexta (Fig. 4). We compared baseline data using the first response to touch (“1st X”) with SUDO methods starting at filaments #3, 4, 5, 6 or 7 (see Table 1 for force values). There were no significant differences in baseline levels from the 1st X method, or SUDO methods 5–7 (P>0.1). In contrast, baseline levels of SUDO3 and 4 were significantly different from the other baseline values (P<0.05). Sensitization was noted 15 min after a pinch using all methods, since all white bars were significantly lower than their counterpart baseline bars (P<0.05). These results suggest that as with the up-and-down method, the 1st X method can be utilized with M. sexta larvae, as can the SUDO method, when the starting filament is determined empirically, as it has been here.
Figure 4. Comparing First response and SUDO methods.
Various methods were assessed from a common data set to determine strike thresholds prior to a pinch (baseline), and 15 min after a pinch to contralateral proleg A6 (pinch). Methods included the first response to a filament (1st X) and SUDO methods starting at filaments #3 through #7 (SUDO3 to SUDO7). Bars are average force required to elicit a strike (mN). The error bars represent the standard errors of the mean. The sample sizes range from 27 to 43. Letters indicate similarities within the baseline groups (p>0.05), based on Two-Way ANOVA with the Holm-Sidak Pairwise Multiple Comparison Method. Pinched groups were not significantly different from one another (p>0.1), but were all significantly lower than their baseline counterparts (p<0.05).
3.3. A noxious pinch induces long-term sensitization
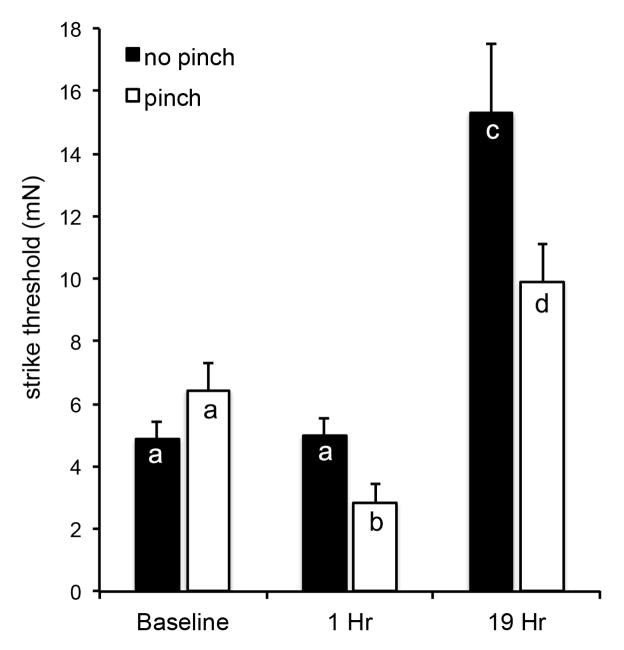
Larvae were assessed for sensitization before and at 1 h and 19 h after receiving a pinch (Fig. 5). When control larvae were tested over a 19 h period, their baseline sensitivity decreased significantly as they aged and grew (P<0.0001). That is, the overall sensitivity of older animals (19 h after initiation of the testing, or 43 h after ecdysis to the 5th larval stage) was reduced compared to young 5th stage larvae, requiring a significantly higher force to elicit a strike. Nevertheless, after a pinch, the pinched larvae became sensitized within 1 h, and remained sensitized 19 h later compared to their control counterparts (P<0.001).
Figure 5. Average threshold of nociceptive sensitivity in Manduca sexta over 19 h.
Animals were assessed using the SUDO method before (no pinch) and 1 and 19 h after a pinch (pinch) to the abdominal proleg 6. Bars are average force required to elicit a strike (mN). The error bars represent the standard errors of the mean. The sample size is 10 per group. Letters indicate similarities within groups (p>0.1), based on Two-Way Repeated Measures ANOVA with the Holm-Sidak Pairwise Multiple Comparison Method.
4. DISCUSSION
Although M. sexta show a number of nocifensive responses to noxious stimuli (Wiel and Weeks 1996; Walters et al., 2001) the strike response was chosen for this assay because of its reliable and robust nature. This behavior is observed as a midline bending of the animal to bring the head towards the noxious stimulus. The strike response occurs as a defense against noxious stimuli such as might occur during predation by birds or parasitic wasps (Walters et al., 2001; van Griethuijsen et al., 2013). To measure this response, researchers originally applied a single von Frey filament (1.5 mN, exerting a pressure of 15 gmm−2 or approximately 1.5×105 Pa) to a sensitized animal, to measure changes in the frequency of defensive strikes, among other behaviors, after noxious stimulation. This resulted in an increased number of strikes and the response was short-lived, only being detectable for about 30 min after the stimulus. This change in behavior is reminiscent of hyperalgesia where pain increases without increasing the force of a touch.
Our results demonstrate that changes in the threshold to elicit a strike behavior can also be measured, using three classical methods, namely the up-and-down method (Crocker and Russell, 1984; Chaplan et al., 1994), the simplified up-down order method (SUDO; Bonin et al., 2014), and the first response method (1st X; Illich and Walters, 1997). All three methods yielded similar results in M. sexta, where we showed that the strike threshold was reduced after application of a noxious pinch. And while sensitivity was reduced in older larvae, perhaps in part due to a thicker cuticle and thicker girth as the insect aged, a single noxious pinch maintained a reduced strike threshold for at least 19 h. The reduced sensitivity of older larvae is not entirely surprising given that 48 h after ecdysis the body weights are nearly doubled compared to fresh L5 larvae (M. Fuse; personal observation), and larvae have been shown to increase their body mass 10,000 fold in only 2 weeks (Lin et al., 2011). Nevertheless, their nociceptors still appear to become sensitized after a noxious insult.
To our knowledge this is the first use of the up-and-down and SUDO methods in an invertebrate. Each method has some strengths and weaknesses. The use of the up-and-down and first response (1st X) methods require variable numbers of touches (from 1 to 15), which could induce some level of habituation, although they capture all larval responses within the broad range of filament forces. In contrast, SUDO requires only 5 touches for each animal but requires an empirically determined starting point, eliminating habituation as an issue, but also producing conservative estimates at the low and high end forces, if the correct starting filament is not used. For instance, with the SUDO 3 method, the highest the 5th test will measure is the force induced by filament #7, and this occurs in 50% of the animals, whereas only 15% score at filament # 7 for SUDO 6 (where the test sequence begins at filament #6 and can go up or down from there). This suggests that SUDO 3 will produce a conservatively low baseline value, where strikes that might normally occur at filaments 8 and up are ignored. Thus both SUDO 6 and SUDO 7 appear to be appropriate for this stage larva, which emphasizes the need to empirically determine a SUDO starting value for different staged larvae. This is strengthened by the fact that the baseline data for SUDO 6 and SUDO 7 are not significantly different from baseline data with the 1st X method. This also suggests that habituation is not an issue with the 1st X method, where the number of tests is often greater than 5.
Using either the up-down or SUDO method, we have shown that mechanical trauma (a pinch) results in sensitization. That is, after tissue damage the threshold sensitivity to a simple touch is decreased much as is noted in allodynia, where otherwise non-noxious stimuli produce painful responses. It will be interesting to determine if these nociceptors can also detect other stimuli such as cold or capsaicin. Polymodal nociceptors are common in vertebrates and invertebrates (e.g. Reviewed by Kumazawa, 1996; Kahn-Kirby and Bargmann, 2006).
The use of these three methods also appears to be substantially more effective at detecting subtle changes in behavioral sensitivity than the previous 16-poke sequence method of Walters et al. (2001). Previously sustained behavioral changes were demonstrated for only 1 h after a noxious stimulus induced by a forceps pinch. With the current methods, a change in the nociceptive threshold after a noxious stimulus was noted for at least 19 h. We suggest that this is long-term sensitization, and can verify it in the future, using protein synthesis inhibitors. Sensitization for 19 h in M. sexta is a significant portion of the larva’s life and provides a large window for assessing the cellular correlates of this neuronal plasticity. Perhaps even more significant is the fact that long-term sensitization occurred with only a single pinch to the body wall. Thus, while this is a relatively short time for long-term sensitization, which has been shown to last days to months (Lewin and Walters, 1999; Gasull et al., 2005) or even a lifetime (Cadet et al., 1995; Walters, 2012), most experimental paradigms required multiple stimuli to transition from short-term to long-term memory (Pinsker et al., 1973; Frost et al., 1985; Castellucci et al., 1989). We have not yet measured responses to a series of noxious stimuli, although the duration of our pinch may result in repetitive firing. Alternatively, the response we note may be an intermediate stage effect, since long-term facilitation is suppressed by both protein and RNA synthesis inhibitors (Ghirardi et al., 1995; Nikitin and Kozyrev, 1993 - as cited in Nikitin and Kozyrev, 2005), while the intermediate stage relies only on translation (Ghirardi et al., 1995). It will be interesting to look at the role of RNA synthesis inhibitors in M. sexta, in this regard.
Being able to assess nociceptive sensitization in another terrestrial insect (e.g. Babcock and Galko, 2009) is a nice bridge to the work conducted in marine invertebrates (e.g. Pinsker et al., 1973; Nikitin and Kozyrev, 2004; Crook et al., 2011) and terrestrial mammals (reviewed by Woolf and Walters, 1991). It is imperative to assess whether similar mechanisms are at work in nociceptive sensitization of invertebrates and vertebrates to understand which mechanisms have been conserved across the more than 500 million years since the ancestors of insects and humans diverged. Moreover, if invertebrates are to become models of human chronic disorders such as allodynia and hyperalgesia, having a quantifiable method of assessing this, similar to the QST methods in humans (reviewed by Backonja et al., 2009; Cruz-Almeida and Fillingim, 2014) is significant. For instance, it will be interesting to determine whether M. sexta larvae respond to classic analgesics using the three methods described here.
HIGHLIGHTS.
Von Frey filaments were used to quantify the defensive strike response in M. sexta.
The first response, up-and-down and SUDO methods were assessed and compared.
All 3 methods measured changes in threshold sensitivity after a noxious pinch.
Nociceptive sensitization after the pinch was determined to last 19 h.
Acknowledgments
We would like to first and foremost thank Louie Ramos for initial testing of many of our methods and training many students. I would also like to thank Tyler Deniston, Alicia Rice, Laura Modilevsky, Christina Orchanian and Tunya Lee Martin for beginning tests of the defensive strike response in the lab. Marissa McMackin was funded by the National Institutes of Health MS/PhD Bridge Fellowship (R25-GM048972) and the Genentech Foundation MS Dissertation Scholarship.
Megumi Fuse was funded by National Institutes of Health - Minority Biomedical Research Support grants (2S06 GM52588-09 and SC2 GM095428-01A1), US Department of Agriculture-National Research Initiative Grant (MF80-2217) and by the SFSU center for Computing for Life Sciences. Dennis Tabuena was funded by the National Institutes of Health RISE Fellowship (R25-GM059298) and the Genentech Foundation MS Dissertation Scholarship. Eric Arreola was funded by the National Institutes of Health RISE Fellowship (R25-GM059298). Matthew Lewin was funded by the Emergency Medicine Foundation Resident Research Grant (EMF 2002-5738424E).
Abbreviations
- L5
fifth larval stage
- F50
force threshold for 50% chance to strike
- SUDO
Simplified Up-and-Down Order method
- filament #
filament number
Footnotes
6. DISCLOSURES
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marissa Zubia McMackin, Email: mzmcmackin@gmail.com.
Matthew R. Lewin, Email: mlewin@calacademy.org.
Dennis R. Tabuena, Email: dennis.r.tabuena2@gmail.com.
F. Eric Arreola, Email: arreola.eric85@gmail.com.
Christopher Moffatt, Email: moffatt@sfsu.edu.
Megumi Fuse, Email: fuse@sfsu.edu.
References
- Alupay JS, Hadjisolomou SP, Crook RJ. Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neurosci Lett. 2014;558:137–142. doi: 10.1016/j.neulet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Babcock DT, Galko MJ. Two sides of the same coin no longer: genetic separation of nociceptive sensitization responses. Commun Integr Biol. 2009;2(6):517–519. doi: 10.4161/cib.2.6.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Shi SJ, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21(18):1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25(7):641–7. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- Bannon AW. Models of pain: hot-plate and formalin test in rodents. Curr Protoc Pharmacol. 2001;Chapter 5(Unit 5.7) doi: 10.1002/0471141755.ph0507s00. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Malmberg AB. Models of nociception: hot-plate tail-flick and formalin tests in rodents. Curr Protoc Neurosci. 2007;Chapter 8(Unit 8.9) doi: 10.1002/0471142301.ns0809s41. [DOI] [PubMed] [Google Scholar]
- Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373. [Google Scholar]
- Binder A, May D, Baron R, Maier C, Tölle TR, Treede RD, Berthele A, Faltraco F, Flor H, Gierthmühlen J, Haenisch S, Huge V, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens S, Uçeyler N, Ufer M, Wasner G, Zhu J, Cascorbi I. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS ONE. 2011;6(3):e17387. doi: 10.1371/journal.pone.0017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Bories CY, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet KA, Peterson KE. A modification of the jump-flinch technique for measuring pain sensitivity in rats. Pharmacol Biochem Behav. 1975;3(1):47–55. doi: 10.1016/0091-3057(75)90079-9. [DOI] [PubMed] [Google Scholar]
- Cadet R, Aigouy L, Woda A. Enhanced nociceptive behaviour following conditioning injection of formalin in the perioral area of the rat. Brain Res. 1995;676(1):189–195. doi: 10.1016/0006-8993(95)00055-u. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, Kandel ER. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219(4583):397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Blumenthal H, Goelet P, Kandel ER. Inhibitors of protein synthesis block long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol. 1989;20:1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Frost WN, Goelet P, Montarolo PG, Schacher S, Morgan J, Blumenfeld H, Kandel ER. Cell and molecular analysis of long-term sensitization in Aplysia. J Physiol. 1986;81(4):349–357. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Copenhaver PF, Truman JW. The role of eclosion hormone in the larval ecdyses of Manduca sexta Insects Lepidoptera moulting. J Insect Physiol. 1982;28:695–701. [Google Scholar]
- Crocker A, Russell R. The up-and-down method for the determination of nociceptive thresholds in rats. Pharmacol Biochem Beh. 1984;21(1):133–136. doi: 10.1016/0091-3057(84)90142-4. [DOI] [PubMed] [Google Scholar]
- Crook RJ, Walters ET. Nociceptive behavior and physiology of molluscs: animal welfare implications. ILAR J. 2011;52(2):185–195. doi: 10.1093/ilar.52.2.185. [DOI] [PubMed] [Google Scholar]
- Crook RJ, Hanlon RT, Walters ET. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J Neurosci. 2013;33(24):10021–10026. doi: 10.1523/JNEUROSCI.0646-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook RJ, Lewis T, Hanlon RT, Walters ET. Peripheral injury induces long-term sensitization of defensive responses to visual and tactile stimuli in the squid Loligo pealeii, Lesueur 1821. J Exp Biol. 2011;214(Pt 19):3173–85. doi: 10.1242/jeb.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Almeida Y1, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15(1):61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- Dixon JW, Mood AM. A method for obtaining and analyzing sensitivity data. J Am Stat Assoc. 1948;43:109–126. [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse M, Truman JW. Modulation of ecdysis in the moth Manduca sexta; the roles of the suboesophageal and thoracic ganglia. J Exp Biol. 2002;205(8):1047–1058. doi: 10.1242/jeb.205.8.1047. [DOI] [PubMed] [Google Scholar]
- Gasull X, Liao X, Dulin MF, Phelps C, Walters ET. Evidence that long-term hyperexcitability of the sensory neuron soma induced by nerve injury in Aplysia is adaptive. J Neurophysiol. 2005;94(3):2218–2230. doi: 10.1152/jn.00169.2005. [DOI] [PubMed] [Google Scholar]
- Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14(11):1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes KE, Klukas KA, Fahrbach SE, Mesce KA. Hormone-dependent expression of Fasciclin ii during ganglionic migration and fusion in the ventral nerve cord of the moth Manduca sexta. J Comp Neurol. 2008;509(3):319–339. doi: 10.1002/cne.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illich PA, Walters ET. Mechanosensory neurons innervating Aplysia siphon encode noxious stimuli and display nociceptive sensitization. J Neurosci. 1997;17(1):459–469. doi: 10.1523/JNEUROSCI.17-01-00459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz-Donsbach J, Levy G. Effect of small variations in heat stimulus temperature on the tail flick response of rats in analgesimetry. J Pharm Sci. 1962;51:185–186. doi: 10.1002/jps.2600510224. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–36. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HT, Kim T, Novotny B, Khan N, Aksamit J, Siegel S, Miranpuri GS, Resnick DK. Thermal hyperalgesia assessment for rats after spinal cord injury: developing a valid and useful pain index. Spine J. 2014;14(6):984–989. doi: 10.1016/j.spinee.2013.09.051. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Seol TK, Lee HJ, Yaksh TL, Jun JH. The effect of intrathecal mu delta kappa and alpha-2 agonists on thermal hyperalgesia induced by mild burn on hind paw in rats. J Anesth. 2011;25(6):884–891. doi: 10.1007/s00540-011-1240-2. [DOI] [PubMed] [Google Scholar]
- Kumazawa T. The polymodal receptor: bio-warning and defense system. Prog Brain Res. 1996;113:3–18. doi: 10.1016/s0079-6123(08)61078-x. [DOI] [PubMed] [Google Scholar]
- Lewin MR. Society for Neuroscience. New Orleans, LA: 2003. Enhanced host defensive behavior suggests a strategy for survival of the parasitoid wasp Cotesia congregata. [Google Scholar]
- Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2(1):18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Wu J, Willis WD. Involvement of cGMP in nociceptive processing by and sensitization of spinothalamic neurons in primates. J Neurosci. 1997;17(9):3293–3302. doi: 10.1523/JNEUROSCI.17-09-03293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HT, Slate D, Paetsch C, Dorfmann L, Trimmer B. Scaling of caterpillar body properties and its biomechanical implications for the use of a hydrostatic skeleton. J Exp Biol. 2011;214:1194–1204. doi: 10.1242/jeb.051029. [DOI] [PubMed] [Google Scholar]
- Merchasin E. MS Thesis. San Francisco State University; 2009. Characterization of nociceptive sensitization in larval Manduca sexta. [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234(4781):1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Neely GG, *, Hess A, *, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, Diatchenko L, Gupta V, Xia CP, Amann S, Kreitz S, Heindl-Erdmann C, Wolz S, Ly CV, Arora S, Sarangi R, Dan D, Novatchkova M, Rosenzweig M, Gibson DG, Truong D, Schramek D, Zoranovic T, Cronin SJ, Angjeli B, Brune K, Dietzl G, Maixner W, Meixner A, Thomas W, Pospisilik JA, Alenius M, Kress M, Subramaniam S, Garrity PA, Bellen HJ, Woolf CJ, Penninger JM. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 2010;143(4):628–638. doi: 10.1016/j.cell.2010.09.047. *equal authorship. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA. Actions of a protein synthesis blocker on the neuronal mechanisms of sensitization in the common snail. Neirofiziologiya. 1993;25(2):109–115. [Google Scholar]
- Nikitin VP, Kozyrev SA. The effects of cAMP on the excitability and responses of defensive behavior command neurons in the common snail evoked by sensory stimuli. Neurosci Behav Physiol. 2000;30(4):441–447. doi: 10.1007/BF02463099. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA. The selective effect of a protein kinase C inhibitor on synaptic plasticity in defensive behavior command neurons during development of sensitization in the snail. Neurosci Behav Physiol. 2004;34(5):423–430. doi: 10.1023/b:neab.0000022625.66506.88. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA. Long-Term Synaptic Facilitation in Defensive Behavior Command Neurons in the Snail During Acquisition of Sensitization Depends on RNA Synthesis. Neurosci Behav Physiol. 2005;35(4):355–362. doi: 10.1007/s11055-005-0032-2. [DOI] [PubMed] [Google Scholar]
- Palovcak E, Delemotte L, Klein ML, Carnevale V. Comparative sequence analysis suggests a conserved gating mechanism for TRP channels. J Gen Physiol. 2015;146(1):37–50. doi: 10.1085/jgp.201411329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Meth. 1999;87(2):182–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Guzman-Karlsson MC, Sweatt DJ. Cellular molecular and epigenetic mechanisms in non-associative conditioning: implications for pain and memory. Neurobiol Learn Mem. 2013;105:133–150. doi: 10.1016/j.nlm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem. 2012;287(36):30743–54. doi: 10.1074/jbc.M112.362194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar P, Kumar A, Bhandari A, Goswami C. Conservation of tubulin-binding sequences in TRPV1 throughout evolution. PLoS One. 2012;7(4):e31448. doi: 10.1371/journal.pone.0031448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Castellucci VF, Kandel ER. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988;240:1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113(2):261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Truman JW, Taghert PH, Reynolds SE. Physiology of pupal ecdysis in the tobacco hornworm Manduca sexta. I. Evidence for control by eclosion hormone. J Exp Biol. 1980;88:327–337. [Google Scholar]
- van Griethuijsen LI, Banks KM, Trimmer BA. Spatial accuracy of a rapid defense behavior in caterpillars. J Exp Biol. 2013;216(3):379–387. doi: 10.1242/jeb.070896. [DOI] [PubMed] [Google Scholar]
- Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987;7(2):400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Injury-related behavior and neuronal plasticity: An evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol. 1994;36:325–427. doi: 10.1016/s0074-7742(08)60307-4. [DOI] [PubMed] [Google Scholar]
- Walters ET. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front Physiol. 2012;3:309. doi: 10.3389/fphys.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Moroz LL. Molluscan memory of injury: evolutionary insights into chronic pain and neurological disorders. Brain Behav Evol. 2009;74(3):206–218. doi: 10.1159/000258667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E, Illich P, Weeks J, Lewin M. Defensive responses of larval Manduca sexta and their sensitization by noxious stimuli in the laboratory and field. J Exp Biol. 2001;204:457–469. doi: 10.1242/jeb.204.3.457. [DOI] [PubMed] [Google Scholar]
- Wiel DE, Weeks JC. Habituation and dishabituation of the proleg withdrawal reflex in larvae of the sphinx hawk Manduca sexta. Behav Neurosci. 1996;110:1133–1347. [PubMed] [Google Scholar]
- Wittenburg N, Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Neurobiol. 1999;96:10477–10482. doi: 10.1073/pnas.96.18.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Walters ET. Common patterns of plasticity contributing to nociceptive sensitization in mammals and Aplysia. Trends Neurosci. 1991;14(2):74–78. doi: 10.1016/0166-2236(91)90024-o. [DOI] [PubMed] [Google Scholar]
- http://www.stoeltingco.com (http://www.stoeltingco.com/media/wysiwyg/58011_Touch_Test_Evaluator.pdf)