Abstract
Introduction
Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) has become the gold standard for surgical weight loss. The success of LRYGB may be measured by excess body-mass index loss (%EBMIL) over 25 kg/m2, which is partially determined by multiple patient factors. In this study, artificial neural network (ANN) modeling was used to derive a reasonable estimate of expected postoperative weight loss using only known preoperative patient variables. Additionally, ANN modeling allowed for the discriminant prediction of achievement of benchmark 50% EBMIL at one year postoperatively.
Methods
Six-hundred and forty-seven LRYGB included patients were retrospectively reviewed for preoperative factors independently associated with EBMIL at 180 and 365 days postoperatively (EBMIL180 and EBMIL365, respectively). Previously validated factors were selectively analyzed, including age; race; gender; preoperative BMI (BMI0); hemoglobin; and diagnoses of hypertension (HTN), diabetes mellitus (DM), and depression or anxiety disorder. Variables significant upon multivariate analysis (P<.05) were modeled by “traditional” multiple linear regression and an ANN, to predict %EBMIL180 and %EBMIL365.
Results
The mean EBMIL180 and EBMIL365 were 56.4%±16.5% and 73.5%±21.5%, corresponding to total body weight losses of 25.7%±5.9% and 33.6%±8.0%, respectively. Upon multivariate analysis, independent factors associated with EBMIL180 included black race (B=−6.3%, P<.001), BMI0 (B=−1.1%/unit BMI, P<.001) and DM (B=−3.2%, P<.004). For EBMIL365, independently associated factors were female gender (B=6.4%, P<.001), black race (B=−6.7%, P<.001), BMI0 (B=−1.2%/unit BMI, P<.001), HTN (B=−3.7%, P=.03) and DM (B=−6.0%, P<.001). Pearson r2 values for the multiple linear regression and ANN models were .38 (EBMIL180) and .35 (EBMIL365), and .42 (EBMIL180) and .38 (EBMIL365), respectively. ANN-prediction of benchmark 50% EBMIL at 365 days generated an area under the curve of 0.78±0.03 in the training set (n=518), and 0.83±0.04 (n=129) in the validation set.
Conclusions
Available at https://redcap.vanderbilt.edu/surveys/?s=3HCR43AKXR, this, or other ANN models may be used to provide an optimized estimate of postoperative EBMIL following LRYGB.
Keywords: bariatric, obesity, gastric bypass, outcomes
Introduction
The incidence of morbid obesity continues to rise, and patients are increasingly turning to surgical weight loss management. Bariatric surgery, while not the first line treatment of obesity, is one of the most effective means of achieving substantial and sustained weight loss. A highly effective bariatric procedure, the Roux-en-Y gastric bypass operation is frequently performed in the U.S [1–3], as it is considered to have the best balance of comorbidity resolution and excess weight loss against complications [4]. The laparoscopic approach to this operation has reduced associated morbidity as well as length of hospital stay [3]. The laparoscopic Roux-en-Y gastric bypass (LRYGB) operation involves exclusion of the stomach, leaving only a fundal pouch to which a cut segment of jejunum (“Roux-limb”) is anastomosed [5, 6]. A biliopancreatic limb with a distal jejunojejunal anastomosis is created as well, allowing for weight loss by multiple mechanisms [4, 6, 7].
The weight loss expectations vary widely among candidates. Some patients may aim to lose a modest amount of body weight to alleviate comorbidities, while others may envision a more significant amount of weight loss. To this effect, the development of a tool to provide a reasonable estimate of postoperative excess body-mass index loss (EBMIL, excess defined as over 25 kg/m2) may be of benefit to surgical candidates and their physicians [8].
There are myriad factors validated in previous studies as independent predictors of weight loss after LRYGB. Some of these factors are related to the technical aspects of the operation itself (i.e., length of the Roux limb), intraoperative findings (i.e. degree of liver fibrosis on intraoperative biopsy), or socioeconomic factors. Preoperative demographic and medical factors have also been identified, including female gender, black race, age at surgery, preoperative weight loss, immediately preoperative BMI (BMI0), diagnoses of hypertension (HTN); diabetes mellitus (DM); depression and anxiety, and iron stores. These have all been externally validated as independent correlates to EBMIL, and were collectively chosen as a panel of comprehensive and easily quantifiable and obtainable variables for review in this study [1, 9–18].
Artificial neural networks (ANN) are advanced, continually adapting computational systems taught to identify complex non-linear relationships among variables correlated with an outcome. Selected independent variables are chosen as the input layer, and an ANN algorithm provides an output layer (dependent variables) [19–23]. The ANN is initially trained using these layers, which are processed through training nodes, and can subsequently be used to predict future outcomes. Its advantages are its ability to continuously adapt its algorithm to new patient information and its superior prognostic ability relative to standard multiple linear regression models. In this study, we aim to devise a web-based tool to predict EBMIL after LRYGB first by identification of independent preoperative predictors of postoperative weight loss in our cohort, with subsequent imputation of those variables into an ANN-based model.
Methods
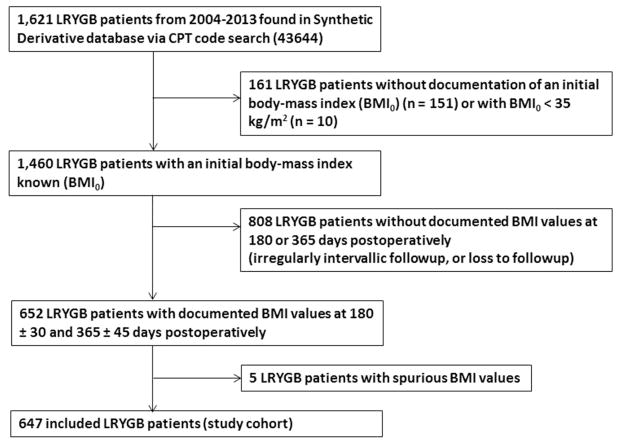
This was a single-institution retrospective cohort study conducted at Vanderbilt University Medical Center, a large bariatric referral center in Nashville, TN. Using the Vanderbilt University Synthetic Derivative, a de-identified mirror of the electronic medical record, 1,621 patients from 2004–2013 were identified via CPT code search (43644, Laparoscopy, surgical, gastric restrictive procedure with gastric bypass and Roux-en-Y gastroenterostomy [roux limb 150 cm or less]) as having undergone LRYGB for bariatric purposes. As all data is scrubbed of patient identifiers, this protocol was approved by the Institutional Review Board with waiving of informed consent. Patients were excluded if they did not have sufficient information in the chart, had an initial BMI < 35 kg/m2, or had clearly spurious values which called the data integrity into question (Figure 1). Six-hundred and forty-seven patients were identified for inclusion, and their demographics were exported to the Research Electronic Data Capture (REDCap) program hosted at Vanderbilt University [24].
Figure 1.
Derivation of the 647 patient study cohort
Demographics and previously identified independent clinical preoperative predictors of EBMIL were chosen for review. These included age at surgery; race; gender; BMI0; pre-operative weight loss; previous diagnosis of HTN, DM, depression or anxiety; and hemoglobin as a surrogate for iron stores. Attribution of comorbidities was based upon having an associated ICD-9 diagnosis documented no later than five days postoperatively. The hemoglobin measurement used was taken as the preoperative value temporally closest to the day of surgery, up to one year prior. The value used to determine whether the patient lost weight preoperatively was the most recent BMI documented between 6–12 months prior to the day of surgery. The primary endpoints were %EBMIL at 180 ± 30 days and 365 ± 45 days postoperatively (EBMIL180 and EBMIL365, respectively). %EBMIL was defined as 100%*(BMI0 − BMIpost-surgery)/(BMI0−25 kg/m2) [8].
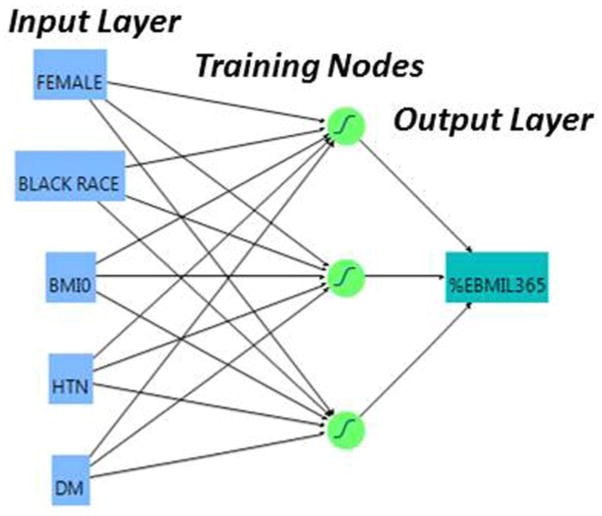
A bivariate linear regression analysis was performed to assess the influence of each variable on EBMIL180 and EBMIL365. Variables for each endpoint that demonstrated a bivariate association (P < 0.1) with EBMIL were entered into a multivariate analysis using the method of least squares, to determine independent association with EBMIL180 and EBMIL365 (P < 0.05). The groups of preoperative variables significant on multivariate analysis represented variables considered in the multiple linear regression models, and as the input layers for ANN180 and ANN365, respectively (Figure 2). The ANN model used was a three-node back-propagation ANN with k-fold validation. Each node was assigned a training value of 0.333, and the model was trained with 3 iterations. The neural network algorithms for ANN180 and ANN365 were derived from 80% of the 647 patients (training set); 20% of the patients were randomly withheld in ANN derivation to comprise a necessary validation set. A full mathematical description of the ANN model has been reviewed previously [19, 21].
Figure 2.
Diagram of the 3-node artificial neural network for prediction of excess body-mass index loss at one year postoperatively (EBMIL365).
Using both the multiple linear regression prediction expressions and the ANN algorithms, actual EBMIL vs. predicted EBMIL plots were generated, using Pearson r2 values of the linear regression lines as the primary measure of goodness of fit. A receiver-operating characteristic (ROC) curve was also generated to assess the ability of ANN365 to predict the achievement of benchmark 50% EBMIL [25]. Measures of central tendency were expressed as mean ± standard deviation. Area under the curve (AUC) values for ROC curves were expressed as AUC ± standard error. Bivariate, multivariate and ANN analysis was performed using JMP Statistical Software (Cary, NC) and GraphPad Prism (La Jolla, CA). P < 0.05 was used to denote statistical significance.
Results
Patient characteristics from the 647 patient cohort are summarized in Table 1. The mean age of the cohort was 47.4 ± 11.0 years. 14.4% were of black race, and 79.6% were female. Among all patients, the average EBMIL180 was 56.4% ± 16.5%, and EBMIL365 was 73.5% ± 21.5%. These values corresponded to total body weight losses of 25.7% ± 5.9% and 33.6% ± 8.0%, respectively. Of note, only 299 patients had BMI values 6–12 months preoperatively. This was due to a combination of poor chart maintenance, decentralized preoperative care as well as a rather stringent six month window chosen for acceptable values, as BMI values beyond one year or within six months were felt more likely to erroneously reflect the preoperative weight trajectory.
Table 1.
Preoperative Clinical Characteristics of the Study Population
| Variable | Patients Analyzed | Patients with Trait (categorical) Mean ± SD (continuous) |
|---|---|---|
| Age at Surgery | 647 | 47.4 ± 11.0 years |
| Female Gender | 647 | 515/647 (79.6%) |
| Black Race | 647 | 93/647 (14.4%) |
| BMI0 | 647 | 47.0 ± 8.5 kg/m2 |
| Hypertension | 647 | 506/647 (78.2%) |
| Diabetes Mellitus | 647 | 331/647 (51.2%) |
| Depression Disorder | 647 | 56/647 (8.7%) |
| Anxiety Disorder | 647 | 97/647 (15.0%) |
| Hemoglobin | 609 | 13.4 ± 1.2 g/dL |
| Preop. Wt. Loss | 299 | 160/299 (53.5%) |
| %EBMIL180 | 647 | 56.4% ± 16.5% |
| %EBMIL365 | 647 | 73.5% ± 21.5% |
SD- standard deviation; %EBMIL180- %excess body-mass index loss (over 25 kg/m2) at 180 days postoperatively; %EBMIL365- %excess body-mass index loss (over 25 kg/m2) at 365 days postoperatively
Results of the bivariate and multivariate analyses for EBMIL180 and EBMI365 are reported in Table 2 and Table 3, respectively. Bivariate analysis of EBMIL180 revealed associations with black race (B = −10.9%, P < .001), BMI0 (B = −1.2%/unit BMI, P < .001), HTN (B = −4.7%, P = .003), DM (B = −5.6%, P < .001) and hemoglobin (B = 1.5%, P = .006). Independent predictors of EBMIL180 were black race (B = −6.3%, P < .001), BMI0 (B = −1.1%/unit BMI, P < .001) and DM (B = −3.2%, P < .004). Results of the bivariate analysis of EBMIL365 revealed associations with female gender (B = 8.0%), black race (B = −12.6%), BMI0 (B = −1.4%), HTN (B = −8.9%) and DM (B = −8.9%), all at P < .001. Independent correlates with EBMI365 were female gender (B = 6.4%, P < .001), black race (B = −6.7%, P < .001), BMI0 (B = −1.2%/unit BMI, P < .001), HTN (B = −3.7%, P = .03) and DM (B = −6.0%, P < .001).
Table 2.
Bivariate and Multivariate Analysis of Factors Associated with Excess Body-Mass Index Loss, 180 days postoperatively
| Variable | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
| ||||
| B (95% CI) | P | B (95% CI) | P | |
| Age (yrs) | −0.03% (−0.14, 0.09) | .64 | - | - |
| Female Gender | 1.6% (−1.6, 4.7) | .95 | - | - |
| Black Race | −10.9% (−14.4, −7.3) | <.001 | −6.3% (−3.3, −9.3) | <.001 |
| BMI0 | −1.2% (−1.3, −1.0) | <.001 | −1.1% (−1.0, −1.2) | <.001 |
| Hypertension | −4.7% (−7.8, −1.6) | .003 | −1.8 (−4.4, 0.8) | .17 |
| Diabetes Mellitus | −5.6% (−8.1, −3.1) | <.001 | −3.2% (−5.3, −1.0) | .004 |
| Depression Disorder | −0.6% (−3.8, 2.6) | .72 | - | - |
| Anxiety Disorder | −0.6% (−4.2, 3.0) | .75 | - | - |
| Hemoglobin | 1.5% (0.4, 2.5) | .006 | 0.2% (−0.7, 1.0) | .67 |
| Preop. Wt. Loss | 0.9% (−3.0, 4.7) | .66 | - | - |
B- Units of %EBMIL180/unit variable (e.g. −0.03% EBMIL180/year) Italics indicate variables included in multivariate analysis; Bold indicates variables included in multiple linear regression and artificial neural network models (P < .05). Multivariable model was a standard least squares fit, n = 609, constant = 110.9%, r2 = .38, F = 75.2, P < .001.
Table 3.
Bivariate and Multivariate Analysis of Factors Associated with Excess Body-Mass Index Loss, 365 days postoperatively
| Variable | Bivariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | P | |
| Age (yrs) | −0.1% (−0.2, 0.06) | .22 | - | - |
| Female Gender | 8.0% (3.9, 12.1) | <.001 | 6.4% (3.0, 9.8) | <.001 |
| Black Race | −12.6% (−17.2, −8.0) | <.001 | −6.7% (−10.6, −2.8) | <.001 |
| BMI0 | −1.4% (−1.5, −1.2) | <.001 | −1.2% (−1.4, −1.1) | <.001 |
| Hypertension | −8.9% (−12.8, −4.9) | <.001 | −3.7% (−7.2, −0.3) | .03 |
| Diabetes Mellitus | −8.9% (−12.2, −5.7) | <.001 | −6.0% (−8.8, 3.2) | <.001 |
| Depression Disorder | −1.9% (−4.0, 7.8) | .40 | - | - |
| Anxiety Disorder | −1.0% (−5.6, 3.7) | .69 | - | - |
| Hemoglobin | 0.9% (−0.4, 2.3) | .18 | - | - |
| Preop. Wt. Loss | 0.7% (−4.4, 5.7) | .79 | - | - |
B- Units of %EBMIL365/unit variable (e.g. −0.1% EBMIL365/year) Italics indicate variables included in multivariate analysis; Bold indicates variables included in multiple linear regression and artificial neural network models (P < .05). Multivariable model was a standard least squares fit, n = 647, constant = 137.1%, r2 = .35, F = 69.9, P < .001.
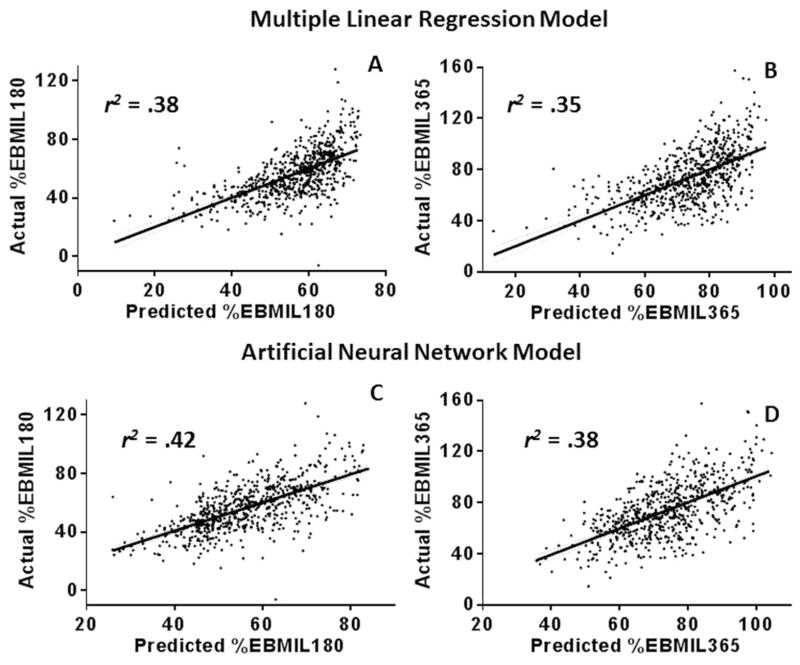
Plots representing actual EBMIL vs. multiple linear regression-predicted EBMIL at both 180 and 365 days postoperatively are shown in Figures 3A and 3B. For EBMIL180 and EBMIL365, the Pearson r2 values of the linear regression lines, a measure of goodness of fit of the model, were .38 and .35, respectively. Plots representing actual EBMIL vs. ANN-predicted EBMIL at both 180 and 365 days postoperatively are shown in Figures 3C and 3D. For EBMIL180 and EBMIL365, the Pearson r2 values of the linear regression lines were .42 and .38, respectively. At both 180 and 365 days postoperatively, the ANN model was more accurate in predicting loss of excess BMI, with a greater Pearson r2 and lower root-mean-square error.
Figure 3.
Actual vs. predicted EBMIL plots for both models, at 180 and 365 days postoperatively
A- Plot of actual vs. multiple linear regression-predicted EBMIL180; r2 = .38, root-mean-square error (RMSE) = 13.0. B- Plot of actual vs. multiple linear regression-predicted EBMIL365; r2 = .35, RMSE = 17.4. C- Plot of actual vs. artificial neural network-predicted EBMIL180; r2 = 0.42, root-mean-square error (RMSE) = 12.6. D- Plot of actual vs. artificial neural network-predicted EBMIL365; r2 = .38, RMSE = 16.9. Linear regression lines with 95% confidence bands are included on all plots (dashed lines).
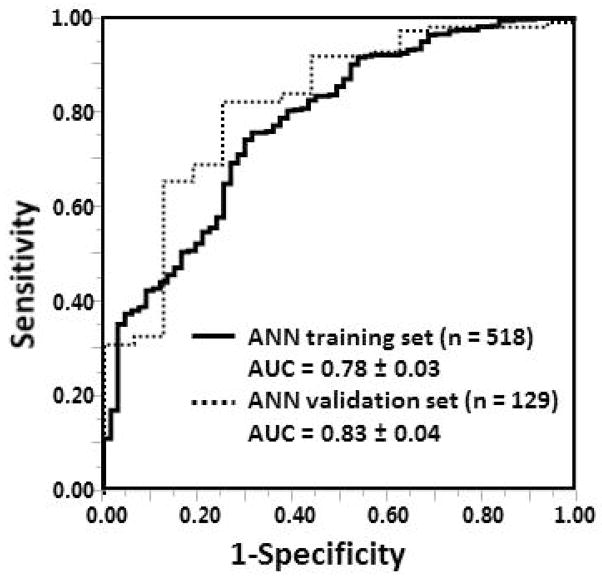
Using the ANN algorithm an ROC curve was generated to assess the ability of ANN365 to predict achievement of benchmark 50% EBMIL. In the cohort, 87% (564/647) of the patients successfully achieved this goal. The ROC curves obtained from training and validation sets of ANN365 analysis in prediction of 50% EBMIL are shown in Figure 5; areas under the curve (AUC) for the training set and validation set were 0.78 ± 0.03 and 0.83 ± 0.04, respectively.
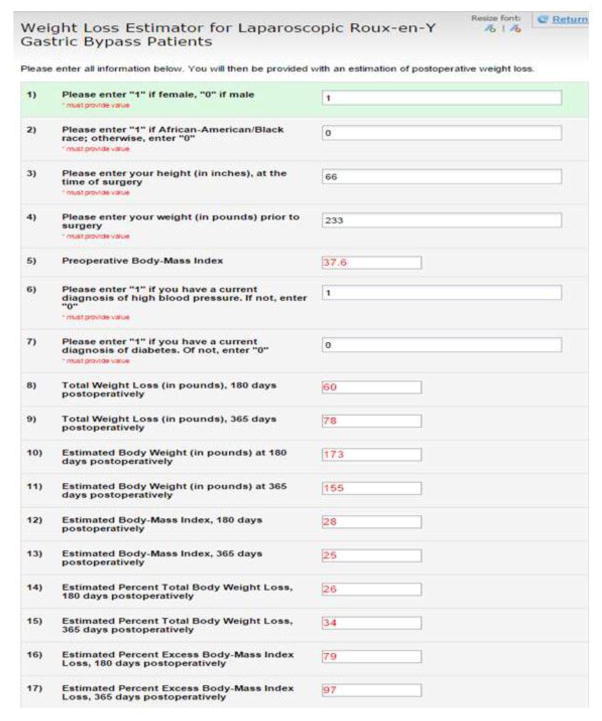
Figure 5.
Demonstration of web-based weight loss estimation tool
The ANN-derived algorithm is available for use at “https://redcap.vanderbilt.edu/surveys/?s=3HCR43AKXR”.
Discussion
ANNs are excellent model systems for prediction of postoperative outcomes, and have been used in general surgery and surgical oncology, among other specialties [22, 26–35]. In bariatric surgery, ANN modeling has been used sparingly. The largest of the few published bariatric ANN studies reported outcomes of laparoscopic adjustable gastric banding in Italy. Using 172 patients, weight loss was predicted on the basis of results from the Minnesota Multiphasic Personality Inventory-2 test of psychopathology, BMI0 and age [21].
We selected the procedure of LRYGB as it represents the gold standard for weight loss. Its role in reduction of BMI, improving comorbidities and decreasing polypharmacy has been well-established [2, 6]. Surgical weight loss is reserved only for those of extreme obesity, with a BMI greater than 40 kg/m2, or greater than 35 kg/m2 with at least one associated weight-related comorbidity, required for candidacy. LRYGB patients have a higher rate of medical and psychiatric comorbidities that the general population, and these additional comorbidities may have significant contributions to the success of these patients in postoperative weight loss. While variance in surgical success can only be partially attributed to preoperative comorbidities, they were nonetheless used along with demographics to develop a robust predictive tool derived from a cohort of over 600 patients, for estimation of EBMIL after LRYGB.
Determination of those variables to examine for this model necessitated thorough review of the literature to identify preoperative predictors of EBMIL after LRYGB. A patient-centered tool for weight loss estimation after bariatric surgery, reported in 2014, found age and preoperative BMI (BMI0) as the most significant predictors of sustained postoperative weight loss [1]. Race has also been shown to predict weight loss, as Coleman et al. recently reported prospective data showing that black race is associated with impaired weight loss in LRYGB [11]. It is a universally accepted recommendation to attempt weight reduction prior to LRYGB. The influence of preoperative weight loss on postoperative weight loss, however, has not been well established. In one study, modest preoperative weight reduction was correlated with sustained weight loss 3–4 years after bypass [12], however, its effect on more immediate weight loss has been somewhat ambiguous. A 2012 systematic review of preoperative predictors of weight loss after bariatric surgery identified seven studies that showed a positive association between preoperative and postoperative weight loss, one showed a negative association, and six did not show an association [14].
Various medical conditions have been reported to have an independent association with postoperative weight loss. Psychiatric disease, particularly depression and anxiety disorders, are common, and are negatively associated with postoperative weight loss in bariatric surgery [14]. In 2014, Still et al. reported observational cohort data in which HTN and DM were considered as measures associated with decreased weight loss [9]. Hemoglobin A1C also had an inverse correlation with weight loss, suggesting that postoperative weight loss is associated with optimal glycemic control [9]. For simplicity, our analysis only considered presence or absence of HTN or DM, and not the extent of disease. Diminished iron stores have demonstrated significance in impairing weight loss after bariatric surgery [9]. For this study, hemoglobin values were chosen as a surrogate for iron stores, as more sensitive measures such as serum ferritin, serum iron, and total iron binding capacity were not sufficiently available [17]. Collectively, the preoperative clinical variables studied is by no means exhaustive, but rather represents a selected set of parameters that in conjunction with behavioral changes may govern the success of the operation [12].
In our cohort, risk factors for impaired EBMIL180 and EBMIL365 on multivariate analysis were found to be black race, higher BMI0 and DM; male gender and HTN were risk factors only for impaired EBMIL365. Using these variables, multiple linear regression functions were obtained for endpoints EBMIL180 and EBMIL365. Pearson r2 values for these models were .38 and .35, for EBMIL180 and EBMIL365, respectively (Figure 3A and 3B). The corresponding Pearson r2 values for the ANN models were .42 and .38 for EBMIL180 and EBMIL365, respectively (Figure 3C and 3D), values that fit the data more closely than the corresponding multiple linear regression model. As the ANN model was superior, its training set algorithm was used to predict 50% EBMIL365, a benchmark sometimes used to herald a successful operation, via generation of an ROC curve (Figure 4).
Figure 4.
Receiver-operating characteristic curve for the 518 patient ANN training cohort (AUC = 0.78 ± 0.03; solid line), and the 129 patient ANN validation cohort (AUC = 0.83 ± 0.04; dashed line) for the model to predict benchmark 50% EBMIL365
Although the ANN model was algorithmically sophisticated, the input factors were easy to quantify. Using only demographics, BMI and basic knowledge of the patient’s medical history, one can easily make an independent determination of estimated EBMIL180 and EBMIL365. This ANN model can be used as a web-based weight loss prediction calculator accessible to patients (currently available at https://redcap.vanderbilt.edu/surveys/?s=3HCR43AKXR; Figure 5), or plausibly incorporated as a platform within an electronic medical record accessible to health care providers. Further, ANNs offer the flexibility to adapt continually to newly input patient data, and to optimize the model’s overall prognostic ability. To the authors’ knowledge, this is the first implementation of neural network modeling for prediction of postoperative weight loss.
This study has several limitations. There is the drawback inherent in the accuracy and bias of data collected retrospectively rather than prospectively. The cohort in this study was also biased by inclusion of only those patients who attended six and twelve month follow-up appointments at Vanderbilt University, perhaps representing a more closely monitored, adherent and motivated group of patients. This study also currently lacks external validity, as the patient cohort underwent institution-specific preoperative and postoperative preparation and follow-up protocols, although somewhat standardized due to the institution’s designation as a Bariatric Surgery Center of Excellence since 2005. Furthermore, the 647 patients were operated on by a relatively small number of bariatric surgeons. In addition, while patients customarily attended 6 and 12 month follow-up appointments, the regularity of these visits beyond this time frame was inconsistent preventing accurate data collection beyond one year. As it is known that weight loss may continue to occur after one year, this study does not allow for the estimation of sustained weight loss. It is our hope that as ANNs are integrated into routine clinical care, this limitation will be overcome. Finally, while ANNs have improved prognostic accuracy, the model does not conveniently express the relative contribution of each variable in predicting the outcome, as opposed to a multiple linear regression analysis. Thus, a “black box” approach in deriving the output variable from input variables in necessitated [21].
In summary, successful postsurgical weight loss is partially dependent on multiple preoperative patient factors. Our cohort demonstrated black race, higher BMI0, DM, HTN and male gender as independent markers for a less successful operation. Using these predictors, an ANN model allows for development of a patient-centered tool with which to obtain an optimized estimate of postoperative EBMIL at 6 and 12 months. This model can be readily translated to a web-based platform as a novel patient-centered tool; alternatively, it may assist bariatric surgeons either as a preoperative screening tool or a means to provide realistic expectations to their patients.
Acknowledgments
Dr. Colleen Brophy, M.D., Professor of Surgery, Vanderbilt University, Vanderbilt University Dept. of Biostatistics
Funding:
Vanderbilt RedCAP: CTSA Award UL1 TR000445 from NCATS/NIH
Footnotes
This manuscript will be presented as a poster (Abstract ID 61988) at the Annual Meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), April 15–18, Nashville TN.
Disclosures:
Eric Wise, Kyle Hocking and Stephen Kavic have no conflict of interest to disclose.
References
- 1.Wood GC, Benotti P, Gerhard GS, Miller EK, Zhang Y, Zaccone RJ, Argyropoulos GA, Petrick AT, Still CD. A patient-centered electronic tool for weight loss outcomes after Roux-en-Y gastric bypass. Journal of obesity. 2014;2014:364941. doi: 10.1155/2014/364941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. The Cochrane database of systematic reviews. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter J, Elliott S, Kaplan J, Lin M, Posselt A, Rogers S. Predictors of hospital stay following laparoscopic gastric bypass: analysis of 9,593 patients from the National Surgical Quality Improvement Program. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2014 doi: 10.1016/j.soard.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. International journal of obesity. 2009;33(Suppl 1):S28–32. doi: 10.1038/ijo.2009.14. [DOI] [PubMed] [Google Scholar]
- 5.Levine MS, Carucci LR. Imaging of bariatric surgery: normal anatomy and postoperative complications. Radiology. 2014;270:327–341. doi: 10.1148/radiol.13122520. [DOI] [PubMed] [Google Scholar]
- 6.Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Seminars in nephrology. 2013;33:75–89. doi: 10.1016/j.semnephrol.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307:R1275–1291. doi: 10.1152/ajpregu.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Laar A. Bariatric Outcomes Longitudinal Database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obesity surgery. 2012;22:1843–1847. doi: 10.1007/s11695-012-0736-7. [DOI] [PubMed] [Google Scholar]
- 9.Still CD, Wood GC, Chu X, Manney C, Strodel W, Petrick A, Gabrielsen J, Mirshahi T, Argyropoulos G, Seiler J, Yung M, Benotti P, Gerhard GS. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity. 2014;22:888–894. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallal RM, Quebbemann BB, Hunt LH, Braitman LE. Analysis of weight loss after bariatric surgery using mixed-effects linear modeling. Obesity surgery. 2009;19:732–737. doi: 10.1007/s11695-009-9816-8. [DOI] [PubMed] [Google Scholar]
- 11.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2014;10:396–403. doi: 10.1016/j.soard.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Alger-Mayer S, Polimeni JM, Malone M. Preoperative weight loss as a predictor of long-term success following Roux-en-Y gastric bypass. Obesity surgery. 2008;18:772–775. doi: 10.1007/s11695-008-9482-2. [DOI] [PubMed] [Google Scholar]
- 13.Alger-Mayer S, Rosati C, Polimeni JM, Malone M. Preoperative binge eating status and gastric bypass surgery: a long-term outcome study. Obesity surgery. 2009;19:139–145. doi: 10.1007/s11695-008-9540-9. [DOI] [PubMed] [Google Scholar]
- 14.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obesity surgery. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JE, King WC, Courcoulas A, Dakin G, Elder K, Engel S, Flum D, Kalarchian M, Khandelwal S, Pender J, Pories W, Wolfe B. Eating behavior and eating disorders in adults before bariatric surgery. The International journal of eating disorders. 2014 doi: 10.1002/eat.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards-Hampton SA, Madan A, Wedin S, Borckardt JJ, Crowley N, Byrne KT. A closer look at the nature of anxiety in weight loss surgery candidates. International journal of psychiatry in medicine. 2014;47:105–113. doi: 10.2190/PM.47.2.b. [DOI] [PubMed] [Google Scholar]
- 17.Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Alimentary pharmacology & therapeutics. 2014;40:582–609. doi: 10.1111/apt.12872. [DOI] [PubMed] [Google Scholar]
- 18.Munoz M, Botella-Romero F, Gomez-Ramirez S, Campos A, Garcia-Erce JA. Iron deficiency and anaemia in bariatric surgical patients: causes, diagnosis and proper management. Nutricion hospitalaria. 2009;24:640–654. [PubMed] [Google Scholar]
- 19.Dumont TM, Rughani AI, Tranmer BI. Prediction of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network: feasibility and comparison with logistic regression models. World neurosurgery. 2011;75:57–63. doi: 10.1016/j.wneu.2010.07.007. discussion 25–58. [DOI] [PubMed] [Google Scholar]
- 20.Lee YC, Lee WJ, Lee TS, Lin YC, Wang W, Liew PL, Huang MT, Chien CW. Prediction of successful weight reduction after bariatric surgery by data mining technologies. Obesity surgery. 2007;17:1235–1241. doi: 10.1007/s11695-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 21.Piaggi P, Lippi C, Fierabracci P, Maffei M, Calderone A, Mauri M, Anselmino M, Cassano GB, Vitti P, Pinchera A, Landi A, Santini F. Artificial neural networks in the outcome prediction of adjustable gastric banding in obese women. PloS one. 2010;5:e13624. doi: 10.1371/journal.pone.0013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoldas O, Tez M, Karaca T. Artificial neural networks in the diagnosis of acute appendicitis. The American journal of emergency medicine. 2012;30:1245–1247. doi: 10.1016/j.ajem.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Presnell SR, Cohen FE. Artificial neural networks for pattern recognition in biochemical sequences. Annual review of biophysics and biomolecular structure. 1993;22:283–298. doi: 10.1146/annurev.bb.22.060193.001435. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction - Response. Circulation. 2007;116:E134–E134. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 26.Debnath J, Chatterjee S, Sharma V. Artificial neural networks in the diagnosis of acute appendicitis: should imaging be a part of it? The American journal of emergency medicine. 2013;31:258–259. doi: 10.1016/j.ajem.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh CH, Lu RH, Lee NH, Chiu WT, Hsu MH, Li YC. Novel solutions for an old disease: diagnosis of acute appendicitis with random forest, support vector machines, and artificial neural networks. Surgery. 2011;149:87–93. doi: 10.1016/j.surg.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Sakai S, Kobayashi K, Toyabe S, Mandai N, Kanda T, Akazawa K. Comparison of the levels of accuracy of an artificial neural network model and a logistic regression model for the diagnosis of acute appendicitis. Journal of medical systems. 2007;31:357–364. doi: 10.1007/s10916-007-9077-9. [DOI] [PubMed] [Google Scholar]
- 29.Gholipour C, Fakhree MB, Shalchi RA, Abbasi M. Prediction of conversion of laparoscopic cholecystectomy to open surgery with artificial neural networks. BMC surgery. 2009;9:13. doi: 10.1186/1471-2482-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gohari MR, Biglarian A, Bakhshi E, Pourhoseingholi MA. Use of an artificial neural network to determine prognostic factors in colorectal cancer patients. Asian Pacific journal of cancer prevention : APJCP. 2011;12:1469–1472. [PubMed] [Google Scholar]
- 31.Biglarian A, Bakhshi E, Gohari MR, Khodabakhshi R. Artificial neural network for prediction of distant metastasis in colorectal cancer. Asian Pacific journal of cancer prevention : APJCP. 2012;13:927–930. doi: 10.7314/apjcp.2012.13.3.927. [DOI] [PubMed] [Google Scholar]
- 32.Dolgobrodov SG, Moore P, Marshall R, Bittern R, Steele RJ, Cuschieri A. Artificial neural network: predicted vs observed survival in patients with colonic cancer. Diseases of the colon and rectum. 2007;50:184–191. doi: 10.1007/s10350-006-0779-8. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed FE. Artificial neural networks for diagnosis and survival prediction in colon cancer. Molecular cancer. 2005;4:29. doi: 10.1186/1476-4598-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng F, Wu Y, Wu Y, Nie G, Ni R. The effect of artificial neural network model combined with six tumor markers in auxiliary diagnosis of lung cancer. Journal of medical systems. 2012;36:2973–2980. doi: 10.1007/s10916-011-9775-1. [DOI] [PubMed] [Google Scholar]
- 35.Faradmal J, Soltanian AR, Roshanaei G, Khodabakhshi R, Kasaeian A. Comparison of the performance of log-logistic regression and artificial neural networks for predicting breast cancer relapse. Asian Pacific journal of cancer prevention : APJCP. 2014;15:5883–5888. doi: 10.7314/apjcp.2014.15.14.5883. [DOI] [PubMed] [Google Scholar]