Abstract
Transcranial infrared laser stimulation is a new non-invasive form of low-level light therapy that may have a wide range of neuropsychological applications. It entails using low-power and high-energy density infrared light from lasers to increase metabolic energy. Preclinical work showed that this intervention can increase cortical metabolic energy, thereby improving frontal cortex-based memory function in rats. Barrett & Gonzalez-Lima (2013) discovered that transcranial laser stimulation can enhance sustained attention and short-term memory in humans. We extend this line of work to executive function. Specifically, we ask whether transcranial laser stimulation enhances performance in the Wisconsin Card Sorting Task (WCST) that is considered the gold-standard of executive function and is compromised in normal aging and a number of neuropsychological disorders. We used a laser of a specific wavelength (1064 nm) that photostimulates cytochrome oxidase—the enzyme catalyzing oxygen consumption for metabolic energy production. Increased cytochrome oxidase activity is considered the primary mechanism of action of this intervention. Participants who received laser treatment made fewer errors and showed improved set-shifting ability relative to placebo controls. These results suggest that transcranial laser stimulation improves executive function and may have exciting potential for treating or preventing deficits resulting from neuropsychological disorders or normal aging.
Keywords: Executive function, low-level light therapy, transcranial infrared laser stimulation, Wisconsin Card Sorting Task, prefrontal cortex, neuroenhancement
Transcranial infrared laser stimulation is a novel, non-invasive low-level light therapy (LLLT2) technique with promising potential for psychological and neurological applications (Gonzalez-Lima & Barrett 2014; Naeser et al. 2014). LLLT consists of using directional low-power and high-fluence monochromatic or quasimonochromatic light from lasers or light-emitting diodes (LEDs) in the red to near-infrared wavelengths to modulate biological functions (Rojas & Gonzalez-Lima 2011). LLLT has been shown to modify neuronal function in cell cultures, animal models, and clinical conditions (Eells et al. 2004; Wong-Riley et al. 2005; Schiffer et al. 2009). It produces a wide range of neurobiological effects including the enhancement of cellular metabolic energy (ATP production) and gene expression (Rojas & Gonzalez-Lima 2013). Recent research suggests that LLLT has exciting potential to enhance cognitive function in humans through transcranial laser stimulation (Barrett & Gonzalez-Lima 2013). This highlights the need for further research into its potential neuro-cognitive enhancing effects and viability as treatment for cognitive dysfunction.
The idea of using red or infrared light to modify cognition in humans is novel, but research over the past decade has elucidated its neurophysiological basis and illustrated its potential for cognitive modulation in animal models. The primary mechanism of action of LLLT appears to be photobiomodulation of mitochondrial cytochrome oxidase (Karu et al. 2005; Wong-Riley et al. 2005; Rojas et al. 2008; Rojas & Gonzalez-Lima 2011). Cytochrome oxidase is the enzyme that catalyzes oxygen consumption in cellular respiration for metabolic energy production (Wong-Riley et al. 2005), and the primary photoacceptor of red to near-infrared light energy (Karu et al. 2005). Previous work has shown that transcranial LLLT can increase cytochrome oxidase activity in the rat brain (Rojas et al. 2008), which can provide neuroprotection and improve behavioral performance (Rojas & Gonzalez-Lima 2011, 2013). In particular, Rojas et al. (2012) demonstrated that transcranial LLLT can improve frontal cortex oxygen consumption and metabolic energy, and thereby increase frontal cortex-based memory functions in rats. These findings in non-human animals suggest that the metabolic energy of tissue exposed to LLLT is enhanced, and that this can result in enhancement of cognitive function.
To date few studies have investigated the use of LLLT as a form of neuromodulation in humans in vivo, but preliminary research suggests it may have a broad range of applications. LLLT has been shown to improve neurological outcome after ischemic stroke (Lampl et al. 2007) and mild traumatic brain injury (Naeser et al. 2014). Schiffer et al. (2009) found that a single LLLT treatment to the forehead using 810 nm LEDs resulted in a significant beneficial effect in patients with major depression and anxiety when measured two and four weeks later. Barrett and Gonzalez-Lima (2013) presented the first placebo-controlled study investigating the effects of transcranial laser stimulation on cognitive function in humans. They showed that transcranial LLLT in healthy young adults improved sustained attention, measured by reaction time in the psychomotor vigilance task (PVT), and short-term memory retrieval, measured by performance on a delayed match-to-sample task (Barrett & Gonzalez-Lima 2013). These findings suggest promising applications of LLLT for enhancing cognitive functions, including treatment or prevention of cognitive and emotional dysfunction in patient populations.
In this study we continued the investigation of LLLT’s capacity for neuromodulation by assessing the effect of transcranial infrared laser stimulation on executive function, an important frontally-based cognitive faculty involved in a wide variety of common cognitive tasks and impaired in a number of neuropsychological disorders. We measured executive function using the Wisconsin Cart Sorting Task (Milner 1963; Heaton, Chelune, Talley, Kay, & Curtiss 1993), arguably the gold standard test of executive function in neuropsychology.
The Wisconsin Card Sorting Task
The WCST has been the standard of neuropsychological test of the prefrontal cortex in humans (Mattay et al. 2003; Monchi et al. 2001; Stuss et al. 2000) and is considered by many to be the gold standard of executive function tests (Delis, Kaplan, & Kramer 2001). The WCST is a complex task that relies on a number of executive cognitive processes, including working memory, inhibition, abstraction, and set-shifting (Miyake et al. 2000; Goldberg et al. 2003; Head et al. 2009; Milner 1963). In the WCST, a participant’s task is to match a test card to one of four reference cards. They are initially told nothing of the classification rule, which is to be acquired through feedback provided after each response. The cards have three dimensions (color, shape, and number), and the rule is to match on one of these dimensions. After achieving 10 correct responses in a row, the rule is unexpectedly changed, requiring the participant to detect the change and adapt to the new task demands. Working memory is involved in maintaining the currently relevant rule, and set-shifting ability is necessary to switch between changing rules, which requires inhibiting the previously relevant rule. The WCST can also be seen as a measure of cognitive flexibility (Buchsbaum, Greer, Chang, & Berman 2005), and so the WCST involves a wide array of the cognitive processes comprising executive function.
The WCST has proven a useful tool in evaluating executive processing deficits in a number of neuropsychological disorders as well as other special populations. For example, deficits are commonly found in patients with Parkinson’s disease (Monchi et al. 2004; Owen et al. 1992), schizophrenia (Egan et al., 2001; Daniel et al. 1991; Berman et al. 1995), Alzheimer's disease (Binetti et al. 1996; Paolo 1996), bipolar disorder (Martinez-Aran et al. 2002; Morice 1990), and in depressed individuals (Davis & Nolen-Hoeksema 2000; Harvey et al. 2004; Channon 1996). There are also well-established effects associated with normal aging (e.g. Fristoe, Salthouse, & Woodard 1997; Head et al. 2009; Salthouse, Atkinson, & Berish 2003).
The WCST was developed as a diagnostic test of frontal cortical pathology. Patients with lesions in the prefrontal cortex (PFC) show impairments in WCST performance (Milner 1963; Stuss et al. 2000; Demakis 2003). Imaging work has also sought to localize the brain regions engaged while performing the WCST and to isolate the areas associated with specific executive functions that are required. A wealth of evidence from fMRI, positron emission tomography (PET), and transcranial magnetic stimulation (TMS) implicate lateral prefrontal cortex in WCST performance (Monchi, et al. 2001, 2004; Nagahama et al. 1996; Ko et al. 2008). Monchi et al. (2001, 2004) found increased activity in mid-dorsolateral prefrontal cortex (areas 9/46) during feedback presentation, and increased activity in mid-ventrolateral prefrontal cortex (areas 47/12) specifically during negative feedback. Konishi et al. (1998) also found set-shifting related activity in dorsolateral prefrontal cortex (DLPFC) (areas 44/45). Ko et al. (2008) found that applying repetitive transcranial magnetic stimulation to the right DLPFC during feedback hindered WCST performance, suggesting that DLPFC plays an integral role in completing this task. Animal work has also implicated DLPFC in set-shifting tasks (Passingham 1972; Dias et al. 1996, 1997).
While other areas have been implicated (e.g., inferior parietal lobule, anterior cingulate cortex, and cerebellum; Buchsbaum et al. 2005), lateral prefrontal cortex is the most consistently reported, and often strongest, area of activation. Many studies report bilateral activation, but activity in the right hemisphere is more consistently implicated in WCST performance (Ko et al. 2008; Konishi et al. 1999). For this reason we chose to target right DLPFC and VLPFC with our LLLT intervention. We applied active LLLT transcranially to two areas of the right forehead, targeting DLPFC and VLPFC, in an 8-minute session before administering the WCST. Our results suggest that LLLT treatment improved overall WCST performance compared to placebo controls, with this being primarily due to an improvement in set-shifting ability.
Methods
Participants
Participants were 30 (13 female; 17 male) undergraduate students who participated for partial course credit. Participants’ mean age was 20.4 (SD = 1.64) years. As discussed below participants were assigned to either an active or a placebo group. The active and placebo groups did not differ in terms of age (20.4 vs. 20.07 years, respectively), t(28) = 0.46, p = 0.65, d = 0.168, or gender (7/15 vs. 6/15 female, respectively), χ2 = 0.136, p = 0.713.
General Procedure
The experimenter obtained informed consent from participants at the beginning of the experimental session. The consent form included details about the safety procedures relevant to the operation of the laser used to conduct the LLLT. Verbal explanation of these procedures was also given. Participants were told that they might be either in the active treatment or placebo treatment groups, and that they would not be told which group they were in. After the experiment was concluded they were informed which group they were in if they wished to know. After the verbal explanation, participants were given the chance to opt out of the experiment with no repercussions, but all participants chose to participate.
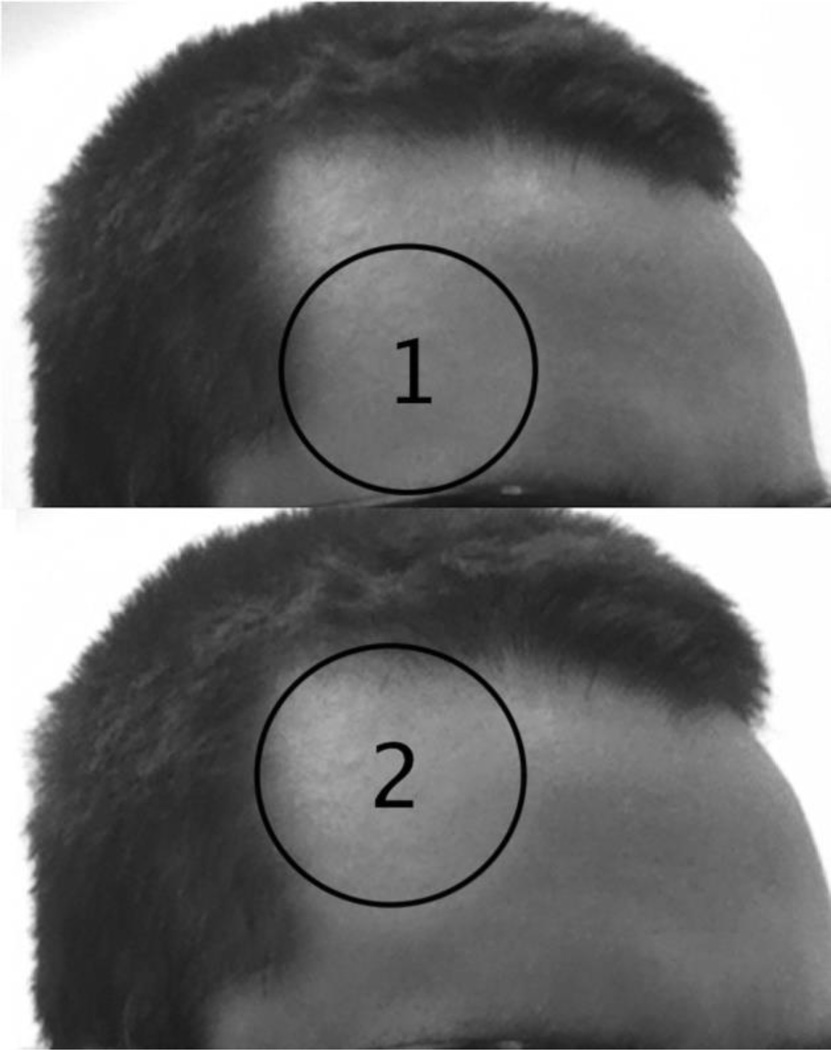
Once consent was given the LLLT session began. Half of the participants (15) received active LLLT administration, and the other half received placebo treatment. Condition assignment was counterbalanced between participants, with every other participant receiving active treatment. The LLLT session lasted 8 minutes, administered in 8 one-minute treatments alternating between two locations on the forehead. Each location was 4-cm in diameter, with little overlap to cover the right lateral forehead in each subject (Figure 1). For the active LLLT group, administration began with location 1 (the lower right portion of the forehead), directly above the eyebrow, switched to location 2 (the upper right side of the forehead), and alternated between the two areas. In reference to the standard 10 – 20 EEG electrode placement system, the area stimulated covered the right frontal polar (FP2) and right frontal (F4) sites.
Figure 1. Locations targeted by LLLT administration.
LLLT was applied to both locations for 4 one-minute treatments each, beginning with the location 1 (the lower location) and alternating between the two locations, for a total of 8 one-minute treatments.
For the placebo group the procedures were identical except that participants received only a brief (5 s) LLLT treatment to the intended site, followed by 55 s of no treatment, during each one-minute treatment session. Thus the placebo group received approximately 1/12th of the cumulative light energy density as the active treatment group. This low energy has been found ineffective to improve cognition (Barrett & Gonzalez-Lima 2013) and it was intended as a sham control to equate subjective experience between the active and placebo groups, as participants often report feeling a slight warm sensation at the start of a treatment session. Because the apparatus emits a sound at the start and end of each session, the laser remained on during the full 60 s session, but was simply directed away from the participant’s forehead toward a designated location on the wall for the remaining 55 s. Previous work has validated the effectiveness of this placebo control procedure (Barrett & Gonzalez-Lima, 2013), showing that participants were at chance when asked to indicate which group they believed they were in, with approximately half of the participants in each group believing they were in the active treatment condition. Immediately following the 8-min placebo or LLLT treatment participants completed a computerized version of the WCST.
LLLT apparatus and procedure
LLLT treatment consisted of applying laser light of a specific wavelength (1064 nm) that intersects with the absorption spectrum of cytochrome oxidase and maximizes tissue penetration (Sommer et al. 2001). Treatment was administered using a laser diode supplied by Cell Gen Therapeutics, LLC (Cell Gen laser, HD Laser Center, Dallas, TX, USA). LLLT received approval by the FDA in 2002 for relief of pain in arthritis, head and neck pain, and carpal tunnel syndrome. Our device has not been evaluated or approved by the FDA for the specific uses tested in this study. Marketing of the Cell Gen laser in the USA is FDA-cleared as safe for various uses on humans, such as for improving circulation, temporary relief of muscle and joint pain, muscle spasm, stiffness associated with arthritis, and relaxation of muscle tissue. The laser received approval from the University of Texas at Austin Laser Safety Program and a standard operating procedure and room for the laser were approved by the University Laser Safety Officer.
During placebo and active treatment, the experimenter and the participant remained inside the locked laser room, with a sign on the outer door indicating that the laser was in use, and wore protective eyewear (900–1000nm: 5+, 1000– 2400 nm: 7+; 2900–10600 nm: 7+) at all times. As an additional precaution participants were instructed to keep their eyes closed while the laser was in use.
The laser wave was continuous (not pulsed) with a uniform circular beam area that measured 13.6 cm2. A button on the handle controlled the onset and offset of the photodiode. Each one-minute treatment cycle was marked by a timer counting down and by a beep from the apparatus. Each of the two forehead locations was exposed to an irradiance (or power density) of 250 mW/cm2 (3,400 mW/13.6 cm2 = 250 mW/cm2) for 4 min (3.4 W×240 s = 816 J/location), which corresponded to a cumulative fluence (or energy density) of 60 J/cm2 (0.25 W/cm2×240 s = 60 J/cm2). The chosen energy density of 60 J/cm2 is the same that showed psychologically beneficial effects in Barrett & Gonzalez-Lima (2013) and Schiffer et al. (2009). At the power level used (3.4 W), this laser dose is safe, exposure to it is not harmful to tissue, and it causes negligible heat and no physical damage. Higher powers (15–20 W) are used clinically by Cell Gen Therapeutics for the treatment of lower back pain, sciatica, and migraine headaches.
Wisconsin Card Sorting Task procedure
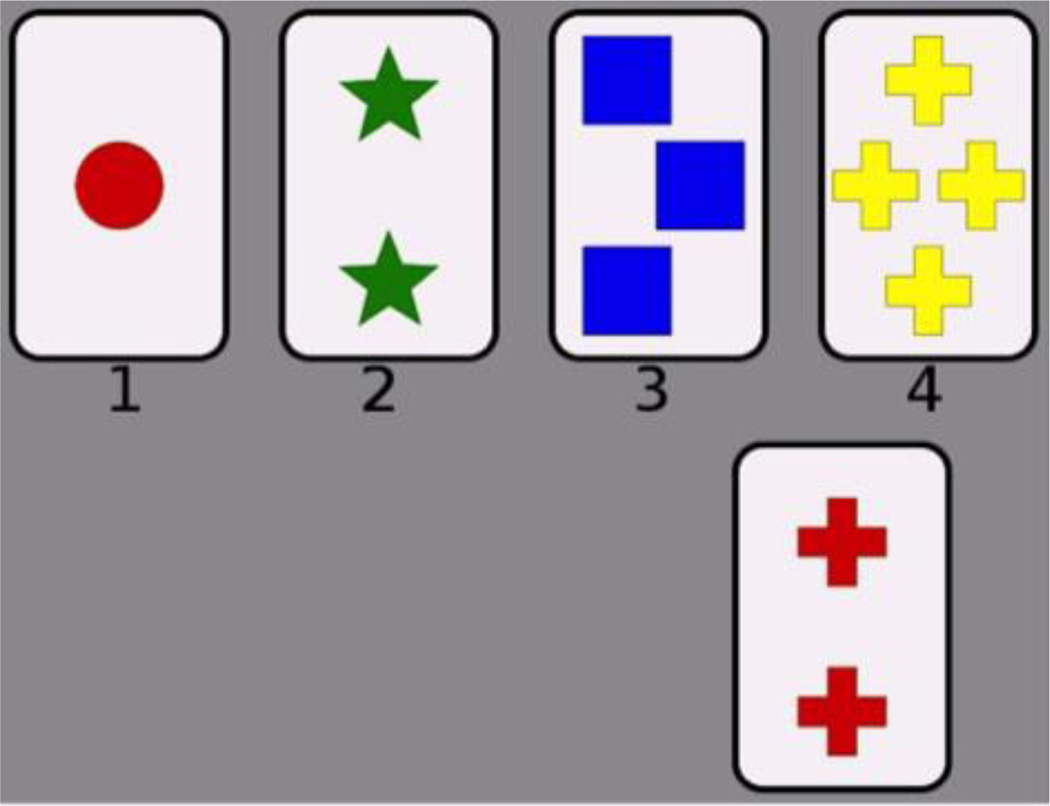
Participants completed a computerized version of the WCST. In the WCST, the participant’s task is to sort a target card into one of four groups. The groups are represented by four reference cards that are fixed and presented at the top of the screen at all times during the experiment (Figure 2). On each trial a target card was presented in the center of the screen, and the participant clicked on one of the four reference cards to sort it into that group. Stimuli consisted of the standard WCST 64-card stimulus set. The set of cards was run through twice for a total of 128 trials. Each card contained elements that varied along three dimensions: color (red, green, yellow, or blue), shape (triangle, star, cross, or circle), and number (1, 2, 3, or 4). The reference cards were one red triangle, two green stars, three yellow crosses, and four blue circles—each differing from the others on every dimension. After a selection, feedback was provided indicating whether the response was “Correct” or “Incorrect”. The correctness of the response was determined by a rule—matching along one of the three dimensions. Starting rule (shape, color, or number) was counterbalanced between participants. Unbeknownst to the participant, after 10 consecutive correct trials the rule changed; the relevant dimension switched to one of the other two dimensions. The task continued in this manner until all 128 trials were completed, which took participants 10 to 15 minutes to complete.
Figure 2. Participant interface for the Wisconsin Card Sorting Task.
The top row contains the four reference cards that are displayed on every trial, and the bottom row shows the target card for an example trial. The subject sorts each target card into one of the four groups represented by the reference cards. Cards vary on color (red, green, blue, or yellow), shape (circle, star, square, or cross), and number (1, 2, 3, or 4).
Results
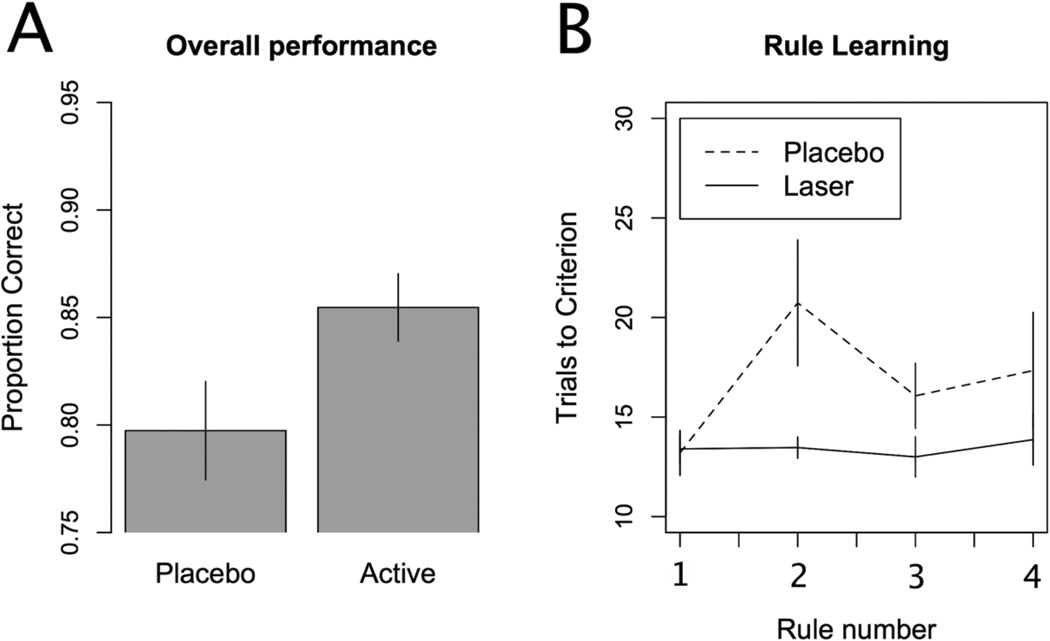
We first compared overall accuracy between the active treatment and placebo groups. The active laser treatment group had a significantly higher overall accuracy than the placebo group (Figure 3a), t(28)= 2.068, p = 0.048, d = 0.755. The active group sorted correctly on 85.5% of trials, and the placebo group sorted correctly on 79.7% of trials. To better understand the nature of this performance difference we then analyzed the number of trials to criterion for the initial and subsequent rules. Figure 3b plots this measure for the first four rules learned, which was the minimum number of rules completed by any participant. A group by rule ANOVA (with participant as an error term) showed a main effect of group, F(1,28) = 5.492, p = 0.026. The main effect of rule, F(3,84) = 1.786, p = 0.156, and the interaction, F(3,84) = 1.603, p = 0.195, were not significant. The two groups did not differ on the number of trials needed to learn the initial rule, t(28) = 0.15, p = 0.882, d = 0.055. However, the active laser treatment group learned the second rule significantly faster than the placebo laser group, t(28) = 2.268, p = 0.031, d = 0.828. Although the numerical learning advantage for the active laser treatment group remained for the third and fourth rules, these differences did not reach statistical significance [t(28) = 1.599, p = 0.121, d = 0.584, and t(28) = 1.087, p = 0.286, d = 0.397, respectively]. We also examined response time data to determine if the performance differences might be accounted for by differing speed-accuracy trade-offs between the two groups. The active (1508 ms) and placebo (1439 ms) did not differ in response times, t(28) = 0.514, p = 0.611, d = 0.188. All reported t-tests are two-tailed tests.
Figure 3. Results.
A) Overall accuracy across all trials for the two groups. The active laser treatment group correctly sorted the cards more often than the placebo group. B) Trials to criterion for each of the first four rules learned. The placebo treatment group took significantly longer to reach criterion on the second rule than the active laser treatment group, suggesting a benefit in set shifting ability in the active treatment group. Error bars represent standard errors.
Discussion
This article reports the first study to investigate the effects of transcranial laser stimulation on executive function using the WCST, which is the gold standard measure of executive function in neuropsychology (Mattay et al. 2003; Monchi et al. 2001; Stuss et al. 2000; Delis, Kaplan, & Kramer 2001; Salthouse, Atkinson, & Berish 2003). We applied LLLT transcranially using an infrared laser diode targeting the lateral prefrontal cortex. Using transcranial infrared laser stimulation in humans to enhance neurometabolic activity as a means of treatment, neuro-enhancement, or neuro-protection is a novel technology with exciting potential. Previous studies have shown increased cerebral metabolic energy production, oxygen consumption and blood flow in animals and humans following transcranial LLLT (Rojas et al. 2008, 2012; Gonzalez-Lima & Barrett 2014; Lampl et al. 2007; Schiffer et al. 2009; Nawashiro et al. 2012). Executive function is an important aspect of cognition that is compromised in a number of neuropsychological disorders such as depression (Davis & Nolen-Hoeksema 2000; Harvey et al. 2004; Channon 1996) schizophrenia (Egan et al. 2001; Monchi et al. 2004; Daniel et al. 1991; Berman et al. 1995), Parkinson’s disease (Monchi et al. 2004; Owen et al. 1992), Alzheimer's disease (Binetti et al. 1996; Paolo 1996), and bipolar disorder (Martinez-Aran et al. 2002; Morice 1990), as well as by normal aging (Head et al. 2009).
Our results demonstrate that WCST performance can be improved with a single 8-minute LLLT session. We take this as further evidence in a growing body of evidence suggesting that LLLT may have neuropsychological applications (Rojas & Gonzalez-Lima 2013; Naeser et al. 2014), and see it as a first step toward investigating its efficacy in the treatment and prevention of conditions compromising executive function. Toward that end a number of important questions remain that must be addressed with future research. While we have shown that transcranial laser stimulation can improve executive function in healthy young adults, its effectiveness in attenuating deficits in executive function resulting from disorders or aging has yet to be investigated in placebo-controlled studies. Another important issue in assessing whether it may serve as an effective clinical treatment is the duration of the effect. If the effect were only momentary, it would prove impractical. Currently little is known about the durations of LLLT’s effects, though previous studies suggest the benefits could last for several weeks. For example, Barrett and Gonzalez-Lima (2013) found a significant benefit as compared to the placebo group in positive and negative affective states in healthy volunteers (n = 40) two weeks after a single laser treatment as described here. In depressed patients (n =10), Schiffer et al., (2009) reported psychological benefits at two and four weeks after a single treatment. Light power density (250 mW/cm2) and energy density (60 J/cm2) used in these two studies were the same we used, but the study by Schiffer et al., (2009) used 810 nm LEDs instead of 1064 nm laser and they did not use a placebo control group. Naeser et al. (2014) used similar LEDs in patients with mild traumatic brain injury (n = 11) for 18 treatments (3/week for 6 weeks). They monitored cognitive performance after one week, and one and two months after the 18th treatment, and reported a significant linear trend for the effect of LED treatment over time for the Stroop test for executive function and the California Verbal Learning Test. While these pioneering studies are promising, there are no placebo-controlled human studies investigating long-term cognitive effects after single or repeated LLLT treatments.
Additionally, we need to investigate to what extent these effects generalize. The wide array of previously reported effects using LLLT suggest that its beneficial outcomes may be rather general, though targeted location is likely an important factor. Various effects may be elicited through targeting different brain regions. But, we may also expect that targeting the lateral prefrontal cortex might have general beneficial effects, as executive function is an important cognitive process involved in myriad tasks. Indeed, the WCST is a complex task involving numerous executive processes. Due to the complex nature of the WCST and the variety of processes involved, the exact mechanism of the improvement in performance is unclear. That performance was higher for the active treatment group on the second, but not the first rule, suggests an improvement in set shifting ability (i.e. cognitive flexibility), but there are other possible explanations. First, the WCST relies on working memory, and it is possible that improved working memory function is responsible for the performance improvement. This would be consistent with the improved working memory performance found in a delayed match-to-sample task after the same infrared laser treatment by Barrett and Gonzalez-Lima (2013). And, while WCST may be seen primarily as a test of cognitive flexibility or set shifting, it is also a learning task. The possibility that the beneficial effect of LLLT administration to the lateral prefrontal cortex could improve other types of learning warrants investigation. For example, an improvement of learning would be consistent with the LLLT findings of Naeser et al. (2014) using the California Verbal Learning Test. As a learning task, the WCST is essentially a rule-based categorization task (Hélie, Paul, & Ashby 2012), and so LLLT treatment may be able to provide a general benefit during category learning. We see this as a particularly promising field of investigation. Current theories of categorization typically posit (at least) two category-learning systems: a frontally-based, explicit system and a striatally-based, implicit system (Ashby & Maddox 2011). The frontal system excels at rule-based tasks, so it is consistent with a dual learning systems theory that enhancing metabolism in the prefrontal cortex could improve learning in the WCST. It remains to be seen whether LLLT targeted at prefrontal cortex would also improve other, non-rule-based categorization, or whether the benefit is specific to rule-based tasks.
Another important question that needs to be investigated in future research is whether there are cognitive costs to enhancing cognition using LLLT. A recent study using transcranial electrical stimulation showed that enhancing one cognitive process may come at the expense of other cognitive processes (Iuculano & Cohen Kadosh 2013). It remains to be seen whether the same type of cost to enhancing a cognitive process occurs with LLLT given the differences in mechanism between the techniques. LLLT enhances metabolic energy in neurons by a cytochrome oxidase photostimulatory mechanism that is fundamentally different than the mechanism of other methods of brain stimulation such as electric or magnetic stimulation. For example, during excitatory electrical stimulation as used for transcranial direct current stimulation (tDCS) there is expenditure of metabolic energy, while effective LLLT doses increase metabolic energy (Gonzalez-Lima & Barrett 2014). Animal studies have shown that LLLT up-regulates the amount of brain cytochrome oxidase for up to two weeks after light stimulation (Rojas et al. 2008), thereby increasing brain capacity to produce metabolic energy to enhance cognitive functions (Rojas et al., 2012).
Conclusion
Our study suggests that transcranial LLLT may have a range of potential benefits for cognitive enhancement, but it also highlights the great need for future research to establish the generalizability, duration, and specific cognitive mechanism of its effects. Future controlled studies should also examine its efficacy as treatment for executive processing and other cognitive dysfunctions in neuropsychological disorders and normal aging. Low-power LED arrays and laser diode sources are compact, portable, commercially available, and have achieved non-significant risk status for human trials by the FDA (Rojas & Gonzalez-Lima 2013). If proven effective LLLT could provide an affordable, safe alternative to current treatment options for cognitive impairment and brain dysfunction.
Footnotes
The authors thank Raj Panesar for help with data collection. FGL gratefully acknowledges support from an institutional research fellowship from the College of Liberals Arts of the University of Texas at Austin. FGL holds the George I. Sanchez Centennial Endowed Professorship in Liberal Arts and Sciences. Research reported in this publication was supported by the National Institute on Drug Abuse at the National Institute of Health (DA032457 to WTM and DA032457-01A1S1 to NJB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing financial interests.
We use the term low-level light therapy by convention to be consistent with previous research employing this technique, but it is important to clarify the technique is used as an experimental manipulation, not a therapeutic intervention, in the current and some previous studies.
Contributor Information
Nathaniel J. Blanco, Department of Psychology, The University of Texas at Austin; Institute for Mental Health Research, The University of Texas at Austin.
W. Todd Maddox, Department of Psychology, The University of Texas at Austin; Institute for Mental Health Research, The University of Texas at Austin; Institute for Neuroscience, The University of Texas at Austin; Center for Perceptual Systems, The University of Texas at Austin.
F. Gonzalez-Lima, Department of Psychology, The University of Texas at Austin Institute for Neuroscience, The University of Texas at Austin; Division of Pharmacology and Toxicology, The University of Texas at Austin.
References
- Ashby FG, Maddox WT. Human category learning 2.0. Annals of the New York Academy of Sciences. 2011;1224:147–161. doi: 10.1111/j.1749-6632.2010.05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Executive dysfunction in early Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 1996;60:91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang W, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: The Wisconsin Card Sorting Test. Journal of Affective Disorders. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. The Journal of Neuroscience. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research. 2000;24:699–711. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis–Kaplan executive function system. New York (NY): Psychological Corporation; 2001. [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sorting Test: restriction to novel situations and independence from ‘on-line’ processing. Journal of Neuroscience. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11:428–436. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P. Executive functions and updating of the contents of working memory in unipolar depression. Journal of Psychiatric Research. 2004;38:567–576. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47:1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual. Odessa (FL): Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- Iuculano T, Kadosh RC. The mental cost of cognitive enhancement. The Journal of Neuroscience. 2013;33(10):4482–4486. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélie S, Paul EJ, Ashby FG. Simulating the effects of dopamine imbalance on cognition: From positive affect to Parkinson’s disease. Neural Networks. 2012;32:74–85. doi: 10.1016/j.neunet.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. Journal of Photochemistry and Photobiology B: Biology. 2005;81:98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the Wisconsin card sorting task during provision of feedback. Journal of Biomedical Imaging. 2008;2 doi: 10.1155/2008/143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nature Neuroscience. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared Laser Therapy for Ischemic Stroke: A New Treatment Strategy Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Penades R, Vieta E, Colom F, Reinares M, Benabarre A, Salamero M, Gasto C. Executive function in patients with remitted bipolar disorder and schizophrenia and its relationship with functional outcome. Psychotherapy and psychosomatics. 2002;71:39–46. doi: 10.1159/000049342. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting: The role of the frontal lobes. Archives of Neurology. 1963;9:90–100. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. The Journal of Neuroscience. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice R. Cognitive inflexibility and pre-frontal dysfunction in schizophrenia and mania. The British Journal of Psychiatry. 1990;157:50–54. doi: 10.1192/bjp.157.1.50. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP, Baker EH. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. Journal of Neurotrauma. 2014;31:1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J. Cerebral activation during performance of a card sorting test. Brain. 1996;119:1667–1675. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomedicine and Laser Surgery. 2012;30:231–233. doi: 10.1089/pho.2011.3044. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Paolo AM, Tröster AI, Blackwell KT, Koller WC, Axelrod BN. Utility of a Wisconsin Card Sorting Test short form in persons with Alzheimer's and Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 1996;18:892–897. doi: 10.1080/01688639608408310. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Non-reversal shifts after selective prefrontal ablations in monkeys (Macaca mulatta) Neuropsychologia. 1972;10:41–46. doi: 10.1016/0028-3932(72)90041-3. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. Journal of Alzheimer's Disease. 2012;32:741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 2011;3:49–67. doi: 10.2147/EB.S21391. http://dx.doi.org/10.2147/EB.S21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochemical Pharmacology. 2013;86:447–457. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. The Journal of Neuroscience. 2008;28:13511–13521. doi: 10.1523/JNEUROSCI.3457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behavioral and Brain Functions. 2009;5:46–58. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. Journal of Clinical Laser Medicine & Surgery. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. The Journal of Biological Chemistry. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]