Abstract
Hairy cell leukemia (HCL) is an indolent B-cell malignancy effectively treated but not often cured by purine analog therapy; after multiple courses of purine analogs, patients can become purine analog resistant and in need of alternative therapies. Complete remission to single-agent purine analog is often accompanied by minimal residual disease (MRD), residual HCL cells detectable by immunologic methods, considered a risk factor for eventual relapse. Several different non-chemotherapy approaches are being used to target relapsed and refractory HCL, including inhibitors of BRAF, but so far only monoclonal antibody (MAb)-based approaches have been reported to eliminate MRD in a high percentage of patients. One of the MAb-based options for HCL currently under clinical investigation involves recombinant immunotoxins, containing a fragment of a MAb and a bacterial toxin. The bacterial toxin, a highly potent fragment from Pseudomonas exotoxin, catalytically ADP-ribosylates elongation factor 2 (EF2), resulting in protein synthesis inhibition and apoptotic cell death. Recombinant immunotoxins tested in HCL patients include LMB-2, targeting CD25, and BL22, targeting CD22. An affinity matured version of BL22, termed moxetumomab pasudotox (formerly HA22 or CAT-8015) achieved high CR rates in phase I, and is currently undergoing multicenter Phase 3 testing. Phase I testing was without dose-limiting toxicity, although 2 patients had grade 2 hemolytic uremic syndrome (HUS) with transient grade 1 abnormalities in platelets and creatinine. Preclinical work is underway to identify residues on moxetumomab pasudotox leading to immunogenicity. Moxetumomab pasudotox is undergoing pivotal testing for relapsed and refractory HCL.
Keywords: Moxetumomab pasudotox, BL22, CD22, CD25, recombinant immunotoxin, Pseudomonas exotoxin
Introduction
Hairy cell leukemia (HCL) was described in 1958 by Bouroncle et al. as an indolent malignancy associated with splenomegaly and pancytopenia, comprising 2% of leukemias [1]. Based on the 2015 leukemia incidence of 54,270 new cases in the US [2], this would translate to about 1100 new cases of HCL/year. The prevalence of HCL is thought to be much higher than the incidence, since chemotherapy is so effective at inducing remission, but often with eventual relapse. Major advances in the 1990s were achieved in the chemotherapeutic management of HCL, particularly with purine analog therapy, proven superior to interferon [3]. Variant HCL (HCLv), recognized since the 1980s, is poorly responsive to purine analogs, and has a slightly different immunophenotype compared to classic HCL [4-12]. Another type of variant of HCL expresses unmutated IGHV4-34 immunoglobulin rearrangements, has an immunophenotype consistent with HCL or HCLv, but regardless responds poorly to purine analogs [13, 14]. In the past 10-15 years, new advances have been made in non-chemotherapy approaches to HCL, both targeting antigens on the cell surface with monoclonal antibody (MAb)-based agents, and targeting pathways found to be important in HCL cells, such as BRAF. One group of MAb-based treatments which are highly cytotoxic to HCL cells and have shown clinical activity in early testing is the recombinant immunotoxins. This review will focus on this type of immunoconjugate and its potential for the treatment of HCL and it variants.
Indications for treatment of HCL
With some variation, the cytopenias considered indicative of treatment in HCL include neutrophil count of <1/nL, hemoglobin of <10 g/dL, or platelets <100/nL [15, 16]. Less stringent criteria including Neutrophils <1.5/nL or hemoglobin <12 mg/dL have also been considered worthy of treatment [3, 17]. Additional eligibility criteria commonly include symptomatic splenomegaly, malignant lymphocytosis >20/nL, enlarging malignant lymph nodes, and frequent infections. Since patients after splenectomy have resolution of cytopenias but progressive increase in malignant lymphocytosis and marrow infiltration, they may not qualify for treatment or clinical trials until far more advanced than patients with spleens. We have therefore in our recent trials allowed patients to qualify for treatment with lymphocytosis of >5/nL rather than requiring patients to wait till >20/nL. For previously treated patients, it is extremely important to determine whether cytopenias are due to chemotherapy toxicity or to relapsed/refractory HCL. It is common for bone marrow biopsies to show significant infiltration of HCL 1-3 months after purine analog treatment, but then show complete remission (CR) by 6 months without additional therapy. It is a common mistake for patients to receive an additional course of purine analog 1-3 month after the first, due to residual cytopenias and HCL still present in the bone marrow. Moreover, patients being treated with new approaches for relapsed/refractory HCL should be enrolled only after sufficient time after the last course of purine analog, so it will be clear that any response after enrollment will be due to the investigational agent, rather to the prior course of purine analog. A progressively increasing HCL count in the peripheral blood documented by flow cytometry is often a good way to confirm that HCL left over from the last therapy will not resolve due to the prior therapy.
Standard treatment of HCL
The purine analogs, first pentostatin, then cladribine, revolutionized the treatment of HCL with 70-90% CR rates and treatment-free intervals exceeding 10 years [3, 15, 18-22]. Cladribine is by far the most commonly used agent since it is given by a single 5-7 day course, whereas pentostatin is given every other week for 3-12 months. The median time to relapse after 1st line purine analog therapy is about 16 years, using follow-up consisting mainly of blood counts [21]. When patients were followed by bone marrow biopsy to determine relapse, the median time to relapse from CR was reported to be under 5 years, specifically in a population (age ≤40 at diagnosis) with high probability of long-term follow-up [23]. However, the median blood counts at relapse were high enough so that many did not require retreatment at the time of relapse. Long term disease-free survival curves fail to show a plateau indicating a cured population [21, 24, 25], and it is unclear what fraction of patients, if any, are actually cured after purine analog therapy. Nine of 19 patients in continuous CR for a median of 16 years, from the original 358-patient group treated at Scripps Clinic, were found to be free of HCL by flow cytometry and IHC studies, indicating that at least some may be cured. Nevertheless, relapse in HCL is common and has resulted an accumulation of relapsed patients requiring additional options.
Goals in the treatment of HCL: response criteria
To evaluate the activity of immunoconjugates in HCL, it is important to understand the response definitions. The main goal in the treatment of HCL, as in all leukemias, is to achieve a CR. From the 1987 Consensus Resolution, CR has been defined as bone marrow and blood free of HCL cells by non-immunologic stains, normalization of organomegaly, and resolution of cytopenias to neutrophils ≥1.5/nL, hemoglobin ≥12 g/dL, and platelets ≥100/nL [26]. Hemoglobin ≥11 g/dL, considered by some to be adequate for females [27], is our criteria for both males and females [16, 28-32], and we have for some protocols dropped the hemoglobin requirement in the presence of iron deficiency (i.e. ferritin < 20 ng/mL). The Consensus Resolution defined partial remission (PR) as ≥50% reduction of palpable hepatosplenomegaly and largest lymph node diameters, normal blood counts sufficient for CR, reduction of HCL marrow infiltration by ≥50%, and ≤5% circulating HCL cells by morphology [26]. While some protocols required CR-level blood counts for PR [3], others required these counts for ‘Good PR’ [27]. Most protocols, including ours, accepted ≥50% improvement in neutrophils, hemoglobin and platelets as sufficient for PR [15-17, 27-32]. For our protocol patients who are transfusion-dependent at baseline, achieving hemoglobin of 9 would satisfy the PR requirement for 50% improvement, even if the baseline hemoglobin was not allowed to drop to 6 g/dL prior to transfusion. However, a patient cannot be considered a major responder until 4 weeks after the last dose of growth factor (G-CSF, GM-CSF, or erythropoietin) or the last transfusion. Many investigators and protocols considered CR and PR based on a single assessment [15, 17], but others [3, 27], including our multiply relapsed protocols, required adequate normal blood counts for at least 4 weeks before performance of a bone marrow to confirm CR. During this 4 week time point, we ignore isolated values inconsistent with CR or PR. If a positive bone marrow is found in a patient who otherwise meets criteria of CR, that patient should be considered in PR. Since we usually do not subject patients to post-treatment bone marrow studies unless CR is being considered, we do not require a bone marrow just for documentation of PR. This is partly due to heterogeneity of bone marrow HCL infiltration in patients with relapsed disease, making this test unreliable to accurately quantify marrow infiltration. To eliminate subjective bias in newer trials, CT or MRI can be used instead of physical exam or ultrasound and if so, we would consider lymph nodes ≤2 cm, and spleen diameter either ≤ 17 cm or >25% decreased from baseline to be consistent with CR. PR by CT or MRI would require ≥50% reduction of abnormal hepatosplenomegaly and products of perpendicular diameters of lymph nodes, or reduction to size consistent with CR.
Assessment of minimal residual disease in HCL
To evaluate the ability of immunoconjugates to resolve minimal residual disease (MRD) in HCL, the standard tests generally used are IHC of the bone marrow biopsy, and flow cytometry of both the blood and bone marrow aspirate. Of these 3, the latter, flow cytometry of the bone marrow aspirate, is by far most sensitive [33, 34]. In fact, since most patients achieving flow-negative bone marrow aspirates have not relapsed despite >5 year follow-up, most of our protocols now define CR for patients with negative bone marrow aspirate flow cytometry and all criteria of CR except for resolution of cytopenias. We believe this is important because many patients who are MRD-free cannot resolve cytopenias due to lasting chemotherapy toxicity and hypersplenism; clearly these patients have better disease-free survival than patients in MRD+ CR with resolved cytopenias. A more sensitive test of MRD was developed which requires the sequence of the immunoglobulin rearrangement and PCR using sequence-specific probe/primer combinations (RQ-PCR). This test can detect a few as 1 HCL cell in 106 normal [35], but would be considered investigational rather than standard at this time.
Introduction to recombinant immunotoxins
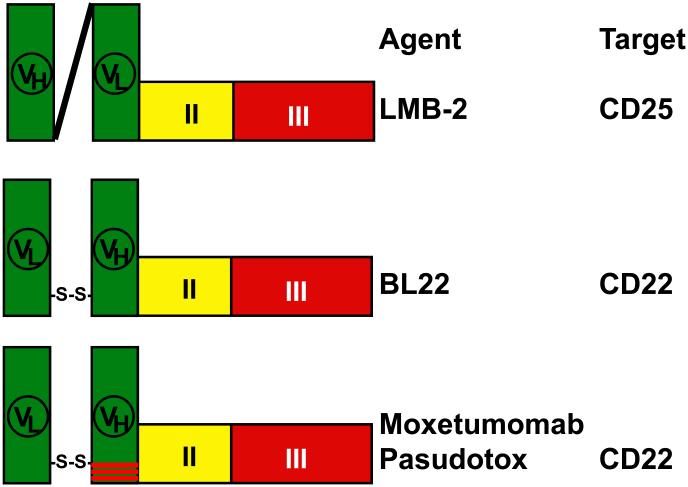
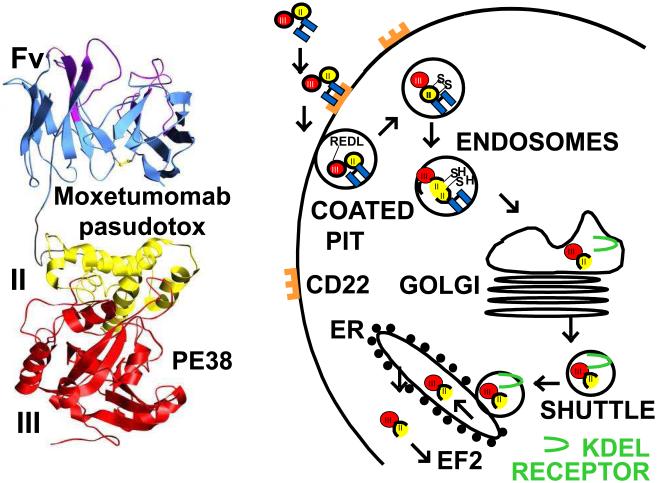
The original immunotoxins were immunoconjugates made by chemically conjugating a cancer cell-binding MAb to a protein toxin [36-38]. Protein toxins included either plant or bacterial toxins, either of which capable of killing a cell with a single molecule reaching the cytosol [39, 40]. The mechanism of cell killing by protein toxins involves catalytic inhibition of protein synthesis, either by removal of Adenine4324 in 28s ribosomal RNA for plant toxins, or by ADP-ribosylation of elongation factor 2 (EF2) for the bacterial toxins diphtheria toxin and Pseudomonas exotoxin [41, 42]. To make targeted protein toxin immunoconjugates smaller and easier to produce in high quantities, fusion toxins were produced by fusing a cell binding ligand to a truncated form of the protein toxin lacking the domain which would bind to normal animal cells [43, 44]. Bacterial toxins are well adapted for this purpose since they are already made by bacteria in single-chain form [45]. Recombinant immunotoxins are recombinant fusion toxins which contain a recombinant Fv fragment of a Mab fused to a truncated protein toxin. The first recombinant immunotoxin made contained a single-chain Fv fragment of the anti-CD25 (anti-IL2-receptor alpha) MAb anti-Tac fused to a 40 kDa fragment of Pseudomonas exotoxin [46]. The resulting molecule anti-Tac(Fv)-PE40, similar in structure to LMB-2 (Figure 1), was highly cytotoxic, attributed to the Fv binding to CD25 followed by internalization, unfolding in an acidic endocytic vesicle [47], proteolytic cleavage between the ligand and toxin by the protease Furin [48-50], reduction of a disulfide bond [51], trafficking of the ADP-ribosylating fragment to the endoplasmic reticulum [52] followed by translocation to the cytosol [53, 54], and finally ADP-ribosylation of EF2 [41, 55], leading to protein synthesis inhibition and apoptotic cell death [56-59]. A model of this intoxication pathway is shown in Figure 2. To minimize improper renaturation of the immunotoxin during production, anti-Tac(Fv)-PE40 was slightly shortened by removal of a disulfide bond within the toxin [60]. The resulting molecule Anti-Tac(Fv)-PE38 was renamed LMB-2 (Figure 1) and eventually entered clinical development for the treatment of CD25+ hematologic malignancies including HCL.
Figure 1. Schematic structures of recombinant immunotoxins.
LMB-2 contains the variable domains (VH and VL) of the anti-CD25 MAb anti-Tac connected by a (G4S)3 peptide linker, with VL fused to PE38. The anti-CD22 recombinant immunotoxins BL22 and moxetumomab pasudotox contain a disulfide-stabilized Fv created by engineering a disulfide bond into the framework regions, replacing Arg44 of VH and Gly100 of VL. Moxetumomab pasudotox differs from BL22 in that amino acids 100, 100a and 100b of VH, in the 3rd complementarity determining region (CDR3) is mutated from SSY to THW, resulting in 14-fold improved binding affinity.
Figure 2. Structure and intoxication of moxetumomab pasudotox.
Left, ribbon diagram of moxetumomab pasudotox showing the Fv and domains II and III of Pseudomonas exotoxin. On the right are steps required for the entry and cell killing. After internalization, the truncated 38 kDa toxin fragment PE38 undergoes proteolysis and disulfide-bond reduction to separate the catalytic domain III from the ligand [48-50]. The carboxy terminal lysine residue is removed [79] resulting in a 35 kDa carboxy terminal toxin ending in REDL. The KDEL receptor transports this fragment from the Golgi to the endoplasmic reticulum (ER) [52], where it translocates to the cytosol, ADP-ribosylates elongation factor 2 [41], and leads to apoptotic cell death [56].
Use of LMB2 in HCL
Although anti-CD25 recombinant immunotoxins leading to LMB-2 were first developed to target adult T-cell leukemia (ATL) [60-63] and chronic lymphocytic leukemia (CLL) [61], LMB-2 was found to be highly cytotoxic toward primary HCL cells ex vivo [64]. As part of a phase 1 trial in hematologic malignancies, LMB-2 was tested in 4 patients with HCL, achieving 1 CR and 3 PRs [28, 29]. All 4 patients had prior cladribine and interferon, and 3 of the 4 had prior splenectomy. The patient without prior splenectomy had CR after cycle 2, and did not require retreatment for relapse until 7 ½ years later. One of 3 patients made neutralizing antibodies. Due to the success of anti-CD22 recombinant immunotoxins for the treatment of HCL, LMB-2 is currently undergoing phase 2 testing only in patients with prior anti-CD22 recombinant immunotoxin, or ineligible for moxetumomab pasudotox, and LMB-2 continues to achieve major responses including CR.
Development of BL22 in HCL
For B-cell malignancies including HCL, CD22 is expressed more widely and at much greater density than CD25 [65, 66]. During preclinical development, chemical immunoconjugates of anti-CD22 Mabs LL2 [67] or RFB4 [68] with truncated Pseudomonas exotoxin were cytotoxic ex vivo to malignant B-cells, but only RFB4 was successful in making a cytotoxic recombinant immunotoxin [69, 70]. BL22, shown in Figure 1, was produced by construction of a disulfide-stabilized Fv of RFB4 fused to PE38, the same truncated form of Pseudomonas exotoxin as LMB-2 [70]. The disulfide-stabilized immunotoxin, modeled in Figure 2, was produced by engineering a disulfide bone within the Fv framework region. This was accomplished by mutating residues in VL and VH to cysteine which were predicted to be separated by the distance of a disulfide bond [71]. As shown in Figure 1, BL22 contains VL disulfide-bonded to a fusion of VH and PE38, but the molecule is still considered recombinant because it forms during renaturation of the crude protein made in E coli, without chemical conjugation. BL22 was cytotoxic toward CD22+ malignant cells ex vivo [72] at concentrations achievable in non-human primates [73].
Clinical development of BL22 in HCL
In phase I testing, BL22 was tested in 31 patients with relapsed/refractory HCL, of whom 28 had classic disease and 3 had HCLv [16, 30]. Attributed to the high CD22 receptor density of CD22 on HCL cells (median ~44,000 sites/cell), and the ability of CD22 to internalize, clinical activity was significant with 19 (61%) CRs and 6 (19%) PRs. All 3 with HCLv achieved CR. Dose-limiting capillary leak syndrome, a common toxicity of immunotoxin chemical conjugates attributed to endothelial damage [74], was not observed in these patients except in one case associated with cytokine release syndrome. Instead, the dose-limiting toxicity of BL22 in HCL was a completely reversible hemolytic uremic syndrome, characterized by transient thrombocytopenia, hemolytic anemia, and renal dysfunction, affecting 13% of patients [30]. Only one patient on this trial was permitted by protocol to receive enough cycles to eradicate MRD by bone marrow aspirate flow cytometry, and this patient has been in continuous MRD-negative CR for nearly 14 years. Since many of the CRs were achieved with a single cycle of BL22, and a single cycle did not cause HUS, phase II testing of BL22 in HCL was undertaken using 1 cycle, with retreatment only if needed to resolve cytopenias. On this trial, 25% of 36 patients achieved CR with 1 cycle, and including the 56% of patients who were retreated, 47% of the 36 achieved CR, with overall response rate (ORR) 72% [31]. Ironically, those with the best outcome on this trial were those who failed to respond well to one cycle and consequently received multiple cycles of BL22, including 2 cycles past CR. Two patients have remain in MRD-negative CR ongoing for 8-10.5 years. On the phase II trial, the rate of HUS was decreased to 6% vs 13% on phase I. The mechanism of HUS with BL22 was felt to be at least partly CD22 mediated since patients treated with PE38-containing immunotoxins targeting antigens other than CD22 do not get HUS. Future development of this agent continued with an affinity matured mutant with more selective binding to CD22.
Development of moxetumomab pasudotox for HCL
To increase the affinity of BL22 for CD22, phage selection was used to select molecules containing random mutations within localized ‘Hot Spots’ within the CDR3 domain of VH. A mutant containing the amino acids THW instead of SSY at position 100, 100A and 100B of VH was determined to have 14-fold improved binding affinity due to lower off-rate [75]. The resulting disulfide-stabilized recombinant immunotoxin HA22 was renamed CAT-8015 and now moxetumomab pasudotox (Figure 2). Preclinical studies of moxetumomab pasudotox showed similar toxicology and improved efficacy compared to BL22 [76]. The new recombinant immunotoxin was prepared for phase I testing, which began in 2007 in patients with multiply relapsed HCL.
Interim phase I results of moxetumomab pasudotox after dose-escalation
Phase I testing in 28 patients with HCL, including 16 at 5-40 ug/Kg every other day for 3 doses (QOD x3), and 12 at 50 ug/Kg QOD x3, showed a CR rate of 46% and ORR 86% [32]. In this trial, patients could be treated with up to 2 cycles after documentation of CR, but had to stop earlier if high levels of neutralizing antibodies or progressive disease were documented. All but one of the 28 patients were retreated and a total of 114 cycles were administered to the 28 patients. CR was documented after 2-5 (median 3) cycles, and a total of 10 consolidation cycles were given to 5 patients. CR did not correlate with the number of cycles of prior purine analog or duration of response to the last purine analog cycle. However, the CR rate was 0 of 7 in patients with prior splenectomy vs 13 (62%) of 21 patients without prior splenectomy (p=0.007). Thus moxetumomab pasudotox may be more appropriate to initiate before splenectomy, possibly because once the spleen is removed, cytopenias resolve and patients become ineligible until bone marrow HCL infiltrations becomes very advanced. With respect to durability of response, 80% of 13 CRs remained in CR for a median of 29 months. Of 8 patients remaining in CR who were evaluated for MRD by flow cytometry of blood and bone marrow biopsy IHC, 7 were MRD-negative. Thus, most of the CRs achieved by moxetumomab pasudotox were MRD-negative by these studies, and MRD negativity was associated with CR durability [32]. Immunogenicity analysis showed neutralizing antibodies in 10 (38%) of 26 evaluable patients, only 1 after cycle 1. No dose-limiting toxicity was observed on this trial. Two patients (7%) had a grade 2 HUS associated with only grade 1 abnormalities of platelets and creatinine, which rapidly recovered with sequelae. Other toxicities were mostly related to mild capillary leak, attributed to the immunotoxin passing through the endothelial cells lining the blood vessels, featuring hypoalbuminemia, edema, hypotension, fatigue, weight gain and proteinuria.
Continued preclinical development targeting CD22
To try and avoid the production of anti-drug antibodies and to allow higher doses to be safely given, a new recombinant immunotoxin (LMB11) targeting CD22 has been developed. It has an Fab instead of an Fv, a deletion of most of domain II including the B and T cell epitopes present in domain II, and 7 point mutations in domain III to suppress B cell epitopes [77]. LMB11 is very cytotoxic to cells from patients with HCL, can be safely given to mice at much higher doses than moxetumomab pasudotox and has produced complete remissions of the Burkitt Lymphoma cell line CA46 growing in immune-deficient mice. Other efforts have been devoted to decreasing immunogenicity by identifying and removing T cell epitopes [78].
Summary
Recombinant immunotoxins targeting CD22 have shown significant clinical utility and a safety profile justifying pivotal clinical testing for treatment of relapsed/refractory HCL. Moxetumomab pasudotox is now undergoing multicenter phase III testing. Although this agent is administered i.v. rather than orally, we believe it has major advantages over other non-chemotherapy approaches, including BRAF inhibition, in its ability to eradicate MRD in a high percentage of patients. Although the value of MRD eradication is debatable in first-line HCL treatment where remission even with MRD is long, in multiply relapsed HCL, removal of MRD appears critical to adequate response durability. The protein engineering which led to the creation of moxetumomab pasudotox is proceeding to identify mechanisms for immunogenicity.
Practice Points.
Hairy cell leukemia is an indolent B-cell malignancy highly response to single agent purine analog therapy, but most patients are left with minimal residual disease.
Relapsed/refractory hairy cell leukemia is a significant problem, as are the poor prognosis variants, all of which may respond very poorly to standard therapies.
Research Agenda.
Both classic and variant hairy cells strongly express CD22 and are thus candidates for recombinant immunotoxin moxetumomab pasudotox targeting this antigen.
Durable complete remission without minimal residual hairy cell leukemia can be achieved with moxetumomab pasudotox, and a multicenter pivotal trial is ongoing.
Acknowledgements
Research in this review was supported in part by the intramural program of the NIH and the Hairy Cell Leukemia Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors are co-inventors on the patent for moxetumomab pasudotox, which has been assigned to the NIH.
References
- 1.Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13:609–630. doi: 10.1182/blood-2016-01-696179. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol. 1995;13:974–982. doi: 10.1200/JCO.1995.13.4.974. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4 Vol. 2. World Health Organization; 2008. [Google Scholar]
- 5.Matutes E, Wotherspoon A, Catovsky D. The variant form of hairy-cell leukaemia. Best Pract Res Clin Haematol. 2003;16:41–56. doi: 10.1016/s1521-6926(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 6.Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4:547–559. doi: 10.1016/0145-2126(80)90066-1. [DOI] [PubMed] [Google Scholar]
- 7.Robak T. Hairy-cell leukemia variant: recent view on diagnosis, biology and treatment. Cancer Treat Rev. 2011;37:3–10. doi: 10.1016/j.ctrv.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Shao H, Calvo KR, Grönborg M, et al. Distinguishing Hairy Cell Leukemia Variant from Hairy Cell Leukemia: Development and Validation of Diagnostic Criteria. Leuk Res. 2012;37:401–409. doi: 10.1016/j.leukres.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matutes E. Immunophenotyping and differential diagnosis of hairy cell leukemia. Hematol Oncol Clin North Am. 2006;20:1051–1063. doi: 10.1016/j.hoc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Venkataraman G, Aguhar C, Kreitman RJ, et al. Characteristic CD103 and CD123 Expression Pattern Defines Hairy Cell Leukemia: Usefulness of CD123 and CD103 in the Diagnosis of Mature B-Cell Lymphoproliferative Disorders. Am J Clin Pathol. 2011;136:625–630. doi: 10.1309/AJCPKUM9J4IXCWEU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasper GA, Arun I, Venzon D, et al. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry B Clin Cytom. 2011;80:83–90. doi: 10.1002/cyto.b.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpe RW, Bethel KJ. Hairy cell leukemia: diagnostic pathology. Hematol Oncol Clin North Am. 2006;20:1023–1049. doi: 10.1016/j.hoc.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Arons E, Suntum T, Stetler-Stevenson M, Kreitman RJ. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood. 2009;114:4687–4695. doi: 10.1182/blood-2009-01-201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119:3330–3332. doi: 10.1182/blood-2011-09-379339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saven A, Burian C, Koziol JA, Piro LD. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–1926. [PubMed] [Google Scholar]
- 16.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the Anti-CD22 Recombinant Immunotoxin BL22 in Chemotherapy-Resistant Hairy-Cell Leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 17.Chadha P, Rademaker AW, Mendiratta P, et al. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine (2-CdA): long-term follow-up of the Northwestern University experience. Blood. 2005;106:241–246. doi: 10.1182/blood-2005-01-0173. [DOI] [PubMed] [Google Scholar]
- 18.Piro LD, Carrera CJ, Carson DA, Beutler E. Lasting remissions in hairy-cell leukemia induced by a single infusion of 2-chlorodeoxyadenosine. N Engl J Med. 1990;322:1117–1121. doi: 10.1056/NEJM199004193221605. [DOI] [PubMed] [Google Scholar]
- 19.Spiers AS, Moore D, Cassileth PA, et al. Remissions in hairy-cell leukemia with pentostatin (2'-deoxycoformycin) N Engl J Med. 1987;316:825–830. doi: 10.1056/NEJM198704023161401. [DOI] [PubMed] [Google Scholar]
- 20.Goodman GR, Burian C, Koziol JA, Saven A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21:891–896. doi: 10.1200/JCO.2003.05.093. [DOI] [PubMed] [Google Scholar]
- 21.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 22.Flinn IW, Kopecky KJ, Foucar MK, et al. Long-term follow-up of remission duration, mortality, and second malignancies in hairy cell leukemia patients treated with pentostatin. Blood. 2000;96:2981–2986. [PubMed] [Google Scholar]
- 23.Rosenberg JD, Burian C, Waalen J, Saven A. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: a single-institution series. Blood. 2014;123:177–183. doi: 10.1182/blood-2013-06-508754. [DOI] [PubMed] [Google Scholar]
- 24.Sawada H, Nishii K, Suzuki T, et al. Autonomic neuropathy in transgenic mice caused by immunotoxin targeting of the peripheral nervous system. J Neurosci Res. 1998;51:162–173. doi: 10.1002/(SICI)1097-4547(19980115)51:2<162::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Goodman GR, Beutler E, Saven A. Cladribine in the treatment of hairy-cell leukaemia. Best Pract Res Clin Haematol. 2003;16:101–116. doi: 10.1016/s1521-6926(02)00089-0. [DOI] [PubMed] [Google Scholar]
- 26.Consensus resolution: proposed criteria for evaluation of response to treatment in hairy cell leukemia. Leukemia. 1987;1:405. [PubMed] [Google Scholar]
- 27.Cheson BD, Sorensen JM, Vena DA, et al. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine via the Group C protocol mechanism of the National Cancer Institute: a report of 979 patients. J Clin Oncol. 1998;16:3007–3015. doi: 10.1200/JCO.1998.16.9.3007. [DOI] [PubMed] [Google Scholar]
- 28.Kreitman RJ, Wilson WH, Robbins D, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–3348. [PubMed] [Google Scholar]
- 29.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin Anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1614–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 30.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 31.Kreitman RJ, Stetler-Stevenson M, Margulies I, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreitman RJ, Wilson W, Calvo KR, et al. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin Cancer Res. 2013;19:6873–6881. doi: 10.1158/1078-0432.CCR-13-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burotto M, Stetler-Stevenson M, Arons E, et al. Bendamustine and Rituximab in Relapsed and Refractory Hairy Cell Leukemia. Clin Cancer Res. 2013;19:6313–6321. doi: 10.1158/1078-0432.CCR-13-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arons E, Margulies I, Sorbara L, et al. Minimal residual disease in hairy cell leukemia patients assessed by clone-specific polymerase chain reaction. Clin Cancer Res. 2006;12:2804–2811. doi: 10.1158/1078-0432.CCR-05-2315. [DOI] [PubMed] [Google Scholar]
- 36.FitzGerald DJP, Waldmann TA, Willingham MC, Pastan I. Pseudomonas exotoxin-Anti-Tac: cell specific immunotoxin active against cells expressing the human T cell growth factor receptor. J Clin Invest. 1984;74:966–971. doi: 10.1172/JCI111516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kronke M, Depper JM, Leonard WJ, et al. Adult T Cell Leukemia: A potential target for ricin A chain immunotoxins. Blood. 1985;65:1416–1421. [PubMed] [Google Scholar]
- 38.Pastan I, Willingham MC, FitzGerald DJ. Immunotoxins. Cell. 1986;47:641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- 39.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 40.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 41.Carroll SF, Collier RJ. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987;262:8707–8711. [PubMed] [Google Scholar]
- 42.Van Ness BG, Howard JB, Bodley JW. ADP-ribosylation of elongation factor 2 by diphtheria toxin. Isolation and properties of the novel ribosyl-amino acid and its hydrolysis products. J Biol Chem. 1980;255:10717–01720. [PubMed] [Google Scholar]
- 43.Lorberboum-Galski H, FitzGerald D, Chaudhary V, et al. Cytotoxic activity of an interleukin 2-Pseudomonas exotoxin chimeric protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988;85:1922–1926. doi: 10.1073/pnas.85.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw JP, Akiyoshi DE, Arrigo DA, et al. Cytotoxic properties of DAB486EGF and DAB389EGF, epidermal growth factor (EGF) receptor-targeted fusion toxins. J Biol Chem. 1991;266:21118–21124. [PubMed] [Google Scholar]
- 45.Kreitman RJ. Getting plant toxins to fuse. Leukemia Res. 1997;21:997–999. doi: 10.1016/s0145-2126(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhary VK, Queen C, Junghans RP, et al. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989;339:394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- 47.Du X, Beers R, FitzGerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and-CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogata M, Fryling CM, Pastan I, FitzGerald DJ. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J Biol Chem. 1992;267:25396–25401. [PubMed] [Google Scholar]
- 49.Fryling C, Ogata M, FitzGerald D. Characterization of a cellular protease that cleaves Pseudomonas exotoxin. Infect Immun. 1992;60:497–502. doi: 10.1128/iai.60.2.497-502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiron MF, Fryling CM, FitzGerald DJ. Cleavage of Pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J Biol Chem. 1994;269:18167–18176. [PubMed] [Google Scholar]
- 51.McKee ML, FitzGerald DJ. Reduction of furin-nicked Pseudomonas exotoxin A: An unfolding story. Biochemistry. 1999;38:16507–16513. doi: 10.1021/bi991308+. [DOI] [PubMed] [Google Scholar]
- 52.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theuer C, Kasturi S, Pastan I. Domain II of Pseudomonas exotoxin A arrests the transfer of translocating nascent chains into mammalian microsomes. Biochemistry. 1994;33:5894–5900. doi: 10.1021/bi00185a029. [DOI] [PubMed] [Google Scholar]
- 54.Theuer CP, Buchner J, FitzGerald D, Pastan I. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc Natl Acad Sci U S A. 1993;90:7774–7778. doi: 10.1073/pnas.90.16.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webb TR, Cross SH, McKie L, et al. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J Cell Sci. 2008;121:3140–3145. doi: 10.1242/jcs.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinkmann U, Brinkmann E, Gallo M, Pastan I. Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc Natl Acad Sci U S A. 1995;92:10427–10431. doi: 10.1073/pnas.92.22.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keppler-Hafkemeyer IJC, Kreitman RJ, Pastan I. Apoptosis induced by immunotoxins used in the treatment of hematologic malignancies. Int J Cancer. 2000;87:86–94. [PubMed] [Google Scholar]
- 58.Decker T, Oelsner M, Kreitman RJ, et al. Induction of Caspase-Dependent Programmed Cell Death in B-Cell Chronic Lymphocytic Leukemia Cells by Anti-CD22 Immunotoxins. Blood. 2004;103:2718–2726. doi: 10.1182/blood-2003-04-1317. [DOI] [PubMed] [Google Scholar]
- 59.Du X, Youle RJ, FitzGerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–3452. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreitman RJ, Batra JK, Seetharam S, et al. Single-chain immunotoxin fusions between anti-Tac and Pseudomonas exotoxin: relative importance of the two toxin disulfide bonds. Bioconj Chem. 1993;4:112–120. doi: 10.1021/bc00020a002. [DOI] [PubMed] [Google Scholar]
- 61.Kreitman RJ, Chaudhary VK, Kozak RW, et al. Recombinant toxins containing the variable domains of the anti-Tac monoclonal antibody to the interleukin-2 receptor kill malignant cells from patients with chronic lymphocytic leukemia. Blood. 1992;80:2344–2352. [PubMed] [Google Scholar]
- 62.Kreitman RJ, Chaudhary VK, Waldmann TA, et al. Cytotoxic activities of recombinant immunotoxins composed of Pseudomonas toxin or diphtheria toxin toward lymphocytes from patients with adult T-cell leukemia. Leukemia. 1993;7:553–562. [PubMed] [Google Scholar]
- 63.Saito T, Kreitman RJ, Hanada S-i, et al. Cytotoxicity of recombinant Fab and Fv immunotoxins on adult T-cell leukemia lymph node and blood cells in the presence of soluble interleukin-2 receptor. Cancer Res. 1994;54:1059–1064. [PubMed] [Google Scholar]
- 64.Robbins DH, Margulies I, Stetler-Stevenson M, Kreitman RJ. Hairy cell leukemia, a B-cell neoplasm which is particularly sensitive to the cytotoxic effect of anti-Tac(Fv)-PE38 (LMB-2) Clin Cancer Res. 2000;6:693–700. [PubMed] [Google Scholar]
- 65.Cordone I, Annino L, Masi S, et al. Diagnostic relevance of peripheral blood immunocytochemistry in hairy cell leukaemia. J Clin Pathol. 1995;48:955–960. doi: 10.1136/jcp.48.10.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robbins BA, Ellison DJ, Spinosa JC, et al. Diagnostic application of two-color flow cytometry in 161 cases of hairy cell leukemia. Blood. 1993;82:1277–1287. [PubMed] [Google Scholar]
- 67.Kreitman RJ, Hansen HJ, Jones AL, et al. Pseudomonas exotoxin-based immunotoxins containing the antibody LL2 or LL2-Fab' induce regression of subcutaneous human B-cell lymphoma in mice. Cancer Res. 1993;53:819–825. [PubMed] [Google Scholar]
- 68.Mansfield E, Pastan I, FitzGerald DJ. Characterization of RFB4-Pseudomonas exotoxin A immunotoxins targeted to CD22 on B-cell malignancies. Bioconj Chem. 1996;7:557–563. doi: 10.1021/bc960043y. [DOI] [PubMed] [Google Scholar]
- 69.Mansfield E, Chiron MF, Amlot P, et al. Recombinant RFB4 single-chain immunotoxin that is cytotoxic towards CD22-positive cells. Biochem Soc Trans. 1997;25:709–714. doi: 10.1042/bst0250709. [DOI] [PubMed] [Google Scholar]
- 70.Mansfield E, Amlot P, Pastan I, FitzGerald DJ. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 71.Reiter Y, Brinkmann U, Kreitman RJ, et al. Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry. 1994;33:5451–5459. doi: 10.1021/bi00184a014. [DOI] [PubMed] [Google Scholar]
- 72.Kreitman RJ, Margulies I, Stetler-Stevenson M, et al. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) towards fresh malignant cells from patients with B-cell leukemias. Clin Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 73.Kreitman RJ, Wang QC, FitzGerald DJP, Pastan I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by Cynomolgus monkeys. Int J Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 74.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 75.Salvatore G, Beers R, Margulies I, et al. Improved Cytotoxic activity towards cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 76.Alderson RF, Kreitman RJ, Chen T, et al. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res. 2009;15:832–839. doi: 10.1158/1078-0432.CCR-08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bera TK, Onda M, Kreitman RJ, Pastan I. An improved recombinant Fab-immunotoxin targeting CD22 expressing malignancies. Leuk Res. 2014;38:1224–1229. doi: 10.1016/j.leukres.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mazor R, Eberle J, Hu X, et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc Natl Acad Sci U S A. 2014;111:8571–8576. doi: 10.1073/pnas.1405153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hessler JL, Kreitman RJ. An early step in Pseudomonas exotoxin action is removal of the terminal lysine residue, which allows binding to the KDEL receptor. Biochemistry. 1997;36:14577–14582. doi: 10.1021/bi971447w. [DOI] [PubMed] [Google Scholar]