Abstract
Hyperperfusion syndrome (HPS) after carotid artery stenting (CAS) may cause hemorrhagic or ischemic events leading to serious sequelae. We previously reported the staged angioplasty (SAP) to prevent HPS. In the present study, we analyzed our treatment results of SAP to know its effectiveness and problems. The study included 43 patients scheduled for SAP in whom preoperative single photon emission computed tomography (SPECT) showed severely impaired cerebral blood flow (CBF). The analyzed subjects were 38 males and 4 females, mean age was 73 ± 8.5 years old. SAP was indicated for the patients whose CBF ratio in the affected/unaffected hemisphere (asymmetry index) was below 0.8, and cerebrovascular reactivity measured by acetazolamide challenge was below 10%. First, percutaneous transluminal angioplasty (PTA) was performed. If PTA was successful, CAS was performed 2 weeks later. If PTA was not successful due to inadequate dilatation or extensive dissection, a stent was placed. SPECT was performed immediately after PTA and CAS to confirm the presence or absence of hyperperfusion phenomenon (HPP) indicating radiologic hyperperfusion. In 39 of 43 patients (91%), SAP was successfully performed and HPP was not observed. On the other hand, in the other four patients (9%), immediate stent placement was added due to inadequate dilatations in three patients and vascular dissection in one. Among 43 candidates for SAP, 41 patients (95.4%) had favorable course, but one hemorrhagic and one ischemic complications were observed after PTA. SAP was a relatively simple procedure, and its clinical results seemed acceptable.
Keywords: staged angioplasty, carotid artery stenosis, stenting, hyperperfusion syndrome
Introduction
Carotid artery stenosis is a part of arteriosclerotic disease, for which the number of treatment has been steadily growing. Carotid artery stenting (CAS) was reported to have equivalent treatment results with carotid endarterectomy (CEA) in two studies of Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE)1) and Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).2) In CAS, embolic and hemorrhagic complications occur. Among them, hyperperfusion syndrome (HPS) is noted in 1.1% of patients after CAS and may result in potentially serious sequelae secondary to intracerebral hemorrhage in 0.7%.3) The cause is considered a transient dysfunction of cerebral vascular autoregulation after revascularization. For HPS after CEA, rigorous postoperative control of blood pressure is appropriate, although some reports showed that it is ineffective after CAS.3) Similarly, HPS after CAS is also known to more likely to develop in preoperative patients with impaired cerebral blood flow (CBF), so we have previously reported staged angioplasty (SAP) for avoidance of this complication.4) This study describes our clinical experience of SAP including hemorrhagic and ischemic complications.
Subjects and Methods
I. Patients
Of 293 patients who underwent CAS between April 2004 and February 2015, 43 patients in whom SAP was performed were included in the study. Subjects are as follows: 38 males and 4 females with mean age of 73 ± 8.5 years old, and 33 symptomatic and 10 asymptomatic lesions (Table 1).
Table 1.
Characteristics of the patients treated with staged angioplasty
| Age, mean ± SD* (y) | 73.3 ± 8.5 |
| Male, n (%) | 38 (88.4) |
| Stenosis, mean ± SD* | 93.0 ± 3.8 |
| Hypertension, n (%) | 35 (81.4) |
| Diabetes, n (%) | 17 (39.5) |
| Hyperlipidemia, n (%) | 19 (44.4) |
SD: standard deviation.
II. Preoperative evaluation
CAS was indicated for patients who had symptomatic lesions with stenosis rate of ≥ 50% or asymptomatic lesions with that of ≥ 80% according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria.1,5) The patients with the stenosis rate of ≥ 80% was subject to preoperative 123I-IMP single photon emission computed tomography (SPECT) according to the report showing that patients with more than 80% of severe ICA stenosis exhibits a drop of CBF and a significant increase of oxygen extraction fraction (OEF) on positron emission computed tomography (PET).6)
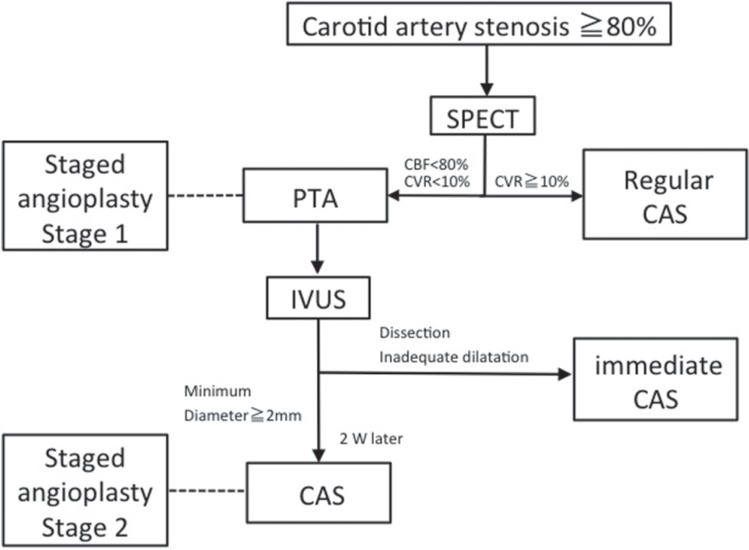
SAP was indicated for patients in whom preoperative cerebral blood test revealed that cerebrovascular reactivity (CVR) was less than 10% and that the CBF ratio of the affected to unaffected hemisphere (asymmetry index: AI) was < 0.8 among these patients (Fig. 1). Currently, we perform SAP only for the patients at high risk of post procedure HPS, whose asymmetry index was no more than 0.8 with CVR of less than 10% on preoperative SPECT. Basically, patients with soft plaques which was shown as high signal intensity on time-of-flight MRA7,8) or with circumferential calcification were excluded from this study.
Fig. 1.
Treatment strategy of carotid artery stenosis. CAS: carotid artery stenting, CBF: cerebral blood flow, CVR: cerebrovascular reactivity, IVUS: intravascular ultrasound, PTA: percutaneous transluminal angioplasty, SPECT: single photon emission computed tomography.
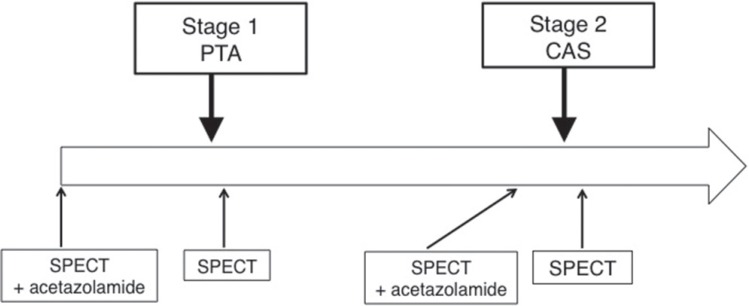
III. Preoperative 123I-IMP SPECT (Fig. 2)
Fig. 2.
Schedule of SPECT. CAS: carotid artery stenting. PTA: percutaneous transluminal angioplasty, SPECT: single photon emission computed tomography.
111MBq of N-Isopropyl-4-Iodoamphetamine (123I- IMP) (Nihon Medi Physics Co. Ltd., Nishinomiya, Hyogo) was intravenously injected twice, once at the time of SPECT scan (t = 0 min) and again at 30 min later. Arterial blood was sampled at 10 min for calibrating the standardized arterial input function. Acetazolamide was administered at 10 min before the second IMP administration. SPECT just after each angioplasty was qualitatively performed under IMP administration without acetazolamide (Diamox) load within 24 hours of the procedure.
Based on the previously mentioned criteria,4) patients whose asymmetry index was less than 0.8 with CVR of less than 10% were judged to be at high risk for post-treatment hyperperfusion phenomenon (HPP). HPP was defined as asymmetrical index of CBF was over 1.2 on SPECT just after angioplasty.
IV. SAP
1. Preoperative antiplatelet therapy
Treatment regimen with two antiplatelet agents composed of aspirin (81–100 mg) and cilostazol (100–200 mg) or clopidogrel (75 mg) were started at least 1 week before percutaneous transluminal angioplasty (PTA). When platelet aggregation test just before PTA indicated that both drugs were ineffective, the drug was switched to another one and/or the dosage was increased. These two drugs were concomitantly used for 3 months after CAS, and then more effective drug in platelet aggregation was continued.
2. SAP stage 1: balloon angioplasty
Proximal protection device such as MO.MA ultra (Medtronic JAPAN Co. Ltd., Tokyo) was used. Each of the patients was systemically heparinized during the operation, and activated partial thromboplastin time (APTT) was controlled at 1.5–2 times that before the procedure. Semi-compliant 2.5 mm balloon catheter was first used. The balloon was inflated at nominal pressure for 30 seconds, and then intravascular ultrasound (IVUS) imaging was performed to confirm whether the minimal lumen diameter was increased to ≥ 2 mm. If successful, the procedure was completed. In case of inadequate dilatation, the balloon was further inflated for additional 2 min at rated burst pressure. In case of inadequate dilatation or extensive dissection, stent placement was added immediately.
3. SAP stage 2: CAS
Basically, CAS was performed at 2 weeks after the PTA. Each of the patients under local anesthesia was systemically heparinized during the operation, and APTT was set at 1.5–2 times that before operation. Following pre-dilatation with a semi-compliant balloon under proximal cerebral protection, a self-expanding nitinol stent was placed, and post-dilatation was additionally performed if needed. After stenting, IVUS was performed to identify the degree of stent apposition and presence of plaque protrusion from the stent strut.
V. Assessment of clinical outcome
We confirmed the presence or absence of HPP or HPS after PTA and CAS. HPS was defined as a condition in which headache, seizures, and cerebral hemispheric symptoms or intracerebral hemorrhage develop after the procedure due to abrupt CBF increase in a patient who had severely impaired CBF. The clinical outcome was assessed by modified Rankin Scale (mRS) on discharge.
VI. Statistical analysis
Descriptive statistical analysis was performed including mean ± standard deviation or median and interquartile range (IQR). Wilcoxon/Kruskal-Wallis test was used to compare the two groups, which found a statistically significant difference between the both groups with P < 0.05. Data were statistically analyzed using JMP 11 software (SAS Institute, Cary, North Carolina, USA).
Representative Case
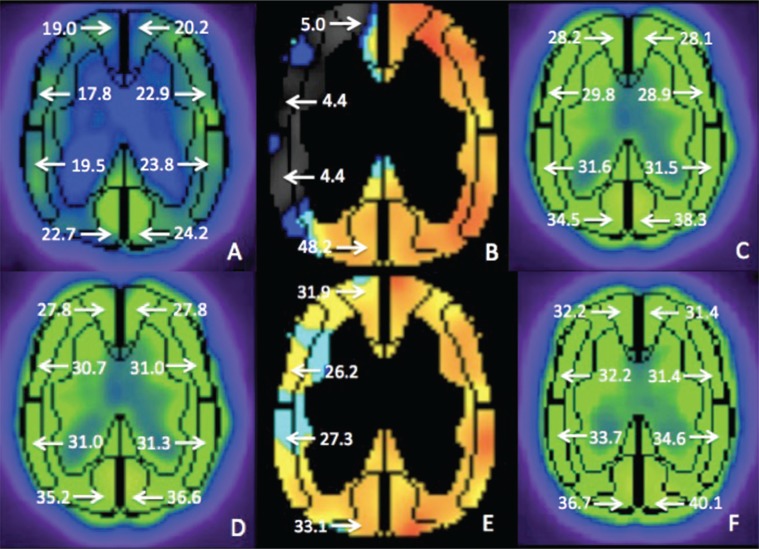
An 82-year-old male had right symptomatic carotid artery stenosis. Preoperative SPECT revealed severely impaired CBF of the right cerebral hemisphere (Fig. 3), and then SAP was scheduled. PTA was performed using the 2.5 mm × 3 cm balloon at 14 atm for 120 seconds under proximal protection, and the lesion was successfully dilated (Fig. 4A, B). After IVUS confirmed that the minimal lumen diameter was ≥ 2 mm, the procedure was finished. SPECT indicated no HPP both after PTA and after CAS performed 2 weeks later successfully.
Fig. 3.
Changes of cerebral blood flow (CBF) on 123I-IMP single photon emission computed tomography (SPECT) in a representative case. Preoperative SPECT showing decrease of rest-CBF (A) and cerebrovascular reactivity (CVR) (B) in the right middle cerebral artery (MCA) territory. SPECT just after stage 1 angioplasty (C) showing no hyperperfusion. SPECT before stage 2 angioplasty showing no decrease of CBF (D), but slight decrease in CVR (E). Postoperative SPECT showing no hyperperfusion after stenting (F).
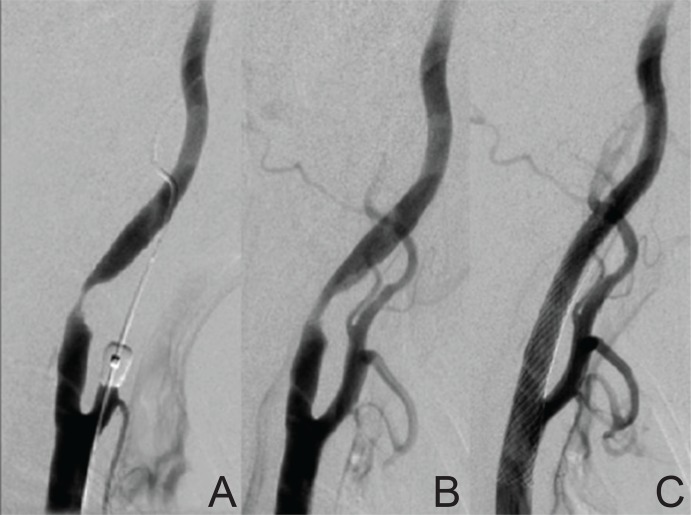
Fig. 4.
The change of angiographic findings before and after staged angioplasty. A: Right carotid angiography showing severe stenosis on the internal carotid artery (ICA) before treatment. B: ICA stenosis was partially dilated by stage 1 angioplasty with an undersized balloon. C: The stenosis was fully dilated by stenting at stage 2 treatment.
Results
I. Clinical results (Table 2)
Table 2.
Summary of staged angioplasty
| Treatment | No. of case | HPP on SPECT | Complication/changes in mRS |
|---|---|---|---|
| Staged angioplasty | 39 (91%) | 0 | Hemorrhage:1/mRS: 0→1 Minor stroke:1/mRS: 0→1 |
| Immediate CAS | 4 (9%) | 2 | 0 |
| Inadequate dilatation | 3 | ||
| Dissection | 1 | ||
| Total | 43 (100%) | 2 | 2 |
CAS: carotid artery stenting, HPP: hyperperfusion phenomenon, mRS: modified Rankin Scale, SPECT: single photon emission computed tomography.
Between April 2004 and February 2015, treatment with this procedure was performed in 43 cases, and 39 (95.4%) were technically successful. Overall, clinical course was uneventful in 41 patients, but one intracranial hemorrhage and one minor stroke were observed. In the patient who showed intracranial hemorrhage, extreme hypertension (> 220 mmHg) was observed after the angioplasty due to abdominal pain caused by bladder cancer. After this, consciousness was gradually disturbed. So, uncontrollable hypertension was considered to be the main reason of the hemorrhage.
In these patients, mRS score was changed from 0 to 2 and from 0 to 1, respectively. No patients who completed SAP experienced hyperperfusion symptoms including headache, seizure, or cerebral hemispheric symptoms after PTA or CAS.
II. Stenosis rate (%)
Median (IQR) stenosis rate (%) decreased from 95 (90–95) at pretreatment to 70 (60–75) after stage 1 procedure and to 0 (0–10) after stage 2.
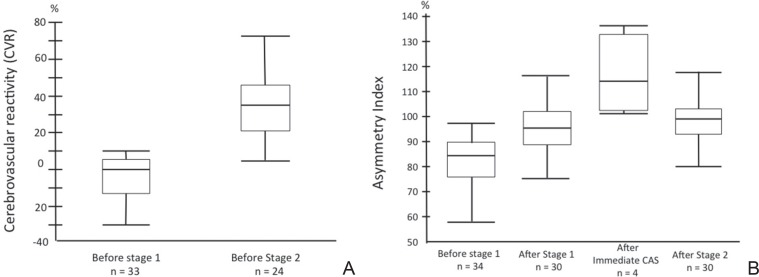
III. CBF change (Fig. 5A, B)
Fig. 5.
A: Changes of cerebrovascular reactivity on single photon emission computed tomography. B: Changes of asymmetry index.
Median (IQR) CVR (%) increased from 0 (−12.3–4.7) before stage 1 to 34.6 (21.5–45.9) before stage 2 procedure. Median (IQR) AI (%) was 84.2 (75.6–89.4) before stage 1, 96.1 (89.4–101.8) after stage 1 procedure, and 99 (91.8–103.6) after stage 2. Immediate CAS group (only 4 cases) median (IQR) was 123 (105–136) after CAS. In the immediate CAS group, the AI value just after CAS was significantly higher than those of SAP (P = 0.0174).
Discussion
To screen patients at high risk for hyperperfusion and treat them uneventfully, we assessed CBF before treatment of the carotid artery stenosis. A patient whose pretreatment CVR was less than 10% and AI was less than 0.8 was defined as a high-risk patient for hyperperfusion. SAP was indicated for these patients. CVR was improved in all the patients after stage 1 angioplasty in SAP groups. Additionally, there was no HPP in the completed SAP group, whereas 2 of 4 cases showed HPP in the immediate CAS group. Taken together, SAP seemed effective to avoid HPP easily and safely in patients with severely impaired CBF.
A possible mechanism avoiding HPS by SAP is as below; the patients with severely impaired CBF due to ICA stenosis were at high risk of post-procedure hyperperfusion in our previous study.4) This means full dilatation of the stenosis in one-stage CAS causes abrupt increase of CBF, leading to hyperperfusion. So if we can dilate the stenosis partially by first angioplasty using an undersized balloon, it may lead to partial increase of CBF, decreasing the incidence of HPS.
Yoshimura et al. compared nine patients who had underwent one-stage CAS with other nine who had underwent SAP in the patients with severely impaired CBF whose CVR was less than 20% before CAS with AI of ≤ 80% (i.e., high-risk group for postoperative hyperperfusion). Results showed that HPP was noted in five of nine and HPS was in one among the one-stage CAS group, whereas neither HPP nor HPS was found in the SAP group. There was a significant difference in incidence of HPP between both groups, which demonstrates the effectiveness of SAP.9) There are other reports regarding revascularization for carotid artery stenosis associated with severely impaired CBF.10–12)
Egashira et al. reported that the stepwise revascularization in which PTA followed by CEA was performed successfully to avoid HPS in the patients with severely impaired CBF.13) Yoshimoto et al. reported that CEA following the superficial temporal-middle cerebral artery (STA-MCA) anastomosis was performed to avoid HPS in 4 patients with severely impaired CBF with CVR of ≤ 10%.14)
There is also a report that the frequency of hemorrhage secondary to hyperperfusion was 1.1–5.0% in CAS.5) Some physicians consider that this method is not necessarily required because of its low frequency. Also, there are negative opinions that two sessions of procedures in SAP increases procedural events. Additionally, an extended interval between PTA and CAS might raise concerns regarding the occurrence of restenosis or ischemic complications. We previously set the interval as for 1 month.7) However, based on our experience that hyperperfusion did not occur even if the interval until CAS was shorter, we recently performed CAS 2 weeks after PTA. The present study experienced no patients who had acute occlusion and/or high-grade restenosis during the time interval between PTA and CAS, but ischemic event was noted in only one patient. There was no increased hemorrhagic and/or ischemic event due to SAP with achievement of safe and easy treatment.
Results of the nationwide questionnaire conducted in 2014 in Japan Society of Neuroendovascular Therapy indicated that the rate of HPS was 6.5% in regular CAS group and 3.2% in SAP group, which suggest SAP was effective in terms of avoiding HPS.
Limitations of the present study are as follows: it was a retrospective analysis, the test and assessment method of CBF varied, definition of the patient selection is to be determined, and its sample size was small.
In Japan, large scale multi-center studies are being currently planned, in which it is expected to more scientifically examine the appropriateness of the treatment as well as the optimal conditions including the time interval between PTA and CAS, size of PTA balloon to be used, and how to combine antiplatelet agents. It is anticipated that the effectiveness of SAP would be investigated through these trials.
Conclusion
SAP was performed in patients with a severely impaired CBF owing to high grade carotid stenosis who were regarded as at high risk of HPS. It is anticipated that the effect of SAP would be tested in larger trials in the near future.
References
- 1). Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators : Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 351: 1493– 1501, 2004. [DOI] [PubMed] [Google Scholar]
- 2). Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, CREST Investigators : Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 363: 11– 23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, Sakai C, Nagata I, Ogawa A, Japanese Society for Treatment at Neck in Cerebrovascular Disease Study Group : Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107: 1130– 1136, 2007. [DOI] [PubMed] [Google Scholar]
- 4). Kaku Y, Yoshimura S, Kokuzawa J: Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 25: 1403– 1408, 2004. [PMC free article] [PubMed] [Google Scholar]
- 5). North American Symptomatic Carotid Endarterectomy Trial Collaborators : Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445– 453, 1991. [DOI] [PubMed] [Google Scholar]
- 6). Hino A, Tenjin H, Horikawa Y, Fujimoto M, Imahori Y: Hemodynamic and metabolic changes after carotid endarterectomy in patients with high-degree carotid artery stenosis. J Stroke Cerebrovasc Dis 14: 234– 238, 2005. [DOI] [PubMed] [Google Scholar]
- 7). Yoshimura S, Yamada K, Kawasaki M, Asano T, Kanematsu M, Takamatsu M, Hara A, Iwama T: High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke 42: 3132– 3137, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Yoshimura S, Yamada K, Kawasaki M, Asano T, Kanematsu M, Miyai M, Enomoto Y, Egashira Y, Iwama T: Selection of carotid artery stenting or endarterectomy based on magnetic resonance plaque imaging reduced periprocedural adverse events. J Stroke Cerebrovasc Dis 22: 1082– 1087, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Yoshimura S, Kitajima H, Enomoto Y, Yamada K, Iwama T: Staged angioplasty for carotid artery stenosis to prevent postoperative hyperperfusion. Neurosurgery 64: ons122– ons128; discussion ons128–ons129, 2009. [DOI] [PubMed] [Google Scholar]
- 10). Oshida S, Ogasawara K, Saura H, Yoshida K, Fujiwara S, Kojima D, Kobayashi M, Yoshida K, Kubo Y, Ogawa A: Does preoperative measurement of cerebral blood flow with acetazolamide challenge in addition to preoperative measurement of cerebral blood flow at the resting state increase the predictive accuracy of development of cerebral hyperperfusion after carotid endarterectomy? Results from 500 cases with brain perfusion single-photon emission computed tomography study. Neurol Med Chir (Tokyo) 55: 141– 148, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Shimamura N, Kikkawa T, Hatanaka M, Naraoka M, Munakata A, Ohkuma H: Dilation of the internal carotid artery at the entrance to the carotid canal following carotid artery stenting predicts post-procedural hyperperfusion. Interv Neurol 2: 1– 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hosoda K, Kawaguchi T, Ishii K, Minoshima S, Shibata Y, Iwakura M, Ishiguro S, Kohmura E: Prediction of hyperperfusion after carotid endarterectomy by brain SPECT analysis with semiquantitative statistical mapping method. Stroke 34: 1187– 1193, 2003. [DOI] [PubMed] [Google Scholar]
- 13). Egashira Y, Yoshimura S, Yamada K, Enomoto Y, Asano T, Iwama T: Stepwise revascularization by carotid endarterectomy after balloon angioplasty for symptomatic severe carotid artery stenosis. Ann Vasc Surg 26: 731.e9– e13, 2012. [DOI] [PubMed] [Google Scholar]
- 14). Yoshimoto T, Shirasaka T, Yoshidumi T, Fujimoto S, Kaneko S, Kashiwaba T: Stepwise revascularization for prevention of postoperative hyperperfusion. Neurol Med Chir (Tokyo) 46: 283– 287; discussion 288–289, 2006. [DOI] [PubMed] [Google Scholar]