Abstract
Partial targeted embolization of the ruptured site of cerebral arteriovenous malformations (AVMs) is considered effective to prevent rebleeding. The site of rupture is usually determined by morphological features, such as an intranidal aneurysm or a venous varix; however, the site can be difficult to identify in high-grade AVM with complicated angioarchitecture. The authors present a case of a 36-year-old woman with high-grade AVM presented with repeated hemorrhage. Cerebral angiography showed intranidal aneurysm, which was considered the ruptured site. The T1-weighted imaging with gadolinium enhancement demonstrated linear enhancement along the outer surface of the thickened wall of the intranidal aneurysm, which could be supplementary information to identify the ruptured site. Obliteration of the intranidal aneurysm was successfully achieved by emergent targeted embolization using N-butyl cyanoacrylate. The patient recovered and regained an independent status. The patient underwent volume-staged radiosurgery and experienced no further hemorrhage during the 26 months follow-up. Targeted embolization of the ruptured site is considered effective to prevent rebleeding in high-grade cerebral AVMs. Wall enhancement of the intranidal aneurysm, in addition to the structural characteristics, could be helpful in identifying the site of rupture embedded in the complicated angioarchitecture.
Keywords: arteriovenous malformation, targeted embolization, magnetic resonance imaging, site of rupture
Introduction
High-grade arteriovenous malformations (AVMs), such as Spetzler–Martin Grades 4 and 5,1) are generally considered difficult to cure using any modalities such as surgery, embolization, and/or radiosurgery.2) However, endovascular treatment potentially offers an advantage over the other two methods because of the ability to immediately target certain areas of an AVM. Partially targeted embolization could be effective in controlling the bleeding point when treating high-grade AVMs; however, it is not curative.3,4) Especially in acutely ruptured AVMs, in which the source of bleeding can be identified, targeted embolization of this compartment may be able to secure the AVM prior to definitive treatment.
Magnetic resonance imaging (MRI) with gadolinium (Gd) enhancement has been shown to be useful in identifying the site of rupture in patients with multiple intracranial aneurysms,5) but has not yet been applied to patients with ruptured AVMs. Here, we report a case of a ruptured high-grade AVM successfully treated with targeted embolization. MRI with Gd enhancement, in addition to the angiographic structural characteristics, could be helpful in identifying the site of rupture embedded in the complicated angioarchitecture.
Case Report
I. History
This patient originally presented with headaches at the age of 11 years in 1986, and an unruptured left parieto-occipital large AVM was detected. Transarterial embolization using polyvinyl acetate was repeated for a total of eight times starting in 1991 at our hospital. She was independent, although she exhibited a mild right hemiparesis postoperatively. She decided not to undergo any further surgical treatment, and the residual lesion was observed conservatively on an outpatient basis. In March 2012, when she was 36 years old, she presented to our hospital with a partial seizure. The seizure was well controlled with anticonvulsant medication. A subsequent workup revealed a Spetzler–Martin Grade 5 AVM with a 7.3-cm maximum diameter in the left parieto-occipital area (Fig. 1A–C). The arterial supply was provided not only by numerous distal cortical branches of the left anterior, middle, and posterior cerebral circulations but also by the meningeal branches. Intranidal aneurysm with bleb on its top was fed by these meningeal branches through marginal tentorial artery and middle meningeal artery. Venous drainage was both superficial and deep, and a large intranidal aneurysm was embedded in the parietal deep white matter. Volume-staged gamma knife radiosurgery was scheduled, and she received a 16 Gy marginal dose to the lower half of the nidus in August 2012. One month later, she experienced sudden progression of right hemiparesis and was transferred to our hospital.
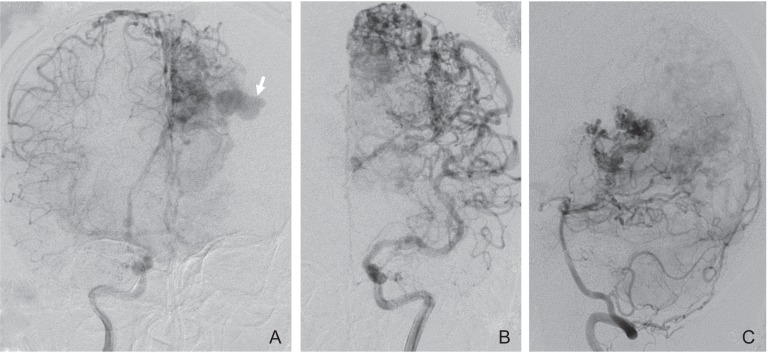
Fig. 1.
Angiograms obtained before hemorrhage. Anteroposterior view of the right internal carotid artery (A), the left internal carotid artery (B), and the left vertebral artery (C) angiogram showing a left parieto-occipital arteriovenous malformation fed by numerous cortical branches with an intranidal aneurysm (arrow).
II. Examination
On arrival, she was alert, and a computed tomography (CT) revealed left parietal intracerebral hemorrhage with intraventricular extension (Fig. 2A). Two hours later, the patient developed a consciousness disturbance, and a second CT revealed an enlargement of the hematoma with acute hydrocephalus. Emergent placement of a ventricular drainage was performed, and her consciousness level improved. On day 2, MRI was performed to evaluate the lesion using a GE 3T Signa HD system (GE Healthcare UK Ltd, Buckinghamshire, England). For the high-resolution T1-weighted imaging before and after intravenous administration of Gd, we used three-dimensional (3D) spoiled gradient-recalled acquisition, repetition time (TR) 8.5 msec, echo time (TE) 1.9 msec, flip angle 15°, matrix 384 × 288, field of view 22 cm, and slice thickness 1.2 mm with zero-fill interpolation. The T1-weighted imaging with Gd enhancement demonstrated linear enhancement along the outer surface of the thickened wall of the intranidal aneurysm (Fig. 3A–C). On day 3, her right hemiparesis suddenly progressed to complete paralysis, and a CT scan revealed rebleeding with an increase in the size of the intracerebral hematoma (Fig. 2B). The intranidal aneurysm was considered the site of rupture, and emergent targeted embolization was performed to prevent further hemorrhage. The intranidal aneurysm was mainly fed by the right middle meningeal artery and the superficial temporal artery through falx (Fig. 4A). These meningeal feeders were embolized using N-butyl cyanoacrylate and micro-coil. The right external and internal carotid angiography after the embolization showed no filling of the intranidal aneurysm (Fig. 4B, C).
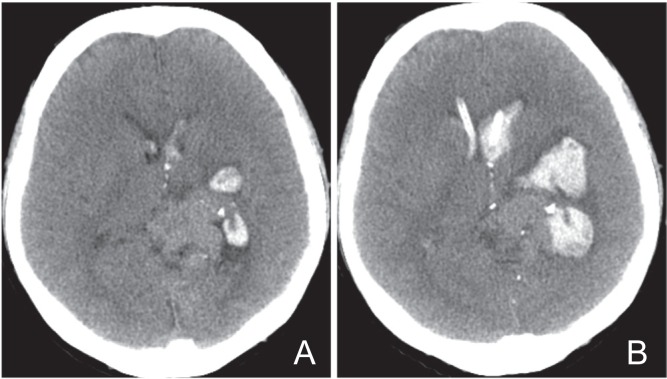
Fig. 2.
A: Computed tomography (CT) on admission showing intracerebral hemorrhage around the left basal ganglia and the left parietal lobe with intraventricular extension. B: CT obtained on day 3 showing the enlargement of the intracerebral hematoma.
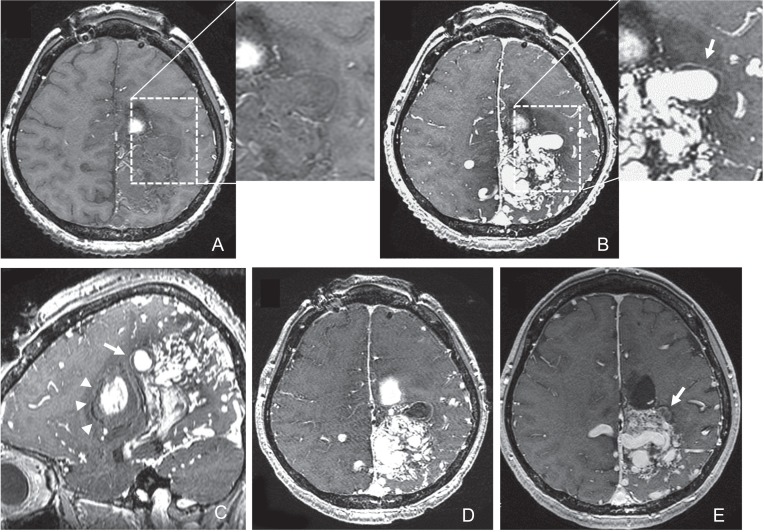
Fig. 3.
Axial non-enhanced (A) and gadolinium-enhanced (B) T1-weighted image obtained on day 2 revealing the linear enhancement of the outer surface of the thickened vessel wall of the intranidal aneurysm (arrows). Sagittal gadolinium-enhanced T1-weighted image (C) showed the intranidal aneurysm located adjacent to the intracerebral hematoma (arrowheads). The outer rim of the hematoma was iso-intensity. Axial gadolinium-enhanced T1-weighted image obtained 5 days after the targeted embolization (D) showing total thrombosis of the intranidal aneurysm. Axial gadolinium-enhanced T1-weighted image obtained 10 months after the targeted embolization (E) showing decrease of the vessel wall enhancement and shrinkage of the intranidal aneurysm (arrow).
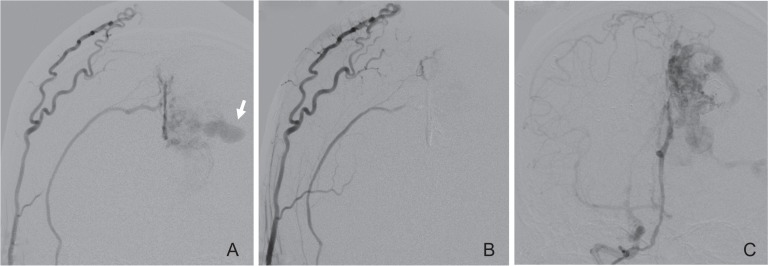
Fig. 4.
A: Right external carotid angiogram obtained before targeted embolization showing that the intranidal aneurysm (arrow) is mainly fed by the right middle meningeal artery through the falx. B: Right external carotid angiogram after the embolization showing no contrast filling of the aneurysm. C: Right internal carotid angiogram after the embolization also showing no contrast filling of the aneurysm.
III. Postoperative course
The patient’s postoperative course was uneventful, and her right hemiparesis gradually improved to an independent status. Postoperative MRI revealed that the intranidal aneurysm had completely thrombosed (Fig. 3D). Follow-up angiography performed 6 months after the embolization confirmed the complete obliteration of the aneurysm. The second session of the volume-staged radiosurgery to the upper one-third of the nidus with the marginal dose of 18 Gy was performed 12 months after the embolization, and the final session to the residual part was scheduled 12 months later. She maintained an independent daily life with mild right hemiparesis and mild aphasia and experienced no further hemorrhage during the 26 months follow-up.
Discussion
Partially targeted embolization has been shown to be effective in treating high-grade AVMs, which have focal weak points, such as intranidal aneurysms or fistulae.3,4,6) In the present case, the intranidal aneurysm was mainly fed by the external carotid system, which was isolated from the internal carotid system. We speculate that feeder embolization of the external carotid system could occlude intranidal aneurysm without affecting hemodynamics of the nidus. Targeted embolization through meningeal branches was successfully performed, and the aneurysm completely thrombosed soon after the embolization. The patient experienced no further hemorrhage and recovered to an independent status. Then, the volume-staged radiosurgery was continued. Targeted embolization is feasible in the setting of a ruptured high-grade AVM if the focal weak point can be identified.
MRI with Gd enhancement was reported useful to identify the site of aneurysmal rupture, using T1-weighted black blood vessel wall sequence.5,7) Matouk et al. investigated 5 patients with aneurysmal subarachnoid hemorrhage, including 3 patients with multiple aneurysms.5) All ruptured aneurysms demonstrated vessel wall enhancement, and none of the associated unruptured aneurysms demonstrated such findings. Nagahata et al. recently investigated 117 consecutive patients with intracranial aneurysms, including 61 ruptured and 83 unruptured aneurysms.7) In this series, strong/faint enhancement of the aneurysm was detected in 73.8/24.6% of the ruptured aneurysms and 4.8%/13.3% of the unruptured aneurysms. These reports might support our idea that the enhancement of the intranidal aneurysm could be supplementary information to identify the ruptured site. Although we did not use the black blood sequence, high-resolution MRI demonstrated linear enhancement of the outer surface of the thickened vessel wall of the intranidal aneurysm, which was adjacent to the hematoma. Physical disruption of the vessel wall and an inflammatory response could explain the thick vessel wall enhancement of the ruptured aneurysms.5,7,8) Such mechanisms could also be applied to the site of rupture in cerebral AVMs. MRI obtained 10 months after the treatment showed decrease of the vessel wall enhancement, which could confirm these mechanisms of vessel wall enhancement of the ruptured site.
This case presented with intracerebral hemorrhage around the intranidal aneurysm. The AVM ruptured at least 3 times within a few days with an associated growing hematoma. Although we did not use the black blood sequence, high-resolution MRI demonstrated linear enhancement of the outer surface of the thickened vessel wall of the intranidal aneurysm, which was adjacent to the hematoma. Considering the rapid deterioration of the patient’s condition and these radiological findings, we decided to perform endovascular embolization targeted to the aneurysm, which effectively prevented further hemorrhage. Although it cannot be generalized, present case indicates the usefulness of wall enhancement finding in identifying the ruptured site of AVMs. This finding may help in the decision-making of the therapeutic approach in ruptured high-grade AVMs, especially regarding targeted embolization performed as acute rescue therapy.
References
- 1). Spetzler RF, Martin NA: A proposed grading system for arteriovenous malformations. J Neurosurg 65: 476– 483, 1986. [DOI] [PubMed] [Google Scholar]
- 2). Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G, Special Writing Group of the Stroke Council American Stroke Association : AHA Scientific Statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 32: 1458– 1471, 2001. [DOI] [PubMed] [Google Scholar]
- 3). Krings T, Hans FJ, Geibprasert S, Terbrugge K: Partial “targeted” embolisation of brain arteriovenous malformations. Eur Radiol 20: 2723– 2731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Le Feuvre D, Taylor A: Target embolization of AVMs: identification of sites and results of treatment. Interv Neuroradiol 13: 389– 394, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Matouk CC, Mandell DM, Gunel M, Bulsara KR, Malhotra A, Hebert R, Johnson MH, Mikulis DJ, Minja FJ: Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 72: 492– 496; discussion 496, 2013. [DOI] [PubMed] [Google Scholar]
- 6). Mjoli N, Le Feuvre D, Taylor A: Bleeding source identification and treatment in brain arteriovenous malformations. Interv Neuroradiol 17: 323– 330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S, Sato S, Mouri W, Saito S, Kayama T: Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8). Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, terBrugge KG, Hans FJ: Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol 7: 547– 559, 2011. [DOI] [PubMed] [Google Scholar]