Abstract
Intragastric (IG) flavor conditioning studies in rodents indicate that isocaloric sugar infusions differ in their reinforcing actions, with glucose and sucrose more potent than fructose. Here we determined if the sugars also differ in their ability to maintain operant self-administration by licking an empty spout for IG infusions. Food-restricted C57BL/6J mice were trained 1 h/day to lick a food-baited spout, which triggered IG infusions of 16% sucrose. In testing, the mice licked an empty spout, which triggered IG infusions of different sugars. Mice shifted from sucrose to 16% glucose increased dry licking, whereas mice shifted to 16% fructose rapidly reduced licking to low levels. Other mice shifted from sucrose to IG water reduced licking more slowly but reached the same low levels. Thus IG fructose, like water, is not reinforcing to hungry mice. The more rapid decline in licking induced by fructose may be due to the sugar's satiating effects. Further tests revealed that the Glucose mice increased their dry licking when shifted from 16% to 8% glucose, and reduced their dry licking when shifted to 32% glucose. This may reflect caloric regulation and/or differences in satiation. The Glucose mice did not maintain caloric intake when tested with different sugars. They self-infused less sugar when shifted from 16% glucose to 16% sucrose, and even more so when shifted to 16% fructose. Reduced sucrose self-administration may occur because the fructose component of the disaccharide reduces its reinforcing potency. FVB mice also reduced operant licking when tested with 16% fructose, yet learned to prefer a flavor paired with IG fructose. These data indicate that sugars differ substantially in their ability to support IG self-administration and flavor preference learning. The same post-oral reinforcement process appears to mediate operant licking and flavor learning, although flavor learning provides a more sensitive measure of sugar reinforcement.
Keywords: Post-oral sugar sensing, Intragastric infusions, Operant licking, Glucose
1. Introduction
The gastrointestinal (GI) tract of mammals has many nutrient sensors for detecting the nutritional quality of ingested food. These nutrient sensors are involved in the regulation of digestion, absorption and metabolism of food as well as in the control of feeding behavior [21]. The role of gut and post-absorptive sensors in the satiation process that terminates feeding bouts has been the subject of extensive research. However, there is also considerable evidence that post-oral nutrient sensors can have an appetite stimulating effect, a process we refer to as appetition [16].
We have investigated post-oral appetition using various flavor conditioning procedures in which animals are trained to associate a novel flavor with intragastric (IG) or intestinal infusions of nutrients. In a typical experiment, rodents lick a sipper spout containing a flavored non-nutritive solution (the CS+; e.g., grape saccharin) and a computer monitoring the licks activates a pump that delivers an IG nutrient infusion (e.g., 16% glucose). In alternative sessions, the rodents lick a different flavored solution (the CS-; e.g., cherry saccharin) that delivers an IG water infusion. The animals’ learned association with the CS+ flavor and IG nutrient is revealed in a subsequent choice test in which they display a preference for the CS+ over the CS- flavor. In some experiments we have observed stimulation of licking within minutes of the first CS+ training session, indicating rapid post-oral detection of the nutrient infusion and activation of brain systems controlling food reward. Other experimental paradigms used to study post-oral nutrient influences on food reward systems include place or spout position preference conditioning in which a distinctive place or spout location is associated with an IG nutrient infusion [3,5,9].
de Araujo and coworkers recently introduced a new method to investigate post-oral nutrient control of food reward that does not involve oral ingestion or specific flavor cues. In their IG self-administration procedure mice are trained to lick a sipper spout to receive IG nutrient infusions. This method is similar to the licking-controlled infusions of flavor conditioning, except that the spout is empty during testing so there is neither flavor nor oral fluid intake. Operant "dry licking" for nutrient self-infusion without feedback from oral ingestion makes it possible to probe the sensitivity of post-oral detection of infused materials. Initial experiments demonstrated that mice adjusted their self-infusion of fat emulsions (Intralipid) to changes in fat energy concentration (0.75 to 3 kcal/ml) such that caloric intakes during 1 h sessions remained relatively constant [4,22]. Operant dry licking for nutrient infusions is not limited to dietary fat: Ferreira et al [4] observed that mice would dry lick for IG glucose infusions at the same caloric densities as used in the fat experiment. We also studied operant dry licking for IG fat and glucose infusions and found that deletion of intestinal GPR40 and GPR120 fatty acid sensors impaired IG fat but not glucose self-administration [19].
The present study further explored the ability of mice to self-infuse nutrients in the absence of oral ingestion. In this case we tested animals with different nutritive sugars. Our IG infusion studies revealed that C57BL/6J (B6) mice display robust CS+ flavor conditioning responses to IG sucrose, glucose and glucose polymers (maltodextrin) but not IG fructose [11,12,15,25–27]. In addition, while IG glucose infusions rapidly stimulated the intake of a CS+, IG fructose infusions were ineffective. These and other findings indicate that flavor conditioning is not determined by energy value per se but by nutrient-specific factors. If operant self-infusion by dry licking is controlled primarily by nutrient energy as previously proposed [4,22], then isocaloric fructose should be as effective as glucose in maintaining this behavior in B6 mice. However, if operant self-infusion behavior is influenced by the same nutrient-specific process that controls post-oral flavor conditioning, then glucose, but not fructose, should support operant dry licking in B6 mice. If this is the case, then mouse strain differences in IG fructose flavor conditioning should also be observed in operant dry licking for fructose infusions. Unlike B6 mice, IG fructose infusions condition flavor preferences in FVB mice, although less so than isocaloric glucose infusions [18]. FVB mice, therefore, should show operant dry licking for IG fructose as well as glucose.
Experiment 1A examined the prediction that operant licking of a dry spout for IG glucose and fructose would differ in B6 mice. Experiment 1B explored the response of glucose-trained mice to changes in glucose concentration and changes in the infused sugar (i.e., to sucrose and fructose). Experiment 2 determined if the operant licking response to fructose differed from that to water and also compared the extinction of glucose-reinforced operant licking when the sugar infusion was replaced by water infusions or no infusions. Experiments 3 and 4 compared operant licking by FVB mice for IG glucose and fructose infusions at 16% and 8% concentrations, respectively.
2. Experiment 1. Operant licking for 16% glucose or fructose in B6 mice
In a previous study of operant dry licking [4], food-restricted B6 mice were trained (1 h/day) to lick a dry sipper spout for IG glucose infusions by "baiting" the spout with a food pellet. After several training sessions, the food was removed from the sipper spout and the mice continued to lick the empty spout for the IG sugar infusions alone. During training and initial empty spout tests dry licks were reinforced with IG infusions of 67.5% glucose and they were subsequently tested with IG infusions of 37.5% and 12.5% glucose (see footnote). We previously reported that B6 mice acquired significant preferences for a CS+ flavor paired with IG infusions of 8%, 16% or 32% glucose infusions whereas 8%, 12% or 16% fructose infusions were ineffective in producing a preference [15,26]. In the present study, therefore, we compared the operant dry licking response to isocaloric 16% glucose and fructose infusions. All mice were first trained to lick a food-baited spout which triggered IG infusions of 16% sucrose. Sucrose was selected as the training sugar because it supports flavor preference conditioning in mice and, being a glucose+fructose disaccharide, it provides post-oral exposure to both monosaccharide sugars [12,14,20].
2.1 Materials and Methods
2.1.1 Animals
Adult male C57BL/6J mice were purchased from Jackson Laboratories. They were singly housed in plastic tub cages kept in a room maintained at 22°C with a 12:12-h light-dark cycle and ad libitum access to chow (LabDiet 5001; PMI Nutrition International, Brentwood, MO) and water. During testing, they were maintained at 90% of ad libitum body weight by feeding them fixed-size chow pellets (0.5 or 1 g, Bio-Serv, Frenchtown, NJ), which allowed for precise adjustment of daily food rations. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.1.2 Surgery
Mice were fitted with IG catheters (0.84 mm OD x 0.36 mm ID, Micro-Renathane tubing, MRE-033; Braintree Scientific, Braintree, MA) while anesthetized with 2% isoflurane inhalation, as previously described [14]. About 10 days after surgery, the mice were briefly (5 min) anesthetized with isoflurane, and tubing was attached to the gastric catheter and then passed through an infusion harness with a spring tether (CIH62; Instech Laboratories, Plymouth Meeting, PA). The tubing and spring were then attached to an infusion swivel mounted on a counterbalanced lever (Instech Laboratories). The body weight of each mouse was measured before and after it was tted with the infusion tether/swivel system; daily body weights were monitored by weighing the mouse with the attached infusion tether/swivel system. Each animal was then returned to a tub cage, and the swivel’s counterbalanced lever was attached above the cage.
2.1.3 Apparatus
IG infusion sessions were conducted in plastic infusion cages (15 x 15 x 32 cm) [11]. The mice licked stainless steel sipper spouts through vertical slots (5 x 20 mm, 32 mm apart) in a stainless steel plate fixed on the wall of the cage. Licking was monitored by an electronic lickometer (ENV-250B, Med Electronics, St. Albans, VT) connected to a computer, which operated syringe pumps (A-99; Razel Scientific, Stamford, CT). The pump infused liquid at a nominal rate of 0.5 ml/min into the gastric catheters as the animals licked the sipper spout, but the animal controlled the overall infusion rate and volume by its licking pattern. The pump was activated for 3 s (delivering 0.025 ml) when a criterion lick was recorded, as described in the Procedure.
2.1.4 Solutions
The solutions were prepared using food-grade sucrose (Domino Foods, Yonkers, NY), fructose and glucose (Honeyville Food Products, Rancho Cucamonga, CA). They were prepared in deionized water on a w/w basis. The 16% solutions provided 0.016 kcal/3 s infusion. Glucose was also prepared at 8% and 32% concentrations.
2.1.5 Procedure
For 2 days the mice were given six 45-mg pellets (grain-based rodent tablet 5TUM, TestDiet, St. Louis, MO) in their home cages to accustom them to the food used to bait the sipper tube. On the second of these days the mice were adapted to the infusion cages in a 1-h session with no food or spout present. The following day chow was removed from the home cages and the mice were given restricted food rations each day to maintain them at 90% of their ad libitum body weight. Daily 1-h sessions were conducted with IG infusions contingent on licking the spout. During the first 6 sessions the mice were trained to lick the dry sipper spout to obtain IG nutrient infusions by placing two 45-mg pellets in the spout. They could contact the first pellet, which protruded slightly from the 1.5 mm hole in the spout tip; the second pellet served as a "weight" to limit movement of the first pellet. Measurements revealed that the animals removed less than 1 mg of food from the pellet during a 1-h session. In the first 2 of these training sessions the 20th lick on the spout was reinforced with a 3-s infusion of 16% sucrose; during infusions additional licks were recorded but had no consequence. The 20th lick after termination of an infusion activated a new 3-s infusion. In the last 4 training sessions only one lick was required per sugar infusion. (The initial 20-lick criterion was due to a programming error; the 1-lick criterion was used in prior studies [4,19,22].) Food rations were provided in the home cage 1 h after the daily sessions.
Following the last 4 training sessions, the mice were divided into two groups matched for sucrose responding, and given 4 test sessions with a clean empty spout to determine their operant licking for IG 16% glucose (n = 12) or fructose (n = 12) infusions in the absence of food stimuli. In these and all subsequent sessions the operant requirement remained at one lick per 3-sec infusion. Thereafter the groups were treated differently. The Fructose group was given IG water infusions in test sessions 5 and 6; the rationale for this is explained below. The Glucose group was studied further to examine shifts in glucose concentration, and then to changes in the infused 16% sugar, each given for 3 consecutive sessions. In Experiment 1B they were shifted to 8% glucose, then to 32%, and then returned to 16% glucose. In Experiment 1C the mice were infused with 16% sucrose, returned to 16% glucose, then given 16% fructose and finally returned to 16% glucose.
2.1.6 Data analysis
Total licks and IG infusions (equivalent to reinforced licks) were stable across the last 4 sessions of the training period with food-baited spouts, so these data were averaged. These training means were compared to the 4 individual test sessions to evaluate changes across testing. The data were evaluated with a mixed model analysis of variance (ANOVA) with a group factor (glucose, fructose) and a repeated measures factor (mean training and tests 1–4). Tests 3 and 4 were averaged for the Fructose group and compared with t-tests to the average of the 2 water infusion sessions. To compare across infusates in the concentration and sugar series for the Glucose group, means of the last 2 sessions of each condition were calculated and compared with one-way ANOVA. To evaluate the delivery of infusions within sessions, mean cumulative infusion curves were generated and infusion rates were also expressed as infusions per 10-min bin. The infusion bin data were analyzed with repeated measures ANOVA. Significant main effects were evaluated with Newman-Keuls tests, and significant interactions with simple main effects tests.
2.2 Results
Experiment 1A
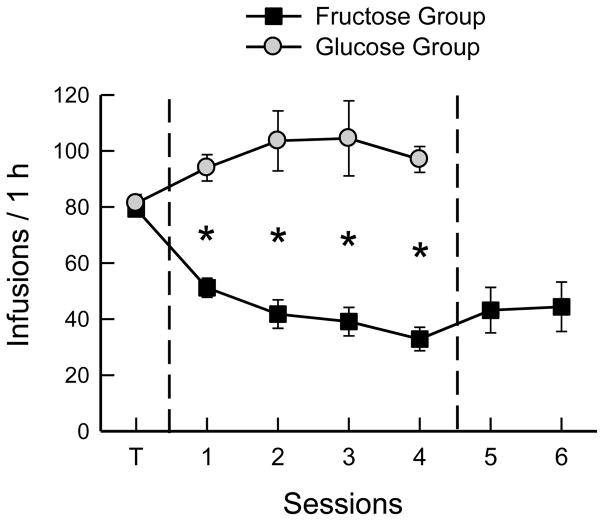
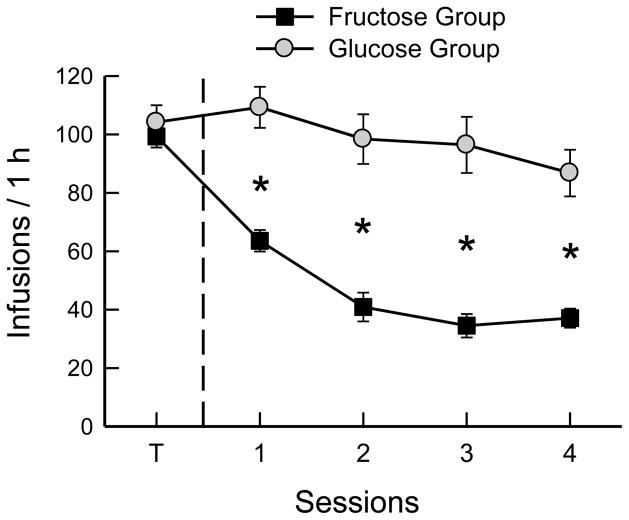
During training with food-baited spouts, the mice earned ~80 sucrose infusions per 1-h session, which provided 1.3 kcal/session. When switched to the empty-spout paired with the new sugar, the Fructose and Glucose groups differed in all 4 test sessions (Fig. 1; F(4,88) = 14.0 for total licks and 15.7 for number of infusions, p < 0.0001). The Fructose mice reduced their licks in the first and second sessions and then maintained their licks at this low level in sessions 3 and 4 (Table 1; F(4,44) = 42.7, p < 0.0001). This resulted in a similar pattern of reduced numbers of infusions (F(4,44) = 34.4, p < 0.0001). The mice averaged 36 infusions of 16% fructose in tests 3 and 4 which provided 0.6 kcal/session. To determine if the fructose infusions were reinforcing the low level of licks, the mice were switched to water infusions for the 2 final sessions. The mice increased their total licks/session from 234.6 in the last 2 fructose tests to 318.4 in the water tests (t(11) = 2.21, p < 0.05), and the number of infusions also increased, although not significantly (p < 0.08).
Fig. 1.
Experiment 1A. Mean numbers of infusions in 1-h sessions of operant licking for sugar solutions. Both groups of B6 mice were trained (T) with food-baited spouts and 16% sucrose infusion and then were shifted to licking on empty spouts. The Glucose group was shifted to 16% glucose infusion, and the Fructose group to 16% fructose infusion for 4 sessions. In addition, the Fructose group was shifted to water infusion in sessions 5 to 6. Significant differences (p < 0.05) between groups are indicated by asterisks.
Table 1.
Total licks (mean and (sem)) by Glucose and Fructose groups during 1-h training and test sessions in Experiments 1 (B6 mice), 3A and 4A (FVB mice).
| Infusate condition | Experiment 1 | Experiment 3A | Experiment 4A | |||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Glucose | Fructose | Glucose | Fructose | |
| Sucrose Baited spout | 766.2 (63.3) | 779.3 (54.6.) | 1134.5 (103.7) | 1146.5 (107.7) | 1741.8 (265.2) | 1777.9 (250.7) |
| Test 1 | 1003.2 (112.1) | 408.7 (34.6) | 1062.2 (112.3) | 419.0 (47.9) | 1030.0 (165.1) | 1010.0 (198.1) |
| Test 2 | 1177.8 (191.0) | 272.5 (57.4) | 1044.0 (147.7) | 243.0 (62.8) | 805.1 (208.4) | 732.6 (161.3) |
| Test 3 | 1177.9 (235.1) | 266.6 (69.2) | 1069.9 (179.1) | 194.0 (41.5) | 946.7 (199.9) | 774.0 (160.6) |
| Test 4 | 1135.6 (97.6) | 202.7 (57.3) | 895.5 (159.0) | 194.9 (35.4) | 753.7 (160.4) | 660.2 (169.8) |
| Experiment 1B | ||||||
| 16% glucose Last 2 days | 1156.8 (148.1) | |||||
| 8% glucose | 1486.7 (137.3) | |||||
| 32% glucose | 550.9 (45.6) | |||||
| 16% glucose | 1136.3 (97.9) | |||||
| Experiment 1C | ||||||
| 16% sucrose | 874.9 (97.8) | |||||
| 16% glucose | 1187.3 | |||||
| Mean last 3 | (94.5) | |||||
| 16% fructose | 538.0 (66.1) | |||||
In contrast to the Fructose mice, the Glucose mice when shifted from baited spout sucrose to empty-spout glucose increased their licks, though not significantly in the first session, and then increased to a stable level in sessions 2 – 4 (Table 1; F(4,44) = 2.94, p < 0.05). This resulted in a similar pattern of increased numbers of infusions (F(4,44) = 2.52, p < 0.054). In sessions 3 and 4, the Glucose mice self-infused 1.6 kcal/session, which was more than twice that of the Fructose mice (t(22) = 7.0, p < 0.001).
Experiment 1B
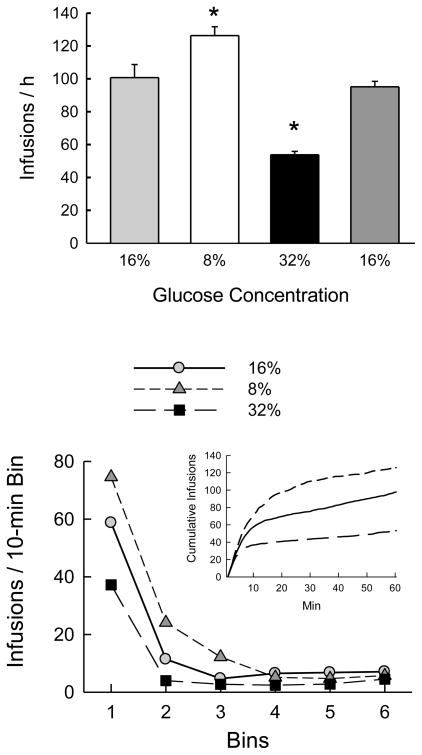
Next the Glucose group was shifted to different concentrations of IG glucose, and means of the last 2 sessions at each concentration were compared. Total licks increased when the 16% glucose concentration was halved to 8% glucose, declined to low levels when the concentration was raised to 32% glucose, and then returned to original levels when 16% glucose infusion was restored (Table 1; F(3,33) = 23.0, p < 0.0001). Numbers of infusions followed the same pattern (Fig. 2; F(3,33) = 44.6, p < 0.0001). The resulting self-infused energy was lower (p < 0.05) for 8% glucose (1.0 kcal/session) than for 16% and 32% glucose (1.6–1.8 kcal/session); the latter did not differ significantly (F(3,33) = 21.6, p < 0.0001).
Fig. 2.
Experiment 1B. Top: Mean numbers of infusions on the last 2 of 3 sessions of operant licking for 16%, 8%, 32% and 16% glucose, in that order. Significant differences (p < 0.05) from the first glucose condition are indicated by asterisks. Bottom: Infusions per 10-min bin during the 1-h sessions. Inset: cumulative infusions during the sessions. Infusions of 8% glucose were greater than those of 16% and 32% in bins 1–3, and 16% exceeded 32% in bins 1 and 2.
The analysis of numbers of infusions in 10-min bins within the sessions (Fig. 2) showed main effects of concentration (F(2,22) = 97.0, p < 0.0001) and bin (F(5,55) = 295.1, p < 0.0001). The latter reflected more infusions in bin 1 than bin 2, and more in bin 2 than in the remaining bins, which did not differ. The significant interaction of concentration and bin (F(10,110) = 27.8, p < 0.0001) was due to significant differences between the concentrations in bins 1, 2 and 3: 8% glucose > 16% > 32% in bins 1 and 2; 8% > 16% and 32% in bin 3. A parallel analysis of kcal infused in 10-min bins showed main effects of concentration (F(2,22) = 48.1, p < 0.0001) and bin (F(5,55) = 236.6, p < 0.0001). The latter reflected greater energy infused in bin 1 than in the remaining bins, which did not differ. The significant interaction of concentration and bin (F(10,110) = 25.5, p < 0.0001) was due to significant differences between the concentrations in bin 1, with energy from 32% glucose > 16% > 8% and bin 6, with 32% > 16% and 8%.
Experiment 1C
In the final series with 16% sugars the Glucose mice were infused with sucrose, glucose, fructose and glucose in that order. Comparison of the last three 16% glucose conditions (including the one that ended Experiment 1B) found no significant differences among them in total licks or infusions, so the mean of the three conditions was used in further analyses. Total licks varied with sugar (F(2,22) = 44.2, p < 0.0001), with more licks for glucose, fewer licks for sucrose, and even fewer for fructose (Table 1). The same pattern was found for numbers of infusions (Fig. 3; F(2,22) = 54.6, p < 0.0001). The total licks and infusions for sucrose did not differ significantly from those in baited spout training with sucrose in Experiment 1A. The analysis of 10-min lick bins (Fig. 3) showed main effects of sugar (F(2,22) = 78.4, p < 0.0001) and bin (F(5,55) = 199.5, p < 0.0001). The latter reflected more licking in bin 1 than bin 2, and more in bin 2 than in the remaining bins, which did not differ. The significant interaction of sugar and bin (F(10,110) = 8.2, p < 0.0001) was due to differences between the sugars in bins 1–4: glucose > sucrose > fructose in bin 1, glucose > fructose in bin 2 and glucose > others in bins 3 and 4.
Fig. 3.
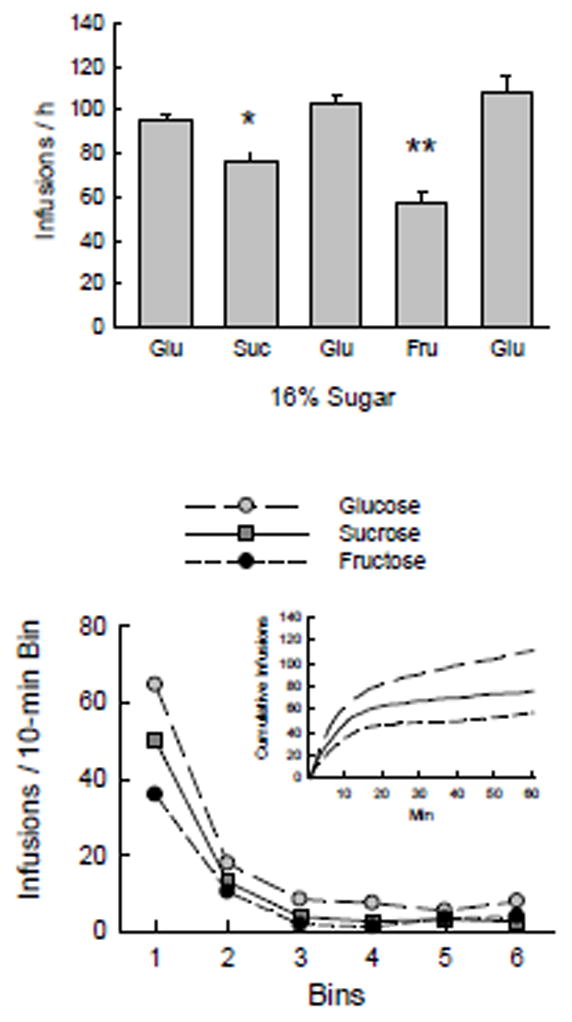
Experiment 1C. Top: Mean numbers of infusions on the last 2 of 3 sessions of operant licking for 16% sucrose, glucose, and fructose. The leftmost column shows the last glucose period of Exp. 1B. The 3 glucose periods did not differ and are averaged for analyses. Significant difference (p < 0.05) between sucrose and glucose infusions indicated by single asterisk, difference (p < 0.05) between fructose and both sucrose and glucose indicated by double asterisks. Bottom: Infusions per 10-min bin during the 1-h sessions. Inset: cumulative infusions during the sessions. Infusions of glucose were greater than those of fructose in bins 1–4, and glucose exceeded sucrose in bins 1, 3 and 4.
2.3 Discussion
Experiment 1A revealed that isocaloric 16% glucose and fructose solutions had opposite effects on operant licking: the glucose infusion increased licks and fructose reduced licks relative to the sucrose training condition. This finding indicates that operant licking was not controlled by the caloric value of the two sugars but rather reflected the differential reinforcement values of glucose and fructose to B6 mice as documented in flavor conditioning studies [15,25,26]. While operant licking was greatly reduced in the fructose group it did not extinguish completely, i.e., decline to zero. To determine if the fructose infusions were reinforcing the residual licking response in sessions 3 and 4, the mice were switched to water infusions in sessions 5 and 6. The mice responded to the change in infusions by increasing rather than decreasing their licking rate. Thus, rather than maintaining operant licking, the fructose infusions appear to be suppressing it. The water infusion data indicate that, once reinforced with sucrose during the training period, dry licking is resistant to complete extinction; this phenomenon was examined further in Experiment 2.
Experiment 1B revealed that the Glucose mice modulated their licking in response to changes in glucose concentration. They increased their licking when the concentration was reduced to 8% and decreased licking when the concentration was increased to 32%. The change in lick response was apparent within the first 10 min of the sessions. These findings are consistent with those previously reported by Ferreira et al. [4] in mice dry licking for IG glucose, although unlike their mice, our animals did not maintain constant caloric intakes. This may be related to the different concentration ranges used in the present study (8% to 32%) and prior study (12.5% to 67.5%). Ferreira et al. [4] hypothesized that concentration-dependent changes in sugar dry licking represented caloric regulation, but the differential licking responses observed here for isocaloric glucose and fructose solutions question this interpretation. Alternatively, mice may lick more for dilute and less for concentrated sugar infusions because of the differential satiating actions of these sugars.
The Glucose mice also adjusted their licking for different sugars presented at 16% concentrations in Experiment 1C. The behavioral change was again rapid, occuring within the first 10 min of the sessions. The finding that the mice licked less for sucrose and even less for fructose than for glucose confirms the findings of Experiment 1A and supports the idea that glucose sensing rather than calorie sensing is the critical determinant of the operant licking response to sugars. The self-infusions of the Glucose mice repeatedly returned to the same level during several presentation of 16% glucose in the concentration and sugar test series, which suggests robust detection of differences in post-oral sugar stimulation. Note though, that the Glucose mice licked more for 16% fructose than did the Fructose mice in Experiment 1A. This indicates that experience with dry licking for IG glucose increases the resistance to extinction of the operant dry licking response.
3. Experiment 2. Operant licking for fructose vs. water and extinction of glucose- reinforced licking in B6 mice
The mice given fructose infusions in Experiment 1 reduced their operant licking to low levels as if the sugar provided no reinforcement. However, the finding that switching the mice from IG fructose to IG water actually increased operant licking suggested that fructose had an inhibitory action on operant licking. Experiment 2A tested this idea by training mice, as in the first experiment, to lick a food-baited spout paired with IG sucrose and then testing their operant licking of an empty spout paired with either IG fructose or IG water. Experiment 2B further investigated extinction of operant dry licking, in this case after mice had been tested with IG glucose, to establish a robust dry licking response. The mice were then subjected to one of two extinction procedures, i.e., they were shifted to no IG infusions or to IG water infusions. This was of interest because in a prior study of operant dry licking for Intralipid [22], mice shifted from fat infusions to no infusions (extinction) showed an initial massive increase in licking with no infusions followed by a gradual return to a level comparable to that displayed when reinforced with IG fat. In contrast, we observed in a different study that mice switched from IG Intralipid infusions to water infusions showed no initial increase in dry licking but only a gradual decline in licking [19].
3.1 Method
Adult male B6 mice were purchased from Jackson Laboratories. Their housing and surgery were the same as in Experiment 1.
Experiment 2A
The mice were trained as in Experiment 1A, ending with 4 sessions in which 1 lick on a food-baited spout triggered infusions of 16% sucrose. They were then divided into groups matched for IG sucrose licking and tested for 6 sessions with licking on an empty spout triggering IG 16% fructose (n = 12) or water (n = 11) infusions. Means of the 4 sucrose training sessions were compared to the individual test sessions.
Experiment 2B
The mice were retrained with food-baited spout and 16% sucrose infusions for 4 sessions, then shifted to an empty spout reinforced by 16% glucose infusions for 6 sessions. The mean licking in the last 2 glucose sessions was used to create two new groups, also matched for prior group membership. Extinction of dry licking was compared in groups infused with water (n = 12) or given no infusion (n = 11). Extinction was evaluated by repeated measures ANOVA comparing the means of total licks of the last 2 glucose sessions with the 8 sessions of water or no infusions.
3.2 Results
Experiment 2A
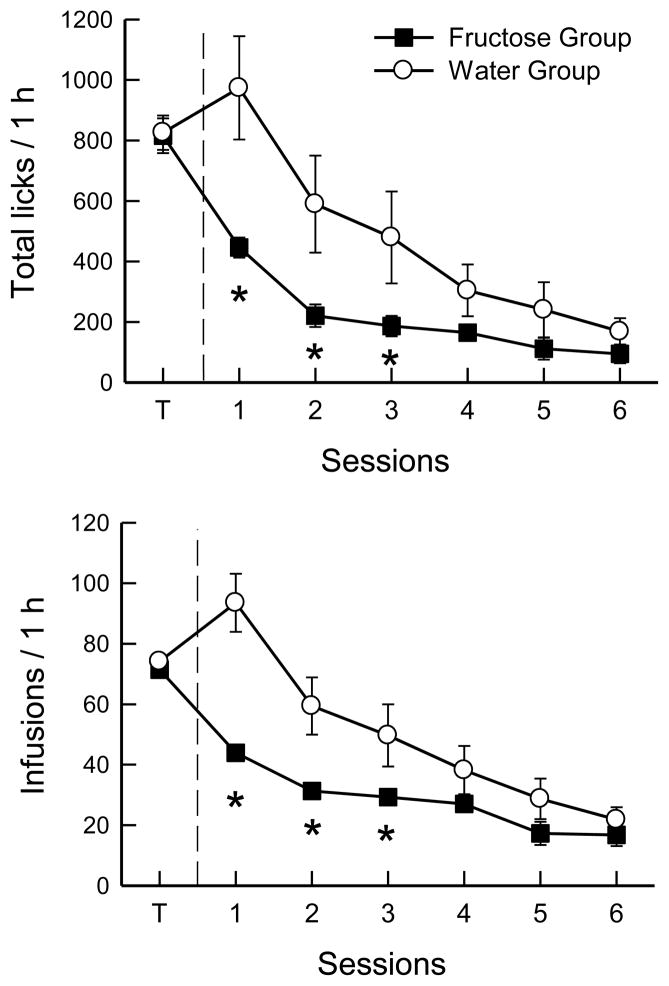
When shifted from baited-spout sucrose, the Water group licked more than the Fructose group (group F(1,21) = 8.0, p < 0.01) and obtained more infusions (group F(1,21) = 14.2, p < 0.01). Overall, licking and numbers of infusions declined over test sessions (F(6,126) = 29.5, 38.5, ps < 0.0001). The groups altered their licking differently (Fig. 4; Group x Session F(6,126) = 3.48, p < 0.01): total licks were greater for water than for fructose in sessions 1–3 but not thereafter. The resulting infusion numbers followed the same pattern (Group x Session F(6,126) = 5.83, p < 0.01). Separate analyses of the groups showed that Fructose licks declined significantly in test session 1, dropped further in session 2 and remained at low levels in the remaining sessions (F(6,66) = 65.9, p < 0.0001); infusion numbers followed a similar pattern, except that they were similar in sessions 2–4 and then declined to a lower level in sessions 5 and 6. In contrast, the decline in licking was not significant until session 3 in the Water group (F(6,60) = 9.8, p < 0.0001), and the number of infusions actually increased on the first water session and then declined more gradually (F(6,60) = 14.9, p < 0.0001).
Fig. 4.
Experiment 2A. Mean numbers of licks (top) and infusions (bottom) in 1-h sessions of operant licking. Both groups of B6 mice were trained (T) with food-baited spouts and 16% sucrose infusion and then were shifted to licking on empty spouts. The Water group was shifted to water infusion, and the Fructose group to 16% fructose infusion. Significant differences (p < 0.05) between groups are indicated by asterisks.
Experiment 2B
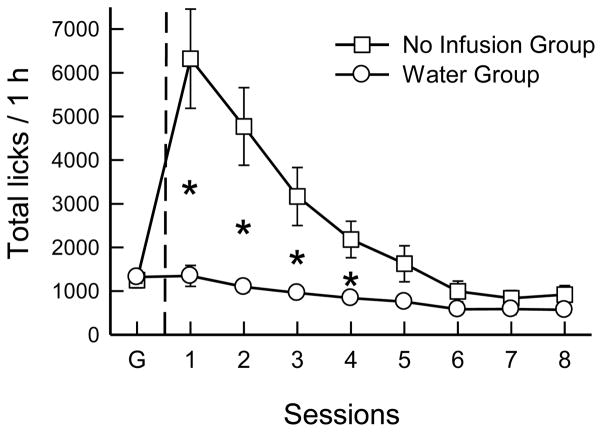
When returned to baited-spout sucrose, the mice resumed licking at the same levels as in Experiment 2A, which yielded similar numbers of infusions. The mice then increased licking and infusions when shifted to empty-spout 16% glucose (F(6,132) = 18.7, 24.6, p < 0.0001). These measures did not change significantly in session 1, and increased thereafter; the last 2 sessions exceeded the first 2, averaging 97 infusions that provided 1.6 kcal/session of glucose. The shift from glucose to extinction produced much more licking in the No Infusion group than in the Water infusion group (Fig. 5; group F(1,21) = 12.9, p < 0.01). This difference was significant in sessions 1–4 but not in sessions 5–8 (Group x Session F(8,168) = 19.7, p < 0.0001). The Water group did not change in the first 2 sessions and then dropped slightly to a lower level of licking in sessions 3–8. In contrast, the absence of infusion resulted in dramatic increases in licking, particularly in the first session when the mice licked 5 times as much as during glucose infusion. This fell to 3.7, and then 2.5 times as much licking as glucose in the next two sessions, and by sessions 7 and 8 the No Infusion group licked 30% less than in the glucose sessions. A separate analysis of the last two glucose and last two extinction sessions indicated that mice licked less in the extinction than in the glucose sessions (F(1,21) = 54.9, p < 0.001) and overall the two groups did not differ in total licks. However, the extinction-induced reduction in total licks was less in the No Infusion group than in Water group (1247.8 to 875.9 vs. 1320.5 to 577.8 licks/session; Group x Session interaction, F(1,21) = 6.54, p < 0.05).
Fig. 5.
Experiment 2B. Mean numbers of licks in 1-h sessions of operant licking. Both groups of B6 mice were tested with empty spouts and 16% glucose (G) infusion. The Water group was shifted to water infusion, and the No Infusion group received no infusions in sessions 1 to 8. Significant differences (p < 0.05) between groups are indicated by asterisks.
3.3 Discussion
As in the first experiment, the Fructose group in Experiment 2A displayed a rapid decline in operant dry licking when switched from food-baited IG sucrose licking to empty spout licking for IG fructose. In contrast, the Water group showed an initial increase in licking and infusions in test 1 and then a more gradual decline in total licks and infusions that reached the levels of the Fructose group by the end of testing. These findings confirm the suggestion from Experiment 1A that IG fructose infusions not only failed to reinforce operant licking but actively inhibited the response, relative to water infusions. This is likely due to the satiating actions of the sugar. As discussed elsewhere, the appetite stimulatory (appetition) and inhibitory (satiation) effects of nutrients are mediated by separate processes, and there is evidence that satiation signals can, in some situations, reduce the post-oral reinforcing actions of nutrients [10,16].
The Water group in Experiment 2B also showed a gradual decline in operant licking when switched from IG glucose to water infusions. In marked contrast, the No Infusion group displayed a dramatic increase in total licks when first switched from IG glucose to no infusions (from 1248 to 6322 licks/session). Their licks then gradually declined to the level of the Water group by extinction tests 7–8. The results obtained with the No Infusion and Water groups are similar to those previously reported for mice trained to dry lick for Intralipid and then switched to no infusion [22] or water infusion [19]. As discussed below, there are interesting similarities and differences in the ingestive responses of the No Infusion mice and sham-drinking rats.
4. Experiment 3. Operant licking for 16% glucose or fructose in FVB mice
In the first two experiments IG glucose supported robust operant dry licking in B6 mice whereas IG fructose was ineffective, just as it fails to condition flavor preferences in this strain. Unlike B6 mice, FVB mice acquired significant preferences for flavors paired with IG 16% fructose infusions, although fructose-based preferences were not as robust as those produced by IG glucose [18]. Based on these findings, we predicted that FVB mice would show operant dry licking for IG fructose. We tested this prediction by training animals to dry lick for IG 16% fructose and glucose as in Experiment 1A. The same FVB animals were then trained in a conditioned flavor preference procedure to evaluate their responses to CS+ flavors paired with IG infusions of 16% fructose or glucose.
4.1 Method
Adult male FVB/NJ mice were purchased from Jackson Laboratories. Details of housing and surgery were the same as in Experiment 1.
Experiment 3A
The mice were trained as in Experiment 1A. After 4 training sessions with a one-lick operant requirement on a food-baited spout paired with 16% sucrose infusions, the mice were divided into matched groups that were given 4 sessions of empty spout licking paired with IG 16% fructose (n = 11) or 16% glucose (n = 10) infusions. Because intakes increased across sucrose sessions, means of the stable last 2 sessions were used for comparison to the individual test sessions.
Experiment 3B
The mice from Exp. 3A were adapted for 2 sessions (1 h/day) to "wet" lick a sipper spout that was attached to a bottle containing 0.2% saccharin (sodium saccharin, Sigma Aldrich, St. Louis, MO). Their licks produced IG infusions of water matched to oral saccharin intake [18]. This was accomplished by adjusting the number of licks required to trigger each 3-sec IG infusion, typically 20–25 licks, individually for each mouse. Licks during an infusion counted toward the lick requirement for the next infusion. The mice then were trained (1 h/day) with a CS- flavored saccharin paired with IG water in sessions 1, 3 and 5, and a CS+ flavored saccharin paired with IG 16% sugar in sessions 2, 4 and 6. The CS flavors were 0.05% cherry and grape Kool-Aid (Kraft Foods, White Plains, NY) and were counterbalanced as CS+ and CS- flavors. Mice received the same sugar as in Experiment 3A. This was followed by a two-bottle choice test (two 1 h/day sessions) between the CS+ and CS- solutions, still paired with their respective infusions. In this test, two infusion pumps containing 16% sugar and water were attached via a Y-connector to each mouse's gastric catheter. The left-right positions of the CS+ and CS- solutions were counterbalanced over training and test sessions. Intakes are expressed as the sum of oral and infused solutions. Intakes were averaged over the 3 training sessions with each CS and over the 2 two-bottle test sessions.
4.2 Results
Experiment 3A
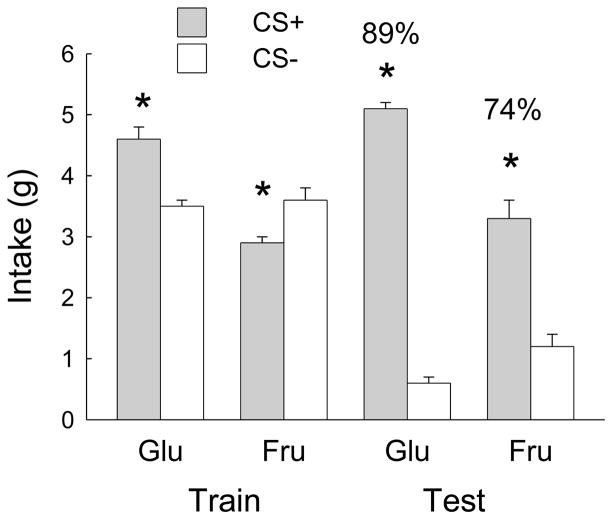
When shifted from baited-spout sucrose to empty spout licking, the Glucose group licked more than the Fructose group (Table 1; Group x Session F(4,76) = 17.6, p < 0.0001) and obtained more infusions (Fig. 6; Group x Session F(4,76) = 13.2, p < 0.0001). In the Fructose group, total licks and infusions dropped in the first test session and then dropped further and stabilized in sessions 2–4 (F(4,40) = 65.4, 64.8, p < 0.0001). Total licks and infusions did not change in the Glucose group when it was shifted from baited-spout licking for sucrose to dry licking for glucose. Sucrose self-infusions averaged 1.7 kcal/session (both groups), and glucose and fructose self-infusions averaged 1.5 and 0.7 kcal/session, respectively, in the two groups (t(19) = 4.84, p < 0.001).
Fig. 6.
Experiment 3A. Mean numbers of infusions in 1-h sessions of operant licking for sugar solutions. Both groups of FVB mice were trained (T) with food-baited spouts and 16% sucrose infusion and then were shifted to licking on empty spouts. The Glucose group was shifted to 16% glucose infusion, and the Fructose group to 16% fructose infusion. Significant differences (p < 0.05) between groups are indicated by asterisks.
Experiment 3B
Mean training and test intakes are shown in Fig. 7. During one-bottle training the Glucose group consumed more in CS+ than CS- sessions, whereas the Fructose group consumed more in CS- than CS+ sessions (Group x CS interaction, F(1,19) = 55.3, p < 0.0001). CS+ licking yielded self-infusion of 1.5 kcal glucose and 0.9 kcal fructose per session (t(19) = 11.1, p < 0.0001). In the two-bottle test, both groups consumed significantly more CS+ than CS- (F(1,19) = 173.3, p < 0.0001). However, the Glucose group consumed more CS+ and less CS- than the Fructose group (Group x CS interaction, F(1,19) = 22.0, p < 0.001). The percent CS+ preference of the Glucose group was greater than that of the Fructose group (89% and 74%, respectively; t(19) = 2.83, p < 0.01).
Fig. 7.
Experiment 3B. Mean intakes (oral + IG) during 1-bottle training and 2-bottle tests for the Fructose and Glucose groups in the conditioned flavor preference procedure. The CS+ and CS- were paired with IG 16% sugar and water infusions, respectively. Numbers atop bars indicate percent CS+ intake, relative to total intake during 2-bottle test. Significant differences (p < 0.05) between CS+ and CS- intakes are indicated by asterisks.
4.3 Discussion
Contrary to our prediction, the FVB Fructose group did not display sustained operant dry licking for IG fructose infusions. Rather, they showed a rapid decline in licking and fructose infusions nearly identical to that of the B6 mice in Experiment 1A. In contrast, the FVB Glucose group showed sustained operant licking for IG glucose infusions and they infused nearly as much sugar as did the B6 mice in the first experiment. The strains differed in that the B6 mice increased their operant licking when shifted from baited spout sucrose to glucose whereas the FVB mice showed no change in operant licking from sucrose training to glucose testing. This strain difference occurred because the FVB mice licked more on the baited sucrose spout than did the B6 mice (Table 1).
While IG fructose failed to reinforce operant licking in Experiment 3A, it conditioned a significant preference for the CS+ flavor in Experiment 3B, albeit weaker that that produced by IG glucose. This confirms prior findings obtained with FVB mice conditioned with IG 16% fructose and glucose [18]. These findings imply that 16% fructose infusions are an effective reinforcer for flavor conditioning but not for operant dry licking. Note, however, that in the flavor conditioning experiment the IG fructose infusions were diluted to 8% fructose in the stomach by the orally consumed CS+ solution. In contrast, the 16% fructose infusions were not diluted in the operant licking experiment. It may be that 16% fructose in the stomach is too concentrated to reinforce operant licking.
5. Experiment 4. Operant licking for 8% glucose or fructose in FVB mice
This experiment determined if FVB mice would dry lick for IG infusions of 8% fructose, a concentration that supports CS+ flavor preference in this strain (Exp. 3B; [18]). The effectiveness of 8% glucose infusions to support dry licking was also investigated.
5.1 Method
Adult male FVB/NJ mice purchased from Jackson Laboratories were trained and tested as in Experiment 3A, except that all sugar infusions (including sucrose) were prepared at 8% concentrations. The same mice were then given flavor preference conditioning as in Experiment 3B but with 8% fructose and glucose.
5.2 Results
Experiment 4A
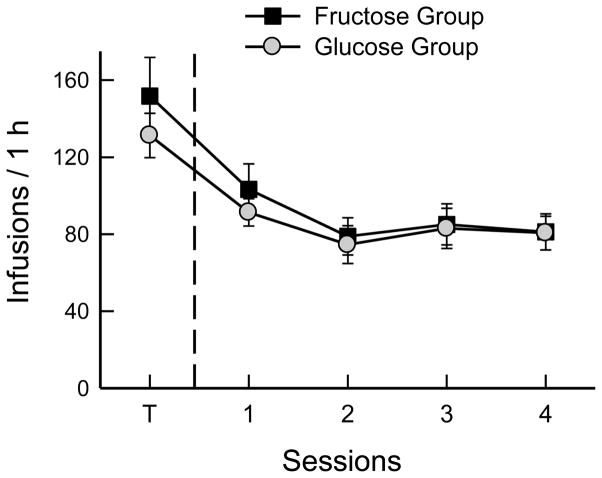
When shifted from baited-spout sucrose to empty spout licking, the Fructose (n=9) and Glucose (n=9) groups showed very similar declines in total licks (Table 1) and sugar infusions (Fig. 8). Total licks and infusion numbers dropped in the first test session and then dropped further and stabilized in sessions 2–4 (F(4,64) = 37.1, 21.5, p < 0.0001). There were no group differences in total licks or self-infusions during training or testing. Sucrose self-infusions averaged 1.2 kcal/session, and fructose and glucose averaged 0.7 kcal/session.
Fig. 8.
Experiment 4A. Mean numbers of infusions in 1-h sessions of operant licking for sugar solutions. Both groups of FVB mice were trained (T) with food-baited spouts and 8% sucrose infusion and then were shifted to licking on empty spouts. The Glucose group was shifted to 8% glucose infusion, and the Fructose group to 8% fructose infusion.
Experiment 4B
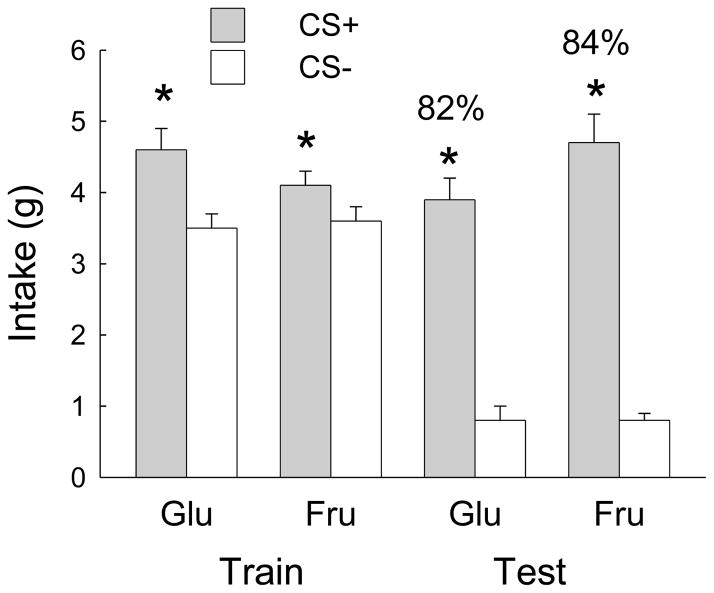
Mean training and test intakes are shown in Fig. 9. During one-bottle training the mice consumed more CS+ than CS- (F(1,16) = 40.5, p < 0.0001). The groups did not differ in overall intakes, but the differences between CS+ and CS- intakes were greater for the Glucose group than the Fructose group (Group x CS interaction, F(1,16) = 5.8, p < 0.05). CS+ licking yielded self-infusion of 0.7 kcal glucose or fructose. In the two-bottle test, the mice consumed significantly more CS+ than CS- (F(1,16) = 104.2, p < 0.0001) and there were no significant group differences in CS intakes or percent CS+ preferences (82% and 84%).
Fig. 9.
Experiment 4B. Mean intakes (oral + IG) during 1-bottle training and 2-bottle tests for the Fructose and Glucose groups in the conditioned flavor preference procedure. The CS+ and CS- were paired with IG 8% sugar and water infusions, respectively. Numbers atop bars indicate percent CS+ intake, relative to total intake during 2-bottle test. Significant differences (p < 0.05) between CS+ and CS- intakes are indicated by asterisks.
5.3 Discussion
This experiment revealed that IG infusions of 8% fructose are no more effective than 16% fructose infusions in reinforcing operant self-infusions in FVB mice. Thus, the findings of Experiment 3 that IG 16% fructose conditioned a flavor preference but did not support operant self-infusions in FVB mice cannot be attributed to the dilution of the IG fructose to 8% in the flavor conditioning experiment. The present experiment further revealed that IG 8% glucose was ineffective in maintaining operant licking in FVB mice. Yet in the flavor conditioning experiment, the 8% fructose and glucose infusions stimulated CS+ intakes during training and induced robust CS+ preferences. Given that the sugar infusions were diluted in the stomach to 4% in the flavor conditioning experiment, the present data and those of Experiment 3 demonstrate that the flavor learning procedure provides a more sensitive assay of IG sugar reinforcement than does the operant licking task.
The FVB mice licked more for the food-baited spout when paired with IG 8% sucrose than when paired with 16% sucrose in the prior experiment (1759.9 vs. 1140.7 licks). This presumably was because the 8% sucrose was less satiating than the 16% sucrose. On the other hand, the mice self-infused 1.2 kcal/session of 8% sucrose compared to 1.6 kcal/session of 16% sucrose. In the shift from 8% sucrose to 8% fructose or glucose, both groups showed declines resembling those of the 16% fructose group, though the licks remained higher than those for 16% sugar. The mice self-infused the same fructose energy, 0.6 kcal, in the 8% and 16% conditions, but glucose energy at 8% was only ~40% of that at 16%. In the flavor conditioning experiment, the two groups self-infused 0.7 kcal sugar/CS+ session.
A new finding of the present experiment is that the FVB mice displayed comparable preferences for the CS+ flavors paired with IG infusions of 8% fructose and glucose, whereas in Experiment 3B and in prior experiments [18] 16% fructose conditioned weaker preferences than did 16% glucose. Thus the reinforcing potency of fructose in FVB mice, unlike that of glucose, declines as concentration increases from 8 to 16%. This may be related to the satiating action and/or malabsorptive effects of concentrated fructose.
The failure of 8% glucose to support operant self-infusions in the FVB mice was unexpected given the robust licking displayed by the B6 mice for 8% glucose in Experiment 1B. Conceivably, FVB mice are less sensitive than B6 mice to the reinforcing value of dilute glucose. Alternatively, 8% glucose may be ineffective in maintaining operant licking in B6 mice when switched from a food-baited sucrose spout, although it will support responding in mice with a well-established operant licking response for 16% glucose.
6. General Discussion
In confirmation of prior studies [4,19], B6 mice licked a dry sipper tube for IG self-infusions of glucose and varied their licking response as a function of sugar concentration to maintain near-equal energy intakes. The new findings here are that the mice did not maintain energy intakes when offered different types of sugars and, most notably, fructose failed to support operant dry licking. The fructose findings are consistent with studies showing that IG fructose, unlike glucose, does not stimulate intake of or preference for a CS+ flavor in B6 mice. These findings demonstrate that operant dry licking and flavor conditioning are not reinforced by signals related to sugar energy per se, but by glucose-specific signals. An unexpected finding was that fructose infusions were also ineffective in supporting operant dry licking in FVB mice even though IG fructose conditions significant flavor preferences in this strain.
6.1 Operant self-infusions in B6 Mice
The B6 mice displayed the greatest differences in operant licking for glucose and fructose, but they also licked less for 16% sucrose than for 16% glucose in Experiments 1A and 1C. The weaker reinforcing effect of sucrose could be attributed to the fact that, when digested, the sugar yields 8% glucose and 8% fructose. Yet, when switched from IG 16% glucose to 8% glucose infusions, the B6 mice increased, not decreased their operant licking rates. Thus, 16% sucrose was not treated as simply a diluted glucose solution. It would seem that the fructose released by the digestion of sucrose suppressed the operant licking response to the disaccharide sugar. This may be because the satiating signals generated by the 16% sucrose infusions were not “diluted” as in the case of the 8% glucose infusions. Evidence that fructose has an inhibitory action on operant licking was provided by the differential licking responses displayed by B6 mice to IG fructose and water infusions. That is, the B6 mice licked more for IG water than they did for IG fructose in the initial water sessions in Experiment 1A and 2A.
The water extinction data show that sugar-reinforced mice significantly decreased their operant licking responses when switched from IG sucrose (Exp. 2A) or IG glucose (Exp. 2B) to IG water. The decline in operant licking over sessions was gradual and the mice never stopped licking, although they might have if the extinction testing was extended beyond 8 sessions. We previously observed a similar partial decline in operant licking in B6 mice dry licking for IG infusions of 5% Intralipid and then switched to water infusions [19]. These findings suggest that once established, the habit of licking a dry sipper tube is quite resistant to complete extinction and/or that the trigeminal (tactile, temperature) stimulation provided by the empty sipper spout has some inherent reinforcement value.
More remarkable than the persistent dry licking displayed by mice switched from IG glucose to water infusions is the profound increase in dry licking displayed by mice in the no infusion condition. These mice increased their licking fivefold when switched from IG glucose infusions to no infusions in Experiment 2B. The dry licking response then rapidly declined but it took 5 sessions to reach the level of the Water group. Similar results were reported for B6 mice switched from IG Intralipid infusions to no infusions [22]. In some respects the no infusion extinction test is analogous to sham-drinking tests conducted in rats. In sham-drinking experiments, rats are typically first trained to “real” drink a sugar solution for several sessions and then switched to sham-drinking sessions in which they drink the same sugar solution which now drains out an open gastric fistula with little or no absorption. The rats respond by substantially increasing their oral sugar intakes in these tests. However, the behavior of rats switched from real to sham-drinking a concentrated (16–32%) sugar solution differs in two important respects from that of the mice switched from dry licking for IG 16% glucose to no infusions. First, while sham-drinking rats increase their sugar intake in the first sham-drinking test, they increase it further in subsequent tests until reaching a peak intake. In contrast, the mice in the present study show peak dry licking responses in the first no infusion session. Second, rats sham-drinking sugar solutions show no decline in intakes with repeated testing, unlike the rapid decline in licking beginning in the second session displayed by mice dry licking with no infusions [2,6,24]. The persistent elevated sham-drinking response to concentrated sugar solutions provides evidence for the inherent rewarding effect of sweet taste. Note that saccharin or dilute (1–6%) sugar solutions do not stimulate excessive intakes in sham-drinking rats [8,23]. However, training rats to associate a flavored saccharin solution (CS+) with IG infusions of 16% glucose enhances the reward value of the solution to that of a concentrated sugar solution (e.g., 16% fructose) [6,7,13]. Furthermore, in an initial sham-drinking test, rats increased their intake of the CS+ flavored saccharin solution to the same level as rats tested with 16% fructose [6]. However, with repeating testing, the sham intakes of the CS+ saccharin solution decreased while sham intakes of fructose increased and remained high. Thus, the behavior of the rats sham-drinking a CS+ flavored saccharin solution previously reinforced with IG glucose infusions is comparable to that of mice licking a dry spout (“sham licking”) that was previously reinforced with IG glucose infusions.
6.2 Operant self-infusions in FVB mice
The FVB mice were like B6 mice in displaying persistent dry licking for IG infusions of 16% glucose but not for 16% fructose. The glucose findings were expected but the fructose findings were not. We previously observed that FVB mice, like B6 mice, display robust flavor conditioning to IG 16% glucose whereas FVB mice, unlike B6 mice, learn to prefer CS+ flavors paired with IG 16% fructose [18]. This was confirmed in the present study, and the FVB mice displayed 74% and 89% CS+ preferences for the CS+ flavors paired with IG 16% fructose and glucose, respectively. The differential operant licking response of the FVB mice to the two 16% sugars may be due to the slower and less potent reinforcing actions of fructose compared to glucose. In our prior study, FVB mice trained to drink a CS+ flavored saccharin solution paired with IG 16% glucose increased their CS+ intake within 10–20 min in the first session and increased their intakes further in subsequent 60-min sessions. In contrast, mice trained with IG 16% fructose displayed no CS+ increases in the training sessions, yet they subsequently displayed a significant CS+ preference, although weaker than that of the glucose mice (67% vs. 92%). It may be that fructose generates no reinforcing signals early within 1-h infusion sessions, and signals generated later or after the session ends may be sufficient to reinforce flavor preferences but not to stimulate CS+ intakes. The sweet taste of the CS+ flavor ensures that fructose-trained mice will continue to lick and receive sugar infusions throughout the training sessions. In the operant dry licking tests, however, the lack of early post-oral reinforcing signals in the empty sipper condition may cause the animals to reduce their licking, and thus their sugar infusions so that the operant licking response is not reinforced. Some data also indicate that the sweet taste of the saccharin increases the salience of the CS+ and enhances its association with post-oral fructose reinforcement. Male rats trained 24 h/day with a CS+ solution paired with IG infusions of 16% fructose developed a significant CS+ preference when the CS+ (e.g., grape Kool-Aid) was sweetened with saccharin but not when it was unsweetened [1].
Dependence of dry licking upon early reinforcement may also account for the failure of 8% glucose infusions to reinforce operant licking in Experiment 4. Although these infusions are sufficient to condition significant preferences for CS+ saccharin solutions, the early reinforcing signals generated by 8% glucose may be insufficient to maintain dry licking in mice switched from a food-baited to an empty spout. Flavor conditioning studies with B6 mice indicate that IG 8% glucose, diluted to 4% in the stomach, conditions CS+ preferences, whereas 4% and 2% glucose infusions, diluted to 2% and 1%, respectively, were ineffective in mice trained in 1 h/day sessions. However, when training sessions are extended to 20 h, the 4%, but not 2% glucose infusions produced significant CS+ preferences [27].
In summary, the present findings support prior results that glucose, like Intralipid, is an effective reinforcer of operant dry licking in mice and that to a certain degree the licking response is modulated by the nutrient density of the solution. However, the reinforcement values of the nutrient solutions are not directly related to caloric density per se, as previously suggested [4], given that isocaloric glucose and fructose differ substantially in their ability to support dry licking. Prior studies indicate that glucose-conditioned flavor preferences are mediated, in part, by intestinal SGLT1 and SGLT3 glucose transporters/sensors and perhaps by GLUT2 transporters [26]. The same sensors may also mediate dry licking for glucose infusions. Other findings implicate intestinal GPR40/GPR120 fatty acid receptors in both Intralipid conditioned flavor preferences and dry licking [17,19]. Our finding that operant dry licking for isocaloric IG infusions of fat and glucose is differentially impaired in mice missing GPR40 and GPR120 receptors provides further evidence for the involvement of nutrient-specific sensors in this behavior [19]. The post-oral process that mediates fructose conditioned flavor preferences in FVB mice remains to be established and an understanding of this process should explain why fructose supports flavor conditioning but not operant licking in these mice.
Highlights.
C57BL/6 mice licked a dry spout for intragastric glucose but not fructose infusions.
They adjusted their dry licking to changes in glucose concentrations and sugar type.
FVB mice also dry licked for glucose but not fructose infusions.
Yet, intragastric fructose conditions flavor preferences in FVB mice.
Operant dry licking reflects nutrient-specific post-oral reinforcement.
Acknowledgments
This research was supported by grant DK031135 from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Martin Zartarian and Kwame McCartney for their expert technical assistance. Portions of these data were presented at the annual meeting of the Society for the Study of Ingestive Behavior, Denver, CO, July 2015.
Footnotes
These glucose concentrations are based on the caloric densities of 2.7, 1.5, and 0.5 kcal/ml reported in [4], assuming 4 kcal/g for glucose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Fructose conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Davis JD, Smith GP. Learning to sham feed: Behavioral adjustments to loss of physiological postingestive stimuli. Am J Physiol. 1990;259:R1228–R1235. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- 3.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira JG, Tellez LA, Ren Z, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavor signaling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumura S, Yoneda T, Aki S, Eguchi A, Manabe Y, Tsuzuki S, et al. Intragastric infusion of glucose enhances the rewarding effect of sorbitol fatty acid ester ingestion as measured by conditioned place preference in mice. Physiol Behav. 2010;99:509–514. doi: 10.1016/j.physbeh.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: I. Intake acceptance and preference analysis. Physiol Behav. 2001;74:481–493. doi: 10.1016/s0031-9384(01)00595-9. [DOI] [PubMed] [Google Scholar]
- 7.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: II. Taste reactivity analysis. Physiol Behav. 2001;74:495–505. doi: 10.1016/s0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- 8.Nissenbaum JW, Sclafani A. Sham-feeding response of rats to Polycose and sucrose. Neurosci Biobehav Rev. 1987;11:215–222. doi: 10.1016/s0149-7634(87)80029-5. [DOI] [PubMed] [Google Scholar]
- 9.Ren X, Ferreira JG, Zhou LH, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sclafani A, Nissenbaum JW, Ackroff K. Learned preferences for real-fed and sham-fed Polycose in rats: Interaction of taste, postingestive reinforcement, and satiety. Physiol Behav. 1994;56:331–337. doi: 10.1016/0031-9384(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 11.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289:R712–720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani A, Ackroff K. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol Behav. 2006;88:88–94. doi: 10.1016/j.physbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Sclafani A, Glass D, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–458. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for post-oral but not oral mediation of fat preferences in the mouse. Am J Physiol Reg Integ Physiol. 2013;305:R1490–R1497. doi: 10.1152/ajpregu.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: strain differences in post-oral sugar reward. Am J Physiol. 2014;307:R1448–R1457. doi: 10.1152/ajpregu.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sclafani A, Touzani K, Ackroff K. Intragastric fat self-administration is impaired in GPR40/120 double knockout mice. Physiol Behav. 2015;147:141–148. doi: 10.1016/j.physbeh.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Zukerman S, Ackroff K. Post-oral glucose sensing, not caloric content, determines sweetener reward in C57BL/6J mice. Chem Senses. 2015;40:245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinert RE, Feinle-Bisset C, Geary N, Beglinger C. Secretion of gastrointestinal hormones and eating control. J Anim Sci. 2013;91:1963–1973. doi: 10.2527/jas.2012-6022. [DOI] [PubMed] [Google Scholar]
- 22.Tellez LA, Ferreira JG, Medina S, Land BB, Dileone RJ, de Araujo IE. Flavor-independent maintenance, extinction, and reinstatement of fat self-administration in mice. Biol Psychiatry. 2013;73:851–859. doi: 10.1016/j.biopsych.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weingarten HP, Watson SD. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav. 1982;28:401–407. doi: 10.1016/0031-9384(82)90131-7. [DOI] [PubMed] [Google Scholar]
- 24.Weingarten HP, Kulikovsky OT. Taste-to-postingestive consequence learning: Is the rise in sham feeding with repeated experience a learning phenomenon? Physiol Behav. 1989;45:471–476. doi: 10.1016/0031-9384(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 25.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol. 2011;301:R1635–R1647. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav. 2013;109:33–41. doi: 10.1016/j.physbeh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]