Abstract
Background
In response to recent studies, a better understanding of the risks of renal complications among African American and biologically-related living kidney donors is needed.
Methods
We examined a database linking U.S. registry identifiers for living kidney donors (1987-2007) to billing claims from a private health insurer (2000-2007 claims) to identify renal condition diagnoses categorized by International Classification of Diseases 9th Revision (ICD-9) coding. Cox regression with left- and right-censoring was used to estimate cumulative incidence of diagnoses after donation, and associations (adjusted hazards ratios, aHR) with donor traits.
Results
Among 4,650 LKD, 13.1% were African American and 76.3% were Caucasian; 76.1% were first-degree relatives of their recipient. By seven years after donation, after adjustment for age and sex, greater proportions of African American compared with Caucasian donors had renal condition diagnoses: chronic kidney disease (12.6% versus 5.6%, aHR 2.32, 95% CI 1.48–3.62), proteinuria (5.7% versus 2.6%, aHR 2.27, 95% CI 1.32–3.89), nephrotic syndrome (1.3% vs 0.1%, aHR 15.7, 95% CI 2.97–83.0), and any renal diagnosis (14.9% vs 9.0%, aHR 1.71, 95% CI 1.23–2.41). While first-degree biological relationship to the recipient was not associated with renal risk, associations of African American race persisted for these conditions and included unspecified renal failure and reported disorders of kidney dysfunction after adjustment for biological donor-recipient relationship.
Conclusions
African Americans more commonly develop renal condition diagnoses after living kidney donation, independent of donor-recipient relationship. Continued research is needed to improve risk stratification for renal outcomes among African American living donors.
Keywords: African Americans, Insurance, Kidney diseases, Living donors, Nephrotic syndrome, Registries
Introduction
In the context of the growing disparity between the need for organ transplants and the deceased donor organ supply, kidney transplantation from living donors has increased markedly over the last several decades (1). The growth in living donation has been accompanied by changes in donor characteristics, including greater racial and ethnic diversity and more unrelated donors (2, 3). Living donors gain no direct medical benefits from donation, and deserve accurate information on health outcomes after donation, tailored when possible to their individual characteristics. Given that most countries including the U.S. do not currently maintain national registries that effectively track long-term health outcomes in living organ donors, much of the information on long-term post-donation outcomes has been drawn from single-center, retrospective studies. However, retrospective studies may be challenged by selection bias, missing data and loss to follow-up (4, 5). Knowledge gaps are particularly notable for African American donors, who to date have been under-represented in large donor cohort studies (6-9).
African American persons in the general population develop end-stage renal disease (ESRD) more often than Caucasian individuals, and recent studies show that these patterns also occur after live kidney donation (10-12). Part of race-related risk may be mediated by comorbidity; for example, we have found that post-donation hypertension and diabetes occur more commonly in African American compared with Caucasian donors (13-15). However, recent literature supports that at least a portion of renal failure previously attributed to hypertensive nephrosclerosis in persons of African descent may be genetically-mediated by coding variants in the gene for a secreted lipoprotein, apolipoprotein L1 (APOL1), and not modifiable by antihypertensive therapy (16, 17). While these genetic variants are rare or absent in other racial groups, homozygosity or compound heterozygosity for APOL1 renal risk alleles is associated with sclerosing glomerulopathies in African Americans, often accompanied by heavy proteinuria (16, 18-21). Notably, causes of renal failure and pre-end stage renal conditions were not described in the prior large registry studies of post-donation ESRD including African Americans (10-12).
Among other live donor subgroups identified as potentially facing increased risks of adverse renal outcomes, emerging data have also generated discussion of possible increased renal risk among donors who are close relatives of their recipients, grounded in concern for heritable renal diseases which may not be manifest at donor evaluation but emerge over time (22-24). Among 1,901 Norwegian living kidney donors followed for a median of 15 years by Mjøen et al, the 9 donors who developed ESRD were all biologically related to their recipients and renal failure was predominantly attributed to immunological diseases (9). All donors in the study by Mjøen et al were Caucasian. In the U.S., African Americans are more commonly related to their recipients than Caucasian American donors (25, 26). However, a recent multi-racial U.S. registry-based study did not detect a significant difference in ESRD incidence over 15 years among donors who were biologically related compared with unrelated to their recipients (12).
To advance understanding of associations of African American race and donor-recipient relationship with renal conditions that may precede ESRD, we recently linked national U.S. donor registry data with administrative claims from a private health insurer and identified racial variation in diagnoses of chronic kidney disease and proteinuria after live kidney donation (13, 14). In the current study, we expanded investigation of provider-reported kidney problems to the broader array of renal diagnosis categories captured in claims data. Our goals were to identify and quantify post-donation diagnoses of renal conditions, with attention to differences among African American compared with Caucasian donors. We also explored potential explanation of racial variation in outcomes by biological relationship to the recipient.
Methods
Data Source and Sample
This study used data from the Organ Procurement and Transplantation Network (OTPN). The OPTN data system includes information on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Study data were assembled by linking OPTN records for living kidney donors with administrative data from a national private health insurer. After approval by HRSA and the Saint Louis University Institutional Review Board, beneficiary identifier numbers from the insurer's electronic databases were linked using names and birthdates to unique OPTN identifiers for living kidney donors. Analyses were performed using Health Information Portability and Accountability Act-compliant, limited datasets with all direct identifiers removed.
We included individuals with records of serving as a live kidney donor in the U.S. between October 1987 and July 2007 and benefits under the participating insurer after donor nephrectomy at some point in May 2000 to December 2007 (the period of available claims data). All study participants were simultaneously enrolled in medical and pharmacy benefits with this insurer exclusively during the study window. The total population in the database numbers six million individuals from across the United States. The total population in the database numbers six million individuals from across the United States. Because of the large sample size, the anonymity of the patients studied, and the non-intrusive nature of the research, a waiver of informed consent was granted per the Department of Health and Human Services Code of Federal Regulations (Title 45, Part 46, Paragraph 46.116).
Definitions of Donor Characteristics and Outcomes
Demographic data from the OPTN at donor nephrectomy included age, gender, race and donor-recipient relationship as reported by the transplant center. First-degree donor-recipient relationship was defined as parent, child, twin or other sibling, whereas any biological relationship also included those defined as “other blood-related relatives”. Times from donation to start and end of captured insurance benefits were based on OPTN-reported donation dates and insurance enrollment records. An index of neighborhood socioeconomic status at the time of donation was computed from U.S. Census data linked by ZIP code, according to methods used by the Agency for Healthcare Research and Quality (27) (for details, please see Supplementary Appendix, SDC 1, Methods).
Non-infectious renal condition diagnoses were defined by identification of billing claims with corresponding diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM)) codes for: acute kidney failure (584.×), proteinuria (791.0, 581.×), nephrotic syndrome (581.×), chronic glomerulonephritis (582.×), nephritis/nephropathy (583.×), chronic kidney disease (585.×), renal failure, unspecified (586), renal sclerosis (587.×) disorders of impaired renal function (588.×), and a composite of any of these diagnoses. Conditions reported in <10 donors (chronic glomerulonephritis (582.×), renal sclerosis (587.×)) were included within the composite endpoint but not reported separately.
Statistical Analyses
Datasets were merged and analyzed with SAS for Windows software, version 9.3 (SAS Institute Inc., Cary, NC). Since windows of captured insurance benefits varied across the sample, Cox regression with left- and right-censoring was used to estimate the cumulative frequency of diagnoses over time after donation, and associations (adjusted hazards ratio, aHR) of donor race, sex and age with the study outcomes. Censoring was applied from donation to claims enrollment and after the end of an individual's claims. Incidence of each renal diagnosis category at 7-years post-donation was computed for the full sample and for African American and Caucasian donors, adjusted to the average sample age and sex distributions at donation. Analyses were repeated including adjustment for first-degree (and then any) biological relationship of the donor with their recipient. Interactions of African American race and biological donor-recipient relationship were also examined.
Results
Demographic Characteristics of the Study Sample
Among 4,650 captured living kidney donors, 13.1% were African American and 76.3% were Caucasian (Table 1). Seventy six percent of donors were first-degree relatives of their recipient and 81% had some form of reported biological relationship. African Americans were more commonly first-degree relatives of (81.5% vs 75.1%, P=0.006), or had any biological relationship with (88% vs 79%, P<0.0001), their recipients than Caucasian donors. Average age at donation was 37.2 years; however, African Americans tended to be younger at donation compared with Caucasian donors (33.9 vs 38.2 years, P<0.001). Median time from donation to end of observation within the insurance data was 7.7 years. Average time to the end of study observation and captured insurance window durations did not differ significantly by donor race. Distributions of demographic characteristics of the linked donor sample were similar to that of all living kidney donors in the OPTN in the period (SDC 2, Table). SES scores were significantly less favorable among African American and Hispanic donors than among Caucasian donors. However, SES scores were similar in the privately-insured donor sample and all U.S. donors with linked SES information, overall and by race (SDC 3, Table).
Table 1.
Baseline characteristics of the study sample of privately-insured living kidney donors, overall and in African American and Caucasian sub-groups.
| Full Study Sample (N=4,650) | African Americans (N=611) | Caucasians (N=3,458) | |
|---|---|---|---|
|
| |||
| Percentage | Percentage | Percentage | |
|
| |||
| Male sex | 45.4% | 42.6% | 45.9% |
| Race | |||
| African American (non-Hispanic) | 13.1% | – | – |
| Caucasian (non-Hispanic) | 76.3% | – | – |
| Hispanic | 8.2% | – | – |
| Other | 2.4% | – | – |
| Biologically related to recipient | 81.2% | 88.2%‡ | 79.3% |
| First-degree relative of recipient | 76.1% | 81.5%* | 75.1% |
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
|
| |||
| Age at donation, yrs | 37.2 (10.0) | 33.9 (9.0)‡ | 38.2 (10.0) |
|
| |||
| Median | Median | Median | |
|
| |||
| Time from donation to end of insurance eligibility, yrs | 7.7 | 7.3 | 8.0 |
|
| |||
| Duration of insurance eligibility in the dataset, yrs | 2.1 | 1.8 | 2.1 |
P-values for differences in trait distributions among African American versus Caucasian donors:
p<0.05–0.0002;
p≤0.0001
Frequency and Variation in Post-donation Renal Conditions according to Race
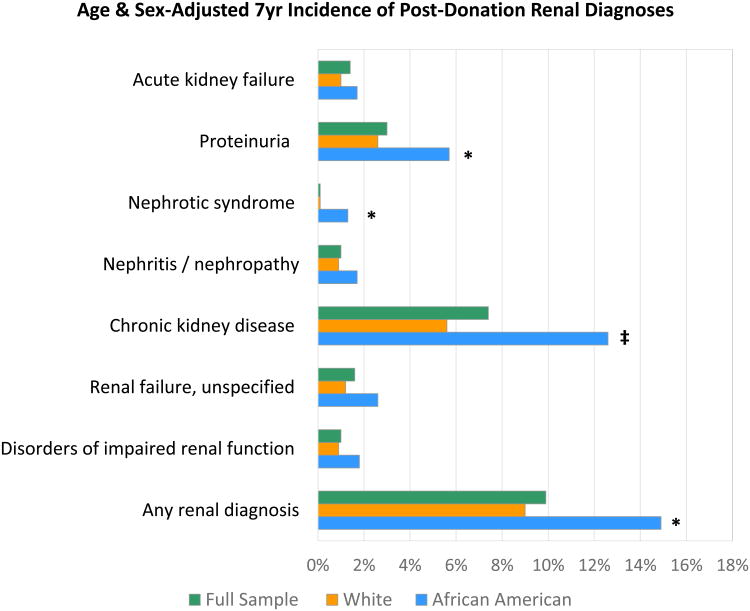
By seven years post-donation, the age- and sex-adjusted incidence of any renal diagnosis was higher among African American compared with Caucasian donors (14.9% vs 9.0%, P=0.002) (Figure 1). In absolute terms, racial differences in the composite outcome were driven largely by significantly higher frequencies of post-donation chronic kidney disease (12.6% vs 5.6%, P<0.0001) and proteinuria (5.7% vs 2.6%, P=0.004) in African American versus Caucasian donors. While uncommon in absolute terms, living donors who developed nephrotic syndrome were predominantly African American (1.3%, vs 0.1% in Caucasian donors, P=0.001).
Figure 1.
Age- and sex-adjusted incidence of renal diagnoses at 7-years post-donation, overall and in African American and Caucasian donors. * p<0.05–0.0001; ‡ p<0.0001
The composite outcome of Any Diagnosis also includes coded chronic glomerulonphritis and renal sclerosis (not shown separately due to low frequencies as indivdiual events).
In relative terms, compared to Caucasians, African Americans experienced 2.3-times the age- and sex-adjusted risks of post-donation proteinuria (aHR 2.27, 95% CI 1.32–3.89) and chronic kidney disease (aHR 2.32, 95% CI 1.48–3.62), as previously reported (13, 14). Notably, African Americans also had a higher likelihood of nephrotic syndrome after donation (aHR 15.7, 95% CI 2.97–83.0) (Table 2). The adjusted risk of any renal diagnosis after donation was 71% higher among African Americans compared with Caucasians (aHR 1.71, 95% CI 1.23–2.41).
Table 2.
Adjusted associations of baseline demographic factors with likelihoods of post-donation renal condition diagnoses in privately-insured living kidney donors.
| Acute kidney failure | Proteinuria§ | Nephrotic Syndrome | Nephritis / nephropathy | |
|---|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Age at donation (per year) | 1.07 (1.03-1.11)† | 1.01 (0.99-1.03) | 1.00 (0.92-1.08) | 1.03 (0.99-1.08) |
| Male Gender | 2.41 (1.03-5.66)* | 1.11 (0.72-1.71) | 1.00 (0.24-4.15) | 0.72 (0.32-1.61) |
| Race | ||||
| Caucasian | Reference | Reference | Reference | Reference |
| African American | 1.69 (0.57-5.66) | 2.27 (1.32-3.89)* | 15.69 (2.97-82.99)* | 1.99 (0.73-5.44) |
| Hispanic | -- | 1.47 (0.67-3.26) | 5.61 (0.49-64.30) | 0.70 (0.09-5.33) |
| Other | 1.93 (0.26-14.60) | 2.77 (0.99-7.72)* | -- | 2.28 (0.30-17.4) |
| Chronic kidney disease§ | Renal failure, unspecified | Disorders of impaired renal function | Any renal diagnosis | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Age at donation (per year) | 1.04 (1.03-1.06)‡ | 1.05 (1.02-1.08)* | 1.02 (0.98-1.07) | 1.04 (1.03-1.06)‡ |
| Male Gender | 1.64 (1.16-2.34)‡ | 2.69 (1.32-5.50)* | 1.33 (0.57-3.11) | 1.28 (1.00-1.65) |
| Race | ||||
| Caucasian | Reference | Reference | Reference | Reference |
| African American | 2.32 (1.48-3.62)‡ | 2.23 (1.00-4.98) | 2.14 (0.77-5.94) | 1.72 (1.23-2.41)* |
| Hispanic | 1.90 (1.05-3.43)‡ | 0.44 (0.06-3.23) | -- | 1.31 (0.82-2.09) |
| Other | 1.74 (0.66-4.76) | -- | 2.55 (0.33-19.74) | 1.51 (0.71-3.22) |
As stage-specific chronic kidney disease coding was not introduced until October 2005, we did not study chronic kidney disease stages as a primary outcome. However, we previously identified associations of African American race with significantly higher adjusted risk of diagnoses of chronic kidney disease stage 3 or higher (aHR 3.60, CI 1.37–9.39, P=0.009) in a subgroup of this sample with benefits after the introduction of stage-specific coding (13); the subanalysis also identified end-stage renal disease in 2 of 271 African American donors (0.7%) as compared with no cases (0%) among 1786 Caucasian donors (P=0.02) (13). Both of the African American patients who developed end-stage renal disease also had diagnoses of nephrotic syndrome.
Racial Variation in Post-donation Renal Conditions after Adjustment for Relationship
First-degree donor-recipient relationship was not associated with significantly increased risk of any of the study outcomes (Table 3); the hazard ratio point estimate for first-degree relatives was greater than 1.0 only for nephritis/nephropathy (aHR 2.79, P=0.17). Associations of African American donor race with post-donation proteinuria (aHR 2.23, 95% CI 1.29–3.82), nephrotic syndrome (aHR 15.51, 95% CI 2.94–81.86) and chronic kidney disease diagnoses (aHR 2.33, 95% CI 1.49–2.64) persisted with similar effect sizes after additional adjustment for first-degree donor-recipient relationship. In addition, African American race was associated with approximately twice the adjusted risks of coded unspecified renal failure (aHR 2.45, 95% CI 1.01–5.02) and disorders of impaired renal function (aHR 1.80, 95% CI 1.29–2.50) after adjustment for first-degree relationship. Patterns were similar with adjustment for any biological donor-recipient relationship. There were no significant interactions of race and first-degree or any biological donor-recipient relationship for the study outcome. SES score was not associated with any study outcome.
Table 3.
Adjusted associations of baseline demographic factors and donor-recipient relationship with post-donation renal condition diagnoses in privately-insured living kidney donors.
| Acute kidney failure | Proteinuria | Nephrotic Syndrome | Nephritis / nephropathy | |
|---|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Age at donation (per year) | 1.06 (1.02-1.11)* | 1.02 (1.00-1.04) | 1.00 (0.92-1.08) | 1.04 (0.99-1.08) |
| Male Gender | 2.53 (1.07-5.94)* | 1.03 (0.67-1.59) | 1.01 (0.25-4.19) | 0.70 (0.31-1.58) |
| Race | ||||
| Caucasian | Reference | Reference | Reference | Reference |
| African American | 1.75 (0.59-5.24) | 2.23 (1.29-3.82)* | 15.51 (2.94-81.86)* | 1.93 (0.70-5.29) |
| Hispanic | -- | 1.45 (0.66-3.21) | 5.46 (0.48-62.57) | 0.69 (0.09-5.30) |
| Other | 2.15 (0.28-16.33) | 2.83 (1.02-7.89)* | -- | 2.14 (0.28-16.35) |
| First-degree relative of recipient | 0.46 (0.20-1.09) | 0.80 (0.47-1.34) | -- | 2.79 (0.65-12.08) |
| Chronic kidney disease | Renal failure, unspecified | Disorders of impaired renal function | Any renal diagnosis | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Age at donation (per year) | 1.05 (1.03-1.07)‡ | 1.05 (1.01-1.08)* | 1.05 (1.04-1.06)‡ | 1.04 (1.03-1.05)‡ |
| Male Gender | 1.54 (1.08-2.19)* | 2.74 (1.34-5.59)* | 1.37 (1.07-1.75) * | 1.29 (1.01-1.66)* |
| Race | ||||
| Caucasian | Reference | Reference | Reference | Reference |
| African American | 2.33 (1.49-3.64)† | 2.45 (1.01-5.02)* | 1.80 (1.29-2.50) † | 1.75 (1.24-2.43)† |
| Hispanic | 1.91 (1.06-3.44)* | 0.44 (0.06-3.28) | 1.07 (0.65-1.78) | 1.32 (0.83-2.11) |
| Other | 1.83 (0.67-5.02) | -- | 1.21 (0.53-2.72) | 1.54 (0.72-3.29) |
| First-degree relative of recipient | 0.83 (0.55-1.26) | 0.64 (0.30-1.35) | 0.85 (0.64-1.14) | 0.76 (0.57-1.02) |
p<0.05–0.002;
p=0.001–0.0002;
p≤0.0001;
-- not evaluable due to lack of events in a stratum
Discussion
Recent studies have identified higher rates of ESRD after live kidney donation among African American compared with Caucasian donors (10-13) and also raise concerns for biologically-related donors (9, 22-24). We studied a linkage of OPTN registry data with administrative billing claims to examine renal conditions identified in the course of clinical care after living kidney donation. By seven years post-donation, 14.9% of African American and 9% of Caucasian living donors had at least one renal diagnosis. In absolute terms, racial differences in the composite outcome were driven largely by significantly higher frequencies of post-donation chronic kidney disease (12.6% versus 5.6%, aHR 2.32) and proteinuria (5.7% versus 2.6%, aHR 2.27) among African American compared with Caucasian donors. While uncommon in absolute terms, living donors who developed nephrotic syndrome were predominantly African American (1.3% vs 0.1%, aHR 15.7). Biological relationship to the recipient was not associated with renal risk in donors, but associations of African American race persisted for these conditions and included unspecified renal failure and reported disorders of kidney dysfunction after adjustment for donor-recipient relationship.
ESRD is more common among African American persons in the general population, and recent studies have shown this pattern also occurs after live kidney donation (10, 11, 13). Further, emerging data suggest a small but significant increase in ESRD rates among African American living donors compared with their own healthy controls, although clinical details regarding the causes of renal failure were not available (12). Review of a small case series of Japanese living donors who developed renal failure found that ESRD was preceded by comorbidities and complications including hypertension, proteinuria, cardiovascular disease and infection (28). Our current study shows that compared with Caucasians, African Americans are more commonly diagnosed with proteinuria, chronic kidney disease and nephrotic syndrome after live kidney donation. Living donors are screened for baseline good health prior to donation, but we previously found that hypertension and diabetes mellitus more commonly affect African Americans than Caucasians after donation (13, 14); onset of these comorbidities after donation may in part mediate post-donation renal risk.
While uncommon, it is notable that post-donation nephrotic syndrome predominantly affected African Americans in our study. Provocative recent literature supports that at least a portion of renal failure previously attributed to hypertensive nephrosclerosis in African Americans may be genetically mediated by coding variants in the APOL1 gene. Heterozygosity for APOL1 variants (G1 or G2) confers resistance to lethal Trypanosoma brucei infections, and these variants are common in populations of sub-Saharan African descent but essentially absent among Caucasians. Although protective against African sleeping sickness, homozygosity or compound heterozygosity for these variants has been associated with focal segmental glomerulosclerosis and HIV-associated nephropathy histopathologies, proteinuria, reduced glomerular filtration rate (GFR), younger age at dialysis and more rapid progression of chronic kidney disease among African Americans in the general population (17, 19, 29, 30). The presence of 2 APOL1 risk alleles in deceased donors was also correlated with nearly 4-times the relative risk of allograft loss (aHR 3.84) compared with 0 or 1 risk alleles (20). A case report of possible APOL1-mediated adverse donor and recipient outcomes after twin-to-twin live kidney donation among young men of Afro-Caribbean descent was recently described (21). In this report, the recipient developed declining GFR, proteinuria and focal segmental glomerulosclerosis by 30 months, followed by allograft failure at 5 years; at 7 years, the donor had low GFR (40 ml/min/1.73 m2) and proteinuria (2.5 g/d). Genotyping after these events revealed compound heterozygosity for APOL1 G1/G2 variants in both brothers. To improve renal-risk stratification and selection of African American potential living kidney donors, use of APOL1 genotyping within the evaluation process has been advocated and is currently used at some centers (16), but the impacts of APOL1 screening on donor exclusion rates and outcomes are not yet defined.
Concern that biologically-related living donors, especially first-degree relatives, may face increased risk of adverse renal outcomes after live kidney donation is a current topic of discussion in the transplant community (22-24). Increased risks of renal failure in close biological relatives of ESRD patients have been observed in population-based and case-control studies among non-donors, regardless of whether recipient ESRD has a known hereditary cause (31-33). In the recent study of Caucasian donors in Norway, the 9 donors who developed ESRD were all biologically related to their recipients and renal failure appeared mainly due to immunological diseases (9). However, 84% study sample were biologically-related, and related donors contributed the longest observation time due to temporal patterns of donor acceptance. As seen in our current and prior studies (25, 26), African Americans more commonly donate to related recipients. But notably, a recent multi-racial U.S. registry-based study did not find a significant difference in ESRD incidence among donors who were biologically related compared with unrelated to their recipients (12). Further, preliminary findings from a cohort of 4,167 dominantly Caucasian donors in the U.S. noted higher incidence of death-censored ESRD among living unrelated donors compared with first-degree relatives who donated to recipients with ESRD from immunologic causes (HR 3.85, 95% CI 1.14–13.04) (34). In the current study, we did not detect significant associations of first-degree or any biological relationship with renal diagnosis categories. We also did not detect interactions of race and relationship, and the renal risk associated with African American race was present and included more diagnostic categories after adjustment for donor-recipient relationship. Racial variation in post-donation renal outcomes appears to be mediated by more than familial risk, and likely reflects a complex array of factors including comorbidities that develop after donation, population genetics, and environmental/lifestyle exposures. It is possible that the donor evaluation and selection process, including renal function measurement, anatomic assessment and screening for proteinuria and hematuria (35, 36), mitigates the renal risk associated with family history of ESRD. In other words, because the predonation evaluation includes comprehensive assessment of renal function, urinalysis, urinary protein excretion, blood pressure and global health history, any potential donor who manifested early kidney disease from a genetic predisposition prior to donation should be excluded from donating; thus, approved donors are a subset of the population with family history of kidney disease who have confirmed excellent renal health after screening. Associations of family history with renal outcomes warrant continued study within the donor population. Importantly, the current study analyzed intermediate-term outcomes, and analyses of lifetime risk according to factors including race and family history are needed.
Frameworks of risk for living donors can include descriptive experience among donors, within-donor subgroup comparisons, and comparisons of donor experience to non-donors (general population or selected) (15). The current study was explicitly designed as a within-donor comparison, and while the design does not quantify the impact of donation itself, there are potential applications of the findings for predonation counseling and postdonation follow-up. In 2013, OPTN/United Network for Organ Sharing (UNOS) implemented a Policy for the Informed Consent of living donors in the U.S. that defines minimum standards for disclosure of possible short- and long-term risks of donation applicable to all donors (36). Because African Americans are not well represented in large single center cohort studies performed to date (6-9), data from these published cohorts may not generalize to African American donors. Our findings support use of race-stratified outcomes information when available in counseling African American potential donors. Our results also reinforce the need for regular follow-up and access to healthcare for all donors, so that any health conditions that develop over time can be recognized and managed early. Gibney et al found that, overall, 18% of living donors in a recent U.S. sample lacked insurance at donation, and insurance access varied demographically, such that 30-40% of young, African American male donors were uninsured (37). Further, followup reporting deficiencies by centers are greater in the groups most likely to lack insurance (5). Donors in our study sample had private insurance during observation, and it is possible that racial disparities in post-donation health outcomes are greater among uninsured donors.
Regarding donor selection, some clinical practice guidelines have recommended more stringent selection criteria for African American potential donors, such as with regard to predonation blood pressure (38, 39). The stringency of donor selection has inherent tensions with the high need for donated organs in the African American population. Compared with Caucasian ESRD patients, African American patients with ESRD have lower access to transplantation including live donor transplants (40, 41). African American transplant candidates are less likely to identify potential living donors, and their potential living donors are less likely to donate for reasons including medical exclusions (42). Race alone should not be used to discourage evaluation for living donation. By identifying and excluding persons with overt predonation comorbidities, donor selection and screening practices have demonstrated value in reducing ESRD rates below rates in the unselected general population across racial groups (11, 12). However, to minimize preventable risk, novel approaches to risk stratifying African American living donors should be pursued.
Limitations of the current study include factors related to the sample and outcome measures. The sample represents privately-insured donors, and findings may not generalize to uninsured living donors or those receiving care under other insurance systems. However, we believe that the most likely direction of bias from exclusion of uninsured/under-insured donors would be an underestimation of medical complications in non-Caucasian donors, as lack of health insurance has been shown to be more common among African American donors (37). Predonation benefits were captured for only a minority of the donors (7.7%), and thus, information on predonation diagnoses was not adequate for inclusion. Due to the nature of OPTN collection of baseline donor data, we also lacked baseline information on clinical parameters such as body mass index sufficient for inclusion. Donors related to their recipient by definition have a family history of ESRD, but we lacked family history information for persons donating to unrelated recipients.
Claims are surrogate measures for diagnoses and the renal condition categories were defined by the ICD-9 coding scheme. Billing claims have been demonstrated to provide sensitive measures of some conditions like diabetes and cardiovascular diagnoses (43, 44), but to under-represent the burden of kidney dysfunction compared to lab-based measures in the general population (45). Coding patterns may differ among donors, and we lacked laboratory data for coding validation. We chose the algorithms based on first submitted diagnostic claims for consistency and comparability with prior studies (13, 14), but the optimal number of claims to confirm renal diagnoses among living donors has not been defined. Given the infrequency of nephrotic syndrome especially among Caucasian donors, the estimate of the relative hazards associated with African American compared with Caucasian race is imprecise. Although some renal diagnosis categories such as nephrotic syndrome should be clinically specific, the clinical importance of categories such as disorders of impaired renal function warrants further study, such as in samples with claims data linked directly to medical records. Nonetheless, the current study demonstrates an aggregate of more frequent renal diagnoses among African American donors that emphasizes a need for continued attention to this important group. Finally, while designed as a within-donor comparison, our study of the spectrum of renal conditions captured in the ICD9 coding system was motivated by recent observation of higher ESRD rates (but without information on causes or other clinical details) among African American donors versus “healthy” African American non-donor controls (12).
In conclusion, we found that compared with Caucasians, African Americans have increased likelihood of renal diagnoses after living kidney donation, driven largely by higher frequencies of post-donation chronic kidney disease and proteinuria. While uncommon, post-donation nephrotic syndrome appears to be predominantly a disease of African American donors. Renal risk associated with African American race was not explained by biological relationship to the recipient alone, as associations persisted and included more categories of renal conditions after adjustment for donor-recipient relationship. Outcomes data specific to African American donors are relevant to tailoring informed consent according to donor demographic traits. These findings also support the need for long-term follow-up and access to healthcare after donation. To improve the counseling, selection and care of living kidney donors, continued efforts to refine risk stratification among African Americans and other higher risk groups should be pursued as an important priority.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK096008. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. An abstract describing portions of this work was presented at the World Transplant Congress, July 29, 2014, San Francisco, CA.
Funding: This work was supported by grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-DK096008 and K24DK101828.
Abbreviations
- aHR
adjusted hazards ratio
- APOL1
Apolipoprotein L1
- ESRD
End-stage renal disease
- GFR
Glomerular filtration rate
- HRSA
Health Resources and Services Administration
- ICD9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
Disclosures: The authors have no conflicts of interest related to this work.
References
- 1.OTPN/HRSA. National Data, Transplants by Donor Type. [Access date: october 7, 2014]; http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp.
- 2.OPTN (Organ Procurement and Transplantation Network) and SRTR (Scientific Registry of Transplant Recipients) Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; [Access date: october 7, 2014]. OPTN / SRTR 2012 Annual Data Report. Kidney Chapter/Live Donation. Available at: http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/01_kidney_13.pdf. [Google Scholar]
- 3.Lentine KL, Segev DL. Health outcomes among non-Caucasian living kidney donors: knowns and unknowns. Transpl Int. 2013;26(9):853. doi: 10.1111/tri.12088. [DOI] [PubMed] [Google Scholar]
- 4.Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin J Am Soc Nephrol. 2006;1(4):885. doi: 10.2215/CJN.00840306. [DOI] [PubMed] [Google Scholar]
- 5.Ommen ES, LaPointe Rudow D, Medapalli RK, Schroppel B, Murphy B. When good intentions are not enough: obtaining follow-up data in living kidney donors. Am J Transplant. 2011;11(12):2575. doi: 10.1111/j.1600-6143.2011.03815.x. [DOI] [PubMed] [Google Scholar]
- 6.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tyden G, Groth CG. Kidney donors live longer. Transplantation. 1997;64(7):976. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier C, Pallet N, Cherqaoui Z, et al. Very long-term follow-up of living kidney donors. Transpl Int. 2012;25(4):385. doi: 10.1111/j.1432-2277.2012.01439.x. [DOI] [PubMed] [Google Scholar]
- 9.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2013 doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 10.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007;84(5):647. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 11.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11(8):1650. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 12.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. Jama. 2014;311(6):579. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363(8):724. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lentine KL, Schnitzler MA, Xiao H, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014;97(3):316. doi: 10.1097/01.TP.0000436731.23554.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentine KL, Schnitzler MA, Garg AX, et al. Understanding Antihypertensive Medications Use after Living Kidney Donation through Linked National Registry and Pharmacy Claims Data. Am J Nephrol. 2014 doi: 10.1159/000365157. In Press. [DOI] [PubMed] [Google Scholar]
- 16.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011;92(7):722. doi: 10.1097/TP.0b013e31822eec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 Gene and Allograft Survival after Kidney Transplantation. Am J Transplant. 2011;11(5):1025. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kofman T, Audard V, Narjoz C, et al. APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis. 2014;63(5):816. doi: 10.1053/j.ajkd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Matas AJ. Transplantation: Increased ESRD and mortality risk for kidney donors? Nat Rev Nephrol. 2014;10(3):130. doi: 10.1038/nrneph.2014.2. [DOI] [PubMed] [Google Scholar]
- 23.Pondrom S. The AJT Report: Studies Confirm Increased Risk of ESRD in Kidney Donors. Am J Transplant. 2014;14:741. [Google Scholar]
- 24.Matas AJ, Wadstrom J, Ibrahim HN. Kidney donation and risk of ESRD. Jama. 2014;312(1):92. doi: 10.1001/jama.2014.5515. [DOI] [PubMed] [Google Scholar]
- 25.Reeves-Daniel A, Bailey A, Assimos D, et al. Donor-recipient relationships in African American vs. Caucasian live kidney donors. Clin Transplant. 2011;25(5):E487. doi: 10.1111/j.1399-0012.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- 26.Lentine KL, Schnitzler MA, Xiao H, et al. Associations of recipient illness history with hypertension and diabetes after living kidney donation. Transplantation. 2011;91(11):1227. doi: 10.1097/TP.0b013e31821a1ae2. [DOI] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality. Creating and Validating an Index of Socioeconomic Status. [Access date: october 7, 2014]; http://www.ahrq.gov/qual/medicareindicators/medicareindicators3.htm.
- 28.Kido R, Shibagaki Y, Iwadoh K, et al. How do living kidney donors develop end-stage renal disease? Am J Transplant. 2009;9(11):2514. doi: 10.1111/j.1600-6143.2009.02795.x. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22(11):2098. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanji Z, Powe CE, Wenger JB, et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol. 2011;22(11):2091. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9(7):1270. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 32.O'Dea DF, Murphy SW, Hefferton D, Parfrey PS. Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. Am J Kidney Dis. 1998;32(5):794. doi: 10.1016/s0272-6386(98)70135-0. [DOI] [PubMed] [Google Scholar]
- 33.Skrunes R, Svarstad E, Reisaeter AV, Vikse BE. Familial Clustering of ESRD in the Norwegian Population. Clin J Am Soc Nephrol. 2014 doi: 10.2215/CJN.01680214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Issa N, Kukla A, Gillingham K, Ibrahim H, Matas AJ. Long-Term Risks for Living Kidney Donors. Am J Transplant. 2014;14:92. S3, Supplement: 2014 World Transplant Congress Abstracts. [Google Scholar]
- 35.Delmonico F. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53. [PubMed] [Google Scholar]
- 36.OPTN (Organ Procurement and Transplantation Network)/UNOS (United Network for Organ Sharing) OPTN Policies, Policy 14: Living Donation. [Access date: october 7, 2014]; http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf.
- 37.Gibney EM, Doshi MD, Hartmann EL, Parikh CR, Garg AX. Health insurance status of US living kidney donors. Clin J Am Soc Nephrol. 2010;5(5):912. doi: 10.2215/CJN.07121009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AST/ASTS/NATCO/UNOS Joint Societies Work Group. Evaluation of the Living Kidney Donor – a Consensus Document from the AST/ASTS/NATCO/UNOS Joint Societies Work Group. [Access date: october 7, 2014]; http://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSurveyExhibit_38.pdf.
- 39.Spanish Society of Nephrology (S.E.N.) and Spanish Transplant Organisation (ONT) recommendations for living-donor kidney transplantation. Nefrologia. 2010;30(Suppl 2):1. [Google Scholar]
- 40.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 41.Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS. Disparities in the utilization of live donor renal transplantation. Am J Transplant. 2009;9(5):1124. doi: 10.1111/j.1600-6143.2009.02620.x. [DOI] [PubMed] [Google Scholar]
- 42.Reeves-Daniel A, Adams PL, Daniel K, et al. Impact of race and gender on live kidney donation. Clin Transplant. 2009;23(1):39. doi: 10.1111/j.1399-0012.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 43.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 44.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4(7):1213. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2439. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.