To the Editor
Human pluripotent stem cell (hPSC)-derived cardiomyocytes are important tools for cardiovascular research and have substantial therapeutic potential. Several efficient differentiation strategies have been devised to generate cardiomyocytes from hPSCs1. Recently we2,3 and Burridge et al.4 published defined, growth-factor free protocols for differentiating hPSCs to cardiomyocytes.
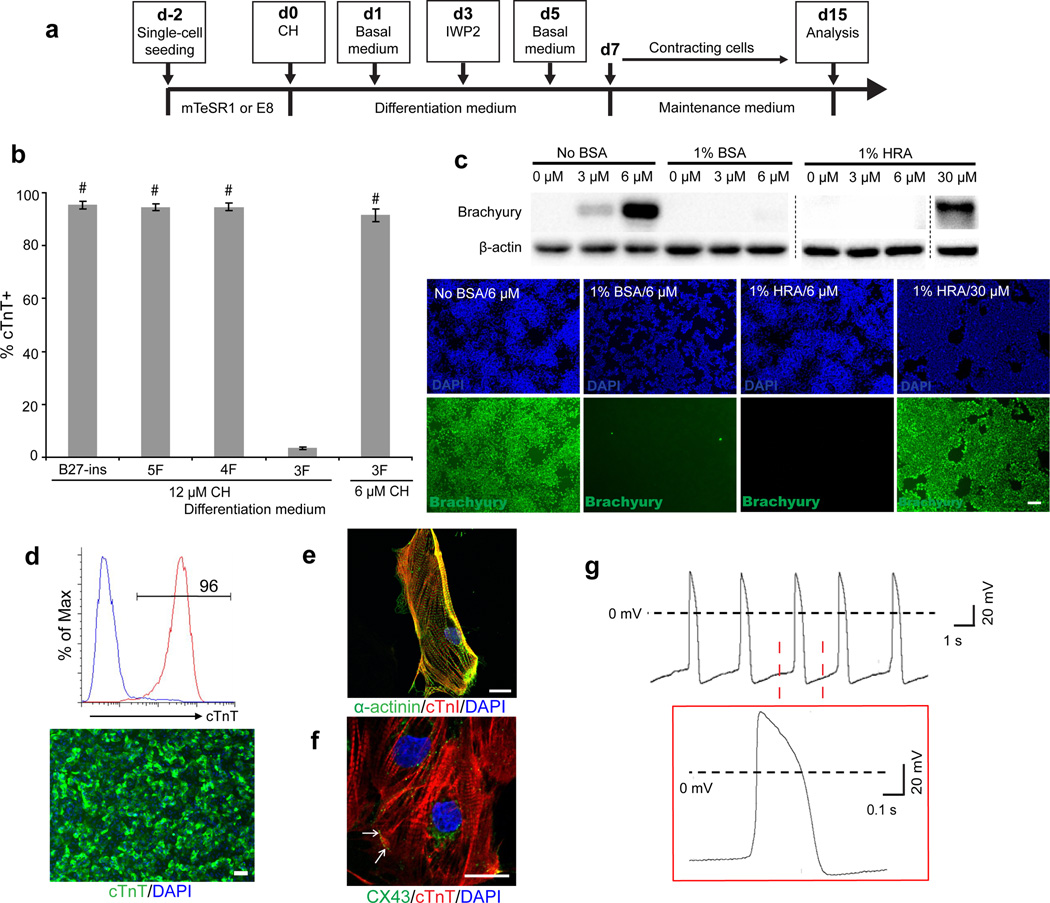
In 2012, we first described the Gsk3 inhibitor and Wnt inhibitor (GiWi) method for robust hPSC differentiation to cardiomyocytes under serum-free and growth factor-free conditions2,3. This GiWi method (Fig. 1A) applies two small molecules at precise developmental stages to sequentially promote mesoderm formation and cardiomyocyte specification2,3. Although the RPMI with B27-ins (B27 without insulin) medium used in the GiWi protocol lacks animal sera and growth factors, the inclusion of bovine serum albumin (BSA) increases the cost and adds xenogenic components. Recently, Burridge et al.4 described modifications to the GiWi method, including replacing B27-ins with recombinant human albumin and L-ascorbic acid 2-phosphate. They reported that albumin and L-ascorbic acid 2-phosphate are necessary for cardiomyocyte differentiation with high yield and purity.
Figure 1.
Albumin is not required for hPSC differentiation to cardiomyocytes. (A) Schematic of the protocol for differentiation of hPSCs to cardiomyocytes via treatment with Wnt-modulating small molecules. (B) Purity, determined by flow cytometry analysis of cTnT expression of cardiomyocytes differentiated from ES03 hESCs in RPMI basal medium supplemented with the indicated components. 5F: transferrin, sodium selenite, progesterone, putrescine, BSA; 4F: sodium selenite, progesterone, putrescine, BSA; 3F: sodium selenite, progesterone, putrescine. Error bars represent standard derivation of five independent biological replicates; #P<0.005, indicated medium versus 3F with 12 μM CH; Student’s t test. (C) Western blot analysis of brachyury expression in ES03 hESCs treated with indicated concentrations of CH in albumin-free or albumin-containing RPMI medium for 24 hours. Immunolabeling for brachyury expression in hPSCs treated with 6 μM CH in albumin-free or albumin-containing RPMI for 24 hours. BSA: bovine serum albumin; HRA: human recombinant albumin. Error bars represent SEM of three independent experiments; Scale bar, 50 μm. (D) Flow cytometry analysis and immunostaining of cTnT expression in cardiomyocytes differentiated from human 19-9-11 iPSCs in RPMI. Scale bar, 100 μm. (E) Coimmunolabeling of cTnI and α-actinin in a single 19-9-11 iPSC-derived cardiomyocyte. Scale bar, 10 μm. (F) Coimmunolabeling of cTnT and CX43 in 19-9-11 iPSC-derived cardiomyocytes. Scale bar, 10 μm. All flow cytometry plots and immunofluorescent images are representative of at least 15 technical replicates from at least 3 independent experiments. (G) A typical action potential of an individual ES03 hESC-derived cardiomyocyte recorded via patch clamp (n=14 cells). The lower inset shows enlarged waveform of a single action potential. Dashed line indicates resting potential 0 mV.
We also simplified the GiWi protocol and developed an albumin-free cardiomyocyte differentiation platform. First, we compared B27-ins (Supplementary Table 1) with other published recipes for cardiomyocyte differentiation1,5–9 and identified five commonly-shared differentiation media supplements (transferrin, sodium selenite, progesterone, putrescine, and BSA). RPMI containing these five components (5F) supported hPSC differentiation to more than 90% cardiac troponin T (cTnT)-expressing cardiomyocytes, comparable to RPMI/B27-ins (Fig. 1B). Removal of transferrin (4F) also produced 90% cTnT+ cells.
However, removal of BSA from 4F medium resulted in virtually no cardiomyocytes. 12 μM CHIR99021 (CH) treatment caused prolific cell death in the absence of BSA. However, 6 μM CH produced more than 90% cTnT+ cells in the absence of albumin (Fig. 1B and Supplementary Fig. 1A). In addition, 2.5 μM IWP2 was sufficient to induce more than 90% cTnT+ cells (Supplementary Fig. 1B), lower than the 5 μM IWP2 required in the presence of BSA. Thus, albumin is not necessary for cardiomyocyte differentiation, and in fact its presence diminishes activity of small molecule agonists and antagonists of Wnt signaling. Basal RPMI lacking supplements supported hPSC differentiation to cardiomyocytes using the GiWi method (Supplementary Fig. 1C). DMEM, DMEM/F12 and MEM also supported cardiomyocyte differentiation, but RPMI outperformed these media (Supplementary Fig. 1D).
6 μM CH in albumin-free RPMI induced robust brachyury expression in hPSCs (Fig. 1C and Supplementary Fig. 2). However, 1% BSA or human recombinant albumin (HRA) completely blocked brachyury expression at CH concentrations up to 6 μM, demonstrating Wnt activation induced by Gsk3 inhibitor treatment is more efficient in media lacking albumin. 30 μM CH induced brachyury expression in medium containing 1% HRA (Fig. 1C).
This albumin-free GiWi (named GiWi2) protocol produced 88–98% cTnT+ cells with yields of greater than 1×106 cardiomyocytes/cm2 in multiple hESC (ES03, ES03-GFP, H9, HS181, H1) and iPSC (19-9-11, 6-9-9, IMR90C4, 19-9-7) lines (Supplementary Table 2, Supplementary Fig. 3). The GiWi2 protocol is equally effective with cells maintained in E8 or mTeSR1 (Supplementary Fig. 4).These cardiomyocytes exhibited spontaneous contraction for more than 8 months (Supplementary Movie S1).
These chemically defined, albumin-free conditions supported cardiac induction from hPSCs based on cTnT (Fig. 1D), cardiac troponin I (Fig. 1E, F) sarcomeric myosin heavy chain, α-actinin, and Nkx2.5 expression (Supplementary Fig. 5). α-actinin showed clear Z-line localization (Fig. 1E) and connexin-43 localized to cell-cell junctions (Fig. 1F). The earliest wave-like spontaneous contractions were observed on day 7, and robust beating was observed by day 10 (Supplementary Movie S2, S3). A representative recording of a ventricular-like action potential is shown (Fig. 1G, Supplementary Table 3). Cardiomyocytes also exhibited spontaneous Ca2+ transients (Supplementary Fig. 6A, B). After 60 days in albumin-free medium, cardiomyocytes generated using the GiWi2 protocol maintained expression of cTnT, α-actinin and connexin-43 (Supplementary Fig. 7).
Taken together, these results demonstrate that the GiWi2 protocol generates more than 90% pure populations of functional cardiomyocytes with a yield of 106 cardiomyocytes/cm2, similar to or exceeding cardiomyocyte production in the presence of albumin2,4. Differences between our study and the Burridge et al.4 report, including the cell density at initiation of differentiation and the exposure windows for GiWi chemicals, may account for their finding that albumin is necessary for efficient cardiomyocyte differentiation. For example, we found optimal Gsk3 inhibitor treatment from days 0-1 and Wnt inhibitor treatment from days 3-5 while Burridge et. al applied the Gsk3 inhibitor from days 0-2 and the Wnt inhibitor from days 2-4. We also found that hPSC treatment with the Gsk3 inhibitor concentration Burridge et al. used caused substantial cell death after 2 days in albumin-free medium. However, reducing the CH concentration and/or treatment time in the absence of albumin permitted efficient mesendoderm induction without cytotoxicity.
We report an efficient, robust, and defined cardiomyocyte differentiation protocol in the absence of albumin. The need to tune small molecule presentation in the absence of albumin might have broader implications on development of other defined, small molecule-based stem cell differentiation systems.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by U.S. National Institutes of Health (NIH) grant R01EB007534. K.K.D. was funded in part by an NIH Chemistry Biology Interface Training Grant (NIGMS T32 GM008505).
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
X.L. and X.B. conceived, performed and interpreted experiments and wrote the manuscript; M.Z., M.W., A.F., C.H., L.B.H., and K.K.D. performed experiments; T.J.K. interpreted experiments; S.P.P. conceived the experiments, provided experimental advice, and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing.
REFERENCES
- 1.Mummery CL, et al. Circ. Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lian X, et al. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian X, et al. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burridge PW, et al. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uosaki H, et al. PLoS One. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami I, et al. Cell Rep. 2012;2:1448–1460. doi: 10.1016/j.celrep.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Hudson J, Titmarsh D, Hidalgo A, Wolvetang E, Cooper-White J. Stem Cells Dev. 2012;21:1513–1523. doi: 10.1089/scd.2011.0254. [DOI] [PubMed] [Google Scholar]
- 8.Xu C, et al. Stem Cells Dev. 2006;15:931–941. doi: 10.1089/scd.2006.15.931. [DOI] [PubMed] [Google Scholar]
- 9.Passier R, et al. Stem Cells. 2005;23:772–780. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.