Abstract
Background
The precise contribution of IORT to the management of locally advanced and recurrent colorectal cancer (CRC) remains uncertain. We performed a systematic review and meta-analysis to assess the value of IORT in this setting.
Methods
Studies published between 1965 and 2011 that reported outcomes after IORT for advanced or recurrent CRC were identified by an electronic literature search. Studies were assessed for methodological quality and design, and evaluated for technique of IORT delivery, oncological outcomes, and complications following IORT. Outcomes were analysed with fixed-effect and random-effect model meta-analyses and heterogeneity and publication bias examined.
Results
29 studies comprising 14 prospective and 15 retrospective studies met the inclusion criteria and were assessed, yielding a total of 3003 patients. The indication for IORT was locally advanced disease in 1792 patients and locally recurrent disease in 1211 patients. Despite heterogeneity in methodology and reporting practice, IORT is principally applied for the treatment of close or positive margins. When comparative studies were evaluated, a significant effect favouring improved local control (OR 0.22; 95% CI=0.05-0.86; p=0.03), disease free survival (HR 0.51; 95% CI=0.31-0.85; p=0.009), and overall survival (HR 0.33; 95% CI=0.2-0.54; p=0.001) was noted with no increase in total (OR 1.13; 95% CI=0.77-1.65; p=0.57), urologic (OR 1.35; 95% CI=0.84-2.82; p=0.47), or anastomotic complications (OR 0.94; 95% CI=0.42-2.1; p=0.98). Increased wound complications were noted after IORT (OR 1.86; 95% CI=1.03-3.38; p=0.049).
Conclusions
Despite methodological weaknesses in the studies evaluated, our results suggest that IORT may improve oncological outcomes in advanced and recurrent CRC.
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related morbidity and mortality, with an estimated 1 million new cases diagnosed annually worldwide [1]. Complete surgical resection remains the cornerstone of treatment in CRC, and in recent years has been increasingly combined with chemotherapy and/or radiotherapy to improve outcomes and diminish local or distant failure. Nevertheless, despite refinements in operative practice and introduction of combined-modality treatment, 5-year overall survival figures seldom exceed 60% in developed countries [2, 3].
Rectal cancer (RC) in particular has a propensity to recur locally. External beam radiotherapy (EBRT) both pre- and post-operatively can improve local control and survival [4-6], and more recently, the additional benefit conferred by multimodality therapy incorporating EBRT and chemotherapy has been confirmed in randomised controlled trials (RCT) [7, 8]. Regardless of these advances, primary locally advanced/unresectable (LARC) and locally recurrent RC (LRRC) pose significant therapeutic challenges. Local relapse occurs in up to 40% of cases of LARC despite chemoradiotherapy and radical resection [9] and previous irradiation limits the scope for further treatment in patients with LRRC, owing to the risk of toxicity to dose-sensitive viscera [9]. In these circumstances aggressive local control still offers the best chance for improved survival and even cure, and it is in this setting that intraoperative radiotherapy (IORT) has been considered as a potentially valuable treatment option in RC.
Intraoperative radiotherapy (IORT) may be defined as the direct application of irradiation to a tumour bed during an operative procedure [10, 11]. This approach permits precise delivery of a single large fraction of radiation (typically 10-20Gy) directly and specifically to high recurrence risk anatomical target areas, while simultaneously displacing and shielding dose-limiting radio-sensitive structures [11, 12]. There is increasing evidence to suggest that inclusion of IORT in the multi-modal treatment of LARC and LRRC can lead to improved local control and survival, however this has not been a universal finding. A limited number of studies have evaluated the role of IORT in locally advanced and recurrent colon cancer (CC), but this approach is more controversial since adjuvant RT is infrequently used in the management of CC.
The purpose of the present review and meta-analysis is to provide a comprehensive and systematic summary of studies reporting outcomes with IORT in the management of primary and recurrent CRC. Where possible, meta-analytic methodology has been applied to summarise and synthesise the available data. Emphasis has been placed on the techniques employed, oncological outcomes, and complications after IORT.
Methods
Identification of studies
An electronic literature search was carried out using MEDLINE (1965 to July 2011), EMBASE (1980 to July 2011), CINAHL (1982 to July 2011) and the Cochrane Library databases. The following medical subject heading (MeSH) terms and key words were used: “radiotherapy”; “intraoperative”; “IORT”; “IOERT”; “HDR-IORT”; “recurrent”; “advanced”; “colorectal cancer”. The “related articles” function was used to broaden the search and all abstracts, studies and citations retrieved were scanned for subject relevance. The latest date of this search was July 2011. All potentially relevant publications were retrieved in full text and formally evaluated for study inclusion. Reference lists of all relevant publications were hand searched for additional studies missed by the search strategy, and this method of cross-referencing was continued until no further relevant publications were identified.
Study inclusion criteria and data extraction
Study methodology was carried out in accordance with the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) recommendations for improving the standard of systematic reviews. [13] Studies that met the following pre-defined criteria were included in the review process: Language: only English language publications were included; Patient population: Studies had to report on outcomes in ≥10 male/female adult patients (≥18 years) with a diagnosis of primary or recurrent CRC having IORT as part of multi-modal treatment involving surgical resection +/− external beam radiotherapy (EBRT) +/− chemotherapy; Type of irradiation: Studies reporting on both high-dose rate intraoperative brachytherapy (HDR-IORT) and intraoperative electron radiation therapy (IOERT) were included; Previous Treatment: Studies reporting on previously irradiated patients with CRC were included; Outcome measures: Studies were included if they reported oncological outcome data (local control rate/overall survival/disease free survival) +/− one or more of the following: (i) functional outcome data (gastrointestinal and urinary); (ii) data on complications associated with IORT.
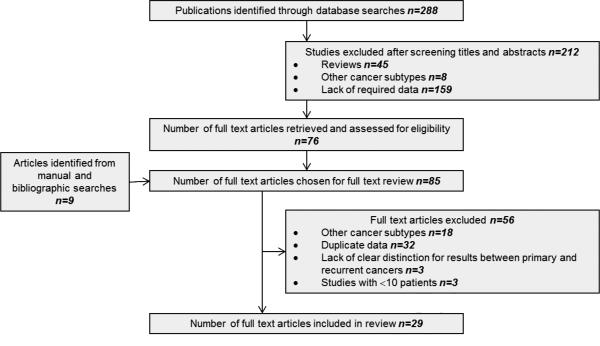
Where multiple studies describing the same patient population were identified, the most recent publication was used unless additional information was imparted by earlier work. Studies reporting oncological outcomes for both primary and recurrent CRC were excluded if results were not reported independently for primary versus recurrent disease. In cases of doubt, authors were contacted for further information to ensure accuracy or for additional data. Figure 1 depicts the screening process for selection of potentially relevant studies. Two reviewers (RM and AHM) independently extracted the following data from all eligible studies according to a predetermined protocol: first author, year of publication, study location, study type, study time-frame, population characteristics, number of subjects, cancer location (colon/rectum/both), primary or recurrent disease, disease stage, type of IORT used, radiation dosage, details of previous treatment (chemotherapy/radiotherapy), follow-up duration, incidence of complications, functional outcome(s), incidence of local/distant recurrence, overall and disease-free survival. Extracted data were entered into a computerised database and cross-checked to reach consensus. Studies were then subgrouped further based on whether they provided data on primary or recurrent CRC. Having met the inclusion criteria, studies were subjected to an assessment of methodological quality and validity and graded on strength of evidence using the revised grading system of the Scottish Intercollegiate Guidelines Network (SIGN). [14]
Figure 1.
Modified PRISMA flow diagram showing study methodology.
Data analysis
Statistical analysis was performed using Comprehensive Meta-analysis Version 2, Biostat (Englewood, NJ). Hazard ratio (HR) was used as the summary statistic for censored outcomes and Odds ratio (OR) for dichotomous variables as described by Tierney and colleagues. [15] A fixed effects model was applied unless there was evidence of heterogeneity. Between-study heterogeneity was evaluated using Q statistics and quantified by the I2 statistic which is independent of the number of studies in the meta-analysis. [16, 17] Where heterogeneity was identified, meta-analyses were performed using the DerSimonian-Laird random-effects model. [18] The P value for overall effect was calculated with the Z test and significance set at P < 0.05. To assess impact of studies with large outlying effects, or those with wide 95% CI on heterogeneity, additional sensitivity analyses were performed after exclusion of these studies. [19] The Q statistic is underpowered and therefore homogeneity of studies was assumed at a P value greater than 0.1. [20] Publication bias was assessed visually with funnel plots and Egger's regression test. [21, 22] No significant publication bias was assumed if the 95% CI included 0. As it was anticipated that margin-positive resections (R1/2) would benefit most from the addition of IORT, where possible, meta-analyses have been conducted using outcome data from non-R0 resections.
Results
Literature search and description of studies
The outlined search strategy identified 288 publications of potential relevance. Following screening of titles and abstracts, 45 reviews and 167 irrelevant studies were excluded, leaving 76 articles that were retrieved in full text. A bibliographic search from these articles identified nine additional studies, providing a total of 85 articles for detailed evaluation. Of these, 56 did not meet inclusion criteria and were withdrawn after evaluation, leaving a total of 29 studies published between 1991 and 2011 that were entered into the review process (figure 1). The total number of patients undergoing IORT from the included studies was 3003 (range 11-607). Studies included in the review comprised 14 prospective studies [23-36] (evidence level 1- and 3) of which two were randomised-controlled trials [29, 32] (evidence level 1-), and 15 retrospective studies [37-51] (evidence level 3 and 2-) including one retrospective multi-national pooled analysis [42] (evidence level 2-).
The indication for IORT was locally advanced CRC [23-32, 37-42] in 1792 patients (61%) and locally recurrent CRC [27, 33-36, 43-51] in the remaining 1211 patients (39%). From studies reporting on locally advanced cancers, 2% of the study population (40/1792 patients) had colon cancer, while 98% (1752/1792 patients) had rectal cancer. For recurrent tumours, 15% (187/1211 patients) had locally recurrent colon cancer, compared with 80% (973/1211 patients) with LRRC. One study provided data on 51 patients with pelvic recurrence following previous CRC resection, but recurrences were not classified distinctly as colonic or rectal in origin (51/1211; 5%) [50]. The technique used for intraoperative irradiation was IOERT in 2765 patients (92%) and HDR-IORT in the remaining 238 patients (8%).
For locally advanced CRC, the indication for IORT was stated in ten of the 16 studies. [23, 25, 27-30, 37, 39-41] In six studies the indication was the finding of residual tumour or close resection margins [23, 27, 28, 30, 37, 40]. A ‘close margin’ was defined as <5mm [23, 37] or <2mm [27, 40]. In two of these six studies no exact distance was assigned to denote a ‘close’ margin [28, 30]. Three studies stated that IORT was applied following all resections with the dose of irradiation increased incrementally in cases of microscopic or macroscopic residual tumour (R0<R1<R2) [25, 39, 41]. In one study IORT was applied to pelvic autonomic nerve plexuses following nerve sparing TME with lateral lymph node dissection [29]. In the six remaining studies the precise rationale for IORT was not disclosed by the authors [24, 26, 31, 32, 38, 42].
The indication for IORT was defined by 11 of the 14 studies reporting on locally recurrent CRC [27, 33, 35, 36, 43, 44, 47-51]. The indication was close or positive margins in 7 studies (positive/<5mm [33, 44]; positive/<2mm [27, 48]; positive/<1mm [50]; positive/<10mm [43]; positive/unspecified ‘close’ [35]). In the remaining four studies, all patients underwent treatment with IORT, with the dose of irradiation increased incrementally in cases of residual tumour (R0<R1<R2) [36, 47, 49, 51]. In three studies no precise rationale was provided for the use of IORT [34, 45, 46].
Techniques
In 23 of the included studies, comprising 93% of the total study population, IORT was delivered using an electron-based device (IOERT) [23, 25, 26, 28-35, 37-39, 41-47, 49, 51]. In four studies, comprising 6% of the total study population, radiotherapy was delivered by high dose-rate brachytherapy (HDR-IORT) [24, 27, 36, 40]. Two studies reported use of both modes of IORT delivery [48, 50].
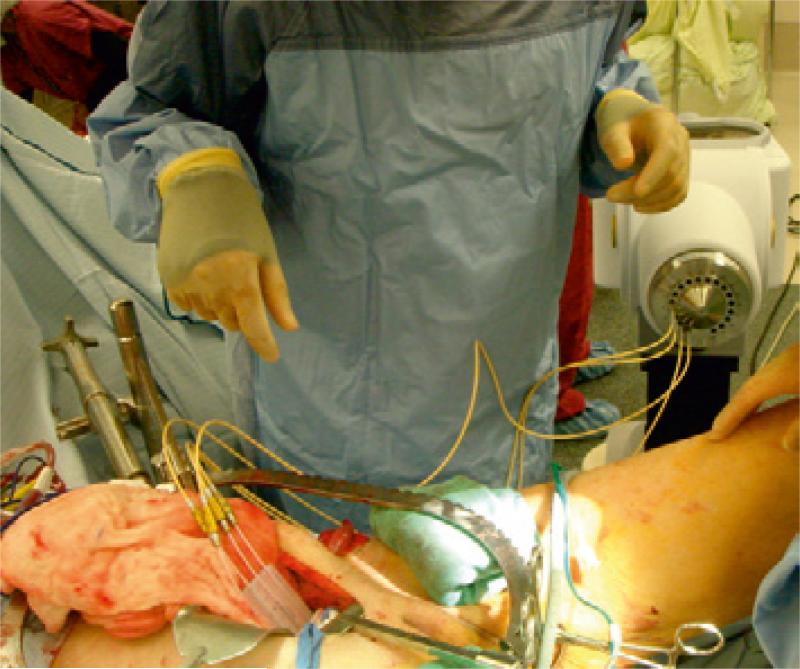
In IOERT, radiation delivery relies on the emission of high-energy electrons from a linear accelerator, with transmission of the electron beam through a rigid conical applicator. [12] In the past, IOERT has required the availability of a dedicated operating room equipped with a stationary linear accelerator and appropriate radiation protection facilities (figure 2) [34, 47], or alternatively, anaesthetised patients have been transferred from the operating room to conventional linear accelerators in shielded bunkers for delivery of IORT. [31, 45] While this latter approach involves less initial costs to individual institutions, it is nevertheless logistically more complex involving the transfer of anaesthetised patients having high-risk surgery often through public corridors in institutions. Additionally, it is also less efficient, as the conventional linear accelerator would remain out of use for a significant length of time, limiting availability for standard radiotherapy patients. More recently, compact and mobile IOERT delivery units have been developed, equipped with portable linear accelerators and self-shielding for improved practicality. [52]
Figure 2.
Stationary linear accelerator for IOERT delivery in dedicated theatre suite.
HDR-IORT relies on gamma emitting radioisotopes such as Iridium-92 (Ir92) transmitted through hollow catheters to a flexible silicone applicator (figure 3). [50] HDR-IORT has similar clinical and radiobiologic effectiveness to IOERT, however the procedure time is generally shorter with IOERT and the amplitude of electron energy that is delivered can be varied, hence both superficial and deep cancerous foci can be irradiated. [12] In contrast, the effective depth of treatment with HDR-IORT is more shallow, and procedure times are generally longer. [12, 53] A further drawback with HDR-IORT is the requirement for radiation shielding, which has been circumvented by the newer self-shielded IOERT devices.
Figure 3.
Delivering HDR brachytherapy to the pelvic side wall using a flexible Harrison-Anderson-Mick applicator.
One important limitation of IOERT is that the rigid applicators used are poorly suited to curved surfaces or narrow spaces. Consequently, delivery to the pelvic sidewall or the prostatic bed may prove challenging. [54] In contrast, HDR-IORT employs a flexible applicator pad that can be customised to fit the shape and size of the intended target area. [54]
From an economic perspective the costs of setting up and implementing an IORT service are high, though not prohibitive, and these will be dependent on a variety of factors including host-institute facilities, choice of delivery platform and anticipated usage. Established facilities could be used for radiotherapeutic treatment in a variety of cancer sub-types to disperse costs over multiple services. In addition, we are witnessing the development of newer, cheaper and more mobile delivery platforms that will facilitate more cost-effective application of IORT technologies. That said, an integrated multidisciplinary approach is critical in the early phases of adoption to ensure efficient service uptake, and to avoid the use of IORT in clinical circumstances where traditional therapeutic approaches would suffice.
Oncological outcomes
For ease of description, the following section presents the results of primary advanced and recurrent CRC separately, with an initial description of the studies identified, followed by a presentation of findings based on levels of evidence.
Primary advanced CRC: Description of studies
Table 1 provides a summary of all studies reporting oncological outcome data for IORT in locally advanced CRC. Sixteen studies were identified giving a total sample size of 1792 patients [23-32, 37-42]. These studies comprised eight prospective case series [23-28, 30, 31] (evidence level 3), five retrospective case series [37-41] (evidence level 3), one retrospective pooled analysis [42] (evidence level 2-) and two randomised controlled trials [29, 32] (evidence level 1-). In 95% of patients the mode of irradiation was IOERT, with HDR-IORT employed in only 5% of reported cases. Only one study described the application of IORT to CC, including 40 cases in this category [30]. Five of the 16 publications failed to clearly define ‘locally advanced’ cancer [23, 24, 26, 27, 38]. The interpretation of this designation from the remaining 11 studies [25, 28-32, 37, 39-42] was highly heterogenous and ranged from T1-T3 tumours with lymph node involvement [28, 29, 32, 39, 41] to tumours with definite evidence of infiltration of surrounding structures and viscera [25, 30].
Table 1.
Studies reporting oncological outcomes following IORT for locally advanced colorectal cancer
| Author, Year, Location | Time frame | N | Cancer type (%) | Clinical T stage (%) | Type of IORT | IORT Dose (Gy) | PR-RT (%) | CT (%) | PO-RT (%) | Follow up (months) | Resection margin | 5-year LC (%) | In-field LC (%) | 5-year OS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Willett et al, 1991, USA [23] ς | 1978-1989 | 42 | R | - | IOERT | 10-20 ϕ | 100 | - | 0 | 26 (6-120) § | R0 R1 R2 |
88 69 50 |
- | - | 53 47 17 |
| Huber et al, 1996, Germany [24] | 1989-1993 | 38 | R | T3 (50) T4 (50) |
HDR-IORT | 15 | 50 | NA (50) A (50) |
50 | 25.5 ‡ | R0/R1/R2 | T3 (84) T4 (90) |
100 | 28 F̵ | - |
| Nakfoor et al, 1998, USA [37] ς | 1978-1996 | 73 | R | - | IOERT | 10-20 ϕ | 100 | - | 0 | 53 ‡ | R0 R1/R2 |
89 65 |
- | - | 63 32 |
| Mannaerts et al, 2000, Netherlands [25] Φ | 1994-1998 | 38 | R | - | IOERT | 10-17.5 ϕ | 100 | - | - | 21 (1-57) § |
R0/R1/R2 | 82* | 92* | 72* | 65* |
| Ratto et al, 2003, Italy [28] ϖ | 1990-1997 | 19 | R | T1-T3 N3 (7) T4Nx (93) |
IOERT | 10-15 ϕ | - | - | - | 74 (27 -120) § |
- | 91 | 95 | 61 | 47 |
| Sadahiro et al, 2004, Japan [26] | 1991-2001 | 99 | R | T1/T2 (29) T3 (59) T4 (12) |
IOERT | 17.3 ‡ | 100 | NA (53) | 0 | 67 Ω | - | 98 | - | 79 | 71 |
| Nuyttens et al, 2004, Netherlands [27] ¡ | 1997-2000 | 18 | R | T1-T4 LN +ve (16) T3N0 (66) T4N0 (16) |
HDR-IORT | 10 | 100 | 0 | 0 | 34 Ω | - | 81* | 94* | 61* | - |
| Diaz-Gonzalez et al, 2006, Spain [38] | 1995-2001 | 115 | R | T1/T2 (8) T3 (95) T4 (12) |
IOERT | 12.5 Ω | 100 | NA (100) A (57) |
0 | 37 (6-83) § |
- | 94* | 98* | 74* | 74* |
| Krempien et al 2006, Germany [39] Φ | 1991-2003 | 210 | R | T1/T2 (6) T3 (72) T4 (22) |
IOERT | 10 (8-18) § |
93 | 93 | 42 | 61 (4-177) § |
R0 R1/R2 |
93 77 |
98 | 74 55 |
68 0 |
| Ferenschild et al, 2006, Netherlands [40] ¡ | 1987-2002 | 30 | R | T3 | HDR-IORT | 10 | 100 | - | 0 | 25 (1-136) § |
R0 R1/R2 |
72 58 |
- | 66 38 |
- |
| Roeder et al, 2007, Germany [41] Φ | 1991-2004 | 243 | R | T1/T2 (14) T3 (66) T4 (20) |
IOERT | 10.4 ‡ | 50 | 36 | 0 | 59 Ω | R0 R1/R2 |
94 72 |
97 | - | - |
| Masaki et al, 2008, Japan [29] | 2000-2007 | 19 | R | T1/T2 (11) T3 (89) |
IOERT | 18-20 ϕ | - | A (37) | - | 34 § | - | 95 | - | 64 | 60 |
| Mathis et al, 2008, USA [30] Φ | 1981-2007 | 146 | C (27) R (73) |
T4 (100) | IOERT | 12.5 (7.5- 25) § |
100 | NA (5) A (40) |
0 | 44 Ω | R0/R1/R2 | 86 | 98 | 52 | 43 |
| Valentini et al, 2009, Italy [31] Φ ϖ | 1991-2006 | 29 | R | T4 (100) | IOERT | 10-15 ϕ | 100 | 100 | 0 | 31 (4-136) § |
R0 | 100 | 100 | - | - |
| Kusters et al, 2010, Netherlands [42] | - | 605 | R | T3 (71) T4 (29) |
IOERT | - | R (36) | A (42) | 0 | - | R0/R1/R2 | 88 | - | 67 | - |
| Dubois et al, 2011, France [32] ¢ | 1993-2001 | 68 | R | T3/T4 (100) | IOERT | 18 Œ | 100 | A (25) | - | 60 (10- 112) § |
- | 92 | 96 | 77 | 62 |
PR-RT pre-operative radiotherapy; CT chemotherapy; PO-RT post-operative radiotherapy; LC local control; OS overall survival; DFS disease-free survival; R rectal; ς all patients having IORT had obvious residual cancer or positive/<5mm margins on frozen section ϕ range reported with no mean/median; § median; ‡ mean; F̵ overall survival reported for T3 and T4 cancers combined; Φ all patients received IORT irrespective of frozen section outcome but dose was increased where margins were positive or close; ϖ overlapping patient populations; * 3-year local control/survival reported; LN lymph node; ¡ IORT used in cases where resection margin was found to be positive or <2mm on intraoperative frozen section.
Ω median reported without range; NA neo-adjuvant chemotherapy; A adjuvant chemotherapy; ¢ randomised controlled trial; Œ one patient received dose of 15Gy.
The dose of IORT applied for cases of locally advanced CRC ranged from 7.5-25 Gy and was stated in all but one of the included studies [42]. In nine studies, IORT doses were reported though no clear rationale was offered with respect to dosing strategy [24, 26-29, 31, 32, 38, 40]. In the remaining six studies, where doses were reported, radiation fractions were incrementally increased according to the extent of residual tumour, with a typical dose of 10-15Gy in cases of microscopic residual tumour (R1) and 15-20Gy where gross residual tumour remained (R2) [23, 25, 30, 37, 39, 41].
Primary advanced CRC: Comparative studies
The highest quality studies were two RCTs which have reported on outcomes from IORT to date [29, 32]. In both of these studies IORT and non-IORT treatment groups were matched for age, gender, tumour stage, type of resection and adjuvant therapy administration. One-hundred and forty-two patients with T3/T4 or lymph node positive disease were randomised to pre-operative EBRT and surgery alone, or pre-operative EBRT, surgery and IORT by Dubois and colleagues [32]. These authors found no statistically significant difference for local control, overall, or disease-free survival between treatment arms. Treatment arms were well-matched in this trial with over 85% of patients having T3/T4 tumours in both groups. However, as 5-year local control exceeded 90% in both treatment groups, it is likely that complete resection was achieved in the majority of cases, potentially minimising the benefit of IORT. The study thus provides compelling evidence against the routine application of IORT in the setting of complete surgical resection. The only other RCT evaluating IORT was conducted by Masaki et al in 2008 [29]. This study compared outcomes between 19 patients with LARC treated with surgery and IORT and 22 patients having surgery alone. No significant difference was observed between the two groups, however the small sample size, and inclusion of patients with T1/T2 disease (albeit with nodal involvement) and absence of any patients with T4 disease, are potential limitations with this trial.
Five other studies have provided non-randomised comparative data for IORT and non-IORT-containing protocols [23, 26, 28, 31, 40]. Patient groups were matched for gender, age, tumour stage, tumour size, procedure type and adjuvant therapy in one of these studies [26]. In the remainder no formal data regarding matching for potential confounding variables was provided [23, 28, 31, 40]. In an early study, Willett et al compared outcomes for 20 patients undergoing R0 resection and IORT, and 18 having R0 resection only for LARC [23]. Although 5-year disease-free survival rates for both groups were similar (53% and 55% in patients receiving and not receiving IORT), dramatically improved local control was noted in patients treated with IORT (88% versus 67% at 5-years). A more recent study by Ferenschild and colleagues found equivalent local control rates in patients undergoing R0 resection for LARC, irrespective of IORT [40]. However, where an R0 resection was not possible, significantly improved local control and overall survival were noted. Similarly Valentini et al analysed outcomes in 78 patients who had undergone pre-operative chemoradiotherapy and R0 resection for T4 tumours of the rectum of whom 29 (37%) received IORT after surgical resection [31]. Overall 5-year local control in this series was 90%. Univariate analysis demonstrated improved local control in patients undergoing R0 resection and IORT compared with those having R0 resection only (100% vs. 81% respectively, p=0.014). On multivariate analysis, IORT was further confirmed as the only variable with a positive predictive value. Likewise Ratto and co-workers also reported significantly improved rates of local control in patients having radical resection and IORT compared with radical resection alone (91% versus 57% at 5 years) [28].
Primary advanced CRC: Non-comparative studies
Of the non-comparative studies, a European multi-national study by Kusters et al is the largest series to date reporting outcomes for patients having IORT as part of multi-modal treatment of LARC [42]. This pooled analysis included a total of 605 patients from four tertiary referral cancer centres with significant experience in IORT. The study population comprised 431 T3 (CRM threatened T3 tumours) and 174 T4 tumours. The treatment protocol in all cases was pre-operative chemoradiation, intended radical resection combined with IORT, and elective adjuvant chemotherapy. Five-year local recurrence, overall survival and cancer-specific survival rates in this study of high risk RC were 12%, 67% and 74% respectively, and compare favourably with non-IORT utilising studies. Additionally, the authors noted that 55% of patients with positive resection margins remained free of local recurrence, supporting the notion that residual tumour cells after an R1 resection may be destroyed by IORT.
Outcomes for 146 patients having IORT as part of multimodal therapy for T4 tumours of the colon (27%) and rectum (73%) were analysed by Mathis et al [30]. Pre-operative EBRT was given to 85% of patients, of whom 90% received concurrent 5-FU based chemotherapy. Five-year overall and disease-free survival was 52% and 43% respectively. The authors found significantly reduced rates of local recurrence within the field of irradiation, compared with out-of-field recurrence in patients having IORT (2% versus 17% respectively). Enhanced local control within the IORT field was also reported by Roeder et al in a study evaluating patterns of failure and local recurrence in 243 patients undergoing total mesorectal excision followed by pre-sacral IOERT for LARC [41]. Fifty percent of patients received pre-operative EBRT, with 80% receiving chemotherapy simultaneously. The overall local control rate was 92% at 5 years, with a local control rate of 97% within the pre-sacral IORT field. Likewise, Diaz-Gonzalez et al reported outcomes in 115 patients with T3/T4 or node positive RC treated with chemoradiotherapy, surgery and IORT [38]. Three-year overall and disease-free survival was 74% and pelvic local control 94%. Pelvic recurrence within the IORT field occurred in only 2 of 115 patients (2%).
Locally recurrent CRC: Description of studies
Table 2 provides a summary of all studies providing oncological outcome data for IORT in locally recurrent CRC. Fourteen studies were identified giving a total sample size of 1211 patients [27, 33-36, 43-51]. These studies comprised five prospective [27, 33-36] and nine retrospective case series [43-51]. IOERT was used in 88% of cases, and HDR-IORT in the remaining. Three studies described delivery of IORT for locally recurrent CC with a total of 187 cases in this category (17%) [45, 46, 49]. The definition, method of diagnosis and work-up, and anatomical classification of local recurrence was highly variable between studies, and 7 studies provided no clear definitions [27, 33, 36, 43, 44, 48, 49].
Table 2.
Studies reporting oncological outcomes following IORT for locally recurrent colorectal cancer
| Author, Year, Location | Time frame | N | Cancer type (%) | Type of IORT | IORT Dose (Gy) | Previous irradiation (%) | PR-RT (%) | CT (%) | PO-RT (%) | Follow up (months) | Resection margin | 5-year LC (%) | In-field LC (%) | 5-year OS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Willett et al, 1991, USA [33] ς | 1978-1987 | 30 | R/RS | IOERT | 15-20 ϕ | - | - | - | - | 30 (2-119) ‡ | R0 R1/R2 |
62 18 |
- | - | 54 6 |
| Suzuki et al, 1995, USA [51] ϖ | 1981-1988 | 42 | R | IOERT | 10-30 ϕ | - | - | - | 98 | 44 (0.5-112) § |
R1 R2 |
60 | - | 19 | - |
| Eble et al. 1998, Germany [34] | 1991-1995 | 31 | R | IOERT | 13.7 (12-20) ‡ | - | 71 | 71 | - | 28 Ω | R0 R1/R2 |
79 65 |
87 | - | 71 29 |
| Valentini et al, 1999, Italy [35] | 1989-1997 | 11 | R | IOERT | 10-15 ϕ | - | - | - | - | 80 (18-120) § | - | 80 | - | 41 | 19 |
| Martinez-Monge et al, 1999, USA [50] | 1989-1997 | 51 | C+R | HDR-IORT (45) IOERT (55) |
15 (10-20) § 15 (10-20) § |
74 54 |
- | 17 32 |
26 21 |
48 (8-92) § | - | 21 40 |
- | 13 8 |
- |
| Lindel et al, 2001, USA [43] ε | 1978-1997 | 49 | R | IOERT | 15 (15-20) ‡ | - | - | 50 | 0 | 4-150 ϕ | R0 R1/R2 |
56 17 |
- | 46 14 |
32 8 |
| Wiig et al, 2002, Norway [44] ς | 1990-1999 | 59 | R | IOERT | 15-20 ϕ | 0 | 100 | - | - | - | R0/R1/R2 | 44 | - | 30 | - |
| Pezner et al, 2002, USA [45] | 1990-2000 | 15 | C (13) R (87) |
IOERT | 15-20 ϕ | - | 73 | 80 | 20 | ≥20 œ | R0/R1/R2 | 25* | 48 | 29* | - |
| Shoup et al, 2002, USA [36] Φ | 1990-2000 | 100 | R | HDR-IORT | 12.5-17.5 ϕ | 50 | 37 | 52 | - | 23.2 Ω | R0/R1/R2 | - | 67 | - | 22 |
| Hashiguchi et al, 2003, Japan [46] | 1987-1999 | 39 | C(8) R (92) |
IOERT | 23 (15-30) ‡ | - | 31 | 36 | 38 | 36 ‡ | R0/R1/R2 R0 |
- 24 |
- | 13 35 |
- 24 |
| Nuyttens et al, 2004, Netherlands [27] ¡ | 1997-2000 | 19 | R | HDR-IORT | 10 | - | 100 | - | 0 | 34 Ω | - | 48 | 79 | 34 | - |
| Dresen et al, 2008, Netherlands [47] Φ | 1994-2006 | 147 | R | IOERT | 10-17.5 ϕ | 53 | 84 | 59 | - | 34 (6-146) § | R0 R1 R2 |
75* 29* 29* |
- | 59* 27* 24* |
- |
| Vermaas et al, 2008[48], Netherlands | 1984-2006 | 11 | R | HDR-IORT (10) IOERT (1) |
10 12.5 |
100 | 55 | - | - | 22 (2-66) § | R0/R1/R2 | 27* | - | 51* | - |
| Haddock et al, 2011, USA [49] Φ ϖ | 1981-2008 | 607 | C (30) R (70) |
IOERT | 15 (7.5-30) § | 45 | 91 | NA (81) A (18) |
7 | 44 Ω | R0 R1 R2 |
79 68 ψ |
- | 46 27 16 |
- |
PR-RT pre-operative radiotherapy; CT chemotherapy; PO-RT post-operative radiotherapy; LC local control; OS overall survival; DFS disease-free survival; ς all patients having IORT had obvious residual cancer or positive/<5mm margins on frozen section R rectum; RS rectosigmoid; ϕ range reported without median/mean; ‡ mean reported; ϖ overlapping patient populations; ε IORT only used where there was tumour adherence or clearance was <1cm; Ω median reported without range; § median reported with range; C colon; œ all patients followed up for longer than 20 months but no mean/median reported; * 3-year local control/survival reported; Φ all patients received IORT irrespective of frozen section outcome but dose was increased where margins were positive or close; ¡ IORT used in cases where resection margin was found to be positive or <2mm on intraoperative frozen section; NA neo-adjuvant chemotherapy; A adjuvant chemotherapy; ψ combined outcomes reported for R1 and R2 resections.
The dose of IORT applied for cases of locally recurrent CRC ranged from 7.5-30Gy and was stated in all included studies. In five studies, IORT doses were reported though no explanation was offered regarding dosing strategy [27, 34, 35, 45, 48]. In the remaining nine studies dosing was dependent on resection margin status, increasing incrementally for R0, R1 and R2 resections respectively [33, 36, 43, 44, 46, 47, 49-51]. In addition three studies stated that higher doses were delivered in previously irradiated patients [36, 49, 50].
Locally recurrent CRC: Comparative studies
Only three comparative studies were identified [35, 44, 51]. Data regarding matching of treatment groups for confounding variables was not provided in any of these reports. A study in 1994 from the Mayo Clinic evaluated outcomes in 106 patients undergoing treatment for LRRC of whom 42 received IORT [51]. Adjuvant EBRT was delivered in 98% of patients receiving IORT compared with 55% in the non-IORT group. The study found a significant improvement in 3-year local control in the IORT group (60% vs. 7%). In addition there was a significant improvement in 5-year survival in patients having IORT as part of treatment (19% vs. 7%). More recently, Valentini et al reported on outcomes in a series of 37 patients with LRRC, 11 of whom received IORT at the time of surgery [35]. All patients in this study received EBRT prior to surgery. A trend towards improved 5-year overall survival in the IORT group was observed (41% vs. 16%) though this was not statistically significant due to the few cases assessed. Significantly improved local control in IORT-treated patients compared with the non-IORT group (79% vs. 23%) was noted however. In a further study, Wiig and co-workers evaluated outcomes in 107 patients with LRRC of whom 59 received IORT [44]. For patients undergoing R0 resection, 5-year survival was equivalent at around 60% in both IORT and non-IORT treatment groups. However, at 5 years, 50% remained free of local recurrence in the IORT group compared to only 30% in the non-IORT group.
Locally recurrent CRC: Non-comparative studies
Ten single institution non-comparative studies evaluated IORT in recurrent CRC (table 2) [27, 33, 34, 36, 43, 45-48, 50]. To date, the largest series has been reported by the Mayo Clinic [49]. Six-hundred and seven patients underwent treatment for local recurrence over a 27 year period, with all cases receiving IORT as a component of treatment. The majority were treated with IOERT. LRRC represented 427 cases (70%), while the remainder were colonic recurrences. In the latter half of the study, re-irradiation with additional EBRT (median dose 30 Gy) was also undertaken in 224 patients prior to radical surgery and IORT. An overall 5- and 10-year survival of 30% and 16% respectively were noted, while 5-year survival as high as 46% was achieved in R0 resections. Long-term survival was also noted in 224 R1 and 156 R2 resections receiving IOERT, with 5-year survival of 27 and 16% respectively, and a remarkable 68% 5-year local control. In a study of 147 patients with LRRC analysed retrospectively, Dresen and co-workers also noted high 5-year local control and overall survival, not just in R0 resections, but also in R1/R2 resections [47]. IORT was delivered to all patients in this study with 10Gy administered to patients with negative margins, 15Gy to those with microscopic positive margins, and 17.5Gy to those with gross residual disease. Re-irradiation with EBRT was also incorporated in selected patients (30.6 Gy five times a week in 17 doses of 1.8Gy) with a history of previous pelvic radiotherapy. Using this aggressive treatment strategy, 5-year local control was achieved in almost one third of R1 and R2 patients, with similar findings for 5-year overall survival also.
Meta-analysis of oncological outcome data for IORT vs. no IORT Local control
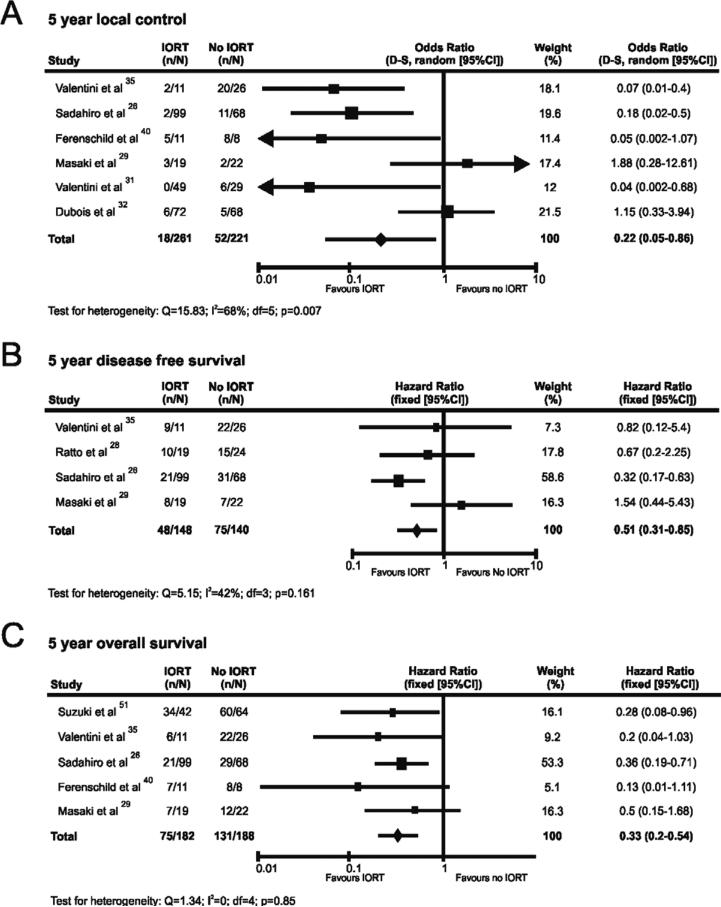
Six comparative studies provided data on the impact of IORT on 5-year local control [26, 29, 31, 32, 35, 40]. A summary of the data and forest plot estimation of effect is shown in figure 4A, with funnel plot analysis depicted in supplementary figure 1. Meta-analysis of the data showed a significant effect favouring improved local control with IORT with a pooled odds ratio of 0.22 (95% CI=0.05-0.86; p=0.03). Between-study heterogeneity was identified (Q=15.83; P=0.007; I2=0.68), which could not be eradicated by sensitivity analyses. No evidence of publication bias was noted (supplementary figure 1).
Figure 4.
Meta-analysis of oncological outcomes following IORT for CRC, showing results for 5 year local control (A); 5 year disease free survival (B); and 5 year overall survival (C). The diamond represents the overall treatment effect, and squares are treatment effects for individual studies with 95% CI indicated by horizontal bars.
Survival analysis
Four comparative studies reported on disease-free survival after IORT (figure 4B) [26, 28, 29, 35]. Meta-analysis of these studies demonstrated a significant benefit from IORT (HR=0.51; 95% CI=0.31-0.85; p=0.009; funnel plot analysis depicted in supplementary figure 2). Overall 5-year survival with or without IORT has been reported by five studies [26, 29, 35, 40, 51]. A summary of the data and forest plot analysis are shown in figure 4C, with funnel plot analysis in supplementary figure 3. Pooled analysis found the HR of 5-year overall survival to be 0.33 (95% CI=0.2-0.54; p=0.001). No significant between-study heterogeneity or evidence of publication bias was identified for either survival measure (supplementary figures 2 and 3).
Complications
Reported short and long-term complications attributable to IORT-containing treatment regimens are summarised in Table 3. The overall complication rate in these studies ranged from 15-59%, and the most frequently reported morbidities were wound related problems, gastrointestinal complications, ureteric obstruction, and neuropathy. Mortality in studies evaluating IORT in locally advanced or recurrent tumours was 0-4.5% and 0-8% respectively. Only one study assessed the impact of IORT dose on incidence and severity of complications [49]. No clear difference in complication profile was apparent between IOERT and HDR-IORT in the studies evaluated.
Table 3.
Short- and long-term complications following IORT for locally advanced CRC
| Author, Year | N | Cancer type (%) | Type of IORT | IORT Dose (Gy) | Overall complication rate (%) | Short-term complications (%) | Long-term complications (%) |
|---|---|---|---|---|---|---|---|
| Willett et al, 1991 [23] | 42 | PR | IOERT | 10-20 ϕ | - | Abscess (5); Fistulae (7) Wound (5); Anastomotic leakage (2) Small bowel obstruction (1) |
Ureteric obstruction (2) Sacral necrosis (2) |
| Willett et al, 1991 [33] | 30 | LRR/RS | IOERT | 15-20 ϕ | - | Fistulae (3) Sacral necrosis (3); Wound (3) |
Pelvic neuropathy (10) |
| Huber et al, 1996 [24] | 38 | PR | HDR-IORT | 15 | 52.6 | Wound (45) Sacral wound dehiscence (21) |
Neurogenic bladder dysfunction (8) |
| Eble et al, 1998 [34] | 31 | LRR | IOERT | 13.7 (12-20) ‡ | - | Wound (13) Abscess (6) |
Ureteric obstruction (6) |
| Nakfoor et al, 1998 [37] | 73 | PR | IOERT | 10-20 ϕ | 15 | - | - |
| Valentini et al, 1999 [35] | 11 | LRR | IOERT | 10-15 ϕ | - | Neuropathy (18) | Ureteric obstruction (9) |
| Lindel et al, 2001 [43] | 49 | LRR/RS | IOERT | 15 (15-20) ‡ | - | Abscess (4) Bladder perforation (2) Small bowel obstruction (6) Deep vein thrombosis (2) |
Ureteric obstruction (4) Pelvic pain (6) Peripheral neuropathy (8) Wound (8) |
| Wiig et al, 2002 [44] | 59 | LLR | IOERT | 15-20 ϕ | - | Anastomotic leak (3) Bladder perforation (5) Abscess (24); Wound (3) Urinary tract infection (27) Deep vein thrombosis (5) |
Wound (10) |
| Pezner et al, 2002 [45] | 15 | LRC (13) LRR (87) |
IOERT | 15-20 ϕ | - | Abscess (13) Fistulae (7) Wound (7) |
Ureteric obstruction (7) Neurogenic bladder (7) Peripheral neuropathy (7) Fistulae (7) |
| Sadahiro et al, 2004 [26] | 99 | PR | IOERT | 17.3 ‡ | - | Anastomotic leak (2) Wound (23) |
Neurogenic bladder (2) |
| Nuyttens et al, 2004 [27] | 37 | LRR (19) PR (18) |
HDR-IORT | 10 | - | Wound (46) Abscess (16) Anastomotic leakage (5) Fistulae (8) |
Neuropathy (14) Chronic diarrhea (3) Chronic pelvic pain (3) Chronic lower limb pain (3) |
| Roeder et al, 2007 [41] | 243 | PR | IOERT | 10.4 ‡ | - | Diarrhea (5); Nausea (4) Haematological derangement (6) Dermatological effects (4); Wound (6) Anastomotic leakage (4) |
Gastrointestinal disturbance (10) Ureteric obstruction (2) Neurogenic bladder (3) |
| Mathis et al, 2008 [30] | 146 | LRC (27) LRR (73) |
IOERT | 12.5 (7.5-25) § | 53 | Anastomotic leak (3) Small bowel obstruction (3) Wound (3) |
Peripheral neuropathy (19) Small bowel obstruction (14) Ureteric obstruction (12) Fistulae (8) Neurogenic bladder (7) Sexual dysfunction (6) Enteritis/proctitis (3) |
| Dresen et al, 2008 [47] | 147 | LRR | IOERT | 10-17.5 ϕ | 59 | Abscess (15); Fistulae (1); Wound (12) Urinary tract infection (3) Urinary retention (20) |
Ureteric obstruction (5) Peripheral Neuropathy (22) |
| Vermaas et al, 2009 [48] | 11 | LRR | HDR-IORT | 12.5 | - | Urinary tract infection (50) Wound (40) |
Ureteric obstruction (10) Pelvic neuropathy (10) |
| Haddock et al, 2010 [49] | 607 | LRC (30) LRR (70) |
IOERT | 15 (7.5-30) § | - | Wound (7) Gastrointestinal fistula/obstruction (3) |
Ureteric obstruction (3) Neuropathy (3) |
| Dubois et al, 2011 [32] | 68 | PR | IOERT | 18 Π| 30 | Anastomotic leakage (9) Re-operation (11) |
Sacral necrosis (1) |
PR primary rectal; LRR locally recurrent rectal; RS rectosigmoid; ϕ range reported without median/mean; ‡ mean reported; § median with range; Œ one patient received 15Gy
Early wound-associated complications including delayed wound healing [23, 24, 27, 30] wound infection [26, 33, 44, 47-49], wound dehiscence [34, 45] and non-specific wound related problems [41] were reported in 13 studies, with an incidence ranging from 3-46%. Delayed sacral osteonecrosis was also reported in two studies [23, 33] with an incidence of 2-3%. In addition, one case of partial sacral necrosis was reported in a recent RCT [32].
Gastrointestinal fistulae were reported as a post-treatment complication in seven studies with an incidence ranging from 1-8% [23, 27, 30, 33, 45, 47, 49]. The dose of IORT used in these studies was variable and ranged from 7.5-30Gy, with no obvious trend towards a higher incidence of this complication in studies that routinely delivered higher radiation fractions. Dresen et al reported a fistula formation rate of 1% with IORT dosing ranging from 10-17.5Gy, while Nuyttens and colleagues using a fixed IORT dose of 10Gy noted an 8% fistula rate [27, 47].
Post-radiation ureteric damage was observed in ten studies with an incidence ranging from 2-12% [23, 30, 34, 35, 41, 43, 45, 47-49]. The IORT dose in these studies ranged from 7.5-30Gy. While previous reports have found increased incidence of ureteric injury with higher radiation dosing [55, 56], few studies included in the present review examined for this.
Plexopathy and neuropathies are recognised late consequences following pelvic irradiation, and 13 studies reported neuropathy as a complication following IORT [24-27, 30, 33, 35, 41, 43, 45, 47-49] with an incidence ranging from 2-22%. Haddock and co-workers demonstrated a dose-dependent relationship, with patients treated with >12.5Gy doses of IOERT having significantly increased incidence and severity of neuropathy compared with those receiving <12.5Gy [49].
Meta-analysis of complications for IORT vs. no IORT
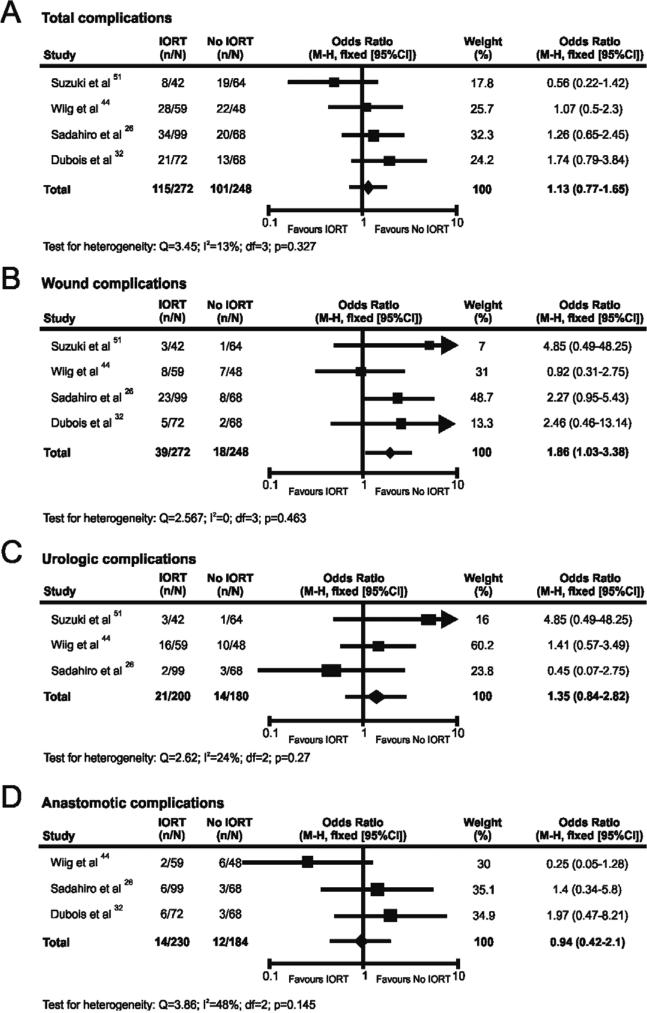
Four studies provided comparative data on the impact of IORT on complications in the post-operative period [26, 32, 44, 51]. Figures 5A-D show the forest plots and estimates of the ORs with CIs of the effect of IORT on total complications (5A), wound complications (5B), urologic complications (5C), and anastomotic complications (5D). No evidence of significant heterogeneity or publication bias was identified (supplementary figures 4 and 5). The pooled estimates demonstrate no overall increase in total, urologic, or anastomotic complications, however a greater number of wound complications was noted after IORT treatment (OR=1.86; 95% CI 1.03-3.38; p=0.049).
Figure 5.
Meta-analysis of complications following IORT for CRC, showing results for total complications (A); wound complications (B); urologic complications (C); and anastomotic complications (D). The diamond represents the overall treatment effect, and squares are treatment effects for individual studies with 95% CI indicated by horizontal bars.
Discussion
Local recurrence and re-recurrence following primary or salvage surgery for CRC present significant challenges. Aggressive multidisciplinary treatment including surgery, chemotherapy, and radiotherapy are critical to achieving local control and improving survival, and the total dose of radiotherapy delivered may be an important determinant of local control in advanced and recurrent tumours [52, 57]. However, the total dose deliverable by EBRT is limited by toxicity to adjacent viscera. It is in this setting that IORT permits direct administration of high-dose radiation to the area at greatest risk following resection. Although limitations in definitions, methodology, and reporting practice preclude definitive conclusions, the present systematic review and meta-analysis finds that IORT is associated with improved local control following resection, and that the benefits are primarily among patients with microscopic (R1) resection margins.
Significant heterogeneity in definitions was noted, in particular for locally advanced disease. Where possible, the effect of radiotherapy was separately evaluated by indication: locally advanced primary vs. recurrent colorectal cancer. Among studies evaluating IORT-containing treatment in locally advanced CRC, 5-year local control rates between 72-100% for R0 resection and 50-77% for R1/R2 resection were observed. Overall morbidity rates were acceptable, although the risk for wound complications appears to be increased with IORT.
A key consideration in the evaluation of IORT is the completeness of tumour resection. The benefit of IORT following R0 resection is a source of debate and potential confounding factor in between-study comparisons. A number of studies have reported equivalent local control in IORT vs. non-IORT groups after R0 resection [23, 40]. In addition, a recent RCT comparing outcomes between IORT and non-IORT containing treatment for LARC reported 5-year local control of 92% and 93% respectively. Though not clearly stated by the authors, these figures suggest that the majority of patients underwent R0 resection, supporting the argument that IORT may not be of benefit after complete tumour resection [32]. Conversely, several groups have reported improved local control even after R0 resection for advanced disease [30, 31]. In cases of incomplete resection, the evidence more consistently shows a trend towards improved local control with IORT [40]. Ferenschild et al reported 5-year local control of 58% and 0% respectively in patients receiving multimodality treatment with and without IORT after R1/R2 resection [40]. The authors suggest that in the setting of microscopic and even macroscopic residual disease, IORT may counter the consistently unfavourable prognosis conferred by an involved resection margin.
Recurrent CRC, and in particular recurrent rectal cancer, presents unique challenges, and achieving an R0 resection is more complex due to disrupted anatomical planes and the confines of the bony pelvis. Local control rates ranged between 56-79% after R0 resection and 18-68% after R1/R2 resection. These figures compare favourably with previous reports in the literature where incidence of re-recurrence frequently exceeds 30% even after R0 resection [47, 58-60]. In a recent update on the Mayo Clinic IORT experience, 607 patients with locally recurrent CRC having IORT as part of multi-modal treatment over a 27 year period were evaluated [49]. Local control was 79% for R0 resections and 68% for R1/R2 resections. In particular the authors draw attention to the 5-year survival of 46% in patients undergoing R0 resection, compared with their earlier published data on non-IORT treated patients, where only 34% achieved 5-year survival [61]. In addition this study reported a median survival of 27 months in patients undergoing R1 resection (224 patients) and 5-year survival of 16% in patients undergoing R2 resection (156 patients) which compared favourably to earlier published results from the same institute, where 5-year survival for patients undergoing palliative resection and EBRT alone was only 7% [51]. Several authors have thus reported a positive impact on local control after IORT when compared to non-IORT treatment groups [35, 44].
The present study represents the first pooled analysis of the effect of IORT on long-term oncological outcome after resection of CRC. Although there are inherent limitations of performing meta-analyses using non-randomised studies, our results indicate that the use of IORT at surgery for locally advanced and recurrent CRC may lead to improved local control and survival, with a more modest but significant effect on disease-free survival also observed. The latter finding is perhaps unsurprising as many authors have noted that distant relapse is a key problem with advanced and recurrent disease, and would likely be uninfluenced by the application of IORT. On the other hand there is a growing body of evidence that contests this suggestion; for example dose intensification studies in head and neck and lung cancer have demonstrated that improvements in radiation fractionation lead to improvements in both local control and 5-year survival [62, 63]. This link between local control and survival may imply that a proportion of metastases arise secondary to ‘in-field’ tumour persistence or recurrence.
There are a number of both limitations and strengths to the present analysis. Most of the included studies were non-randomized comparisons, often with limited sample sizes, and were heterogeneous with respect to tumour location, primary or recurrent indication, completeness of operative resection, and measured outcome. Most were conducted in large tertiary cancer centres with differing patient selection, staging and treatment protocols. In most studies examined, IORT has been administered as a component of multi-modality treatment including EBRT +/− concurrent chemotherapy and surgery, making it difficult to draw definitive inferences about the independent contribution of IORT. In addition, re-irradiation with hyperfractionated EBRT after previous pelvic radiotherapy is increasingly performed in specialist centres, and is a source of further heterogeneity [49, 64]. Furthermore, the included studies describe outcomes in patient series’ spanning over large time-frames, during which there will undoubtedly have been changes in operative practice, staging approaches and adjuvant therapy regimes. Clearly our findings must be interpreted within these limitations. However, in light of the scarcity of high quality evidence in this field, synthesized evidence from a systematic review and meta-analysis of non-randomized studies represents the most informative means of evaluation. Furthermore, where possible, we specifically evaluated outcome data from non-R0 resection, the most common indication for IORT. Due to the limitations of the source data, our meta-analysis included all patients with CRC who underwent comparative evaluation of IORT without stratification for advanced primary vs. recurrent disease. As patients with recurrent disease are more likely to have been previously irradiated, receive less EBRT prior to the resection in question, and have a greater likelihood of disease recurrence, this may bias the results. Nevertheless, the question of whether IORT contributes to better outcomes is unlikely to be addressed in the near future by higher quality randomized controlled studies due to the large numbers required (ideally of cases with non-R0 resection) and the likely low number of events to study (namely in-field recurrence), hence the current studies are likely to represent the best available evidence from which to draw conclusions.
Overall, when considering the data for both locally advanced and recurrent CRC, the utility of IORT is supported by three key observations. Firstly, there is positive long-term prospective data emerging from internationally renowned referral centres. In addition, a low rate of tumour recurrence within the IORT radiation field and convergence of survival curves between R0 and R1/2 resections with IORT seen in studies with long-term data, provide further support for the notion that IORT to cases with a close or involved margin may offer an oncological advantage. Indeed, although large high-quality RCT data is lacking, this is analogous to radical resection of colorectal liver metastases which also has not been subjected to evaluation by RCT but is now a standard of care. RCT evidence may not be forthcoming in the study of IORT. Moreover, an RCT with a non-IORT arm may well be deemed unethical in many units currently delivering IORT. Different methodological approaches such as cluster non-randomised controlled studies may enable overcoming this limitation in future work however.
A number of questions remain regarding the use of IORT including patient selection, the ideal zone of irradiation, radiation dosing, and the optimal ratio of EBRT to IORT. In addition, an important challenge on the horizon will be to identify better methods of limiting side-effects, and avoidance of over-treatment in patients with radiation-insensitive tumours. Appropriate patient selection is essential and novel strategies for identifying radio-sensitive tumours and directly targeting radioresistance mechanisms are currently the focus of investigation in a series of clinical and pre-clinical trials [65-67]. These strategies will help enhance the oncological benefit conferred by IORT in more precisely stratified patient groups. In addition, as functional imaging modalities become more sophisticated, it may become possible to identify pre-cancerous, architecturally bland tumour-adjacent tissues that are functionally deemed ‘recurrence-prone’ on the basis of radio-biological features. Furthermore, cost-effective, high-throughput technologies for profiling DNA, RNA, protein, and metabolites in biofluid and tumour samples will allow more accurate prediction of likelihood of recurrence, facilitating selection of patients that will benefit most from IORT. In addition molecular profiling techniques are emerging with the ability to provide clinically relevant biological information in the seconds-to-minutes time-scale [68, 69], meaning that they could be used to evaluate tissues ‘on-table’ at the time of surgery to determine likely radiotherapeutic response. These exciting developments are slowly revolutionising established treatment pathways, and raise hopes for a future with more personalised radiation therapy.
Conclusion
In patients with complex CRC, an aggressive loco-regional approach with addition of IORT to conventional multi-modality treatment strategies can aid in local disease control and further broadens our array of therapeutic modalities with acceptable morbidity. Future studies should more rigorously identify eligible patients by clearly defined primary indication (e.g. primary vs. recurrent; R0 vs. R1 vs. R2; transparent descriptions of locally advanced primary tumours) and with matched neo-adjuvant and adjuvant protocols, in order to more precisely determine the incremental contribution made by IORT. In light of the small numbers of cases treated by most individual institutions, organized multicentre collaboration should be pursued.
Supplementary Material
Synopsis.
The addition of intraoperative radiotherapy (IORT) to conventional treatment for locally advanced and recurrent colorectal cancer may improve oncological outcomes. The present study provides a systematic review and meta-analysis of the available evidence on the role of IORT in this setting.
Acknowledgements
AHM is supported by grant funding from Cancer Research UK (C28503/A10013). GJC is supported by grant funding from the US National Cancer Institute (K07-CA1333187).
Sources of funding: Cancer Research UK
Role of the funding source: The funding sources had no role in the collection, analyses, interpretation of data, writing of the report, or decision to publish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol 2007. 8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 4.Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 5.Camma C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 6.Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007:CD002102. doi: 10.1002/14651858.CD002102.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 9.Harrison LB, Minsky BD, Enker WE, et al. High dose rate intraoperative radiation therapy (HDR-IORT) as part of the management strategy for locally advanced primary and recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:325–330. doi: 10.1016/s0360-3016(98)00211-9. [DOI] [PubMed] [Google Scholar]
- 10.Goldson AL. Preliminary clinical experience with intraoperative radiotherapy. J Natl Med Assoc. 1978;70:493–495. [PMC free article] [PubMed] [Google Scholar]
- 11.Gunderson LL. Rationale for and results of intraoperative radiation therapy. Cancer. 1994;74:537–541. doi: 10.1002/1097-0142(19940715)74:2<537::aid-cncr2820740202>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Skandarajah AR, Lynch AC, Mackay JR, et al. The role of intraoperative radiotherapy in solid tumors. Ann Surg Oncol. 2009;16:735–744. doi: 10.1245/s10434-008-0287-2. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–336. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Patsopolous NA EE, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;38:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song F, Sheldon TA, Sutton AJ, et al. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–151. doi: 10.1177/016327870102400203. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol. 1991;9:843–849. doi: 10.1200/JCO.1991.9.5.843. [DOI] [PubMed] [Google Scholar]
- 24.Huber FT, Stepan R, Zimmermann F, et al. Locally advanced rectal cancer: resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy. Dis Colon Rectum. 1996;39:774–779. doi: 10.1007/BF02054443. [DOI] [PubMed] [Google Scholar]
- 25.Mannaerts GH, Martijn H, Crommelin MA, et al. Feasibility and first results of multimodality treatment, combining EBRT, extensive surgery, and IOERT in locally advanced primary rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:425–433. doi: 10.1016/s0360-3016(99)00492-7. [DOI] [PubMed] [Google Scholar]
- 26.Sadahiro S, Suzuki T, Ishikawa K, et al. Preoperative radio/chemo radiotherapy in combination with intraoperative radiotherapy for T3-4Nx rectal cancer. Eur J Surg Oncol. 2004;30:750–758. doi: 10.1016/j.ejso.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Nuyttens JJ, Kolkman-Deurloo IK, Vermaas M, et al. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:106–112. doi: 10.1016/s0360-3016(03)01494-9. [DOI] [PubMed] [Google Scholar]
- 28.Ratto C, Valentini V, Morganti AG, et al. Combined-modality therapy in locally advanced primary rectal cancer. Dis Colon Rectum. 2003;46:59–67. doi: 10.1007/s10350-004-6497-1. [DOI] [PubMed] [Google Scholar]
- 29.Masaki T, Takayama M, Matsuoka H, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg. 2008;393:173–180. doi: 10.1007/s00423-007-0260-8. [DOI] [PubMed] [Google Scholar]
- 30.Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg. 2008;248:592–598. doi: 10.1097/SLA.0b013e318187ed4a. [DOI] [PubMed] [Google Scholar]
- 31.Valentini V, Coco C, Rizzo G, et al. Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: a prospective evaluation of a single institutional experience. Surgery. 2009;145:486–494. doi: 10.1016/j.surg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol. 2011;98:298–303. doi: 10.1016/j.radonc.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for recurrent locally advanced rectal or rectosigmoid carcinoma. Cancer. 1991;67:1504–1508. doi: 10.1002/1097-0142(19910315)67:6<1504::aid-cncr2820670607>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol. 1998;49:169–174. doi: 10.1016/s0167-8140(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 35.Valentini V, Morganti AG, De Franco A, et al. Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma: prognostic factors and long term outcome. Cancer. 1999;86:2612–2624. doi: 10.1002/(sici)1097-0142(19991215)86:12<2612::aid-cncr5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Shoup M, Guillem JG, Alektiar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585–592. doi: 10.1007/s10350-004-6250-9. [DOI] [PubMed] [Google Scholar]
- 37.Nakfoor BM, Willett CG, Shellito PC, et al. The impact of 5-fluorouracil and intraoperative electron beam radiation therapy on the outcome of patients with locally advanced primary rectal and rectosigmoid cancer. Ann Surg. 1998;228:194–200. doi: 10.1097/00000658-199808000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Gonzalez JA, Calvo FA, Cortes J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys. 2006;64:1122–1128. doi: 10.1016/j.ijrobp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Krempien R, Roeder F, Oertel S, et al. Long-term results of intraoperative presacral electron boost radiotherapy (IOERT) in combination with total mesorectal excision (TME) and chemoradiation in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:1143–1151. doi: 10.1016/j.ijrobp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum. 2006;49:1257–1265. doi: 10.1007/s10350-006-0651-x. [DOI] [PubMed] [Google Scholar]
- 41.Roeder F, Treiber M, Oertel S, et al. Patterns of failure and local control after intraoperative electron boost radiotherapy to the presacral space in combination with total mesorectal excision in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67:1381–1388. doi: 10.1016/j.ijrobp.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Kusters M, Valentini V, Calvo FA, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol. 2010;21:1279–1284. doi: 10.1093/annonc/mdp501. [DOI] [PubMed] [Google Scholar]
- 43.Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83–87. doi: 10.1016/s0167-8140(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 44.Wiig JN, Tveit KM, Poulsen JP, et al. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? A prospective study. Radiother Oncol. 2002;62:207–213. doi: 10.1016/s0167-8140(01)00486-8. [DOI] [PubMed] [Google Scholar]
- 45.Pezner RD, Chu DZ, Ellenhorn JD. Intraoperative radiation therapy for patients with recurrent rectal and sigmoid colon cancer in previously irradiated fields. Radiother Oncol. 2002;64:47–52. doi: 10.1016/s0167-8140(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 46.Hashiguchi Y, Sekine T, Kato S, et al. Indicators for surgical resection and intraoperative radiation therapy for pelvic recurrence of colorectal cancer. Dis Colon Rectum. 2003;46:31–39. doi: 10.1007/s10350-004-6493-5. [DOI] [PubMed] [Google Scholar]
- 47.Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol. 2008;15:1937–1947. doi: 10.1245/s10434-008-9896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermaas M, Nuyttens JJ, Ferenschild FT, et al. Reirradiation, surgery and IORT for recurrent rectal cancer in previously irradiated patients. Radiother Oncol. 2008;87:357–360. doi: 10.1016/j.radonc.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 49.Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143–150. doi: 10.1016/j.ijrobp.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Monge R, Nag S, Martin EW. Three different intraoperative radiation modalities (electron beam, high-dose-rate brachytherapy, and iodine-125 brachytherapy) in the adjuvant treatment of patients with recurrent colorectal adenocarcinoma. Cancer. 1999;86:236–247. doi: 10.1002/(sici)1097-0142(19990715)86:2<236::aid-cncr7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939–952. doi: 10.1002/1097-0142(19950215)75:4<939::aid-cncr2820750408>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 52.Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol. 2007;25:971–977. doi: 10.1200/JCO.2006.10.0255. [DOI] [PubMed] [Google Scholar]
- 53.Hu KS, Enker WE, Harrison LB. High-dose-rate intraoperative irradiation: current status and future directions. Semin Radiat Oncol. 2002;12:62–80. doi: 10.1053/srao.2002.28666. [DOI] [PubMed] [Google Scholar]
- 54.Calvo FA, Meirino RM, Orecchia R. Intraoperative radiation therapy first part: rationale and techniques. Crit Rev Oncol Hematol. 2006;59:106–115. doi: 10.1016/j.critrevonc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Miller RC, Haddock MG, Petersen IA, et al. Intraoperative electron-beam radiotherapy and ureteral obstruction. Int J Radiat Oncol Biol Phys. 2006;64:792–798. doi: 10.1016/j.ijrobp.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Shaw EG, Gunderson LL, Martin JK, et al. Peripheral nerve and ureteral tolerance to intraoperative radiation therapy: clinical and dose-response analysis. Radiother Oncol. 1990;18:247–255. doi: 10.1016/0167-8140(90)90060-a. [DOI] [PubMed] [Google Scholar]
- 57.Gunderson LL, Shipley WU, Suit HD, et al. Intraoperative irradiation: a pilot study combining external beam photons with “boost” dose intraoperative electrons. Cancer. 1982;49:2259–2266. doi: 10.1002/1097-0142(19820601)49:11<2259::aid-cncr2820491110>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 58.Akasu T, Yamaguchi T, Fujimoto Y, et al. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol. 2007;14:74–83. doi: 10.1245/s10434-006-9082-0. [DOI] [PubMed] [Google Scholar]
- 59.Asoglu O, Karanlik H, Muslumanoglu M, et al. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33:1199–1206. doi: 10.1016/j.ejso.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:447–454. doi: 10.1245/s10434-006-9256-9. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki K, Dozois RR, Devine RM, et al. Curative reoperations for locally recurrent rectal cancer. Dis Colon Rectum. 1996;39:730–736. doi: 10.1007/BF02054435. [DOI] [PubMed] [Google Scholar]
- 62.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 63.Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol. 1999;52:137–148. doi: 10.1016/s0167-8140(99)00087-0. [DOI] [PubMed] [Google Scholar]
- 64.Das P, Delclos ME, Skibber JM, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys. 2010;77:60–65. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 65.Coquery N, Pannetier N, Farion R, et al. Distribution and radiosensitizing effect of cholesterol-coupled Dbait molecule in rat model of glioblastoma. PLoS One. 2012;7:e40567. doi: 10.1371/journal.pone.0040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorgensen TJ. Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther. 2009;8:665–670. doi: 10.4161/cbt.8.8.8304. [DOI] [PubMed] [Google Scholar]
- 67.Kelley MR, Fishel ML. DNA repair proteins as molecular targets for cancer therapeutics. Anticancer Agents Med Chem. 2008;8:417–425. doi: 10.2174/187152008784220294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirnezami R, Kinross JM, Vorkas PA, et al. Implementation of molecular phenotyping approaches in the personalized surgical patient journey. Ann Surg. 2012;255:881–889. doi: 10.1097/SLA.0b013e31823e3c43. [DOI] [PubMed] [Google Scholar]
- 69.Schafer KC, Szaniszlo T, Gunther S, et al. In Situ, Real-Time Identification of Biological Tissues by Ultraviolet and Infrared Laser Desorption Ionization Mass Spectrometry. Anal Chem. 2011;83:1632–1640. doi: 10.1021/ac102613m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.