Abstract
The brain represents only a small portion of the body mass and yet consumes almost a quarter of the available energy, and has a limited ability to store energy. The brain is therefore highly dependent on oxygen and nutrient supply from the blood circulation, which makes it vulnerable to vascular pathologies. Key vascular determinants will ensure proper brain maturation and function: the establishment of vascular networks, the formation of the blood-brain barrier, and the regulation of blood flow. Recent evidence suggests that the phenomenon of neurovascular coupling, during which increased neural activity normally leads to increased blood flow, is not functional until few weeks after birth, implying that the developing brain must rely on alternative mechanisms to adequately couple blood supply to increasing energy demands. This review will focus on these alternative mechanisms, which have been partly elucidated recently via the demonstration that neural activity influences the maturation of cerebrovascular networks. We also propose possible mechanisms underlying activity-induced vascular plasticity.
Keywords: cerebrovascular patterning, neural activity, neurovascular unit, brain, endothelial cells
Graphical abstract
I. Control of cerebrovascular patterning by neural activity
I.a. Meeting energy demands: neurovascular interactions in the mature versus immature brain
In order to function properly, the brain relies heavily on the delivery of oxygen and nutrients from the blood stream (Attwell and Laughlin, 2001; Peters et al., 2004), requiring an adequate matching between metabolic demands of neural cells and blood supply. In the central nervous system (CNS), neural and vascular cells form a functionally integrated network, whereby neural activity and vascular dynamics are tightly coupled (Hamel, 2006; Lecrux and Hamel, 2011).
The anatomical substrate of neurovascular interactions in the brain is known as the ‘neurovascular unit’ (NVU), a complex multicellular system where neurons, astrocytes, microglia, pericytes and endothelial cells communicate to control the diameter of brain vessels and ensure an adequate delivery of oxygen and nutrients to neural tissues through the blood stream (Attwell et al., 2010; Cauli and Hamel, 2010; Chen et al., 2014; Fernandez-Klett et al., 2010; Hall et al., 2014; Hamel, 2006; Howarth, 2014; Lecrux and Hamel, 2011; Lo and Rosenberg, 2009; Petzold and Murthy, 2011). The NVU is also the anatomical substrate of the blood-brain barrier (BBB), a system which provides a tightly controlled environment, free of various toxins, pathogens, and with adequate chemical composition, for proper brain function (Andreone et al., 2015; Ben-Zvi et al., 2014; Saunders et al., 2014).
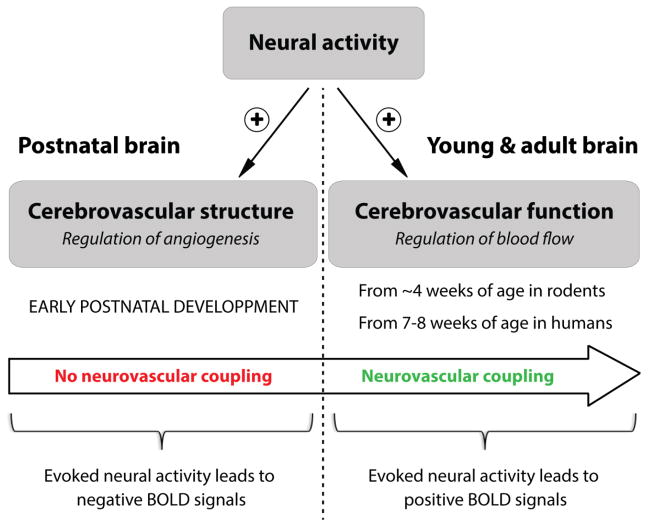
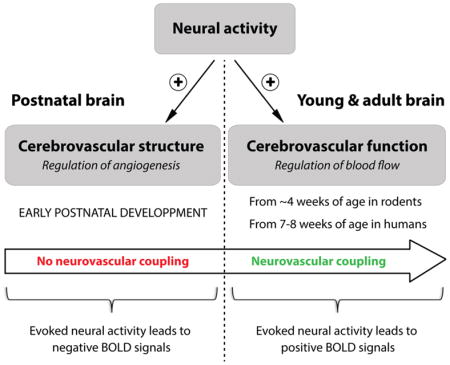
In the mature brain, the functional coupling between neural activity and cerebral blood flow (CBF) has been known for more than a century (Roy and Sherrington, 1890), as recently revisited (Sandrone et al., 2014). The increase in CBF following neural activity, also known as “neurovascular coupling”, has far-reaching implications in health and disease (Cauli and Hamel, 2010; Drake and Iadecola, 2007; Iadecola, 2004; Zlokovic, 2010), and represents the basis of functional brain imaging using blood oxygen level-dependent (BOLD) signals (Devor et al., 2007; Devor et al., 2005; Hillman, 2014). In the immature brain, however, recent studies in rodents and humans have shown that the phenomenon of neurovascular coupling is not functional until few weeks after birth. While in adults sensory stimulation leads to a positive BOLD signal, reflecting a local increase in CBF, the identical stimulus in newborn infants or rat pups was shown to result in an inverted response with negative BOLD signals (Anderson et al., 2001; Born et al., 2002; Kozberg et al., 2013; Muramoto et al., 2002; Yamada et al., 2000). In these studies, negative BOLD signals were suggested to result from either decreased perfusion or increased oxygen consumption in response to sensory stimulation. The absence of a neurovascular coupling response to neuronal activation implies that, during early postnatal development, the immature brain must rely on alternative mechanisms to adequately match oxygen and nutrients supply with increasing energy demands. One potential mechanism during postnatal development could be the control of cerebrovascular patterning by neural activity.
I.b. Activity-induced vascular plasticity during postnatal development
Both the nervous and vascular systems comprise highly branched and complex networks, and their patterning is initiated during development in a highly stereotyped fashion that is controlled by genetic programs, as reviewed elsewhere (Adams and Eichmann, 2010; Andreone et al., 2015; Carmeliet and Tessier-Lavigne, 2005; Tam and Watts, 2010). However, both networks exhibit a certain degree of plasticity and undergo dynamic remodeling after birth (Norman and O’Kusky, 1986). As early as embryonic day 10.5 (E10.5), the neural environment plays a critical role in the initial ingression and pruning/stabilization of blood vessels (Daneman et al., 2009; Haigh et al., 2003; Hogan et al., 2004). Along development, multiple cell types including neuroblasts, neuroepithelial radial glia, pericytes, microglia and astrocytes associate with blood vessels and influence their density/branching patterns (Arnold and Betsholtz, 2013; Lee and McCarty, 2014; Ma et al., 2012; Ma et al., 2013). For instance, reducing the proliferation of radial glial or astroglial progenitors during embryonic development led to a severe reduction in vascular density and branching frequency in the CNS at peri-and post-natal stages (Ma et al., 2012; Ma et al., 2013).
Whether electrical activity of neural cells influences the postnatal maturation of cerebrovascular networks remained elusive and controversial until recently. Almost thirty years ago, William T. Greenough and colleagues postulated that, during postnatal development, the brain adapts to increased metabolic demands by creating new vessels (Black et al., 1990; Black et al., 1987; Black et al., 1991). These milestone studies introduced the concept of vascular remodeling during maturation of the brain, however they did not establish a direct link between neural activity and vascular patterning after birth.
From studies in the rat cerebral cortex, the unique prevailing view was that requirements from expanding neural tissues influence the maturation of underlying capillary networks (Black et al., 1987; Sirevaag et al., 1988), and that high metabolic activity correlates with higher vascular density (Riddle et al., 1993). Moreover, several studies proposed the existence of anatomical relationships between neuronal and vascular modules within cortical columns in the rat somatosensory cortex (Cox et al., 1993; Patel, 1983). Such anatomical parallelism suggests that neuronal and vascular modules may instruct each other to build a precise wired network for optimized local interactions, similar to the neurovascular congruency observed in the peripheral nervous system (Mukouyama et al., 2002). However, it was later demonstrated in the same species that cortical microvascular domains do not display any direct topological relationship with underlying columns (Woolsey et al., 1996). In line with this observation, recent studies using novel imaging and computational techniques, with three-dimensional (3-D) reconstructions of cerebrovascular networks, further demonstrated that the microvascular topology does not match the neuroarchitecture in the mouse cerebral cortex (Blinder et al., 2013; Lacoste et al., 2014; Tsai et al., 2009). Thus, in light of the fact that cortical columns are shaped after birth by neural activity (Erzurumlu and Kind, 2001; Li et al., 2013a; Narboux-Neme et al., 2012), it is possible that vascular network structure can also be influenced by neural activity.
The concept of neural activity-induced cerebrovascular plasticity during postnatal development was first introduced by earlier studies which postulated that sensory stimulation had a positive effect on brain angiogenesis (Argandona and Lafuente, 1996; Argandona and Lafuente, 2000; Black et al., 1987; Sirevaag et al., 1988). Therefore, after birth, sensory-related neural activity may refine cerebrovascular networks into their mature form, as it does for neuronal circuits (Katz and Shatz, 1996; Zhang and Poo, 2001). With the ability to simultaneously visualize and analyze neuronal and vascular modules, the direct effect of sensory neural activity on postnatal cerebrovascular development in the healthy brain was recently demonstrated in a study from our laboratory (Lacoste et al., 2014). We found that vascular density and branching, as well as endothelial cell proliferation, were decreased in layer IV of the primary somatosensory cortex when sensory input was reduced either by a complete deafferentation, by a genetic impairment of neurotransmitter release at thalamocortical synapses or by a selective reduction of sensory-related neural activity. In contrast, enhancement of sensory inputs led to an increase in vascular density and branching. Therefore, sensory-related neural activity appears necessary for vascular patterning, and changes in neural activity are sufficient to trigger changes in vascular structure. This implies that the postnatal maturation of brain vascular networks not only relies on angiogenic programs, but is also influenced by environmental stimuli.
Under pathological conditions in which neural activity is affected, the brain vascular structure may be regulated differently, particularly when these conditions occur during critical developmental periods. Excessive neural activity following hyperactivation of sensorimotor systems was recently shown to impair cerebrovascular network formation during a critical postnatal time (Whiteus et al., 2014). Whiteus et al. found a severe reduction of angiogenesis in the cerebral cortex following either intense locomotor exercise, persistent auditory stimulation, or following chemically-induced seizures in mice. This led the authors to propose that excessive neural activation during early childhood may trigger long-term deficits in microvascular networks with important consequences for brain function. In the adult rat brain however, previous studies with such hyperactivation paradigms evidenced increased angiogenesis in the cerebellum following vigorous locomotor exercise (Isaacs et al., 1992) or in the hippocampus after electroconvulsive seizures (Newton et al., 2006), thus emphasizing the difference between the “immature” and the “mature” brain in terms of vascular plasticity. Importantly, this angiogenic capability of the adult brain might be of interest in ischemic conditions such as stroke. Indeed, it has been demonstrated that angiogenesis is increased in the penumbra of the ischemic adult mouse barrel cortex following enhancement of sensory-related neural activity by whisker stimulation (Whitaker et al., 2007), an effect which involves vascular endothelial growth factor (VEGF)/VEGFR2 signaling (Li et al., 2011) and which can be amplified by inhibition of de novo cholesterol synthesis by statins (Zhang et al., 2012).
II. Possible mechanisms underlying activity-induced cerebrovascular plasticity
II.a. What cell types could be involved?
The question remains whether neural activity affects angiogenesis directly via neurotransmitter and/or growth factor release by incoming axons, or indirectly via local pathways activated following neural activation that involve various cellular components of the NVU (Table 1).
Table 1.
Contribution of different cell type in the neurovascular unit to cerebrovascular development.
| Cell type | Released factors | Effect on vascular patterning | References |
|---|---|---|---|
| Pericytes | Ang1 | Vessel stabilization | Suri et al., 1996 |
| Astrocytes | VEGF-A | Pro-angiogenic |
Stone et al., 1995 Munzenmaier & Harder, 2000 Zhang & Harder, 2002 Potente et al., 2003 Pozzi et al., 2005 West et al., 2005 Li et al., 2013 |
| EETs | |||
| Shh | |||
| Ang1 | Vessel stabilization | ||
| Neurons | Ang1 | Vessel stabilization |
Cao et al., 2004 Joyal et al., 2014 |
| VEGF-A | Pro-angiogenic | ||
| Microglia | TNFα | Pro-angiogenic |
Stefater et al., 2011 Arnold & Betsholtz, 2013 Li et al., 2014 |
| VEGF-C,D | Pro-/anti-angiogenic | ||
| Wnt5a, Wnt11 | Anti-angiogenic |
Ang1, angiopoietin-1; EETs, epoxyeicosatrienoic acids; Shh, sonic hedgehog; VEGF, vascular endothelial growth factor; Wnt, wingless integration site.
Neurons
In the coupling between neural activity and vascular function in the cerebral cortex, the main neuronal players are neurons projecting from subcortical regions (e.g. basal forebrain, locus coeruleus) to the cortex (Cauli et al., 2004; Hamel, 2006), as well as local neurons (Cauli and Hamel, 2010; Lecrux et al., 2011). The cerebral cortex is densely innervated by projection neurons that release neurotransmitters such as acetylcholine (ACh, basal forebrain), noradrenaline (NA, locus coeruleus), serotonin (5-HT, raphe nuclei), or glutamate (Glu, thalamus). We recently demonstrated that proliferation of endothelial cells was decreased in layer IV of the barrel cortex following reduction of somatosensory inputs, pointing to a possible role of thalamocortical neurotransmission in the control of cortical angiogenesis (Lacoste et al., 2014). Local cortical pyramidal (excitatory) neurons and inhibitory interneurons are also recruited by somatosensory inputs (Lecrux et al., 2011), and in turn release vasoactive mediators which control vascular tone (Cauli and Hamel, 2010; Drake and Iadecola, 2007). However, the question remains whether these neural modules also release angiogenesis regulators upon neural activity changes. Interestingly, it has been shown that neuronal expression of VEGF increases in the hippocampus of rats subjected to environmental enrichment (Cao et al., 2004). More recently, two reports using the postnatal mouse retina as a model system for studying neuro-vascular crosstalk identified novel neuronal signaling pathways that govern CNS angiogenesis (Joyal et al., 2014; Okabe et al., 2014). Joyal et al. demonstrated that a subset of retinal neurons (the ganglion cells) express a G protein-coupled receptor known as F2rl1 (or Par2) which, upon agonist stimulation, relocates to the nucleus via a microtubule-dependent shuttle to induce Vegfa expression, promoting neovascularization. Okabe et al. demonstrated that VEGFR2 is predominantly expressed by retinal neurons. Upon binding to neuronal VEGFR2, VEGF protein is engulfed in the cell while its receptor is being endocytosed, a mechanism that allows titration of extracellular VEGF to regulate vascularization around retinal neurons. Additionally, neurons communicate with other cell types of the NVU (i.e., glial cells and pericytes) and might therefore indirectly influence vascular plasticity, as detailed below.
Glial cells
Astrocytes are well positioned to mediate the effects of neural activity, being in close contact with both cerebral synapses and vessels. They express different classes of glutamate receptors and project specialized extensions (‘endfeet’) around vessels. It is well accepted that astrocytes respond to glutamate (Glu), via metabotropic glutamatergic receptors, by a rise in intracellular calcium ([Ca2+]i) from internal stores (Lind et al., 2013; Winship et al., 2007; Zonta et al., 2003), triggering signaling cascades which lead to the production of glial messengers involved in the control of CBF (Howarth, 2014), including epoxyeicosatrienoic acids (EETs) (Alkayed et al., 1996b). In addition to their role as vasodilators (Alkayed et al., 1996a; Alkayed et al., 1997), EETs are pro-angiogenic lipids. Indeed, in vitro evidence demonstrated that EETs are as potent as VEGF in inducing endothelial cells proliferation and tube formation (Munzenmaier and Harder, 2000; Potente et al., 2003; Pozzi et al., 2005; Zhang and Harder, 2002). More recently, a recent genetic study demonstrated that astrocytes are essential for the normal postnatal development of cerebral cortex vasculature (Ma et al., 2012). In addition, astrocytes are well known to release pro-angiogenic VEGF in vivo (Stone et al., 1995; West et al., 2005), and they can also promote angiogenesis via release of sonic hedgehog (shh) in ischemic conditions in vitro (Li et al., 2013b). Future studies should investigate the precise mechanisms through which neural activity controls the release of astroglial angiogenesis modulators in vivo, and their effects on cerebrovascular patterning. The other glial cell type of the brain, microglia, could also be involved in activity-induced vascular plasticity. Microglia are considered to be the brain’s resident macrophages (Neumann et al., 2009). However, microglia also play critical roles in postnatal brain maturation during vascular development (Arnold and Betsholtz, 2013) and in experience-dependant neuronal plasticity during critical periods (Tremblay and Majewska, 2011; Tremblay et al., 2011). Microglia appear associated with blood vessels as early as E10.5 when vessels start to ingress into the neuroepithelium (Ginhoux et al., 2010) and they promote vascular branching in the embryo (Fantin et al., 2010) as well as postnatally (Kubota et al., 2009). Recent studies suggested that microglia regulate vascular branching and proliferation via release of soluble mediators which either promote or inhibit vascular network formation (Arnold and Betsholtz, 2013; Stefater et al., 2011). More recently, microglia have been shown to facilitate angiogenesis in vitro via release of tumor necrosis factor-α (TNF-α), which upregulates endothelial ephrin-A3 and ephrin-A4 (Li et al., 2014). Moreover, microglia are endowed with neurotransmitter receptors including iono-and metabo-tropic Glu receptors (Pocock and Kettenmann, 2007) and are reactive to neural activity (Hung et al., 2010; Wake et al., 2009). However, it remains to be determined whether microglial angiogenesis regulators are released upon neuronal activation.
Pericytes
Long considered as support cells for the endothelium, pericytes are also involved in the proper vascularization of the brain (ElAli et al., 2014), in the formation and maintenance of the blood-brain barrier (Armulik et al., 2010; Daneman et al., 2010; Mae et al., 2011), and in the regulation of capillary diameter in response to neural activity (Fernandez-Klett et al., 2010; Hall et al., 2014; Hamilton et al., 2010; Itoh and Suzuki, 2012). Whether pericytes also play a role in activity-induced cerebrovascular structural plasticity needs to be clarified, but several lines of evidence advocate in favor of such possibility. Pericytes and endothelial cells interact via signalling pathways which are instrumental for vascular network formation, as extensively reviewed elsewhere (Armulik et al., 2005; ElAli et al., 2014). To only give one example, angiopoietin-1 secreted by pericytes binds to the endothelial-specific Tie2 receptor to promote maturation and stabilization of the microvascular endothelium (Suri et al., 1996). Interestingly, pericytes are sensitive to neurotransmitters such as glutamate, noradrenalin and acetylcholine (Hall et al., 2014; Peppiatt et al., 2006; Puro, 2007; Wu et al., 2003), and they are responsive to electrical stimulation (Peppiatt et al., 2006). Moreover, like in astrocytes, neurotransmitters cause a rise in [Ca2+]i in pericytes (Kawamura et al., 2004; Kawamura et al., 2003). In microvessels of the postnatal brain, the [Ca2+]i elevation induced in pericytes by synaptic release of Glu was shown to result in nuclear accumulation of NFATc3, a transcription factor involved in vascular development and maturation (Filosa et al., 2007). Future studies could investigate the ability of pericytes to secrete angiogenesis regulators upon neural activity changes.
II.b. What endothelial gene(s) could be involved?
As best illustrated by the NVU, activity-dependent regulation of vascular structure is an integrative process recruiting broad cellular networks. Knowing that the endothelium represents the ultimate effector undergoing structural changes, future studies could focus on investigating the genes that are regulated within endothelial cells upon modulation of neural activity. This approach is timely, since technological advances in gene analysis allow for a better understanding of cerebrovascular cell transcriptome (Ozkan et al., 2012; Zhang et al., 2014). Such approaches could be used to investigate candidate genes and identify new molecular players of vascular plasticity.
One endothelial-specific gene whose upregulation has already been linked to neural activity is named “vascular early response gene”, or Verge (Mirza et al., 2013; Regard et al., 2004). In developing tissues, Verge is constitutively expressed (mRNA and protein) in the endothelium and is associated with angiogenesis, whereas in the adult brain it is regulated as an immediate early gene induced by electrical or chemical seizures and by focal ischemia. In cultured endothelial cells, Verge is induced by growth factors and hypoxia. Since Verge induction is associated with remodeling of the actin cytoskeleton, the authors proposed that Verge might play a role in activity-dependent changes of brain vasculature (Regard et al., 2004). It would be interesting to test whether changes in Verge expression occur in the cerebral cortex following neuronal activation in a physiological context, and assess the effects of its modulation on cerebrovascular plasticity.
Other candidate genes that might be involved in activity-induced vascular plasticity include genes postnatally expressed in the endothelium and known to affect vascular remodeling. For instance, homeobox transcription factors are promising candidates involved in the control of vascular remodeling (Gorski and Walsh, 2000). Expression of members of HOX A, HOX B, and HOX D clusters has been detected in endothelial cells and involved in the balance between resting and angiogenic state of the endothelium. HOX D3 is highly expressed in proliferating endothelial cells that are induced to form tubes in vitro (Boudreau et al., 1997), and constitutive expression of HOX B3 in the chick chorioallantoic membrane leads to increased angiogenesis (Myers et al., 2000). Receptor neuropilin-1 (Nrp1) and its co-receptor VEGFR2 (Kdr) have recently been associated to postnatal angiogenesis and vascular remodeling after ischemic challenge (Gelfand et al., 2014). Another interesting candidate is Bai1 (brain-specific angiogenesis inhibitor-1), a G-protein coupled receptor present in vessels of the mouse brain throughout development with higher expression at the tips of angiogenic sprouts (Ozkan et al., 2012). Interestingly, while endothelial-specific expression of Bai1 has to be confirmed, Bai1 appears predominantly expressed in dense microvascular networks of the cerebral cortex at P14 (Ozkan et al., 2012), which is an age when cerebrovascular growth and remodeling are very active (Lacoste et al., 2014). Future studies could investigate whether candidates or new endothelial genes are regulated by neural activity in vivo during postnatal development.
II.c. Could hypoxia be involved?
As mentioned earlier in this review, the absence of neurovascular coupling responses is characteristic of the early postnatal brain. Evoked neural activity in the immature brain results in decreased perfusion and/or increased oxygen extraction, underlying negative BOLD signals (Anderson et al., 2001; Born et al., 2002; Kozberg et al., 2013; Muramoto et al., 2002; Yamada et al., 2000). Therefore, in the absence of blood flow regulation, it is possible that the developing brain recruits alternative mechanisms to meet its increasing metabolic needs. Indeed, after birth, the maturation of neuronal networks involves energy-consuming processes such as neurogenesis, synaptogenesis, maturation of astrocytes, and changes in brain cytoarchitecture. Thus, in the young brain, neuronal network activation might generate a local and transient hypoxic state. Interestingly, hypoxia-induced growth factors represent an important driving force of vascular development during embryogenesis (Haigh et al., 2003; James et al., 2009; Provis et al., 1997; Raab et al., 2004; Stone et al., 1995) but also during postnatal cerebrovascular remodeling (Rey and Semenza, 2010), particularly in pathological conditions such as ischemia and cancer (Silpanisong and Pearce, 2013). Local reduction in oxygen tension leads to activation of transcription factors ‘hypoxia-inducible factors’ (HIFs) that signal in the nucleus following heterodimerization. Activated HIFs regulate the expression of virtually all the key angiogenic factors, including VEGF, angiopoietin-2 and placental growth factor upon binding to their hypoxia response elements (Jiang et al., 1997; Rey and Semenza, 2010; Semenza et al., 1997). Future studies could investigate whether sensory stimulation, which leads to increased vascular density and branching in the cerebral cortex (Lacoste et al., 2014), is due to a local hypoxic state that triggers hypoxia-induced angiogenic factors.
III. Future questions
While the postnatal maturation of brain vascular networks begins to draw most attention, it remains unknown whether the emergence of neuronal function influences brain angiogenesis before birth. In the embryo, before maturation of sensory organs, neural activity exists and has been identified as a spontaneous phenomenon contributing to the refinement of CNS connectivity (Katz and Shatz, 1996; Meister et al., 1991; Weliky and Katz, 1999). Yet, whether this type of electrical activity influences cerebrovascular patterning during embryogenesis remains to be determined. A recent study investigating stimulus-induced vascular remodeling described a melanopsin-dependent fetal light response regulating the regression of hyaloid vessels in the developing mouse eye (Rao et al., 2013). However, the contribution, if any, of stimulus-independent neural activity to cerebrovascular patterning before birth remains an uncharted territory, raising the need for future investigation. Moreover, it is also not known whether postnatal vasculogenesis might be regulated by neural activity in the brain. In this context, postnatal recruitment of bone marrow-derived endothelial progenitor cells and their incorporation to growing vessels (Ribatti et al., 2001) may also be investigated following modulation of neural activity. Future studies could also investigate the influence of neural activity on cerebrovascular patterning during adulthood. Knowing that environmental changes can affect the neuronal connectivity in the mature brain (Bavelier et al., 2010; May, 2011), they might as well affect vascular networks. In line with this hypothesis, studies in adult rodents have shown that whisker stimulation can enhance angiogenesis in the penumbra surrounding the ischemic core following middle cerebral artery occlusion in adult mice (Li et al., 2011; Whitaker et al., 2007), suggesting that sensory stimulation might be beneficial in stroke. Other questions remain unanswered, for instance concerning the indirect effect of CBF on brain angiogenesis. Knowing that mechanical forces affect angiogenesis (Hoefer et al., 2013), it is also possible that the regulation of CBF by neural activity remotely influences cerebrovascular patterning. As already assessed in vitro and in skeletal muscle (Hansen-Smith et al., 2001; Wilkins et al., 2014), future investigations will be needed to shed light on stretch-induced angiogenesis in the brain.
IV. Conclusion
Evidence gathered in this review highlight the role of neural activity in promoting the maturation of cerebrovascular networks during postnatal development. We propose a new model in which, in the absence of coupling between neural activity and cerebral blood flow, the immature brain might recruit alternative mechanisms around microvessels in order to meet the increasing metabolic demands triggered by developing neural tissues (Figure 1). This model, which involves different cell types in the NVU, as well as their ability to regulate vascular growth and plasticity, opens a new area in neurovascular research.
Figure 1. A new model for the role of neural activity in postnatal cerebrovascular development.
In the mature brain, a functional coupling between increased neural activity and increased cerebral blood flow (“neurovascular coupling”) ensures proper brain function. In the immature brain, however, the phenomenon of neurovascular coupling is not functional until few weeks after birth. Here, we propose a model in which, in the absence of coupling between neural activity and cerebral blood flow, the immature brain might recruit alternative mechanisms around microvessels in order to meet the increasing metabolic demands triggered by developing neural tissues
Highlights.
Neural activity controls cerebral blood flow in the mature brain
Neural activity is not coupled to cerebral blood flow in the early postnatal brain
Neural activity controls cerebrovascular patterning in the early postnatal brain
A model is proposed to explain how metabolic needs are met early after birth
Acknowledgments
We thank Dr. Edith Hamel and members of the Gu lab for constructive comments on the manuscript. This work was supported by the Mahoney postdoctoral fellowship, the Goldenson postdoctoral fellowships (B.L.), and by the following grants to C.G.: Harvard/MIT Joint Research Grants Programm in Basic Neuroscience, NIH grant R01NS064583, and NIH’s Director Pioneer Award DP1 NS092473.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Baptiste Lacoste, Email: Baptiste_Lacoste@hms.harvard.edu.
Chenghua Gu, Email: Chenghua_Gu@hms.harvard.edu.
References
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996a;271:H1541–6. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–72. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996b;27:971–9. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Schneider KC, Gore JC, Ment LR. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Andreone BJ, Lacoste B, Gu C. Neuronal and Vascular Interactions. Annu Rev Neurosci. 2015 doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argandona EG, Lafuente JV. Effects of dark-rearing on the vascularization of the developmental rat visual cortex. Brain Res. 1996;732:43–51. doi: 10.1016/0006-8993(96)00485-4. [DOI] [PubMed] [Google Scholar]
- Argandona EG, Lafuente JV. Influence of visual experience deprivation on the postnatal development of the microvascular bed in layer IV of the rat visual cortex. Brain Res. 2000;855:137–42. doi: 10.1016/s0006-8993(99)02361-6. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013;5:4. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–11. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–5. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Black JE, Zelazny AM, Greenough WT. Capillary and mitochondrial support of neural plasticity in adult rat visual cortex. Exp Neurol. 1991;111:204–9. doi: 10.1016/0014-4886(91)90008-z. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–97. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Miranda MJ, Larsson HB, Lou HC. Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR) Magn Reson Imaging. 2002;20:199–205. doi: 10.1016/s0730-725x(02)00469-1. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–64. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–9. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SB, Woolsey TA, Rovainen CM. Localized dynamic changes in cortical blood flow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. J Cereb Blood Flow Metab. 1993;13:899–913. doi: 10.1038/jcbfm.1993.113. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–9. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci U S A. 2005;102:3822–7. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–52. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15:6453–74. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–95. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–5. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFATc3 nuclear accumulation in pericytes from cortical parenchymal microvessels. Am J Physiol Cell Physiol. 2007;293:C1797–805. doi: 10.1152/ajpcell.00554.2006. [DOI] [PubMed] [Google Scholar]
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, Gu C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. Elife. 2014:3. doi: 10.7554/eLife.03720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–72. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–41. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006;100:1059–64. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Smith F, Egginton S, Zhou AL, Hudlicka O. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvasc Res. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–81. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276–83. doi: 10.1093/cvr/cvt089. [DOI] [PubMed] [Google Scholar]
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–13. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Chansard M, Ousman SS, Nguyen MD, Colicos MA. Activation of microglia by neuronal activity: results from a new in vitro paradigm based on neuronal-silicon interfacing technology. Brain Behav Immun. 2010;24:31–40. doi: 10.1016/j.bbi.2009.06.150. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab. 2012;32:1167–76. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–41. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–60. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, Sapieha P, Hamel D, Sanchez M, Zaniolo K, St-Louis M, Ouellette J, Montoya-Zavala M, Zabeida A, Picard E, Hardy P, Bhosle V, Varma DR, Gobeil F, Jr, Beausejour C, Boileau C, Klein W, Hollenberg M, Ribeiro-da-Silva A, Andelfinger G, Chemtob S. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 2014;20:1165–73. doi: 10.1038/nm.3669. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol. 2004;561:671–83. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–99. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozberg MG, Chen BR, DeLeo SE, Bouchard MB, Hillman EM. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci U S A. 2013;110:4380–5. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, da Costa LF, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–47. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, McCarty JH. Inducible gene deletion in glial cells to study angiogenesis in the central nervous system. Methods Mol Biol. 2014;1135:261–74. doi: 10.1007/978-1-4939-0320-7_22. [DOI] [PubMed] [Google Scholar]
- Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013a;79:970–86. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214:503–13. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu DX, Li MY, Qin XX, Fang WG, Zhao WD, Chen YH. Ephrin-A3 and ephrin-A4 contribute to microglia-induced angiogenesis in brain endothelial cells. Anat Rec (Hoboken) 2014;297:1908–18. doi: 10.1002/ar.22998. [DOI] [PubMed] [Google Scholar]
- Li Y, Xia Y, Wang Y, Mao L, Gao Y, He Q, Huang M, Chen S, Hu B. Sonic hedgehog (Shh) regulates the expression of angiogenic growth factors in oxygen-glucose-deprived astrocytes by mediating the nuclear receptor NR2F2. Mol Neurobiol. 2013b;47:967–75. doi: 10.1007/s12035-013-8395-9. [DOI] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci U S A. 2013;110:E4678–87. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40:S2–3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7:e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 2013;11:e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae M, Armulik A, Betsholtz C. Getting to know the cast -cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–4. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15:475–82. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–43. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mirza MA, Capozzi LA, Xu Y, McCullough LD, Liu F. Knockout of vascular early response gene worsens chronic stroke outcomes in neonatal mice. Brain Res Bull. 2013;98:111–21. doi: 10.1016/j.brainresbull.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–7. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Muramoto S, Yamada H, Sadato N, Kimura H, Konishi Y, Kimura K, Tanaka M, Kochiyama T, Yonekura Y, Ito H. Age-dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. J Comput Assist Tomogr. 2002;26:894–901. doi: 10.1097/00004728-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Myers C, Charboneau A, Boudreau N. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J Cell Biol. 2000;148:343–51. doi: 10.1083/jcb.148.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci. 2012;32:6183–96. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–95. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Girgenti MJ, Collier EF, Duman RS. Electroconvulsive seizure increases adult hippocampal angiogenesis in rats. Eur J Neurosci. 2006;24:819–28. doi: 10.1111/j.1460-9568.2006.04958.x. [DOI] [PubMed] [Google Scholar]
- Norman MG, O’Kusky JR. The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol. 1986;45:222–32. [PubMed] [Google Scholar]
- Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M, Kubota Y. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014;159:584–96. doi: 10.1016/j.cell.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Ozkan A, Bicer A, Avsar T, Seker A, Toktas ZO, Bozkurt SU, Basak AN, Kilic T. Temporal expression analysis of angiogenesis-related genes in brain development. Vasc Cell. 2012;4:16. doi: 10.1186/2045-824X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U. Non-random distribution of blood vessels in the posterior region of the rat somatosensory cortex. Brain Res. 1983;289:65–70. doi: 10.1016/0006-8993(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28:143–80. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–97. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–25. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6-and 8,9-epoxyeicosatrienoic acids (5,6-and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–46. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–68. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–6. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JB, Scheek S, Borbiev T, Lanahan AA, Schneider A, Demetriades AM, Hiemisch H, Barnes CA, Verin AD, Worley PF. Verge: a novel vascular early response gene. J Neurosci. 2004;24:4092–103. doi: 10.1523/JNEUROSCI.4252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, Roncali L, Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100:157–63. doi: 10.1016/s0925-4773(00)00522-0. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–15817. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrone S, Bacigaluppi M, Galloni MR, Cappa SF, Moro A, Catani M, Filippi M, Monti MM, Perani D, Martino G. Weighing brain activity with the balance: Angelo Mosso’s original manuscripts come to light. Brain. 2014;137:621–33. doi: 10.1093/brain/awt091. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Mollgard K, Bauer HC. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci. 2014;8:404. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor. 1. Kidney Int. 1997;51:553–5. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- Silpanisong J, Pearce WJ. Vasotrophic regulation of age-dependent hypoxic cerebrovascular remodeling. Curr Vasc Pharmacol. 2013;11:544–63. doi: 10.2174/1570161111311050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Res. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–5. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–2. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–9. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–70. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–62. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–11. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JR, Pike DB, Gibson CC, Kubota A, Shiu YT. Differential effects of cyclic stretch on bFGF-and VEGF-induced sprouting angiogenesis. Biotechnol Prog. 2014 doi: 10.1002/btpr.1883. [DOI] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–72. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–60. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003;284:H2083–90. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Muramoto S, Kimura K, Tanaka M, Yonekura Y, Ishii Y, Itoh H. A milestone for normal development of the infantile brain detected by functional MRI. Neurology. 2000;55:218–23. doi: 10.1212/wnl.55.2.218. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–64. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl):1207–14. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci. 2014;34:11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang S, Wang B, Sun B, Li W, Lu X, Ding X. Atorvastatin and whisker stimulation synergistically enhance angiogenesis in the barrel cortex of rats following focal ischemia. Neurosci Lett. 2012;525:135–9. doi: 10.1016/j.neulet.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]