Abstract
Major vaccine safety controversies have arisen in several countries beginning in the last decades of 20th Century. Such periodic vaccine safety controversies are unlikely to go away in the near future as more national immunization programs mature with near elimination of target vaccine-preventable diseases that result in relative greater prominence of adverse events following immunizations, both true reactions and temporally coincidental events. There are several ways in which vaccine safety capacity can be improved in the future to potentially mitigate the impact of future vaccine safety controversies. This paper aims to take a “lifecycle” approach, examining some potential pre- and post-licensure opportunities to improve vaccine safety, in both developed (specifically U.S. and Europe) and low- and middle- income countries.
Keywords: vaccine safety, AEFI, sustainability, capacity building, LMICs
Introduction
As we approach halfway through the Decade of Vaccines (1), the US faced its largest measles outbreak since the disease was “eliminated” in 2000. (2) This outbreak occurred mostly among unvaccinated children, some of whose parents' decision to avoid vaccination may have been influenced by earlier (subsequently disproven and retracted) hypothesized safety concerns about linkage between vaccination and autism, (3-6) and who may be clustered geographically. (7) The resurgence of this vaccine-preventable disease (VPD) is but the latest unfortunate impact of vaccine safety controversies that have arisen in several countries beginning in the last decades of 20th Century (8-11). While the specific circumstances of each occurrence may be unique, the convergence of multiple larger forces may also play a role (Table 1) (12). Such periodic vaccine safety controversies are unlikely to go away in the near future as those factors are unlikely to change. Nevertheless, there are several ways in which vaccine safety capacity can be improved in the future to potentially mitigate the odds and impact of future vaccine safety controversies. This paper aims to take a “lifecycle” approach (13), examining some potential pre- and post-licensure opportunities to improve vaccine safety, in both developed and low- and middle- income countries (LMIC).
Table 1. Some factors whose recent convergence may increase the risk of vaccine safety controversies (12, 123, 124).
| 1) Lower tolerance for immunization risks: Medical intervention, including immunizations, aims to minimize the risks while maximizing the benefits. Because most immunizations are administered to healthy persons (frequently babies) to prevent illness, are commonly recommended for near-universal use, and are often legally required for school entry, the tolerance for risks is much lower than most other medical interventions (generally targeted at treating ill patients). |
| 2) Increasing maturity of National Immunization Programs (NIP): Most NIPs are maturing with high coverage and effectiveness of most commonly used immunizations, resulting in (near) elimination of their target vaccine-preventable disease (VPD) (125)but also an increased number of adverse events following immunizations (AEFI; both true reactions and temporally coincidental events). |
| 3) Limited clinical trial sample size: The inherent limits of sample size in pre-licensure clinical trials usually means rare, AEFIs are likely to be detectable only in post-licensure settings once larger populations are immunized (126); |
| 4) New vaccines and complex schedules: The biotechnology revolution triggered by our ability to sequence the genome of any microorganism has enhanced our ability to develop and introduce new vaccines (121, 122); each of whom, however, needs to be added to an already complex routine immunization schedule, thereby raising concerns in some parents about “over-immunization” of their babies (123). Furthermore, pre-licensure clinical trials often involve looking at a single vaccine and comparator control vaccine or placebo, or a minimal number of simultaneous vaccines. This is not necessarily how vaccines are administered in children in practice, where many vaccines might be co-administered. So the pre-licensure trials might not be representative of how vaccine is administered to children in practice and does not completely address the issue of simultaneous vaccination and the safety of the schedule (81). |
| 5) Evolving Parenting: Post-modern parenting aiming to make optimal informed risk-benefit decision for their own child may overemphasize perceived vaccine risks in absence of wild disease, thereby delay (“hesitate”) or increase use of exemptions to mandated immunizations (123, 124); |
| 6) Diverging risk-benefit: For vaccines that confer herd immunity, there is an inherent divergence between societal and individual risk-benefit at high vaccine coverages (127); |
| 7) Time lag between hypothesis and evidence: Hypothesis testing validation studies of rare vaccine safety associations are difficult, costly, time consuming, and can usually tackle only one or small number of AEFI's at a time. Even under optimal circumstance, months but typically years are usually needed for expert consensus to develop based on appropriately gathered scientific evidence. Therefore there is an inherent mismatch in timespan between the questions (and expectations) raised by rapid and unpredictable communications via 24/7 mass media and social network apps on cell phones and scientific process that allow vaccine safety controversies to amplify (124, 128). |
1. History of vaccine safety
Before we peer into the future, however, it is instructive to review the history [Table 2] and relative prominence of vaccine safety through the lens of evolution of immunization programs (Figure 1) (14). For most national immunization programs (NIP) and most VPD they target, the vast majority of the time since inception has been spent in Stage 1 (pre-vaccine introduction) or Phase 2 (increasing vaccine coverage); phases where the number of VPD vastly exceed that of adverse events following immunizations (AEFI; both true vaccine reactions and temporally coincidental events). As sustained high vaccine coverage with an effective vaccine nearly eliminates a target VPD, however, the number of serious AEFI may gain in prominence relative to the VPD with several possible outcomes. Routine smallpox vaccination was discontinued (beginning with the USA and UK in 1971 before global eradication in 1979) when the risk of AEFI outweighed that from imported smallpox (15, 16); stopping the use of a vaccine eradicates its AEFI (Figure 1 Phase 5). A similar rationale underlies the switch from live oral to inactivated polio vaccine in the USA (17) and elsewhere (18) when the risk of vaccine-derived paralytic polio exceeded that from wild polio.
Table 2. Some highlights in the history of vaccine safety in the U.S., pre- and post- National Childhood Vaccine Injury Act (NCVIA) of 1986.
|
Figure 1.
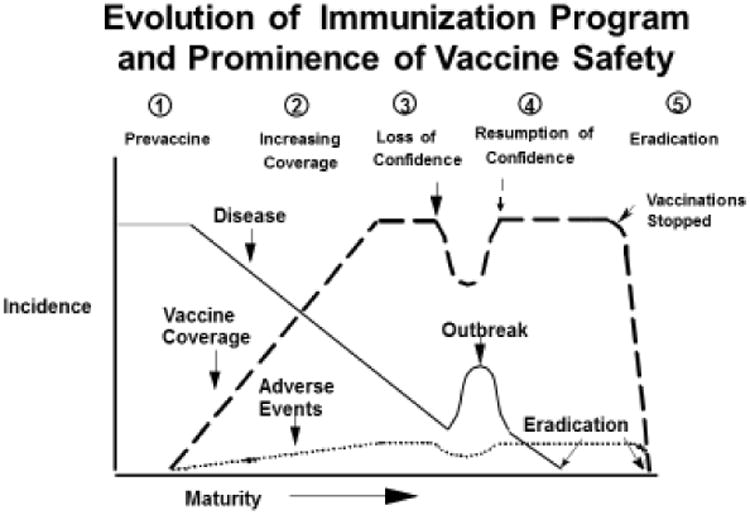
Potential stages in the evolution of immunization program, showing the dynamics of the interaction between vaccine coverage, disease incidence and vaccine adverse events, as the program matures from pre-vaccine to disease eradication.(14)
A different outcome occurred for pertussis, however. Beginning in the 1970's for countries such as Sweden, Japan, and U.K., the success of whole cell pertussis (wP) vaccination in near elimination of pertussis resulted in increased attention on the high rate of wP-associated AEFI (fever and neurologic events), media attention, loss of confidence in wP with major drop in vaccine coverage, and resurgence of pertussis (Figure 1 Phase 3) (8). In the US, similar forces resulted instead in an increase in lawsuits and price of vaccine, loss of vaccine manufacturers, and eventual passage of the National Childhood Vaccine Injury Act (NCVIA) in 1986. (19) The development and adoption of safer (though in retrospect, also likely shorter duration of effectiveness (20)) acellular pertussis (aP) vaccines resulted in resumption of confidence with continued high vaccine coverage and control of pertussis (Figure 1, Stage 4).
The need to meet NCVIA's mandates for better scientific evidence for decision making on vaccine injury compensation helped to spur the development of several key vaccine safety capacity and infrastructure in the U.S. in the 1990's (Table 2). As NIPs mature globally, they are or will face similar challenges and needs for better vaccine safety data in their populations; therefore, efforts to establish similar vaccine safety capacity in other developed and, as described below, increasingly LMIC has begun.
Looking ahead, since few VPD are eradicable 1 (21), most NIP will continue to confront a challenging (and unstable) situation of relatively high AEFI vs. VPD (pre-Stage 3). One possible “stabilizing” solution is to harness the scientific data inherent in AEFI to potentially immunize safer by identifying new contraindications or develop safer vaccines. Distinguishing a true vaccine reaction from all the coincidental AEFI, (22) let alone understanding the pathogenesis has always been challenging. This paper lays out some steps to fulfilling the potential for improving the science of vaccine safety in an era of large datasets, genomics, personalized medicine, and multi-national collaborative studies, and thereby enhance the sustainability of successful NIP.
2. Opportunities to enhance vaccine safety in regulatory setting
Starting with the Biologics Control Act of 1902 in the U.S. (in response to 13 deaths in recipients of tetanus-contaminated diphtheria antitoxin) (23), many of the lessons learnt from vaccine safety crises subsequently have been codified by national regulatory authorities (NRA) [or the World Health Organizations (WHO) for the countries without NRAs] for the licensure and procurement of safe and effective vaccines of high quality (24). The continued evolution and modernization of these regulations (or guidance documents) for new biotechnologies used in vaccine development and production are crucial to preventing future vaccine safety problems (25). For example, the US Food and Drug Administration (FDA) just issued a draft guidance on Current Good Manufacturing Practice Requirements for Combination Products such as a vaccine in a micro-needle device (26). The European Medicines Agency issued its guidelines on live recombinant viral vectored vaccines in 2011 applicable to several Ebola vaccine candidates (27). The WHO issued its guidance on regulatory risk evaluation on finding an adventitious agent in a marketed vaccine in 2014 (28). Additional opportunities for enhancing regulatory science have recently been summarized (29, 30).
More challenging are the tasks facing NRAs in LMIC's. Many are relatively newly established and may have limited experience and/or expertise, especially in highly subspecialized biotechnologies (24, 25). Once pre-qualified by WHO, manufacturers in these countries are now providing much of the vaccines used globally (31). The NRA's providing oversight over these manufacturers may need technical assistance to ensure these vaccines meet good manufacturing practice and other global standards. Groups such as the Developing Country Vaccine Regulators Network (DCVRN) and African Vaccine Regulatory Forum (AVAREF) may help bridge some of the identified gaps (32).
3. Opportunities to enhance the evaluation of vaccine safety in pre-licensure setting
Recent efforts have helped improve the evaluation of vaccine safety in the prelicensure setting. Better standardization and use of case definitions for AEFIs (e.g., as organized via the Brighton Collaboration) and toxicity grading scales allow greater accuracy and comparability needed for scientifically rigorous studies of vaccine safety (33). The Brighton Collaboration has also developed guidelines for standardized assessment of the safety elements in protocols of clinical trials investigating vaccines (34). For viral vector vaccines, a working group has been formed to anticipate and standardize assessment of potential safety issues, thereby facilitating their eventual public acceptance when licensed (35). Sponsors of new candidate viral vector vaccines can facilitate transparent discourse among key stakeholders by completing its standardized template with key considerations for risk/benefit assessment (36).
Data Safety Monitoring Boards (DSMB) can be improved by ensuring they include persons with safety (i.e., pharmacovigilance and pharmacoepidemiology - not just infectious disease epidemiology) skills. The cumulative safety experience of a new candidate vaccine from all trials should be available to the DSMB, not just the limited experience from the current trial underway (37). In addition to the principal investigator making an assessment of the possible causal relationship between a candidate vaccine and an AEFI, clinical trials could be organized within healthcare organizations that maintain large linked databases. This would potentially allow seamless capture of safety data from trial subjects over longer periods as well as combined analyses with evolving postmarketing data (38).
4. The future of post-licensure vaccine safety: United States perspective
The United States has a well-developed post-licensure vaccine safety monitoring infrastructure that largely resides in the US Department of Health and Human Services, which includes passive surveillance through the Vaccine Adverse Event Reporting System (VAERS) (14), active surveillance using large-linked electronic health record databases in the Vaccine Safety Datalink (VSD) (39) and the Postlicensure Rapid Immunization Safety Monitoring (PRISM) systems (40) and clinical research activities through the Clinical Immunization Safety Assessment (CISA) Project (41). Vaccine manufacturers might also conduct post-licensure safety studies as part of postmarketing requirements or commitments in accordance with Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act (42). The Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) are responsible for post-licensure monitoring of vaccines and CDC and FDA systems provide the foundation for safety surveillance in the United States. Post-licensure safety data are routinely presented to CDC's Advisory Committee on Immunization Practices (ACIP) (43) and FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) (44) and Pediatric Advisory Committee (45) for consideration during policy deliberations and in the context of regulatory decision making.
Advances in information technology and biomedical science, modifications and expansion to the US immunization program, and evolving parent and patient safety concerns necessitate that CDC and FDA rapidly adapt to the changing public health and regulatory environments. Selected examples of anticipated future issues as well as current and planned vaccine safety initiatives are described below.
4.1 Harnessing new information technology
An important opportunity for public health surveillance in the information age is to take full advantage of advances in modern technology (46). These advances facilitate information capture through enhanced reporting, information linkage, and improved pattern recognition. When put into full production, the technological enhancements described here have the potential to substantially improve the efficiency, timeliness and quality of vaccine safety surveillance.
Despite the proliferation of electronic healthcare data, lags in data processing ensure that VAERS remains the fastest link between an astute observer of a sentinel AEFI and public health and regulatory authorities. Thus, VAERS has added importance in the first few weeks of a situation such as an influenza pandemic when large numbers of persons may be immunized over a short time. Currently underway is a joint CDC-FDA initiative to transform VAERS from a legacy paper-based system into a modern information system that maximizes online reporting capability (47, 48) and electronic data interchange (49, 50). In a separate initiative, CDC is investigating the utility and feasibility of incorporating specialized computer algorithms into electronic health records to facilitate physician identification and reporting of adverse events to VAERS directly from electronic health record systems using standardized electronic messages – a form of enhanced passive surveillance (51, 52). Additionally, exploratory work using mobile technologies like handheld devices and smart phones to facilitate vaccine safety surveillance and research has shown promise (53, 54).
Another important technological advance is statistical techniques using complex software programs that allow large volumes of data to be rapidly analyzed to identify unusual and unexpected patterns. Empirical Bayesian data mining to detect disproportional reporting has already been incorporated into routine VAERS surveillance (55-57) and natural language processing (58) and text mining (59) hold early promise for rapid case classification in both active and passive surveillance. In the VSD system, rapid cycle sequential analytic methods have been developed to conduct near real-time (weekly) monitoring for pre-specified conditions following individual vaccines and types of vaccine (39, 60-62). As part of the process, advanced statistical techniques have been developed and are routinely used to monitor influenza vaccine safety during each influenza season and to monitor newly recommended vaccines during the uptake period (63-65). Rapid cycle analysis was particularly valuable in monitoring the safety of 2009 influenza A (H1N1) monovalent vaccine during the large-scale pandemic influenza vaccination program in the Unites States (66). Within the PRISM system, a new effort is underway to detect AEFI of potential concern without pre-specification (67). PRISM health outcome data can be hierarchically organized using the Agency for Healthcare Research and Quality's Multi-Level Clinical Classification software (68) and TreeScan is a statistical method that can be used to analyze such data (69). The PRISM pilot improves upon the underlying statistical method (70) through the use of unconditional and conditional variants of a self-controlled tree-temporal scan statistic. If successful, this method will enable population-based safety signal identification. This would represent a significant new capability since to date PRISM has primarily been used for signal strengthening and validation. All of these technological improvements will continue to require experienced pharmacovigilance and pharmacoepidemiology staff who can critically review pattern recognition output within relevant medical and public health context.
4.2 Vaccination in pregnancy
Vaccination in pregnancy is unique in that the benefits might potentially extend to the pregnant woman and the developing fetus, as well as to the newborn infant as a result of antibody transfer (71). Pregnancy is typically an exclusion criterion for pre-licensure clinical trials, so intensive safety monitoring is critical following recommendations to vaccinate during pregnancy. Currently in the United States, only two vaccines are routinely recommended for all pregnant women, inactivated influenza and combined tetanus, diphtheria and pertussis (Tdap) vaccines (72, 73). Although ACIP has considered available scientific evidence prior to making recommendations, (74-76) data on the safety of vaccination in the early stages of pregnancy or just prior to conception, and for repeat vaccination at short intervals – as in the case of the Tdap recommendation – are limited. Safety monitoring is becoming increasingly important as uptake of these vaccines in pregnancy increases and investigational vaccines for use in pregnancy, such as group B streptococcus and respiratory syncytial virus vaccines, are developed (77, 78). FDA's new Pregnancy and Lactation Labeling Rule, effective June 30, 2015, enhances the level of detail communicated within Pregnancy, Lactation, and new Females and Males of Reproductive Potential sections of the package insert (79). The agency expects that including information derived from scientifically acceptable pregnancy exposure registries in prescription drug and vaccine labeling may encourage additional data collection (80). Effective use of surveillance systems that rely on large linked electronic health record databases that enable rapid comparisons and estimates of risk of adverse pregnancy outcomes will be critical to building and maintaining confidence in expanded maternal vaccination programs moving forward.
4.3 Complex safety issues beyond single vaccines
In 2013 the Institute of Medicine (IOM) issued a report on the safety of the childhood immunization schedule (81). The IOM did not find any evidence to suggest that the immunization schedule was not safe; however, there was acknowledgement that limited evidence was available on the safety of the immunization schedule as a whole and parents and other stakeholders viewed this as an important concern. Additionally, the IOM concluded that using randomized clinical trials or prospective cohort studies to address this issue would be unethical or not feasible. In its report, the IOM identified CDC's VSD as the best surveillance system to conduct research to evaluate the safety of the immunization schedule. Given the challenges of evaluating the safety of the immunization schedule in a highly vaccinated population (82) and differences in healthcare utilization based on vaccination status (83), developing and exploring the use of new and innovative methodologies will be critical, particularly for studying rare outcomes with potentially long time periods between exposure and onset.
4.4 New manufacturing technologies and delivery systems
We are living in an era of rapid advances in biotechnology and biomedical science. DNA vaccines, recombinant vector vaccines and other novel types of vaccines, adjuvants and delivery systems are in various stages of development and testing (84-88). Although these new types of vaccines and delivery systems have great potential, there is not an existing body of knowledge on their general safety as there is for the older, more conventional vaccine products currently in use. Appropriate monitoring and post-marketing safety studies will be necessary to ensure the safety of new vaccines. Monitoring for rare adverse events that may not have been identified in pre-licensure clinical trials will be particularly important to guide appropriate public health action to ensure safety, if necessary.
4.5 Systems biology and personalized medicine
Systems biology involves incorporating elements from the fields of cell biology, proteomics, genomics, immunology, computational modeling and others to “… understand functioning at the level of the organism, tissue, or cell” (89). By contrast, personalized or precision medicine “… uses an individual's genetic profile to guide decisions made in regard to the prevention, diagnosis, and treatment of disease” (90). In the context of vaccine safety, applying knowledge gained from basic science, clinical research and systems biology to direct patient care might eventually lead to routine screening tests or other procedures that guide healthcare providers' vaccination decisions in order to maximize benefit and minimize adverse reactions at the individual patient level. The logical extension is to harness systems biology and genetic research to guide vaccine development, possibly even the development of personalized vaccines for individual patients based on a unique genetic profile (91-93). Although these concepts might seem futuristic, it is interesting to note that advancing precision medicine was highlighted in President Barack Obama's 2015 State of the Union Address (94). There is reason to be optimistic that the field of vaccinology could benefit from a concerted focus on systems biology research, which in turn could be applied to personalized medicine.
5. European Perspective
In the European Union (EU), new vaccines are introduced according to centralized regulatory procedures, which implies that they are licensed with common indications for their use across member countries. Although many countries use similar immunization schedules and may also use the same commercial products, they may concurrently administer different vaccines, measure vaccine coverage in different ways or in different age groups and have different systems for monitoring adverse events (95).
Several countries in the EU, such as United Kingdom, Denmark, Sweden, Finland, Germany and the Netherlands have well established academic and/or public health capacity for active surveillance of vaccine safety and each single country contributes significantly to science, regulatory and public health issues. However, approaches tended to be based on national data only, were highly variable across member states, and dependent on national budgets. To help address this fragmentation, the European Center of Disease Control and Prevention (ECDC) funded the VAESCO II project (Vaccine Adverse Event Surveillance and Communication project) (95). VAESCO attempted to translate the experience of the VSD Project in the United States to the European context. It also capitalized on the landmark EU-Adverse Drug Reaction (ADR) system, which was started to improve the scale and level of drug safety surveillance assessments for small molecules by pooling databases across European national jurisdictions (96).
The safety of the 2009 H1N1 pandemic influenza vaccine was assessed in an unprecedented fashion in the EU for VAESCO by federating a variety of health care databases. As a result, the background rates of 12 adverse events of interest were assessed in eight countries in four months. Case control studies and self-controlled case series analyses on the association between the pandemic influenza vaccine and Guillain-Barré syndrome were conducted with data from seven countries. Consequently, expert committees at the European Medicines Agency (EMA) were able to be informed on a timely basis (97-100). The association between pandemic influenza vaccine and narcolepsy was assessed by changes in population-based incidence rates and case control studies using data from eight countries (98, 99). All studies were designed to enable pooling of individual-level patient data. They also used a common protocol, standardized methods, shared information technology infrastructure for international data transfer (in line with the European data protection directive), and a central data management and analysis team.
Lessons learned from the VAESCO experience identified five main issues as critical to the future development of a successful European collaboration in vaccine safety monitoring and research: sustainability, governance, purpose, structure, and ethical and administrative approvals (95). The VAESCO project ended in 2012 while the narcolepsy signal was still incompletely evaluated; this was due to resource constraints at ECDC, which was funded through the European Commission. This experience highlights the need for long-term commitment and sustainability planning if countries are to perform ongoing, coordinated signal assessment and state of the art research. Another lesson around safety monitoring of pandemic influenza vaccine in Europe underscored a need to allow for transparent interaction between public (e.g. public health organizations), and private (e.g., vaccine manufacturers) stakeholders. The existing governance at the time did not allow the vaccine manufacturer to participate in the research, thereby compelling the manufacturer to conduct its own research in Canada in order to comply with European regulatory obligations.
To help address this issue, the Innovative Medicines Initiative (IMI) in 2012 called for a public-private partnership on development of a framework for rapid monitoring of vaccine benefits and risk. Consequently, the Accelerated Development of VAccine beNefit-risk Collaboration in Europe (ADVANCE; www.advance-vaccines.eu) consortium was formed in October 2013 for five years. The ADVANCE framework is being developed for wide adoption and implementation by all stakeholders, including ECDC, national public health bodies, the EMA, national regulatory agencies, health ministries, insurance companies, vaccine manufacturers, healthcare providers, and the general public. Bringing together these diverse groups is challenging. But doing so around a neutral platform offered by IMI will allow ADVANCE to develop a blueprint for a sustainable and rapid system to monitor benefits and risks of vaccines in Europe.
In summary, the active surveillance programs to monitor the safety of the 2009 pandemic H1N1 influenza vaccines (requested by ECDC, EMA, academics and national public health institutes) changed the paradigm in Europe in terms of innovation, research scale, size of population monitored, degree of collaboration, standardization of methods, and sophistication of shared research infrastructures. Looking ahead, these programs could leverage the infrastructures and methods for distributed networks and methods that have also been built in Europe to address similar needs to improve drug safety methods and collaborations (96).
6. Monitoring vaccine safety in low- and middle-income countries (LMIC)
The Expanded Programme on Immunization (EPI) launched in 1974 has transformed vaccination coverage in LMIC (101). Through a systematic effort based on tailored strategies and implementation of adaptive vaccine delivery tools, EPI has made it possible to reach children in the most remote parts of the world. This success required addressing complex supply chain issues, while managing procurement, negotiating costs, and establishing effective information systems. As a result, millions of untimely deaths are now prevented every year. At the turn of the 21st century, EPI expanded the range of VPD by introducing new vaccines in LMIC (102). An increasing number of vaccine products are now available, some developed primarily for use in LMIC and several available from manufacturers (103).
6.1 Passive surveillance of AEFI
Not surprisingly, the “maturation” of EPIs in LMICs with better VPD control is also resulting in increased attention on possible AEFI's (14). These concerns require adequate and timely evidence to help NIP managers address those risks, propose sound responses to decision-makers, and maintain communities' trust in immunization services (104).
In 2012, WHO and its partners developed a strategy to help LMIC establish and strengthen their vaccine safety monitoring systems. The Global Vaccine Safety Blueprint (GVSB) (105) proposes three strategic goals: 1) establish minimal vaccine safety capacity in all countries; 2) provide enhanced capacity when vaccines are manufactured in-country, or products are introduced in populations; and 3) provide a technical support network. GVSB implementation is ensured by a network of technical partners that participate in the Global Vaccine Safety Initiative (GVSI) (106), which offers a common platform to partners interested in furthering vaccine safety systems. It align their activities (107) and compiles them in a portfolio (108).
Currently, a majority of LMIC do not have the elements of minimal vaccine safety capacity in place as reflected by the very low number of AEFI reported annually (109). However, several countries have recently made remarkable progress through the application of a five-step capacity-building model of benchmarking, assessment, planning, implementation, and monitoring (110). In addition, the introduction of new vaccines presents opportunities for conducting enhanced AEFI monitoring, as was the case with meningococcal A conjugate vaccine in sub-Saharan Africa (111). The eventual availability of more complex products, such as an adjuvanted malaria (112) vaccine or viral vectors that express dengue (113) or Ebola virus (114) antigens, will require additional scrutiny in the post-clinical stages. Early planning for such scenarios which engages a broad array of stakeholders is underway through the Council for International Organizations of Medical Sciences Vaccine Safety Working Group (115). Full-scale introduction of these vaccines will undoubtedly provide opportunities for furthering the GVSI and help garner the political support required to implement its strategies.
6.2 Active surveillance of AEFI
As noted above, several new vaccines targeting infectious tropical and subtropical diseases (e.g., dengue) are in advanced stages and may be licensed in the near future. These vaccines have the potential to be widely used (116) since most children live in LMIC and immunizations are among the most effective health interventions. Many of these new vaccines may also be introduced, possibly exclusively, in LMIC where capacity for program evaluation and safety monitoring post-licensure has been relatively weak -- without similar data from high resource countries (117). While passive surveillance is important to generate and detect new safety signals, active surveillance systems need to be developed and maintained to better quantify risk associations between an administrated vaccine and a potential AEFI.
Ideally and in a long-term perspective, active surveillance systems for vaccine safety monitoring should be well integrated in national health systems. Initially, the focus might be building on existing infrastructure and capacity so that it can reliably identify AEFIs. Therefore, the identification and evaluation of existing longitudinal demographic, health and vaccination program information and data collection systems (e.g., birth cohorts, morbidity surveillance, and vaccination registries) are crucial. The utility of these systems for vaccine safety assessment purposes can be evaluated by trying to replicate already known positive adverse event associations (e.g., febrile seizures following measles vaccination).
The results of such evaluations can inform the investments needed to adapt existing infrastructures and capacity for an integrated active vaccine safety surveillance system. These investments need to target: 1) demographic surveillance systems (i.e., to generate reliable population denominator, socioeconomic data), 2) morbidity and health outcome registries (i.e., to collect potential AEFI and monitor incidence rates of events of interest in vaccinated and unvaccinated persons), and 3) vaccination registries (i.e., to relate potential adverse events to exposure and to monitor vaccination administration). All three information categories need to be linked with a unique identifier and integrated in an active vaccine safety surveillance system.
On a regional level, the International Network for the Demographic Evaluation of Populations and Their Health (INDEPTH) member centers (118) are examples of infrastructure where an active vaccine safety system could be integrated. The centers have extensive experience with epidemiologic studies. Many INDEPTH centers are already collecting, managing, and analyzing linkable household individual and clinic-based health and demographic surveillance system (HDSS) data. Several centers are also collecting vaccine exposure information and are located in south Asia and Africa where new vaccines are likely to be introduced. The coverage and longevity of INDEPTH would facilitate monitoring and assessment of rare safety concerns requiring large populations with enough follow-up person-time. Alternatively, population-based surveillance monitoring for potential AEFI requiring hospitalization could also be established (119).
The establishment and sustainability of active vaccine safety surveillance systems in LMIC will need substantial investments in linkable demographic, heath outcome, and vaccination administration information systems. Hopefully the key stakeholders (e.g., national public health and regulatory institutions, academia, industry, WHO, and private donors investing in the development and administration of vaccines) will help bring this vision into reality (120).
7. Conclusion
Among various promethean human endeavors, immunizations are remarkable for their aim to alter the ecologic relationship between Homo sapiens and the targeted infectious disease species in human's favor. We are in a unique moment in the history of Homo sapiens as the species attempt to shift from wild VPD-induced immunity (with high price paid in morbidity and mortality) historically to vaccine-induced immunity prospectively. The formidable task of building the infrastructure needed for sustainable maintenance of vaccine-induced immunity for each successive birth cohort globally has just begun. Such an infrastructure should include robust vaccine safety systems needed to timely detect, assess and if needed, minimize the risks of immunizations. Several key elements of the vaccine safety system are in place in some developed countries. Hopefully with adequate resource investment, they can serve as the foundations of an eventual global system as the remaining countries (including many LMIC's) build theirs as biotechnology continues to bring new vaccines from dream to reality.(84, 121, 122)
Acknowledgments
The authors would like to thank the following individuals for their assistance: Vidisha Singh for references, Charles LeBaron for helpful comments, and the many colleagues, past, present, and future, who have enhanced vaccine safety globally.
Abbreviations
- ACIP
Advisory Committee on Immunization Practices
- ADR
Adverse Drug Reaction
- ADVANCE
Accelerated Development of VAccine beNefit-risk Collaboration in Europe, AEFI, Adverse events following immunizations
- aP
Acellular pertussis
- AVAREF
African Vaccine Regulatory Forum
- AVAREF
African Vaccine Regulatory Forum
- CDC
US Centers for Disease Control and Prevention
- CISA
Clinical Immunization Safety Assessment
- DSMB
Data Safety Monitoring Boards
- ECDC
European Center of Disease Control and Prevention
- EMA
European Medicines Agency
- EPI
Expanded Programme on Immunization
- EU
European Union
- FDA
US Food and Drug Administration
- GBS
Guillain-Barre Syndrome
- GVSB
Global Vaccine Safety Blueprint
- GVSI
Global Vaccine Safety Initiative
- HDSS
Health and demographic surveillance system
- IMI
Innovative Medicines Initiative
- INDEPTH
International Network for the Demographic Evaluation of Populations and Their Health
- IOM
Institute of Medicine, LMIC, Low and middle income countries
- MSAEFI
Monitoring System for Adverse Events Following Immunizations
- NCVIA
National Childhood Vaccine Injury Act
- NIP
National immunization programs
- NRA
National regulatory authorities
- PRISM
Postlicensure Rapid Immunization Safety Monitoring Systems
- SIGN
Safe Injection Global Network
- Tdap
Combined Tetanus, diphtheria, and pertussis
- VAERS
Vaccine Adverse Event Reporting System
- VAESCO
Vaccine Adverse Event Surveillance and Communication
- VRBPAC
Vaccines and Related Biological Products Advisory Committee
- VPD
Vaccine Preventable Diseases
- VSD
Vaccine Safety Datalink
- WHO
World Health Organization
- wP
Whole cell pertussis
Footnotes
Even the few VPD that are eradicable, it may be unwise to stop these immunizations in an era of bioterrorism and recreate conditions similar to the devastations wreaked by the introduction of diseases like measles and smallpox into various isolated populations by great explorers beginning in the 16th Century.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the U.S. Food and Drug Administration and the World Health Organization.
Conflicts of interest: MS is leading a research group that occasionally conducts research for pharmaceutical companies including Boeheringer, Pfizer, Novartis, Eli Lilly. These are not related to this paper and grants all arrive at Erasmus University Medical Center. None for the other co-authors.
References
- 1.Moxon ER, Das P, Greenwood B, Heymann DL, Horton R, Levine OS, et al. A call to action for the new decade of vaccines. Lancet. 2011;378(9788):298–302. doi: 10.1016/S0140-6736(11)60766-6. [DOI] [PubMed] [Google Scholar]
- 2.Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K. Measles outbreak - California, December 2014-February 2015. MMWR Morbidity and mortality weekly report. 2015;64(6):153–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–41. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 4.Retraction--Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 2010;375(9713):445. doi: 10.1016/S0140-6736(10)60175-4. [DOI] [PubMed] [Google Scholar]
- 5.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–9. doi: 10.1016/j.vaccine.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 6.Maglione MA, Das L, Raaen L, Smith A, Chari R, Newberry S, et al. Safety of vaccines used for routine immunization of U.S. children: a systematic review. Pediatrics. 2014;134(2):325–37. doi: 10.1542/peds.2014-1079. [DOI] [PubMed] [Google Scholar]
- 7.Lieu TA, Ray GT, Klein NP, Chung C, Kulldorff M. Geographic clusters in underimmunization and vaccine refusal. Pediatrics. 2015;135(2):280–9. doi: 10.1542/peds.2014-2715. [DOI] [PubMed] [Google Scholar]
- 8.Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, et al. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. 1998;351(9099):356–61. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 9.Balinska MA. Hepatitis B vaccination and French Society ten years after the suspension of the vaccination campaign: how should we raise infant immunization coverage rates? Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2009;46(3):202–5. doi: 10.1016/j.jcv.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Jegede AS. What led to the Nigerian boycott of the polio vaccination campaign? PLoS medicine. 2007;4(3):e73. doi: 10.1371/journal.pmed.0040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker CI, Snape MD. Pandemic influenza A H1N1 vaccines and narcolepsy: vaccine safety surveillance in action. The Lancet Infectious diseases. 2014;14(3):227–38. doi: 10.1016/S1473-3099(13)70238-X. [DOI] [PubMed] [Google Scholar]
- 12.Hickler B, Guirguis S, Obregon R. Vaccine Special Issue on Vaccine Hesitancy. Vaccine. 2015;33(34):4155–6. doi: 10.1016/j.vaccine.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Salmon DA, Pavia A, Gellin B. Editors' introduction: Vaccine safety throughout the product life cycle. Pediatrics. 2011;127(Suppl 1):S1–4. doi: 10.1542/peds.2010-1722U. [DOI] [PubMed] [Google Scholar]
- 14.Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, et al. The Vaccine Adverse Event Reporting System (VAERS) Vaccine. 1994;12(6):542–50. doi: 10.1016/0264-410x(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 15.Lane JM, Millar JD. Routine childhood vaccination against smallpox reconsidered. The New England journal of medicine. 1969;281(22):1220–4. doi: 10.1056/NEJM196911272812205. [DOI] [PubMed] [Google Scholar]
- 16.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication Geneva: World Health Organization. 1988:1460. [Google Scholar]
- 17.Prevots DR, Burr RK, Sutter RW, Murphy TV Advisory Committee on Immunization P. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49(RR-5):1–22. quiz CE1-7. [PubMed] [Google Scholar]
- 18.Patel M, Zipursky S, Orenstein W, Garon J, Zaffran M. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines. 2015;14(5):749–62. doi: 10.1586/14760584.2015.1001750. [DOI] [PubMed] [Google Scholar]
- 19.Smith MH. National Childhood Vaccine Injury Compensation Act. Pediatrics. 1988;82(2):264–9. [PubMed] [Google Scholar]
- 20.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. 2013;131(6):e1716–22. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 21.Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 1993;42(RR-16):1–38. [PubMed] [Google Scholar]
- 22.Baxter P. Pertussis vaccine encephalopathy: ‘;Oh! Let us never, never doubt’. Developmental medicine and child neurology. 2010;52(10):883–4. doi: 10.1111/j.1469-8749.2010.03781.x. [DOI] [PubMed] [Google Scholar]
- 23.Lilienfeld DE. The first pharmacoepidemiologic investigations: national drug safety policy in the United States, 1901-1902. Perspectives in biology and medicine. 2008;51(2):188–98. doi: 10.1353/pbm.0.0010. [DOI] [PubMed] [Google Scholar]
- 24.Baylor NW, Marshall VB. Regulation and testing of vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th. Edinburgh: Saunders; 2012. pp. 1427–46. [Google Scholar]
- 25.Nunnally BK, Turula VE, Sitrin RD. Vaccine analysis : strategies, principles, and control. 1st. New York, NY: Springer; 2014. [Google Scholar]
- 26.U.S. Food and Drug Administration (FDA) 21 CFR Part 4 [Docket No. FDA-2009-N-0435]. Current Good Manufacturing Practice Requirements for Combination Products. Federal Register. 2013 Aug 11;2015:4307–23. [Google Scholar]
- 27.Committee for Medicinal Product for Human Use (CHMP) Guideline on quality, non-clinical and clinical aspects of live recombinant viral vectored vaccines London: European Medicines Agency. 2010 [updated 24 June 2010]. EMA/CHMP/VWP/141697/2009:[Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/08/WC500095721.pdf.
- 28.World Health Organization (WHO) World Health Organization technical report series. 993. 2015. WHO Expert Committee on Biological Standardization; pp. 63–87. [Google Scholar]
- 29.Elmgren L, Li X, Wilson C, Ball R, Wang J, Cichutek K, et al. A global regulatory science agenda for vaccines. Vaccine. 2013;31(Suppl 2):B163–75. doi: 10.1016/j.vaccine.2012.10.117. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration (FDA) CBER Strategic Plan for Regulatory Science and Research. 2012 [updated 2012; cited 2015 August 11]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/ScienceResearch/UCM303542.pdf.
- 31.Pagliusi S, Leite LC, Datla M, Makhoana M, Gao Y, Suhardono M, et al. Developing Countries Vaccine Manufacturers Network: doing good by making high-quality vaccines affordable for all. Vaccine. 2013;31(Suppl 2):B176–83. doi: 10.1016/j.vaccine.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 32.Belgharbi L, Dellapiane N, Wood DJ. Regulation of vaccines in developing countries. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. 6th. Edinburgh: Saunders; 2012. pp. 1454–63. [Google Scholar]
- 33.U.S. Food and Drug Administration. Center for Biologics Evaluation and Research. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007 [updated 09/15/2014; cited 2015 August 11]. Available from: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm.
- 34.Bonhoeffer J, Imoukhuede EB, Aldrovandi G, Bachtiar NS, Chan ES, Chang S, et al. Template protocol for clinical trials investigating vaccines--focus on safety elements. Vaccine. 2013;31(47):5602–20. doi: 10.1016/j.vaccine.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen RT, Carbery B, Mac L, Berns KI, Chapman L, Condit RC, et al. The Brighton Collaboration Viral Vector Vaccines Safety Working Group (V3SWG) Vaccine. 2015;33(1):73–5. doi: 10.1016/j.vaccine.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monath TP, Seligman SJ, Robertson JS, Guy B, Hayes EB, Condit RC, et al. Live virus vaccines based on a yellow fever vaccine backbone: standardized template with key considerations for a risk/benefit assessment. Vaccine. 2015;33(1):62–72. doi: 10.1016/j.vaccine.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen RT, Glanz J, Vellozzi C. Pharmacoepidemiology studies of vaccine safety. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th. Chichester, West Sussex, UK: Wiley-Blackwell; 2012. pp. 423–68. [Google Scholar]
- 38.Black S. Perspectives on the design and analysis of prelicensure trials: bridging the gap to postlicensure studies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33(Suppl 4):S323–6. doi: 10.1086/322568. [DOI] [PubMed] [Google Scholar]
- 39.McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32(42):5390–8. doi: 10.1016/j.vaccine.2014.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen M, Ball R, Midthun K, Lieu TA. The Food and Drug Administration's Post-Licensure Rapid Immunization Safety Monitoring program: strengthening the federal vaccine safety enterprise. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):291–7. doi: 10.1002/pds.2323. [DOI] [PubMed] [Google Scholar]
- 41.LaRussa PS, Edwards KM, Dekker CL, Klein NP, Halsey NA, Marchant C, et al. Understanding the role of human variation in vaccine adverse events: the Clinical Immunization Safety Assessment Network. Pediatrics. 2011;127(Suppl 1):S65–73. doi: 10.1542/peds.2010-1722J. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Food and Drug Administration (FDA) Guidance for Industry: Postmarketing Studies and Clinical Trials – Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act. 2011 [updated April 2011; cited 2015 August 11]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM172001.pdf.
- 43.Smith JC. The structure, role, and procedures of the U.S. Advisory Committee on Immunization Practices (ACIP) Vaccine. 2010;28(Suppl 1):A68–75. doi: 10.1016/j.vaccine.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Food and Drug Administration (FDA) Charter of the Vaccines and Related Biological Products Advisory Committee. 2014 [updated January 9 2014; cited 2015 August 11]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm129571.htm.
- 45.U.S. Food and Drug Administration (FDA) Charter of the Pediatric Advisory Committee to the Food and Drug Administration. 2014 [updated September 18, 2014; cited 2015 August 11]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/ucm116525.htm.
- 46.Savel TG, Foldy S Centers for Disease Control and Prevention (CDC) The role of public health informatics in enhancing public health surveillance. MMWR Surveill Summ. 2012;61(Suppl):20–4. [PubMed] [Google Scholar]
- 47.Shimabukuro T. The Vaccine Adverse Event Reporting System (VAERS) form Version 2.0 (proposed) National Vaccine Advisory Committee meeting September 9, 2014: National Vaccine Program Office/HHS. [cited 2015 August 11]. Available from: http://www.hhs.gov/nvpo/nvac/meetings/upcomingmeetings/NVAC%20Meeting%20Materials/tab_7.3_shimabukuro_vaers_2_0_enote.pdf.
- 48.U.S. Centers for Disease Control and Prevention (CDC) Request for Comment on Draft Vaccine Adverse Event Reporting System (VAERS) 2.0 Form. Federal Register. 2014;79(226):69853–4. Docket No. CDC–2014–0015. [Google Scholar]
- 49.U.S. Food and Drug Administration (FDA) 21 CFR Parts 310, 314, 329, and 600. [Docket No. FDA–2008–N–0334]. RIN 9010–AF96. Postmarketing Safety Reports for Human Drug and Biological Products; Electronic Submission Requirements. Federal Register. 2014;79(111):33072–92. [PubMed] [Google Scholar]
- 50.U.S. Food and Drug Administration (FDA) [Docket No. FDA–2001–D–0067 (Formerly Docket No. 2001D–0185)]. Draft Guidance for Industry on Providing Submissions in Electronic Format—Postmarketing Safety Reports. Federal Register. 2014;79(111):33200–1. [PubMed] [Google Scholar]
- 51.Hinrichsen VL, Kruskal B, O'Brien MA, Lieu TA, Platt R. Using electronic medical records to enhance detection and reporting of vaccine adverse events. J Am Med Inform Assoc. 2007;14(6):731–5. doi: 10.1197/jamia.M2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker MKD, Mazza M, et al. Adult and Pediatric Vaccines at IDWeek 2013. Vol. 2013 San Francisco, CA: Oct 3, 2013. Automated Detection and Reporting of Vaccine Adverse Events: ESP-VAERS. [Google Scholar]
- 53.Vaccine Adverse Event Reporting System (VAERS) Application for Mobile Devices: Small Business Innovation Research/Small Business Technology Transfer. 2013 [cited 2015 August 11]. Available from: http://www.sbir.gov/sbirsearch/detail/679697.
- 54.Stockwell MS, Broder K, LaRussa P, Lewis P, Fernandez N, Sharma D, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr. 2014;168(3):211–9. doi: 10.1001/jamapediatrics.2013.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat. 1999;53:177–90. [Google Scholar]
- 56.Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf. 2002;25(6):381–92. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 57.Martin D, Menschik D, Bryant-Genevier M, Ball R. Data mining for prospective early detection of safety signals in the Vaccine Adverse Event Reporting System (VAERS): a case study of febrile seizures after a 2010-2011 seasonal influenza virus vaccine. Drug Saf. 2013;36(7):547–56. doi: 10.1007/s40264-013-0051-9. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Hripcsak G, Markatou M, Friedman C. Active computerized pharmacovigilance using natural language processing, statistics, and electronic health records: a feasibility study. J Am Med Inform Assoc. 2009;16(3):328–37. doi: 10.1197/jamia.M3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Botsis T, Buttolph T, Nguyen MD, Winiecki S, Woo EJ, Ball R. Vaccine adverse event text mining system for extracting features from vaccine safety reports. J Am Med Inform Assoc. 2012;19(6):1011–8. doi: 10.1136/amiajnl-2012-000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(Suppl 1):S45–53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- 61.Lieu TA, Kulldorff M, Davis RL, Lewis EM, Weintraub E, Yih K, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Medical care. 2007;45(10 Supl 2):S89–95. doi: 10.1097/MLR.0b013e3180616c0a. [DOI] [PubMed] [Google Scholar]
- 62.McClure DL, Xu S, Weintraub E, Glanz JM. An efficient statistical algorithm for a temporal scan statistic applied to vaccine safety analyses. Vaccine. 2012;30(27):3986–91. doi: 10.1016/j.vaccine.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 63.Greene SK, Kulldorff M, Lewis EM, Li R, Yin R, Weintraub ES, et al. Near real-time surveillance for influenza vaccine safety: proof-of-concept in the Vaccine Safety Datalink Project. American journal of epidemiology. 2010;171(2):177–88. doi: 10.1093/aje/kwp345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM Group VSDRCAIW. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine. 2012;30(11):2024–31. doi: 10.1016/j.vaccine.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 65.Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29(46):8279–84. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 66.Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH, et al. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. American journal of preventive medicine. 2011;41(2):121–8. doi: 10.1016/j.amepre.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Yih WKNM, Maro J, Baker M, Balsbaugh C, Brown J, Cole D, Dashevsky I, Kulldorff M. Mini-Sentinel Cber/Prism Methods Protocol: Pilot Of Self-Controlled Tree-Temporal Scan Analysis For Gardasil Vaccine, Version 2.0. 2015 [Google Scholar]
- 68.HCUP. Clinical Classifications Software: Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2015. [Google Scholar]
- 69.Kulldorff M. TreeScan: Software for the Tree-Based Scan Statistic. 2014 Available from: www.treescan.org.
- 70.Kulldorff M, Dashevsky I, Avery TR, Chan AK, Davis RL, Graham D, et al. Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf. 2013;22(5):517–23. doi: 10.1002/pds.3423. [DOI] [PubMed] [Google Scholar]
- 71.Swamy GK, Garcia-Putnam R. Maternal immunization to benefit the mother, fetus, and infant. Obstet Gynecol Clin North Am. 2014;41(4):521–34. doi: 10.1016/j.ogc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morbidity and mortality weekly report. 2015;64(30):818–25. doi: 10.15585/mmwr.mm6430a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.U.S. Centers for Disease Control Prevention (CDC) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morbidity and mortality weekly report. 2013;62(7):131–5. [PMC free article] [PubMed] [Google Scholar]
- 74.Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, Nohynek H, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32(52):7057–64. doi: 10.1016/j.vaccine.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 75.Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal Influenza Immunization and Birth Outcomes of Stillbirth and Spontaneous Abortion: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 doi: 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- 76.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897–904. doi: 10.1001/jama.2014.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rasmussen SA, Watson AK, Kennedy ED, Broder KR, Jamieson DJ. Vaccines and pregnancy: past, present, and future. Semin Fetal Neonatal Med. 2014;19(3):161–9. doi: 10.1016/j.siny.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Faucette AN, Unger BL, Gonik B, Chen K. Maternal vaccination: moving the science forward. Hum Reprod Update. 2015;21(1):119–35. doi: 10.1093/humupd/dmu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.U.S. Food and Drug Administration (FDA) Pregnancy and Lactation Labeling Final Rule. 2014 [updated 12/04/14; cited 2015 August 11] Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm.
- 80.U.S. Food and Drug Administration (FDA) Draft Guidance for Industry: Pregnancy, Lactation, and Reproductive Potential: Labeling for Human Prescription Drug and Biological Products. 2014 [updated Dec 2014; cited 2015 August 11]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM425398.pdf.
- 81.Institute of Medicine (IOM) The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. Washington (DC): National Academies Press (US); 2013. [PubMed] [Google Scholar]
- 82.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M (CDC) CfDCaP. National, state, and selected local area vaccination coverage among children aged 19-35 months - United States, 2013. MMWR Morbidity and mortality weekly report. 2014;63(34):741–8. [PMC free article] [PubMed] [Google Scholar]
- 83.Glanz JM, Newcomer SR, Narwaney KJ, Hambidge SJ, Daley MF, Wagner NM, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167(3):274–81. doi: 10.1001/jamapediatrics.2013.502. [DOI] [PubMed] [Google Scholar]
- 84.Nabel GJ. Designing tomorrow's vaccines. The New England journal of medicine. 2013;368(6):551–60. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines. 2011;10(4):539–50. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 86.Saroja C, Lakshmi P, Bhaskaran S. Recent trends in vaccine delivery systems: A review. Int J Pharm Investig. 2011;1(2):64–74. doi: 10.4103/2230-973X.82384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–93. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czerkinsky C, Cuburu N, Kweon MN, Anjuere F, Holmgren J. Sublingual vaccination. Hum Vaccin. 2011;7(1):110–4. doi: 10.4161/hv.7.1.13739. [DOI] [PubMed] [Google Scholar]
- 89.Wanjek C. Systems Biology as Defined by NIH: An Intellectual Resource for Integrative Biology. The NIH Catalyst. 2011;19(6):1–12. [Google Scholar]
- 90.U.S. National Institutes of Health (NIH) National Human Genome Research Institute; Talking Glossary of Genetic Terms: Personalized Medicine. Available from: http://www.genome.gov/glossary/index.cfm?id=150. [Google Scholar]
- 91.Kennedy RB, Poland GA. The top five “game changers” in vaccinology: toward rational and directed vaccine development. OMICS. 2011;15(9):533–7. doi: 10.1089/omi.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.U.S. Food and Drug Administration (FDA) Paving the Way for Personalized Medicine: FDA's Role in a New Era of Medical Product Development. 2013 [updated October 2013; cited 2015 August 11]. Available from: http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PersonalizedMedicine/UCM372421.pdf.
- 93.Oberg AL, McKinney BA, Schaid DJ, Pankratz VS, Kennedy RB, Poland GA. Lessons learned in the analysis of high-dimensional data in vaccinomics. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Remarks by the President in State of the Union Address. 2015 Jan 20; [cited 2015 August 11]. Available from: http://www.whitehouse.gov/the-press-office/2015/01/20/remarks-president-state-union-address-january-20-2015.
- 95.Destefano F, Vellozzi C. Facilitators Report, Lessons learned exercise, ECDC Vaccine Adverse Event Surveillance and Communication: (VAESCO II) project, Stockholm: European Centers for Disease Control and Prevention. 2009 [cited 2015 August 11]. Available from: http://vaesco.net/vaesco/news/extras/01/text_files/file/VAESCO%20facilitator%20report%20lessons%20learned%20fxd%20CVupdated_August312012%20.pdf.
- 96.Trifiro G, Coloma PM, Rijnbeek PR, Romio S, Mosseveld B, Weibel D, et al. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: why and how? Journal of internal medicine. 2014;275(6):551–61. doi: 10.1111/joim.12159. [DOI] [PubMed] [Google Scholar]
- 97.Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M, et al. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. Bmj. 2011;343:d3908. doi: 10.1136/bmj.d3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.European Centre for Disease Prevention and Control (ECDC) Narcolepsy in association with pandemic influenza vaccination – a multi-country European epidemiological investigation. Stockholm: ECDC; [updated September 2012; cited 2015 August 11]. Available from: http://vaesco.net/vaesco/results/main/04/text_files/file/ECDC%202012%20VAESCO%20Narco%20report%20FULL.pdf. [Google Scholar]
- 99.Wijnans L, Lecomte C, de Vries C, Weibel D, Sammon C, Hviid A, et al. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2013;31(8):1246–54. doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 100.Romio S, Weibel D, Dieleman JP, Olberg HK, de Vries CS, Sammon C, et al. Guillain-Barre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe. PloS one. 2014;9(1):e82222. doi: 10.1371/journal.pone.0082222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitchell V, Dietz VJ, Okwo-Bele JM, Cutts FT. Immunization in Developing Countries Vaccines. 6th. Philadelphia: Elsevier Saunders; 2013. pp. 1369–94. [Google Scholar]
- 102.Mahoney RT, Maynard JE. The introduction of new vaccines into developing countries. Vaccine. 1999;17(7-8):646–52. doi: 10.1016/s0264-410x(98)00246-1. [DOI] [PubMed] [Google Scholar]
- 103.Kaddar M, Milstien J, Schmitt S. Impact of BRICS' investment in vaccine development on the global vaccine market. Bulletin of the World Health Organization. 2014;92(6):436–46. doi: 10.2471/BLT.13.133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Black S, Zuber PL. Global trends and challenges in vaccine safety. Pediatric Health. 2009;3:329–35. [Google Scholar]
- 105.World Health Organization (WHO) Global Vaccine Safety Blueprint. Geneva: Department of Immunization, Vaccines and Biologicals; 2012. [Google Scholar]
- 106.World Health Organization (WHO) The Global Vaccine Safety Initiative (GVSI): WHO. [updated November 2014; cited 2015 August 11]. Available from: http://www.who.int/vaccine_safety/initiative/en/
- 107.Maure CG, Dodoo AN, Bonhoeffer J, Zuber PL. The Global Vaccine Safety Initiative: enhancing vaccine pharmacovigilance capacity at country level. Bulletin of the World Health Organization. 2014;92(9):695–6. doi: 10.2471/BLT.14.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.World Health Organization (WHO) Switzerland: WHO; 2013. Global Vaccine Safety Initiative portfolio of activities. [updated June 5, 2015; cited 2015 August 11]. Available from: http://www.who.int/vaccine_safety/news/highlight_3/en/ [Google Scholar]
- 109.World Health Organization (WHO) Geneva: Department of Immunization, Vaccines and Biologicals; 2012. Global Vaccine Safety Blueprint: The Landscape Analysis. [cited WHO/IVB/12.04 August 11, 2015]. 94]. Available from: http://extranet.who.int/iris/restricted/bitstream/10665/70919/1/WHO_IVB_12.07_eng.pdf?ua=1. [Google Scholar]
- 110.World Health Organization (WHO) Strengthening national regulatory authorities. 2012 [updated February 6, 2012; cited 2015 August 11]. Available from: http://www.who.int/immunization_standards/national_regulatory_authorities/strengthening/en/
- 111.Diomande FV, Vannice KS, Ouandaogo CR, Keita M, Djingarey MH, Mbakuliemo N, et al. Lessons learnt from enhancing vaccine pharmacovigilance activities during MenAfriVac™ introduction in African countries, 2010 to 2013. CID. 2015 doi: 10.1093/cid/civ599. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.World Health Organization (WHO) Global Advisory Committee on Vaccine Safety: Preparing for malaria vaccine introduction. Wkly Epid Rec. 2015;90:18–20. [Google Scholar]
- 113.World Health Organization (WHO) Global Advisory Committee on Vaccine Safety: Preparing for dengue vaccine introduction. Wkly Epid Rec. 2015;90:17–8. [Google Scholar]
- 114.The Lancet. An Ebola vaccine: first results and promising opportunities. Lancet. 2015 doi: 10.1016/S0140-6736(15)61177-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 115.Council for International Organizations of Medical Sciences (CIOMS) 2013 Available from: http://www.cioms.ch/
- 116.Alonso PL, de Quadros CA, Robert M, Lal AA. Decade of Vaccines. Editorial. Vaccine. 2013;31(Suppl 2):B3–4. doi: 10.1016/j.vaccine.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 117.Izurieta HS, Zuber P, Bonhoeffer J, Chen RT, Sankohg O, Laserson KF, et al. Roadmap for the international collaborative epidemiologic monitoring of safety and effectiveness of new high priority vaccines. Vaccine. 2013;31(35):3623–7. doi: 10.1016/j.vaccine.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 118.Indepth Network: Better Health Information for Better Health Policy. Available from: http://www.indepth-network.org/
- 119.Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barre Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–58. doi: 10.1016/j.vaccine.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 120.Black S, Mulholland K, Halsey NA. Developing sustainable funding for vaccine safety infrastructure. Vaccine. 2010;28(5):1133–4. doi: 10.1016/j.vaccine.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 121.U.S. National Institutes of Health (NIH) 2012 NIH publication no 11-7778. NIH, NIAID; 2012. The Jordan report: accelerated development of vaccines. [Google Scholar]
- 122.Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12288–93. doi: 10.1073/pnas.1402981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Human vaccines & immunotherapeutics. 2013;9(8):1755–62. doi: 10.4161/hv.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Human vaccines & immunotherapeutics. 2013;9(8):1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization. 2008;86(2):140–6. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ellenberg SS, Foulkes MA, Midthun K, Goldenthal KL. Evaluating the safety of new vaccines: summary of a workshop. American journal of public health. 2005;95(5):800–7. doi: 10.2105/AJPH.2004.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fine PE, Clarkson JA. Individual versus public priorities in the determination of optimal vaccination policies. American journal of epidemiology. 1986;124(6):1012–20. doi: 10.1093/oxfordjournals.aje.a114471. [DOI] [PubMed] [Google Scholar]
- 128.Francois G, Duclos P, Margolis H, Lavanchy D, Siegrist CA, Meheus A, et al. Vaccine safety controversies and the future of vaccination programs. Pediatr Infect Dis J. 2005;24(11):953–61. doi: 10.1097/01.inf.0000183853.16113.a6. [DOI] [PubMed] [Google Scholar]
- 129.Seeff LB, Beebe GW, Hoofnagle JH, Norman JE, Buskell-Bales Z, Waggoner JG, et al. A serologic follow-up of the 1942 epidemic of post-vaccination hepatitis in the United States Army. The New England journal of medicine. 1987;316(16):965–70. doi: 10.1056/NEJM198704163161601. [DOI] [PubMed] [Google Scholar]
- 130.Nathanson N, Langmuir AD. The Cutter Incident. Poliomyelitis Following Formaldehyde- Inactivated Poliovirus Vaccination in the United States during the Spring of 1955. I. Background. American journal of hygiene. 1963;78:16–28. doi: 10.1093/oxfordjournals.aje.a120327. [DOI] [PubMed] [Google Scholar]
- 131.Nathanson N, Langmuir AD. The Cutter Incident. Poliomyelitis Following Formaldehyde- Inactivated Poliovirus Vaccination in the United States during the Spring of 1955. Ii. Relationship of Poliomyelitis to Cutter Vaccine. American journal of hygiene. 1963;78:29–60. doi: 10.1093/oxfordjournals.aje.a120328. [DOI] [PubMed] [Google Scholar]
- 132.Offit PA. The Cutter incident, 50 years later. The New England journal of medicine. 2005;352(14):1411–2. doi: 10.1056/NEJMp048180. [DOI] [PubMed] [Google Scholar]
- 133.Wilson GS. The hazards of immunization. x. London: Athlone P; 1967. p. 324. [Google Scholar]
- 134.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. American journal of epidemiology. 1979;110(2):105–23. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 135.Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)--United States, 1991-2001. MMWR Surveill Summ. 2003;52(1):1–24. [PubMed] [Google Scholar]
- 136.Stetler HC, Mullen JR, Brennan JP, Livengood JR, Orenstein WA, Hinman AR. Monitoring system for adverse events following immunization. Vaccine. 1987;5(3):169–74. doi: 10.1016/0264-410x(87)90094-6. [DOI] [PubMed] [Google Scholar]
- 137.Faich GA. Adverse-drug-reaction monitoring. The New England journal of medicine. 1986;314(24):1589–92. doi: 10.1056/NEJM198606123142427. [DOI] [PubMed] [Google Scholar]
- 138.Niu MT, Erwin DE, Braun MM. Data mining in the US Vaccine Adverse Event Reporting System (VAERS): early detection of intussusception and other events after rotavirus vaccination. Vaccine. 2001;19(32):4627–34. doi: 10.1016/s0264-410x(01)00237-7. [DOI] [PubMed] [Google Scholar]
- 139.Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, et al. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI) Vaccine. 2002;21(3-4):298–302. doi: 10.1016/s0264-410x(02)00449-8. [DOI] [PubMed] [Google Scholar]
- 140.Chen RT, Glasser JW, Rhodes PH, Davis RL, Barlow WE, Thompson RS, et al. Vaccine Safety Datalink project: a new tool for improving vaccine safety monitoring in the United States. The Vaccine Safety Datalink Team. Pediatrics. 1997;99(6):765–73. doi: 10.1542/peds.99.6.765. [DOI] [PubMed] [Google Scholar]
- 141.Ball LK, Evans G, Bostrom A. Risky business: challenges in vaccine risk communication. Pediatrics. 1998;101(3 Pt 1):453–8. doi: 10.1542/peds.101.3.453. [DOI] [PubMed] [Google Scholar]
- 142.Gust DA, Gangarosa P, Hibbs B, Pollard R, Wallach G, Chen RT. National Immunization Information Hotline: Calls concerning adverse events, 1998-2000. Journal of health communication. 2004;9(5):387–94. doi: 10.1080/10810730490503487. [DOI] [PubMed] [Google Scholar]
- 143.Howson CP, Howe CJ, Fineberg HV, editors. Adverse Effects of Pertussis and Rubella Vaccines: A Report of the Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines. Washington (DC): National Academy Press; 1991. [PubMed] [Google Scholar]
- 144.Stratton KR, Howe CJ, Johnston RB, editors. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Washington (DC): National Academy Press; 1994. [PubMed] [Google Scholar]
- 145.Institute of Medicine (IOM) Immunization Safety Review 2001-2004: National Academy Press (US) [updated 7/24/2013; cited 2015 August 11]. Available from: http://www.iom.edu/activities/publichealth/immunizationsafety.aspx.
- 146.Stratton K, Ford A, Rusch E, Clayton EW, editors. Adverse Effects of Vaccines: Evidence and Causality. Washington (DC): National Academy Press; 2011. [PubMed] [Google Scholar]
- 147.Farrington P, Pugh S, Colville A, Flower A, Nash J, Morgan-Capner P, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet. 1995;345(8949):567–9. doi: 10.1016/s0140-6736(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 148.Hviid A. Postlicensure epidemiology of childhood vaccination: the Danish experience. Expert Rev Vaccines. 2006;5(5):641–9. doi: 10.1586/14760584.5.5.641. [DOI] [PubMed] [Google Scholar]
- 149.Pasternak B, Feenstra B, Melbye M, Hviid A. Improving Vaccine Safety Through a Better Understanding of Vaccine Adverse Events. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dourado I, Cunha S, Teixeira MG, Farrington CP, Melo A, Lucena R, et al. Outbreak of aseptic meningitis associated with mass vaccination with a urabe-containing measles-mumps-rubella vaccine: implications for immunization programs. American journal of epidemiology. 2000;151(5):524–30. doi: 10.1093/oxfordjournals.aje.a010239. [DOI] [PubMed] [Google Scholar]
- 151.Freitas DR, Moura E, Araujo G, Cardoso A, Scheidt P, Ferraz E, et al. Investigation of an outbreak of hypersensitivity-type reactions during the 2004 national measles-mumps-rubella vaccination campaign in Brazil. Vaccine. 2013;31(6):950–4. doi: 10.1016/j.vaccine.2012.11.095. [DOI] [PubMed] [Google Scholar]
- 152.Ouandaogo CR, Yameogo TM, Diomande FV, Sawadogo C, Ouedraogo B, Ouedraogo-Traore R, et al. Adverse events following immunization during mass vaccination campaigns at first introduction of a meningococcal A conjugate vaccine in Burkina Faso, 2010. Vaccine. 2012;30(Suppl 2):B46–51. doi: 10.1016/j.vaccine.2011.12.112. [DOI] [PubMed] [Google Scholar]
- 153.Hutin YJ, Chen RT. Injection safety: a global challenge. Bulletin of the World Health Organization. 1999;77(10):787–8. [PMC free article] [PubMed] [Google Scholar]
- 154.Ali M, Canh GD, Clemens JD, Park JK, von Seidlein L, Minh TT, et al. The use of a computerized database to monitor vaccine safety in Viet Nam. Bulletin of the World Health Organization. 2005;83(8):604–10. [PMC free article] [PubMed] [Google Scholar]
- 155.Pool V, Braun MM, Kelso JM, Mootrey G, Chen RT, Yunginger JW, et al. Prevalence of anti-gelatin IgE antibodies in people with anaphylaxis after measles-mumps rubella vaccine in the United States. Pediatrics. 2002;110(6):e71. doi: 10.1542/peds.110.6.e71. [DOI] [PubMed] [Google Scholar]
- 156.Kramarz P, DeStefano F, Gargiullo PM, Davis RL, Chen RT, Mullooly JP, et al. Does influenza vaccination exacerbate asthma? Analysis of a large cohort of children with asthma. Vaccine Safety Datalink Team. Archives of family medicine. 2000;9(7):617–23. doi: 10.1001/archfami.9.7.617. [DOI] [PubMed] [Google Scholar]
- 157.Ball R, Shadomy SV, Meyer A, Huber BT, Leffell MS, Zachary A, et al. HLA type and immune response to Borrelia burgdorferi outer surface protein a in people in whom arthritis developed after Lyme disease vaccination. Arthritis and rheumatism. 2009;60(4):1179–86. doi: 10.1002/art.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.White OJ, McKenna KL, Bosco A, HJvdB A, Richmond P, Holt PG. A genomics-based approach to assessment of vaccine safety and immunogenicity in children. Vaccine. 2012;30(10):1865–74. doi: 10.1016/j.vaccine.2011.12.118. [DOI] [PubMed] [Google Scholar]
- 159.Kaslow RA, Sullivan-Bolyai JZ, Hafkin B, Schonberger LB, Kraus L, Moore MJ, et al. HLA antigens in Guillain-Barre syndrome. Neurology. 1984;34(2):240–2. doi: 10.1212/wnl.34.2.240. [DOI] [PubMed] [Google Scholar]


