Abstract
Mucins are large glycoproteins expressed on the epithelia that provide a protective barrier against harsh insults from toxins and pathogenic microbes. These glycoproteins are classified primarily as being secreted and membrane-bound; both forms are involved in pathophysiological functions including inflammation and cancer. The high molecular weight of mucins is attributed to their large polypeptide backbone that is extensively covered by glycan moieties that modulate the function of mucins and, hence, play an important role in physiological functions. Deregulation of glycosylation machinery during malignant transformation results in altered mucin glycosylation. This review describes the functional implications and pathobiological significance of altered mucin glycosylation in cancer. Further, this review delineates various factors such as glycosyltransferases and tumor microenvironment that contribute to dysregulation of mucin glycosylation during cancer. Finally, this review discusses the scope of mucin glycan epitopes as potential diagnostic and therapeutic targets.
Keywords: Mucin, Glycan, Cancer, O-glycosylation, Carbohydrate antigen, Glycosyltransferase
Graphical Abstract
1. Introduction
Mucins are a family of glycosylated proteins with high molecular weight and complex molecular organization. So far, 21 mucin genes have been identified. Based on their structural organization, mucins are mainly classified as secreted and membrane-bound forms [1, 2]. Both of these mucin classes are involved in epithelial cell homeostasis where they act as a protective shield against severe environmental insults from toxins and pathogenic microbes [3]. MUC2, MUC5AC, MUC5B, MUC6, MUC7, and MUC19 are secreted mucins, which lack transmembrane domains and have a common von Willebrand factor D domain (vWF-D) and C terminus cysteine knot domain that are required for their oligomerization [4]. The other important class of mucins is the transmembrane mucins, which include MUC1, MUC3, MUC4, MUC12, MUC16, and MUC17. These transmembrane mucins are anchored to the cell surface via their transmembrane domains and are involved in various signaling pathways through their short cytoplasmic tails [5].
The structural complexity of these secreted and transmembrane glycoproteins is due to their large polypeptide chain and various post-translational modifications such as glycosylation, sulfation, and phosphorylation [6–8]. Glycosylation is one of the major post-translational modifications that defines these mucins and affects their function. Mucins can be either O-glycosylated or N-glycosylated. Both forms of glycosylation occur in distinct subcellular compartments. They differ by the amino acid involved and the covalent attachment of carbohydrates [9]. N-glycosylation of mucins is initiated in the endoplasmic reticulum (ER) by the action of UDP-GlcNAc phosphotransferases, whereas mucin type O-glycosylation occurs in the Golgi apparatus and is mediated by a family of enzymes known as UDP-GalNAc: polypeptide GalNAc-transferases (GalNAc-Ts/GALNTs) [10, 11]. Notably, O-glycosylation is the major post-translational modification of mucins. It occurs predominantly in the central tandem repeat domain, which is common to all mucins and rich in proline, serine, and threonine (PTS) residues [12].
Carbohydrate structures located on mucin polypeptides dictate their biochemical and biophysical characteristics. These structures also determine the biological functions of mucins. For instance, mucin glycans act as a steric barrier that prevents microorganisms from attacking the underlying epithelium surface [6, 13]. Indeed, some of the glycan structures are also involved in mediating cellular interactions like those with leukocytes [14]. Importantly, this review discusses the pathobiological significance of altered mucin glycosylation in cancer and focuses on the functional implications of oligosaccharide structures displayed by mucins during cancer progression. It also discusses regulation of mucin glycosylation by several factors such as glycosyltransferases and the tumor microenvironment. Finally, the potential involvement of these mucin-associated glycans in diagnostics and therapeutics is discussed.
2. Mucin glycosylation
Glycosylation is the principal post-translational modification and hallmark of mucins. Mucins undergo both O- and N-linked glycosylation, depending on the amino acid on which glycans are added. In the case of N-glycosylation, carbohydrate chains are attached to the amidic nitrogen of asparagine, whereas O-glycosylation is defined by the addition of carbohydrate chains to the hydroxyl group of serine or threonine [15, 16]. Both forms of glycosylation occur in distinct cellular regions and contribute to the biochemical, biophysical, and functional properties of mucins [9].
2.1. N-linked glycosylation
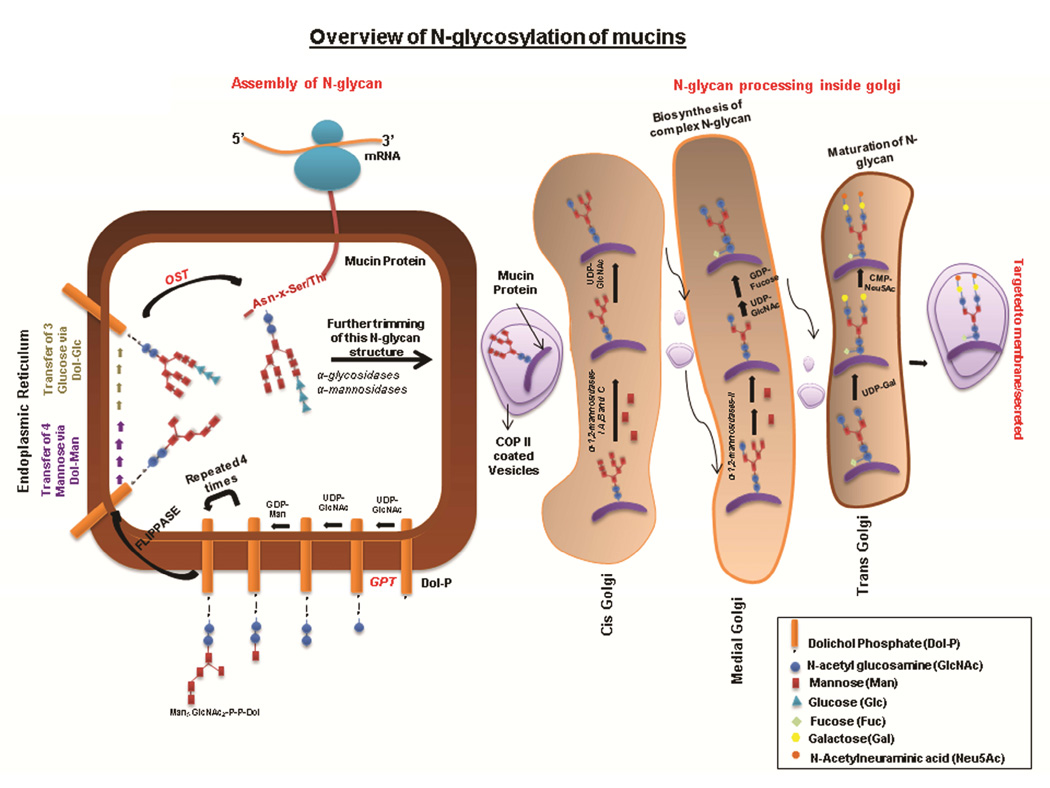
Protein N-glycosylation is initiated by the transfer of N-acetyl glucosamine phosphate (GlcNAc-P) to the polyisoprenol lipid precursor, dolichol phosphate (Dol-P), which results in the formation of dolichol pyrophosphate N-acetyl glucosamine (Dol-P-P-GlcNAc) (Figure 1). This occurs on the cytoplasmic face of the ER and is mediated by the action of UDP-N-acetylglucosamine-dolichyl-phosphate N-acetylglucosaminephosphotransferase (DAPGT1/GPT). Following this event, another molecule of N-acetyl glucosamine (GlcNAc) and five mannose (Man) residues are transferred sequentially by specific glycosyltransferases to generate Man5GlcNAc2-P-P-Dol. Subsequently, the dolichol-linked glycan precursor translocates to the ER lumen by the action of the enzyme flippase. Further, the transfer of four mannose and three glucose (Glc) glycans from Dol-P-Man and Dol-P-Glc, respectively, completes the synthesis of the N-glycan precursor Glc3Man9GlcNAc2-P-P-Dol. This structure is then transferred en bloc by oligosaccharyl transferase (OST) to the asparagine residue in one of the prominent N-glycosylation sites of the nascent protein (Asp-X-Ser/Thr) [15] A series of enzymatic processing reactions inside the ER trim this 14 sugar N-glycan structure, following which the nascent glycoprotein is transported to the Golgi apparatus by COPII-coated vesicles [17, 18]. In cis Golgi, further processing involves the removal of mannose residues by means of mannosidases. The processed protein is then transferred to the medial Golgi, where oligomannose (containing high mannose), complex-type (comprising several saccharides including N-acetylglucosamine) and hybrid N-glycans (mixture of oligomannose and complex N-glycans) are synthesized. In Trans Golgi, complex and hybrid N-glycan structures are further elongated by the addition of several repeats of N-acetyllactosamine (LacNAc-3Galβ1-4GlcNAcβ1-). Finally, these elongated structures are capped by the addition of residues like sialic acid, N-acetylglucosamine, and fucose [11].
Figure 1. N glycosylation of mucins.
N-glycosylation is initiated in ER by the transfer of GlcNAc from UDP-GlcNAc to the lipid molecule known as Dol-P. Further, one molecule of UDP-GlcNAc and four molecules of Man are transferred sequentially on GlcNAc-P-P-Dolichol resulting in the formation of Man5GlcNAc2-P-P-Dol. This is followed by the translocation of this oligosaccharide-linked Dolichol to the lumen of ER. Next, the successive addition of four molecules of mannose and three molecules of glucose forms Glc3Man9GlcNAc2P-P-Dol, which is transferred en bloc by OST to an Asn residue of the mucin molecule. N-glycans linked to the mucin molecule are further trimmed inside ER and processed in the Golgi apparatus (complex N-glycan synthesis, elongation and capping), which gives rise to mature N-glycans linked to the mucin molecule.
The number of N-glycosylation sites varies among mucin family members. For example, MUC1 has five potential N-glycosylation sites, whereas MUC4 has more than 10 putative N-glycosylation sites [19, 20]. N-glycosylation sites in different mucins contribute to protein stability, folding, and trafficking. For example, in the secreted mucin MUC2, Asker et al. showed the N-glycosylation to be critical for its proper folding inside the ER, which ensures its correct dimerization [21]. They demonstrated this using pulse chase studies on MUC2 in tunicamycin-treated LS14T colon carcinoma cells; tunicamycin is an N-glycosylation inhibitor. In the absence of N-glycans, the formation of disulfide-linked dimers via the cysteine knot domain of MUC2 was reduced due to incorrect folding of MUC2 apomucin. Further, non-glycosylated MUC2 was not transported to the Golgi apparatus and was subsequently degraded in the ER [21]. Importantly, transport of MUC2 to the Golgi apparatus allows for its O-glycosylation and multimerization, which is followed by its packaging into secretory vesicles [22, 23]. Thus, N-glycans allow for correct dimerization of MUC2 in the ER, which is followed by assembly and secretion of MUC2. In addition to its role in protein folding and dimerization, N-glycosylation has been been shown to play an important role in the surface localization of the membrane mucin MUC17 [24]. Taken together, the above studies highlight the critical role of N-glycans in regulating the biological functions of mucins.
2.2. O-linked glycosylation
Although more complex and less understood than N-linked glycosylation, O-glycosylation is the major post-translational modification that occurs in mucins (Figure 2). O-glycan chains attached to mucin polypeptide (apomucin) comprise various monosaccharides such as N-acetylgalactosamine, galactose, N-acetylglucosamine, sialic acid, and fucose. Arrangement of these sugars on mucins adds to the structural diversity of oligosaccharides (as outlined in Figure 2) that dictate the biophysical and functional characteristics of mucins. O-glycosylation of mucins is a multistep process that begins after the glycoproteins are exported to the Golgi apparatus by vesicle-mediated transport [9, 25].
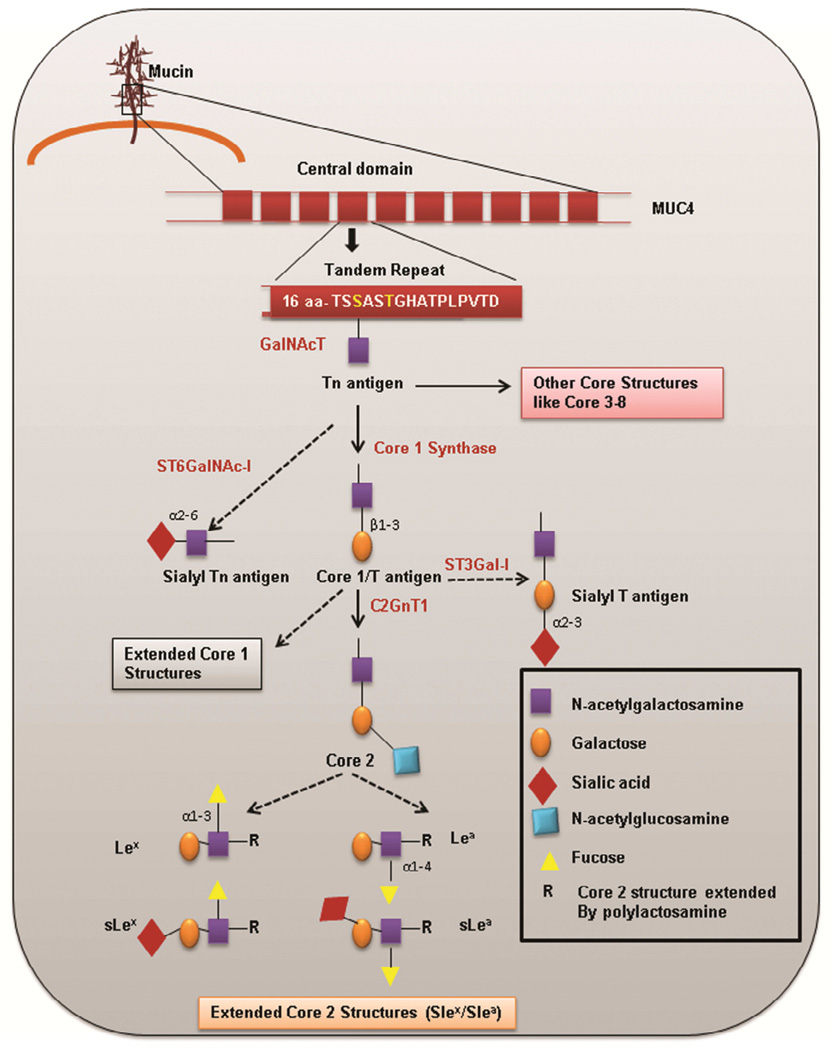
Figure 2. Overview of mucin type O glycosylation.
O-glycosylation is initiated by the activity of ppGalNAcTs, which attaches an N-acetylgalactosamine to Ser/Thr (highlighted in yellow) amino acid residues in the tandem repeat region, resulting in the formation of a Tn antigen. This Tn antigen formed can be extended by activity of C1GALT1 to form the T/Core 1 antigen. Alternatively, Tn can be modified by sialyltransferase to form sialyl Tn (sTn). The Core 1 antigen can be further elongated to form the Core 2 structure. Further, Core 2 structure is extended to several structures, including Lex, sLex, Lea, and sLea. Tn can also be extended by activity of other glycosyltransferases to form several other core structures (i.e., Cores 3 – 8).
First, the assembly of O-glycans is initiated by the addition of the sugar N-acetylgalactosamine (GalNAc) to the serine/threonine amino acid in the PTS region. This occurs through the activity of a superfamily of enzymes known as GalNAc-Ts [26]. The first O-glycan formed by this family of enzymes is the Tn (GalNAc-α1-O-Ser/Thr) antigen [27]. Based on earlier in vitro studies performed by Yoshida et al. in 1990s, it is established that efficient transfer of GalNAc occurs mainly to the threonine residue [28]. To date, 20 GalNAcTs genes have been identified. These genes catalyze the initiation of mucin type O-glycosylation. Despite the functional redundancy among GalNAc-Ts, different substrate specificity and tissue-specific expression of these enzymes have been reported [26]. In an attempt to understand the mechanism that confers substrate specificity to these distinct GalNAc-Ts, one study by Kinarsky et al. investigated the role of the polypeptide conformation in substrate recognition by GalNAc-Ts. Using series of synthetic MUC1 glycopeptides and recombinant GalNAc-Ts, Kinarsky et al. proposed that the glycosylation pattern confers a particular conformation recognizable by a GalNAc-T-specific catalytic site [29]. Notably, a characteristic feature of this class of glycosyltransferases is their lectin domain, which helps them bind to existing GalNAc residues on mucins. This binding increases their catalytic efficiency and allows them to complete the initiation of glycosylation prior to elongation of GalNAc residues [30]. The first sugar, GalNAc (Tn) is then processed by the activity of various glycosyltransferases that add distinct glycans. For instance, GalNAc can be elongated by specific glycosyltransferases that add several monosaccharides, giving rise to various core structures. To date, eight core structures (Core 1 – 8) have been recognized. Biosynthesis of the Core 1 structure is initiated by the addition of galactose sugar to GalNAc by β1, 3 linkage. This reaction is catalyzed by T-synthase/Core 1 synthase (C1GALT1) that gives rise to a disaccharide structure known as Core 1/T antigen (Thomsen-Friedenreich antigen-Galβ1-3GalNAc-α1-O-Ser/Thr). The key molecule that regulates the activity of C1GALT1 is an ER chaperon molecule Cosmc (Core 1 β3-Gal-T-specific molecular chaperone), which associates with C1GALT1, thereby preventing its ubiquitin-mediated proteasomal degradation [31]. Mutations in the Cosmc protein lead to the inactivation of C1GALT1, which is associated with Tn syndrome [32]. In addition to extending to the Core 1 carbohydrate structure, the first sugar, GalNAc, can also be elongated by addition of N-acetylglucosamine to form a disaccharide Core 3 carbohydrate structure. Both the Core 1 and Core 3 structures can be elongated to form trisaccharide Core 2 and Core 4 carbohydrate structures, respectively. This is brought about through the addition of N-acetylglucosamine by family of enzymes known as β1,6-N-acetylglucosaminyltransferases [33]. The resultant core structure depends on the relative activity of glycosyltransferase. These O-glycans can also be extended by addition of several residues of poly-N-acetyllactosamine. Further, these oligosaccharide chains can be terminally glycosylated by sialylation, fucosylation, or sulfation. Terminal glycosylation often results in the formation of several carbohydrate epitopes such as Lewis antigen x (Lex), Lewis antigen a (Lea), sialylated Lewis antigen x (sLex), and sialylated Lewis antigen a (sLea), which are commonly expressed by carcinoma mucins (Figure 2) [34].
In addition to elongation to several core structures, the first sugar (Tn antigen) can also be sialylated by α2, 6-sialyl transferases (ST6GalNAc1) to yield the disaccharide Sialyl-Tn (sTn-Neu5Acα2-6GalNAcα-O-Ser/Thr). Additionally, T antigen can also be modified by sialylation to trisaccharide, sialyl-T (sT) antigen. Sialic acid can be linked either by alpha-2,3 linkage to galactose (NeuAcα2-6(Galβ1-3)GalNAc-O-Ser/Thr) via ST3Gal1 or by alpha-2,6 linkage to N-acetylgalactosamine via ST6GalNAc1 [35]. De-sialylation of T antigen (NeuAcα2-3Galβ1-3(NeuAcα2-6) GalNAc- Ser/Thr), in which both galactose and N-acetylgalactosamine are sialylated, can also occur. These sialyltransferases compete with other glycosyltransferases and prevent elongation of glycans. Such hypoglycosylated structures (sTn, sT) that arise in pathological conditions like cancer are usually not seen in healthy tissues [36].
The extent of O-glycosylation is determined by the length and the number of PTS repeats, which are distinct to each mucin and can vary in the same mucin due to gene polymorphisms [12]. Additionally, O-glyosylation is influenced by variations in the peptide sequence of PTS repeats. For instance, replacement of proline with threonine in the PTS repeat of MUC1 results in an additional site of O-glycosylation [37]. A large number of PTS-rich tandem repeats in mucins allows for extensive glycosylation such that the polypeptide backbone adopts a bottle brush-like conformation. The complex glycan structures surrounding these mucins have been shown to contribute to viscoelastic properties of mucus and also protect the apomucin backbone from attack by proteases. Additionally, the diverse array of glycans on mucins that line different intestinal regions dictate their interactions with the gut microbiome [38]. Specifically, it has been shown that the oligosaccharide composition of mucins varies in different intestinal regions, in which the sialic acid content increases from the ileum to the colon, whereas the fucose content decreases [39]. This may also explain specific colonization of microbiota in intestine. Further, several peripheral oligosaccharide structures on mucins, such as Lex and sLex, serve as ligands for bacterial fimbrial adhesins, thereby trapping and preventing them from infecting the underlying epithelia [40].
3. Role of mucin and its aberrant glycosylation in cancer
3.1. Role of mucins in cancer pathogenesis
Aberrant expression of mucins is observed in various malignancies, wherein they play an essential role in cancer pathogenesis [1, 41]. For example, MUC1 is involved in breast cancer pathogenesis as it affects several signaling pathways that influence disease aggressiveness. The C-terminal subunit of MUC1, also known as MUC1-C, acts as an oncoprotein through its interaction with various receptor tyrosine kinases such as EGFR and ErbB2, which leads to the activation of PI3K–Akt and MEK-ERK signaling pathways in breast cancer [42, 43] . Further, this transmembrane mucin is also found to be overexpressed in ovarian cancer and various gastrointestinal malignancies including pancreatic cancer [44–46]. Similarly, MUC4, another transmembrane mucin, is implicated in the pathobiology of cancers including pancreatic, breast, lung, and cervical cancers. Several studies from our lab have well established the involvement of MUC4 in certain aspects of cancer progression including metastasis, evasion of apoptosis, and induction of drug resistance [47–49]. Specifically, we have shown that MUC4 can promote breast and pancreatic cancer metastasis [48, 49]. Interestingly, in the case of ovarian cancer, MUC4 also plays a role in cancer stem cell maintenance via stabilization of HER2 [50]. MUC4 has also been shown to interact with and stabilize HER2 in pancreatic cancer cells, but whether or not this interaction is involved in cancer stem cells (CSCs) remains to be examined [51].
The largest transmembrane mucin identified to date is MUC16, also known as CA-125, which exhibits pro-tumorigenic and pro-metastatic properties [52]. In ovarian cancer, MUC16 is deregulated and plays an important role in metastasis by protecting the tumor cells from cytotoxic responses from natural killer (NK) cells [53, 54]. Further, studies from our lab indicate the involvement of MUC16 in pancreatic cancer progression and in breast cancer proliferation [55, 56]. In pancreatic cancer cells, MUC16 has been reported to interact with the GPI-linked protein Mesothelin (MSLN), which leads to activation of MMP-7, an extracellular matrix remodeling enzyme, thereby resulting in enhanced pancreatic cancer cell motility and invasion [57]. In addition to MUC16, aberrant expression of MUC13 is also observed in many epithelial cancers but has not been significantly examined [58].
Along with transmembrane mucins, expression of secreted mucins is also dysregulated in various malignancies. Specifically, MUC5AC is one of most studied secreted mucins and is shown to be upregulated in various cancers including colorectal and pancreatic cancer [59]. Differential expression of another secreted mucin, MUC5B, is also observed in malignancies such as breast and colorectal cancer [43, 60]. MUC2 is equally important as it has been associated with colorectal cancer and is typically downregulated during cancer progression [61]. Overall, compounding evidence helps to confirm that mucins play an important role in the pathobiology of various malignancies.
3.2 Aberrant glycosylation of mucins in cancer
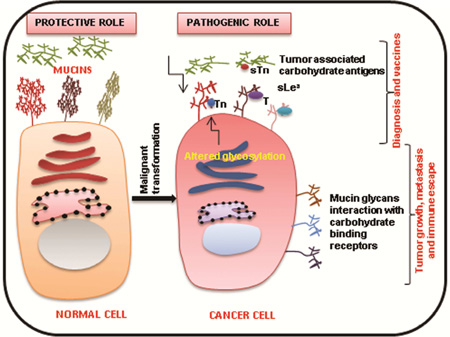
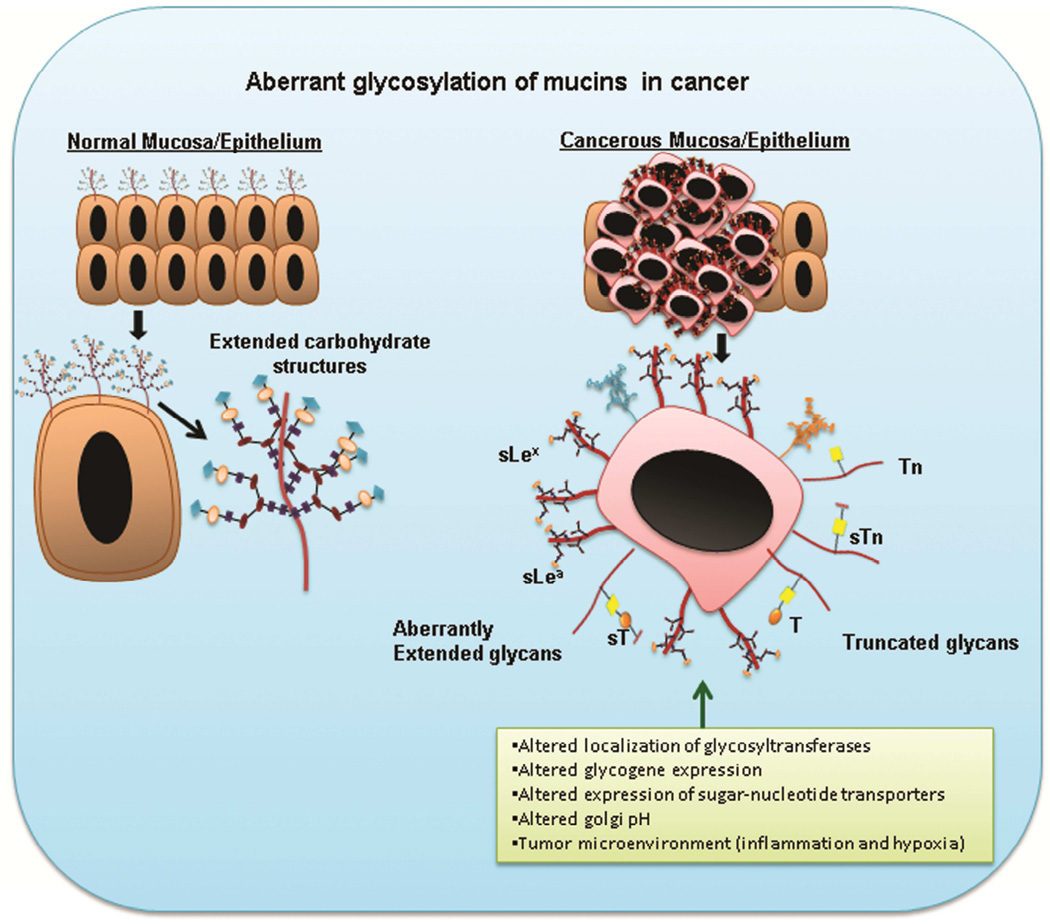
Alterations in the composition and structure of mucin glycans have been observed in various malignancies [62]. During carcinogenesis, neo glycan structures emerge on various glycoproteins including mucins. These atypical glycan forms can be due to incomplete glycosylation that results in truncated structures (Tn, sTn, T, ST) or aberrant extension of glycan chains (sLex, sLea) [36, 63, 64] (Figure 3). Expression of these truncated carbohydrate antigens are generally observed in embryonic tissues, and hence they are also known as oncofetal antigens [65]. Moreover, several examples of such glycan alterations in carcinoma mucins have been reported in literature. For instance, mucins with reduced Core 1 and Core 3 structures, along with increased expression of truncated glycans such as Tn and sTn, have been observed in intestinal, colon, and pancreatic cancers [35, 66].
Figure 3. Aberrant glycosylation of mucins during cancerous conditions.
Differential glycosylation of mucins occur in malignant conditions that result in truncated glycan structures such as Tn, STn, and T, and several Core 2 extended structures such as sLex and sLea. This aberrant glycosylation is a result of several factors such as altered glycogene expression, mislocalization of glycosyltransferases, abnormal Golgi pH, and the tumor microenvironment.
Evidence for the abnormal carbohydrate structures on mucins emerged from earlier studies that demonstrated the increased expression of Thomsen–Friedenreich antigens (T/TF) on the surface mucins in colonic adenocarcinoma and ulcerative colitis, compared to the normal colon [67]. Likewise, dramatic changes in mucin glycosylation have been reported in colonic adenocarcinoma, compared to normal colon. Terminal sialic acids of colonic mucins are generally O-acetylated under normal conditions, which prevents degradation of the mucins from the sialidase activity of enteric bacteria [63, 68]. Acetylation of the sialylated glycan structures on cancer cells also blocks their interaction with cognate receptors, such as selectins and siglecs present on endothelial and immune cells [68]. Malignant transformation of colon tissue is marked by significant decrease in O-acetylation of these sialic acid residues. This was shown by isolating mucin fractions from human colonic carcinoma cell lines representative of different stages of disease progression (from adenoma to cancer). The decrease in acetylation is associated with exposure of glycan structures such as sLex and sTn, which are masked by acetylation of sialic acid in normal condition [69].
Unusual glycan structures formed on glycoproteins during cancer are involved in its progression. The role of these aberrant glycans is well documented in cancer cell adhesion, motility, invasion, and altering the interaction of cancer cells with other cells, such as lymphocytes and endothelial cells [35]. Apart from the pathologic role of these atypical mucin glycans, these aberrant glycan structures also constitute tumor-associated carbohydrate antigens (TAA), which are extensively used as diagnostic markers.
4. Pathologic role of mucin glycans in cancer
Accumulating evidence suggests that deregulated expression of mucin glycans can significantly influence the function of the mucins in pathological conditions.
4.1. Mucin glycans mediated cellular interactions
The glycophenotype of cell surface mucins dictates both homotypic and heterotypic cellular interactions. These glycan moieties on mucins serve as ligands for various carbohydrate-binding proteins, such as galectins, selectins, and siglecs, and mediate diverse biological processes including cell adhesion, migration, trafficking, and inflammation [35]. All of these glycan binding proteins comprise the lectin family and are expressed by specific subsets of cells such as immune cells and endothelial cells.
4.1.1. Galectins
Cancerous conditions are often accompanied by altered expression of beta galactoside-binding lectins, also known as galectins [70, 71]. So far, 15 galectin members have been discovered and are involved in wide range of biological processes. Specifically, the role of galectins in immunomodulation, regulating cell proliferation, and apoptosis has been well elucidated [72]. Galectin 1 and 3 are among the most characterized galectins and are upregulated in several malignancies. Several studies have identified their roles in tumor angiogenesis and metastasis. [73–75]. Various mucins display galactose-rich oligosaccharides on their surface and hence serve as binding partners for galectins. The importance of galectin-mucin interactions is reflected by the ocular epithelial barrier, where interaction of MUC4 and MUC16 with galectin-3 maintains the integrity of the mucosal barrier that prevents infection of underlying eye epithelium [76]. Further, galectin-mucin glycan interactions play an important role in tumor metastasis, which is further described in this review.
4.1.2. Selectins
Selectins are adhesion molecules that recognize galactose-containing carbohydrates on their cognate ligands [77]. Depending on the cell types on which they are expressed, selectins are classified into E-selectin (endothelial cells), L-selectin (leukocytes), and P-selectin (platelets). These selectins recognize sialylated/sulfated lewis antigens (such as sLex, sLea) on mucin glycoproteins, which are highly expressed on cancer cells [78]. The interaction of secreted and surface mucins with selectins on endothelial cells, platelets, and leukocytes is illustrated in colon carcinoma [79]. Interaction of carcinoma mucins with selectins on these components of the blood stream allow cancer cells to extravasate and metastasize to the target organs. Also, the interaction of mucins with selectins on platelets has been shown to cause aggregation of platelets, which results in the formation of structures known as microthrombi. This may also explain excessive coagulation (Trousseau syndrome) generally observed in mucinous adenocarcinomas such as mucinous ovarian and breast carcinomas, which are marked by excessive production of mucins in the bloodstream [80].
4.1.3. Siglecs (Sialic acid-binding immunoglobulin (Ig)-type lectins)
Sialic acid-binding lectins, or siglecs, are expressed by some hematopoietic cells such as macrophages (Siglec-1/sialoadhesin) and B cells (Siglec-2/CD22). Siglecs play an important role in mediating cell-cell interactions and modulating the immune response [81, 82]. To date, 10 different siglecs have been identified [82]. Siglecs can interact with sialic acid groups present on the non-reducing ends of glycoproteins such as mucins. This siglec-glycoprotein interaction has been described between Siglec-1 (sialoadhesin) on tumor-infiltrating macrophages and MUC1 (presenting sialylated glycan structures) on breast carcinoma cells. However, the biological role of this interaction remains unclear [83]. Further, Siglec-9, which is expressed on surface of immune cells such as B-cells, monocytes, and NK cells, has also been shown to interact with transmembrane mucins including MUC16 and MUC1 [84, 85].
4.2. Functional consequences of mucin glycotope-mediated cellular interactions
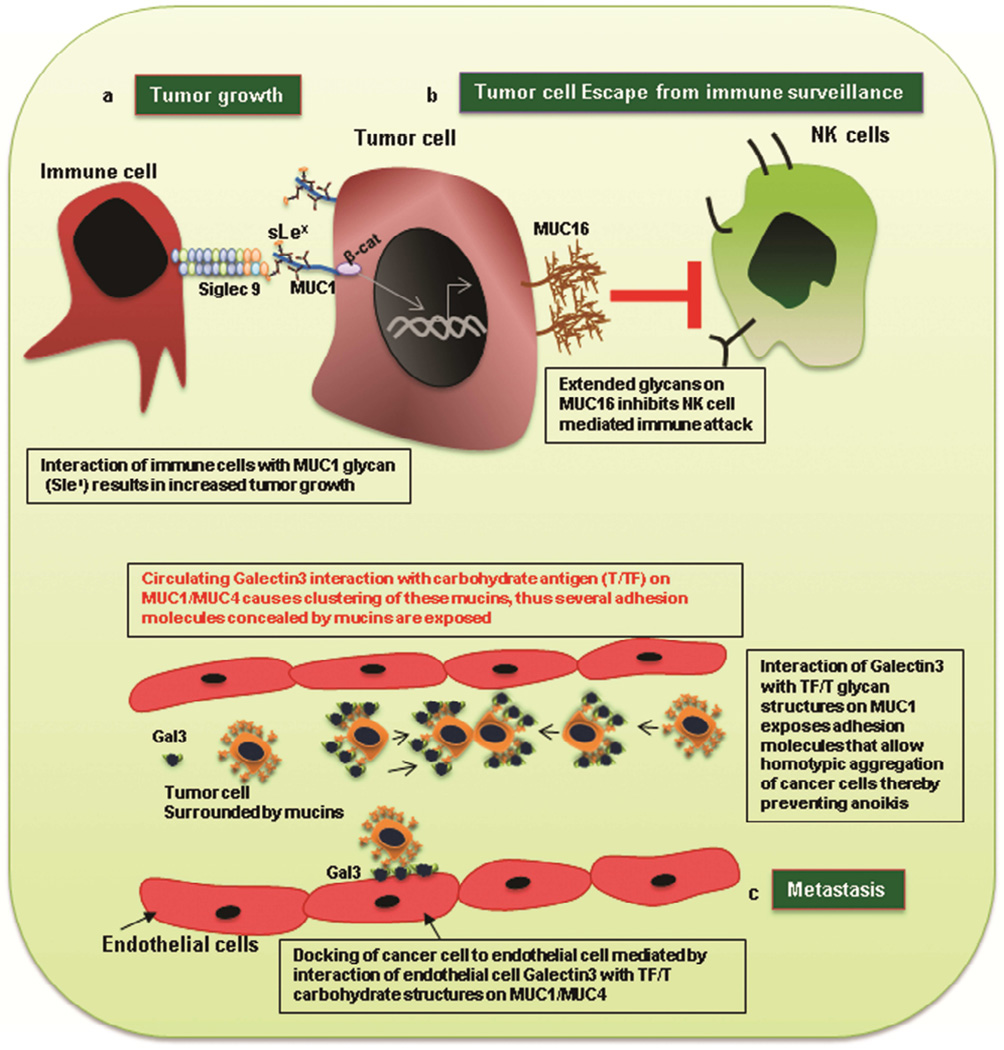
The interactions of mucin glycans with sugar-binding proteins have been shown to affect several aspects of tumor progression including tumor growth, escape of immune surveillance, and metastasis (Figure 4).
Figure 4. A schematic representation of mucin glycans mediated cellular interactions.
a) Aberrant glycan structures (e.g., sLex) on MUC1 mediate tumor growth by binding to siglec-9 on immune cells. This binding induces the translocation of beta catenin to the nucleus of cancer cells and results in cell growth. b) Extensive glycosylation of MUC16 helps tumor cells escape immune attack via NK cells. c) Interaction between galectin-3 and the T antigen on MUC1 and MUC4 results in clustering of these mucins towards one side of the cell (polarization), which exposes adhesion molecules concealed earlier by the presence of mucins around the entire cell surface. Exposure of adhesion molecules allow cancer cells to interact with endothelial cells thereby mediating extravasation leading to metastasis. Also exposure of adhesion molecules causes homotypic interactions among cancer cells, which results in the formation of aggregates and prevents their anoikis.
4.2.1. Mucin Glycans in tumor growth
Sialoglyans expressed on mucins have been shown to affect tumor growth. For instance, MUC1 sialoglycans, such as sLex, are shown to mediate its binding to siglec-9, which is commonly expressed by immune cells. Preferential interaction of MUC1 with Siglec-9 was seen using pull-down studies with several soluble recombinant siglec molecules (Siglec-1, 3, 5 and 9). The functional role of this interaction was established using a co-culture model, in which HCT116 (colon cancer cells) and HEK-239 that were stably transfected with MUC1cDNA and Siglec-9cDNA, respectively were used. Co-incubation of these cells resulted in recruitment of β-catenin to MUC1-C domain. Further, ligation of MUC1 (on 3T3 cells) with Siglec-9 triggered translocation of beta-catenin from cytoplasm to nucleus that was accompanied by elevated expression of c-myc and increased proliferation of 3T3/MUC1 cells [84] (Figure 4a).
4.2.2 Role of mucin glycans in evading immune surveillance
Although tumor tissues are characterized by the infiltration of immune cells, such as macrophages, natural killer cells, and cytotoxic T cells, tumor cells still effectively escape the immune system. This is due to the activation of various immunosuppressive pathways by the tumor cells themselves [86]. A considerable body of evidence suggests that cancer cells escape the immune response through the involvement of their surface glycan epitopes. Elongation of mucin glycans beyond the Tn antigen alters their susceptibility towards NK-and cytotoxic T lymphocyte-mediated killing [87] (Figure 4b). This has been shown in breast (T47D) and pancreatic cancer cells (Capan1), wherein knockout of Cosmc protein (chaperone for C1GALT1) inhibited glycan elongation beyond Tn, which consequently increased their sensitivity towards immune attack via NK cell-mediated antibody dependent cell cytotoxicity (ADCC) and cytotoxic T lymphocytes. Further, a reverse correlation was observed between MUC1/MUC16 expression and immune killing, which suggests that upregulated expression of these mucins along with their glycan elongation beyond Tn is involved in immune evasion [87].
4.2.3. Mucin Glycotope-mediated cancer metastasis
In order to successfully metastasize, cancer cells must leave their primary site of origin by degrading the extracellular matrix. Following this degradation, the cancer cells enter the blood stream (i.e., intravasation), wherein they are recognized as circulating tumor cells. After being brought into the endothelia of target organs, these cells extravasate (i.e., leave the blood vessel) and colonize in secondary sites [88]. Several studies highlight the important roles played by aberrant glycans on mucins during various stages of the metastatic cascade. In 2010, it was shown that altered glycans on MUC1 affect invasion and migration of breast cancer cells by influencing the interaction of MUC1 with CIN85, an adaptor protein involved in invasion and cytoskeletal alterations [89]. This was demonstrated by overexpressing glycosyltransferases ST6GalNAc1 and ST3Gal1, which are involved in the biosynthesis of carbohydrate antigens, sTn and sT respectively. Specifically, increased interaction of CIN85 and MUC1 was observed with increased expression of sTn, whereas this interaction was not altered with increased expression of sT [90]. This suggests that the hypoglycosylated form of MUC1 (MUC1-sTn) can promote MUC1’s interaction with CIN85 that results in increased migration and invasion. Further, elevated expression of STn on the surface of MUC1 in gastrointestinal cancer cells is also associated with increased intraperitoneal metastasis. However, the exact mechanism underlying intraperitoneal metastasis remains undefined.
Overexpression of mucin-associated glycans, such as sLex, has also been implicated in metastasis. This involves interaction of the sLex glycan of mucin with E and P selectins on endothelial and platelet cells, respectively [91]. In addition to selectins, interaction of mucin glycans with circulating/membrane-bound galectins also leads to enhanced metastasis. As shown by our lab, MUC4 interacts with Galectin-3 via T antigens on its surface [92]. This Galectin-3 binding results in MUC4 clustering (MUC4 cell surface polarization) that exposes adhesion molecules, such as integrins, that were concealed by MUC4. This facilitates the attachment of tumor cells to endothelial cells, which is essential for the extravasation of circulating tumor cells (Figure 4c). Similarly, elevated levels of circulating Galectin 3 in several cancers have also been shown to interact with Thomsen–Friedenreich (TF/T) antigens on MUC1, which expose surface ligands (which were earlier shielded by presence of extensive glycans on MUC1), like CD44, to endothelial cells. Hence, the interaction of mucins with galectins leads to metastasis by eliminating the “shield effect” of mucins, which prevents the adhesion of cancer cells to endothelial cells (Figure 4c) [73, 93]. This galectin3-MUC1 (TF) interaction also induces MUC1 cell surface polarization, which results in exposure of adhesion molecules that were previously shielded by MUC1. This allows homotypic interaction among cancer cells and increases the survival of these cancer cells in the blood stream (Figure 4c) [94].
Apart from O-glycan structures, N-glycans on mucins such as MUC16 are also implicated in facilitating peritoneal metastasis via their interaction with mesothelin expressed by mesothelial cells lining the peritoneal cavity [95].
4.2.4. Role of glycosylation in membrane trafficking of mucins
Most of the aforementioned processes mediated by mucin glycans, including metastasis and tumor growth, necessitate the membranous localization of these glycoproteins. Both N-and O-linked oligosaccharides for certain mucins contain tremendous information for their apical sorting. This has been well described for MUC1 using Madin-Darby canine kidney (MDCK) cells. Here, it was demonstrated that O-glycosylated tandem repeats on MUC1 serve as an apical sorting signal [96]. Several studies suggest the involvement of these O- and N-glycomoieties in the membrane trafficking of their carrier protein. This was shown using Benzyl 2-acetamido-2-deoxy-3-O-D-galactopyranoside (BGN) and tunicamycin, which are commonly used drugs that interfere with O- and N-glycan biosynthesis, respectively. Tunicamycin is a uridine analog that inhibits N-glycan biosyntheis by preventing the formation of a pentasaccharide core (Man3GlcNAc2), whereas BGN inhibits O-glycosylation in a competitive fashion by acting as a structural analog of GalNAc-α-1-O-serine/threonine [97]. For example, incubation of human colorectal adenocarcinoma cells (HT29) with BGN decreased the secretion and membrane targeting of MUC1 [98]. Consistent with this study, treatment of endometrial and breast cancer cells with these inhibitors also reduced the expression of MUC1 on their surface [99]. These studies highlight the essential role played by mucin glycosylation in their membrane trafficking. However, the possible roles of glycans in apical sorting of other mucins, such as MUC4 and MUC16, remain to be investigated.
While glycosylation affects trafficking, altered cellular trafficking can also affect mucin glycosylation. This concept was demonstrated for MUC1, wherein secreted and membrane-shed MUC1 probes have been shown to display altered glycosylation profiles. Specifically, the secreted MUC1 form (MUC1-S) exhibited more Core 2 glycan structures, whereas membrane-shed form was rich in sialylated Core 1 glycan structures [100].
4.2.5 Mucin glycans and cancer stem cells
Recent studies have established the critical role of mucins in self-renewal of cancer stem cells (CSCs). Because altered mucin glycosylation is implicated in cancer growth and metastasis, it is possible that CSCs have an increased tendency for altered glycosylation of mucins. However, few studies suggest the altered glycosylation of mucins in highly tumorigenic cells like CSCs. Studies from our lab utilizing ovarian cancer cells that ectopically overexpressed MUC4 demonstrated slight decrease in the molecular weight of MUC4 in the enriched side-population (CSC population) as compared to non-side population [50]. Altered glycosylation is a plausible mechanism for such an observation, but this remains to be investigated. Interestingly, another study reported the existence of hypoglycosylated MUC1 in CSCs in MCF-7 breast cancer cells [101]. Still, more comprehensive studies can decipher the role of mucin glycosylation in development and maintenance of CSCs.
5. Regulation of Mucin glycosylation
Given the functional significance of aberrant mucin glycans in cancer, it is important to understand how mucin glycosylation is regulated. Several factors influence the differential expression of mucin glycans during carcinogenesis. These include alterations in glycogene expression, altered localization of the glycosyltransferases, aberrant activity of respective glycosyltransferases/glycosidases, changes in the pH of the Golgi apparatus, efficiency of the sugar nucleotide transporters, and physiological alterations in the tumor microenvironment such as hypoxia and inflammation [102].
5.1. Mucin glycosyltransferases and glycosidases
Glycogenes consist of either glycosyltransferases that are responsible for synthesizing glycan structures or glycosidases that catalyze the release of glycans from glycoproteins and glycolipids. These glycosyltransferases and glycosidases are essential components of the cellular glycosylation machinery. The relative level and activity of glycosyltransferases and glycosidases dictate the glycan profiles of glycoproteins like mucins, and any imbalance results in aberrant glycosylation [103].
5.1.1 Altered glycogene expression during cancer
Diversity in the expression of glycosyltransferases and glycosidases in a cell manifests in the variety of glycans displayed by mature mucins [104]. In general, cancer-associated glycan expression is regulated by myriad of glycogenes, some of which are transcriptionally repressed while others are induced during malignant transformation [105, 106]. Further, genetic and epigenetic silencing mechanisms are involved in the inactivation of many glycogenes during carcinogenesis. Such alterations in glycogenes profoundly change glycosylation patterns such that glycans that exist in normal conditions are modified (truncated/extended). For instance, normal pancreatic and biliary tract epithelia express disialyl Lewis A. This carbohydrate determinant is lost during oncogenic transformation of these tissues due to the epigenetic silencing of the glycogene ST6GALNAC6 that synthesizes this antigen. In turn, loss of this glycogene is associated with elevated expression of SLea. Notably, SLea is one of the carbohydrate antigens associated with mucins and plays a significant role in diagnosis of several gastrointestinal cancers including pancreatic cancer [107, 108]. Further, in colon cells, activity of core-3 synthase is suppressed by promoter methylation, and its ectopic overexpression reduces cancer cell motility, suggesting that cancer cells downregulate this enzyme to enhance their metastatic potential [109]. Furthermore, loss of the Sda antigen in gastrointestinal malignancies has been reported, which is attributed to hypermethylation of CpG islnds in the promoter region of β1, 4-N-acetylgalactosaminyltransferase (β1, 4GalNAcT2/ B4GALNT2) [110]. The Sda antigen is mainly expressed in glycolipids (oxyntic mucosa) and glycoproteins/mucins (colonic mucosa). B4GALNT2 catalyzes the last step in biosynthesis of the Sda carbohydrate antigen by an addition of N-acetylgalactosame (in the β1, 4 linkage) to terminal galactose of α2, 3-sialylated N-acetyllactosaminic units [111, 112]. B4GALNT2 and Fucosyltransferases (FucTs) compete for α2, 3-sialylated N-acetyllactosaminic units and form Sda and sLex/sLea, respectively. The absence of this glycogene favors the expression of the sLex and sLea antigens due to competition between β4GALNT2 and FucTs. In turn, sLex and sLea serve as ligands for selectins and are involved in metastasis. Stable expression of β4GALNT2 glycosyltransferase in colorectal cancer cells (HT29, LS174T) and gastric cancer cells (KATOIII) leads to reduced expression of sLex and sLea and decreased metastasis [113, 114]. These studies suggest that gastrointestinal cells downregulate β4GALNT2 (i.e., decrease Sda) during malignant transformation, which is accompanied by compensatory increases in sLex and sLea due to increased activity of FucTs, thus allowing for cancer cells to metastasize.
In addition to the enzymes involved in the synthesis of terminal carbohydrate antigens and core glycan structures, expression of glycosyltransferases involved in initiation of O-glycosylation is also regulated by epigenetic mechanisms. In 2013, Keita et al. showed that the O-glycosylation-initiating enzyme GALNT3 is hypomethylated and overexpressed in epithelial ovarian tumors [115]. Further, Wang et al. showed that knockdown of this enzyme is associated with increased adhesion and decreased MUC1 stability, which suggest that the involvement of GALNT3 in ovarian cancer progression possibly via aberrant MUC1 glycosylation [116]. Thus, there is direct evidence that the epigenetic regulation of glycogenes can significantly contribute to cancer progression and metastasis.
In addition to epigenetic mechanisms, researchers have also reported the post-transcriptional regulation of glycosyltransferases. For example, during melanoma metastasis, the expression of the O-glycosylation-initiating GalNAc transferase, GALNT7, is down regulated by the increased expression of miR-30b/30d. Knockdown of miR-30b/30d restores GALNT7 expression, results in decreased cellular invasion, and induces IL-10, which is an immunosuppressive cytokine [117].
5.1.2. Localization of glycosyltransferases in normal and cancer cells
Various glycosylating enzymes are distributed non-uniformly inside the Golgi apparatus, with each compartment expressing a unique set of glycosyltransferases. Several factors, including intrinsic structural features, dictate the localization of these glycosylating enzymes on the Golgi apparatus. These glycosyltransferases are predominantly type II integral proteins that are organized into N-terminal, cytoplasmic tail, transmembrane and the stem regions (CTS), which are involved in directing their localization to the Golgi apparatus [118]. Specifically, the transmembrane domain of α-2, 6-sialyl transferase affects its distribution in the Golgi. For example, mutagenesis studies have revealed that the length of the transmembrane domain serves as a sorting signal, and varying its length alters the ability of the Golgi apparatus to retain the trans Golgi enzyme α-2, 6-sialyl transferase [119]. The compartmental identity of medial Golgi enzymes like mannosidase II and MGAT1 (α-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase) is maintained through their oligomerization, which is mediated by their luminal regions [120]. The differential ratio of glycerophospholipids and sphingolipids among individual Golgi compartments also accounts for non-homogenous distribution of these enzymes, with sphingolipids:glycerophospholipids being highest in trans Golgi and lowest in cis [118, 121, 122]. The relative position of these glycosyltransferases inside the Golgi apparatus determines the glycan structure that is produced by the cells. Aberrant glycosylation in various pathological states can also be attributed to anomalous localization of glycosyltransferases.
5.2. Nucleotide transporters in glycosylation
Altered efficiency and expression of sugar nucleotide transporters also influence glycosylation of carcinoma mucins [123]. Transport of sugars from the cytoplasm to the ER and Golgi apparatus, where glycosylation occurs, is facilitated by nucleotide transporters that line the membranes of these organelles [124]. A series of nucleotide sugar transporters are available to transport these sugars to the Golgi apparatus, thus influencing glycosylation. Some of the examples of these transporters are UDP-galactose (UDP-Gal), UDP-N-acetylglucosamine (UDP-GlcNAc) and CMP-sialic acid (CMP-SA).
In colorectal carcinoma, which involves lymph nodes and distant metastases, increased mRNA expression of UDP-Gal has been reported. Likewise, the availability of galactose sugar via UDP-Gal transporter influences the biosynthesis of several carbohydrate structures involved in metastasis, such as Thomsen–Friedenreich (T/TF), sLea, and sLex antigens. For example, when colon cancer cells (SW1083, SW480) were transfected with UDP-Gal Transporter cDNA, increased expression of the Thomsen–Friedenreich antigen was observed. Transfection of this cDNA in SW80 cells also induced expression of other carbohydrate determinants such as sLea and sLex. Further, the transfected cells showed increased adhesion to E-selectin, which suggests increased metastatic behavior of UDP-Gal-transfected cells [125].
A study by Yusa et al. revealed altered glycosylation in colon cancer cells due to epigenetic silencing of the diastrophic dysplasia sulfate transporter (DTDST). This transporter is involved in formation of 6 sulfo-sialyl–Lewis x structure by sulphation of the terminal sLex glycan structure. Expression of 6 sulfo-sialyl–Lewis x is seen in normal colonic epithelial cells but undetectable in colon cancer cells. Further, epigenetic silencing of DTDST results in insufficient sulfation and increased expression of sLex [126]. Studies have also shown that in HeLa cells the silencing of nucleotide transporters CMP-sialic acid and GDP-fucose, which provide terminal sugars, is associated with increased ER stress along with decreased synthesis and secretion of glycoproteins.
5.3. Effect of tumor microenvironment on Mucin glycosylation
In addition to the aforementioned cell-intrinsic determinants, various factors in the tumor microenvironment like hypoxia and inflammation can also modulate glycans on carcinoma mucins.
5.3.1. Hypoxia-induced glycogene expression
A hypoxic microenvironment prevails in the advancing tumor niche. Hypoxia induces neo-angiogenesis, directs the intracellular metabolism to anaerobic glycolysis, and increases the metastatic potential of the cancer cells [127]. Such hypoxic conditions also result in altered glycan structures due to transcriptional deregulation of various glycogenes including sialyl transferase, FucTs (Fucosyltransferases), and sugar transporters. Certainly, hypoxic conditions activate hypoxia-induced factors (HIFs) that result in increased expression of sLea and sLex in colon cancer cells. These selectin ligands were increased due to the increased expression of UDP-galactose transporter-1 expression (UGT1), sialyltransferase (ST3Gal-I), and FucT-7 [128]. Abnormal glycan structures with increased N-glycosyl sialic acid residues are also prevalent in hypoxic conditions. This is attributed to Sialin, a sialic acid transporter that is activated under hypoxic conditions and promotes the incorporation of the N-glycolyl neuraminic acid (Neu5Gc) in mucin-type glycans [129]. Like N-acetylneuraminic acid, N-glycolyl neuraminic acid is also a sialic acid molecule; however, it cannot be synthesized in humans and is incorporated into glycoproteins via dietary absorption [130, 131]. Notably, hypoxia induces a specific set of glycans under malignant versus nonmalignant conditions, such as ischemic disorders, suggesting the distinct role of glycans under different pathological conditions [105].
5.3.2. Inflammation and altered glycosylation
Inflammation is associated with several pathological conditions, including Helicobacter pylori infection, inflammatory bowel disease (IBD), and cancer, and can lead to altered glycosylation of many proteins [132–134]. For instance, inflammation is associated with changes in surface glycans of lymphocytes, resulting in an increase in sulfated carbohydrate epitopes, such as 6-sulpho-sLex, that mediate transient interactions with selectins on endothelial cells. This in turn facilitates the homing of activated lymphocytes to inflamed sites [135, 136]. Similarly, inflammatory conditions have also been shown to modulate the glycan profile of various mucins in cancer cells [137]. This has been described by Haab et al. at the Van Ander Research Institute, wherein by stimulating pancreatic cancer cells (Aspc-1, BxPC-3 and SU.86.86) with various inflammatory cytokines, such as IFNγ, IL-1α and TNFα, significant changes were observed in mucin glycosylation and expression. Using immunoprecipitation and antibody microarrays, Haab et al. demonstrated that TNFα and IFNγ-stimulated BxPC-3 cells display elevated CA 19-9 (sLea) levels on MUC1 and MUC5AC. Further, elevated levels of terminal sugars such as GlcNAcβ, GalNAcα, GalNAcβ, and lactosamine were observed on MUC16 in MIA PaCa pancreatic cancer cells [138]. These experiments highlight the effect of inflammation on mucin glycans with respect to cancer, although much remains to be discovered regarding the underlying mechanisms involved in inflammation-mediated glycan changes in mucins. Interestingly, studies by Kreisman et al. described the crosstalk between inflammation and glycosylation, in which they showed that many glycosylation changes can also contribute to inflammation [139]. As such, further research will be intriguing to determine whether such an autocrine loop exists between mucin glycosylation and inflammation in cancer.
5.4. Impaired mucin glycosylation with elevated pH
The ability of the Golgi apparatus to facilitate essential cellular functions, such as protein folding, glycosylation, sorting, and trafficking, relies on its luminal pH. An aberration in this pH interferes with these events, resulting in several abnormalities seen in pathological conditions like cystic fibrosis and cancer [140, 141]. While an altered glycosylation profile of mucins in several cancers is attributed to mislocalization of glycosyltransferases, evidence also supports the involvement of an altered Golgi pH in aberrant glycosylation. For instance, in breast and colorectal cancer cells, increased Golgi pH is associated with elevated expression of the T antigen, a Core 1 carbohydrate structure usually associated with carcinoma mucins [142]. However, the exact mechanism by which these changes occur remains unclear.
6. Diagnostic and prognostic significance of mucin glycans
Altered expressions of mucin glycans are the hallmark of several malignancies, including pancreatic, breast, gastric, colon and lung cancer [143]. Because these unusual glycan structures are generally not seen in normal tissues, mucin glycotopes can potentially serve as efficient diagnostic tools. It is well accepted that early diagnosis is the key for improving the clinical outcome of malignancies and mandates the development of efficient diagnostic tools. Given the non-invasive nature and relative cost effectiveness of serum biomarkers, they are clinically acceptable and preferred over other diagnostic modalities like a CT scan, MRI, and endoscopy. In fact, aberrant mucin glycans released by tumor cells into the bloodstream have been the basis of several clinically approved serodiagnostic tests for certain malignancies [144–146]. For example, Cancer Antigen 125 (CA-125), used in the diagnosis of ovarian cancer, is an epitope present on MUC16 [147]. Similarly Cancer Antigen 15-3 (CA15-3), used to monitor breast cancer, is an epitope on MUC1 [148]. Further, several glycan epitopes have been approved by the FDA as biomarkers both for diagnosis and to monitor therapeutic responses. A well-documented example of such a marker is CA19-9, which possesses clinical utilities to detect various gastrointestinal cancers and is often used to diagnose pancreatic cancer [149]. This serological assay detects aberrant changes in sLea tetrasaccharide, which is highly expressed on mucins. Likewise, the CA72-4 assay is used to detect elevated levels of sTn, another carbohydrate antigen associated with mucins in several epithelial cancers [150]. However, it is important to note that while several glycan markers are used in clinics, they are often inefficient due to lack of specificity. This necessitates the development of a targeted diagnostic approach that is directed towards detection of glycan changes on certain mucins that carry these altered glycans.
Due to extremely low level of glycans during early disease stages, rapid hepatic clearance, and complexities associated with their characterization, the discovery of glycan biomarkers is challenging [151]. As an alternative, a few studies have provided encouragement for the identification of glycan biomarkers by detecting anti-glycotope autoantibodies. For example, during the onset of tumorigenesis, the immune system recognizes altered glycan epitopes as neoantigens and elicits humoral responses that result in the generation of autoantibodies. These autoantibodies are highly specific and display exclusive affinity toward the host antigen as the disease progresses. These two unique features poise autoantibodies as an effective diagnostic tool for the early detection of cancer, as well as for monitoring the disease status. Importantly, the presence of these autoantibodies towards glycopeptide regions (Tn-MUC1, sTn-MUC1) of aberrantly glycosylated MUC1 has been demonstrated in breast, ovarian, and prostate cancer [152].
In 2001, Pederson et al. used seromic profiling of colon cancer patients to demonstrate the existence of autoantibodies directed towards Tn and sTn epitopes, and T antigens on MUC1, MUC4, and other mucins [153]. Among these autoantibodies, the IgG type in the serum of cancer patients specifically reacted with MUC1 glycopeptides, while there was no reactivity with sera from healthy and IBD controls. On the other hand, the IgM type in the serum showed reactivity with all known mucins associated with Core 3 glycans. Thus, it can be deduced that the IgG type autoantibodies are highly specific to MUC1 and can be used to discern between cancer and IBD patients. Further, the serum samples of cancer patients contained increased levels of anti-MUC4 IgA type autoantibodies. The study was also extended to secreted mucins such as MUC2, MUC6, MUC7, and MUC5AC; however, it should be noted that the membrane mucins MUC1 and MUC4 carry the most prominent tumor-associated glycan epitopes [6, 153].
In 2012, Pinto et al. attempted to detect the combination of mucins and their associated glycans using a proximity ligation assay (PLA). Specifically, using a set of samples from different types of adenocarcinomas, including colon, lung, breast, and ovarian cancer cells, they suggested that this in situ technique can detect aberrant mucin glycoforms with increased sensitivity and specificity compared with assays that detect only O-glycans or mucins [154]. However, further studies with an increased cohort size are needed to confirm these aberrant glycoforms as cancer biomarkers.
7. Glycans as therapeutic targets
The presence of glycans on various biomolecules underscores their functional importance. Some of these molecules, including mucins, are localized on the cell surface and modulate cellular functions during physiological and pathological conditions [6]. More specifically, mucin glycans are involved in tumor cell proliferation, invasion, hematogenous metastasis, and angiogenesis. Thus, glycans are very attractive targets for cancer treatment.
7.1 Mucin glycan/glycopeptide vaccines
A major limitation in targeting mucins peptides is the normal homeostatic role played by their secreted and membrane-bound forms. However, mucin glycopeptide-based vaccines may offer an added advantage in that they limit undesirable effects on normal epithelial tissues due to the distinct glycan patterns in mucins of tumor cells compared to normal cells. Unusual glycomotifs on mucins are recognized by the immune system, but they are poorly immunogenic. Therefore, administration of synthetic glycans along with carrier proteins can be used as anti-cancer vaccines to boost the immune response [155]. One such example is the sTn-KLH vaccine, which was used in phase III clinical trials for breast cancer patients with metastatic disease and has been shown to induce both humoral and cellular immune responses [156, 157]. Another example of glycan vaccines are multi-copy multivalent vaccines, in which a polymerised Tn carbohydrate antigen is conjugated with gold nanoparticles. In vivo experiments on New Zealand white rabbits using this vaccine have demonstrated significant immune response along with cross-reactivity towards the Tn antigen on various mucins [158].
In addition to glycan vaccines, in 2009 Kaiser et al. designed a MUC1-based glycopeptide vaccine by linking the sialyl-Tn-MUC1 (glycopeptide antigen) to tetanus toxoid. This vaccine induced a very strong and selective immune response in mice [159]. Similarly, because the MUC1 glycopeptide is poorly immunogenic, Westerlind et al. designed a synthetic vaccine in 2008 that consists of a MUC1 glycopeptide along with a T helper (TH) peptide to activate humoral immunity [160]. To stimulate immune response, Cai et al. constructed an oligovalent vaccine in 2011 by conjugating a MUC1 glycopeptide with toll-like receptor 2 (TLR2) lipopeptide ligands using click chemistry [161]. In 2014, Abdel-Aal et al. generated a MUC1 tripartite vaccine using a MUC1 glycopeptide, TH peptide, and TLR2/9 agonist, which was found to elicit an improved immune response [162].
Currently, our lab is investigating MUC4-associated glycan epitopes in various cancers for their use in vaccine development. Understanding the glycan alterations on mucins in cancer is expected to lead to the development of novel diagnostic and therapeutic targets. Additionally, generating vaccines carrying both N- and O-glycosylated mucin peptides could be an effective therapy against various cancers. Further, it is important to investigate the possibility of MUC16 as a vaccine candidate, as it is highly overexpressed in pancreatic, breast, and ovarian cancers [52]. Finally, considering that multiple mucins are involved in malignant conditions, multivalent vaccines that target many mucins could also be of great therapeutic potential.
7.2 Glycosyltransferase inhibitors and glycomimetics as therapeutic agents
A significant number of studies have been performed that use glycans as therapeutic targets have been performed. The role of several core glycan structures that build the O-glycans have been studied extensively by several investigators. In addition to core glycans, the terminal glycans on mucins are also uniquely important due to their primary interactions with other proteins [85]. Sialic acid and fucose are the common glycan structures observed on the terminal branches of N- and O-glycans of mucins. Interestingly, sialylation of mucin glycans has been shown to modulate several functions and play a major role in cancer metastasis by aiding the cancer cells in detaching from the primary tumor [163]. In particular, selectin ligands (e.g., sLex and sLea) carry α2, 3-linked sialic acid in their terminal regions and are involved in metastasis. This further highlights the importance that sialylation has on mucin-associated glycans in cancer. A family of enzymes known as sialyltransferases catalyzes the addition of sialic acid groups to mucin glycans. Given the role of the terminal sialic acid in mucin glycans (sTn, sLex, SLea, and ST) in tumor growth and metastasis, several inhibitors that target sialyltransferases have been developed. One example of such an inhibitor is soyasponin-I, which selectively inhibit α2, 3-sialyltransferase activity. Hsu et al. showed that treatment of metastatic breast cancer cells (MBA-231cells) with soyasponin-I significantly decreased their migration [164]. In 2010, Chiang et al. reported on the lead-derived compound AL10, which is a novel inhibitor of sialyltransferase. AL10 was shown to inhibit both α2, 3 sialyltransferase and α2, 6-sialyltransferase activity. Further, AL10 shown to inhibit metastasis in vivo by metastatic lung cancer cells (A549 cells) [165]. While the effect of these inhibitors on the sialylation of carcinoma mucins has not been addressed, collectively these studies suggest that the potential use of sialyltransferases inhibitors as an effective cancer treatment approach [166].
Several studies have documented the significant role played by terminal fucose on glycoproteins to mediate cellular adhesion. Fucose constitutes many terminal glycan structures, as such as Lex and sLex antigens. The involvement of these antigens on mucins in cancer pathogenesis is well elucidated. The process of addition of terminal fucose, also known as fucosylation is catalyzed by family of enzymes known as fucosyltransferases (FucTs) [167]. Recently a study has reported the pharmacologic potential of cell permeable inhibitors of both FucTs and sialyltransferases. These are fluorinated analogs of fucose and sialic acids and have been shown to inhibit fucosylation and sialylation in vitro [168, 169] .
Ernst and Magnani examined the potential of glycomimetic drugs to block selectin interaction with the endogenous glycans [170]. Acetylated derivatives of GlcNAcβ3GalβO-Nap and Galβ4GlcNAcβ1O-Nap were used as decoys to disrupt the biosynthesis of natural ligands for selectins. Similarly, Marathe et al. evaluated the utility of acetylated F-4 GlcNAc as an inhibitor of lectin-mediated ovarian tumor cell adhesion [171]. This study indicates that fluorinated GalNAc metabolically alters glycan structures on mucins. Specifically, the glycans of leukocyte PSGL-1 are altered, which reduces cell binding to selectins and inhibits the biosynthesis of chondroitin sulfate. Further, acetylated F-4GalNAc exhibits superior metabolic inhibitory activity compared to the GalNAcα-OBenzyl inhibitor. Additionally, significant efforts have also been invested to synthesize/isolate natural products that can function as competitive inhibitors against their native substrates. Several of these small molecules that mimic the glycan epitope are currently undergoing clinical trials [172]. Even with this progress, further research is needed to identify effective glycan targets on mucins that aid in the progression of disease. Their discoveries will provide the platform for designing the systematic synthesis of therapeutic reagents.
8. Conclusion and perspectives
The glycosylation of mucins not only regulates their stability but also modulates their function. As discussed in this review, mucin glycosylation is altered during neoplastic transformation and cancer progression. This results in the expression of several truncated glycan epitopes on carcinoma mucins that are not seen in their normal counterparts. Currently, these carbohydrate structures also known as tumor associated carbohydrate antigens are the focus of translational research and have been utilized clinically for diagnosis/prognosis of several cancers. However, much improvement is required in order to generate diagnostic tools that offer greater sensitivity and specificity. This can potentially be achieved by detecting glycan changes on specific mucins. Further, in addition to their uses as biomarkers, significant efforts are also being made in designing glycan/glycopeptide vaccines.
Differential glycosylation of mucins during cancer is caused by several factors including genetic and epigenetic mechanisms, dysregulated glycosylation machinery, and the tumor microenvironment. Altered expression of specific glycosyltransferases leads to the formation of specific glycan structures (Tn, sTn, sLex, sLea) that are involved in cancer growth and metastasis due to several mechanisms. This altered expression provides a greater opportunity to screen for the specific inhibitors of glycosyltransferases. Knowledge of the specificities of these enzymes and their tissue distribution is important for drug discovery, especially for those enzymes involved in altering the glycan signature during cancer progression.
Considering the implications in cancer and other diseases, considerable interest has been generated to explain the role of glycans in the versatile functions of the mucins. While great strides have been made, further research is needed to identify the functional glycotopes on the surface of mucins that are responsible for cancer progression. To gain deep insight into the involvement of mucin glycans in cancer, we suggest the development of animal models of cancer with modulated expression of glycosyltransferases such as GALNTs, C1GALT1, and sialyltransferase. These improved models have the strong potential to help elucidate the functional significance of mucin glycosylation in cancer and lead to the identification of useful prognostic, diagnostic, and therapeutic targets.
Highlights.
Mucins are high molecular weight proteins that are aberrantly glycosylated in cancer.
Deregulated expression of mucin glycans alters biological properties of mucins.
Mucin glycans are involved in cancer growth and metastasis.
Glycans on mucins are clinically important as diagnostic markers.
Further research is needed to target specific glycans on mucins.
Acknowledgements
We would like to thank Ms. Melody Montgomery, Editor, for professionally editing this manuscript. Further, we acknowledge the invaluable support from Ms. Kavita Mallya. The authors in this article work were supported by grants from National Institutes of Health (UO1 CA111294, P50 CA127297, U54 CA163120, RO1 CA183459, RO1 CA195586, K22 CA175260 and P20 GM103480).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
I will greatly appreciate if you can exclude Dr. Kermit L. Carraway (Department of Cell Biology and Anatomy, University of Miami School of Medicine, FL) and Dr. Isabelle van Seuningen (INSERM, Lille, France) from reviewing this article due to conflict of interest.
References
- 1.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J. Biol. Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 5.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 7.Cullen PJ. Post-translational regulation of signaling mucins. Curr. Opin. Struct. Biol. 2011;21:590–596. doi: 10.1016/j.sbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Klinken BJ, Van der Wal JW, Einerhand AW, Buller HA, Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44:387–393. doi: 10.1136/gut.44.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011;3:a005199. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev. Biochem. Mol. Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 11.Pamela Stanley, Harry Schachter, and Naoyuki Taniguchi, Chapter 8 N-Glycans. Essentials of Glycobiology. (2nd edition) 2009 [Google Scholar]
- 12.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resto VA, Burdick MM, Dagia NM, McCammon SD, Fennewald SM, Sackstein R. L-selectin-mediated lymphocyte-cancer cell interactions under low fluid shear conditions. J. Biol. Chem. 2008;283:15816–15824. doi: 10.1074/jbc.M708899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 16.Inka Brockhausen, Harry Schachte, and Pamela Stanley, Chapter 9 O-GalNAc Glycans. Essentials of Glycobiology. (2nd edition) 2009 [Google Scholar]
- 17.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Ruddock LW, Molinari M. N-glycan processing in ER quality control. J. Cell Sci. 2006;119:4373–4380. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- 19.Parry S, Hanisch FG, Leir SH, Sutton-Smith M, Morris HR, Dell A, Harris A. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology. 2006;16:623–634. doi: 10.1093/glycob/cwj110. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Wang TT, DeMaria G, Sheehan JK, Kesimer M. Mapping the protein domain structures of the respiratory mucins: a mucin proteome coverage study. J. Proteome. Res. 2012;11:4013–4023. doi: 10.1021/pr300058z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J. Biol. Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 22.Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJ, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backstrom M, Ambort D, Thomsson E, Johansson ME, Hansson GC. Increased understanding of the biochemistry and biosynthesis of MUC2 and other gel-forming mucins through the recombinant expression of their protein domains. Mol. Biotechnol. 2013;54:250–256. doi: 10.1007/s12033-012-9562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho JJ, Jaituni RS, Crawley SC, Yang SC, Gum JR, Kim YS. N-glycosylation is required for the surface localization of MUC17 mucin. Int. J. Oncol. 2003;23:585–592. [PubMed] [Google Scholar]
- 25.Marth JD. Complexity in O-linked oligosaccharide biosynthesis engendered by multiple polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 1996;6:701–705. doi: 10.1093/glycob/6.7.701. [DOI] [PubMed] [Google Scholar]
- 26.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida A, Suzuki M, Ikenaga H, Takeuchi M. Discovery of the shortest sequence motif for high level mucin-type O-glycosylation. J. Biol. Chem. 1997;272:16884–16888. doi: 10.1074/jbc.272.27.16884. [DOI] [PubMed] [Google Scholar]
- 29.Kinarsky L, Suryanarayanan G, Prakash O, Paulsen H, Clausen H, Hanisch FG, Hollingsworth MA, Sherman S. Conformational studies on the MUC1 tandem repeat glycopeptides: implication for the enzymatic O-glycosylation of the mucin protein core. Glycobiology. 2003;13:929–939. doi: 10.1093/glycob/cwg109. [DOI] [PubMed] [Google Scholar]
- 30.Raman J, Fritz TA, Gerken TA, Jamison O, Live D, Liu M, Tabak LA. The catalytic and lectin domains of UDP-GalNAc:polypeptide alpha-N-Acetylgalactosaminyltransferase function in concert to direct glycosylation site selection. J. Biol. Chem. 2008;283:22942–22951. doi: 10.1074/jbc.M803387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aryal RP, Ju T, Cummings RD. The endoplasmic reticulum chaperone Cosmc directly promotes in vitro folding of T-synthase. J. Biol. Chem. 2010;285:2456–2462. doi: 10.1074/jbc.M109.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014;14:63–81. doi: 10.3233/CBM-130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran DT, Ten Hagen KG. Mucin-type O-glycosylation during development. J. Biol. Chem. 2013;288:6921–6929. doi: 10.1074/jbc.R112.418558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho JJ, Siddiki B, Kim YS. Association of sialyl-Lewis(a) and sialyl-Lewis(x) with MUC-1 apomucin ina pancreatic cancer cell line. Cancer Res. 1995;55:3659–3663. [PubMed] [Google Scholar]
- 35.Hauselmann I, Borsig L. Altered Tumor-Cell Glycosylation Promotes Metastasis. Front Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules. 2012;2:435–466. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelmann K, Baldus SE, Hanisch FG. Identification and topology of variant sequences within individual repeat domains of the human epithelial tumor mucin MUC1. J. Biol. Chem. 2001;276:27764–27769. doi: 10.1074/jbc.M103187200. [DOI] [PubMed] [Google Scholar]
- 38.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal. Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta JP, Michalski JC. Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 2003;278:46337–46348. doi: 10.1074/jbc.M302529200. [DOI] [PubMed] [Google Scholar]
- 40.Corfield AP. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK, Mucins in the pathogenesis of breast, cancer: implications in diagnosis. prognosis and therapy. Biochim. Biophys. Acta. 2011;1815:224–240. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng J, Wang L, Chen H, Li L, Ma Y, Ni J, Li Y. The role of tumour-associated MUC1 in epithelial ovarian cancer metastasis and progression. Cancer Metastasis Rev. 2013;32:535–551. doi: 10.1007/s10555-013-9423-y. [DOI] [PubMed] [Google Scholar]
- 45.Hinoda Y, Ikematsu Y, Horinochi M, Sato S, Yamamoto K, Nakano T, Fukui M, Suehiro Y, Hamanaka Y, Nishikawa Y, Kida H, Waki S, Oka M, Imai K, Yonezawa S. Increased expression of MUC1 in advanced pancreatic cancer. J. Gastroenterol. 2003;38:1162–1166. doi: 10.1007/s00535-003-1224-6. [DOI] [PubMed] [Google Scholar]
- 46.Wang RQ, Fang DC. Alterations of MUC1 and MUC3 expression in gastric carcinoma: relevance to patient clinicopathological features. J. Clin. Pathol. 2003;56:378–384. doi: 10.1136/jcp.56.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br. J. Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, Chakraborty S, Jain M, Pai P, Smith LM, Lele SM, Batra SK. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS. One. 2013;8:e54455. doi: 10.1371/journal.pone.0054455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rachagani S, Macha MA, Ponnusamy MP, Haridas D, Kaur S, Jain M, Batra SK. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis. 2012;33:1953–1964. doi: 10.1093/carcin/bgs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponnusamy MP, Seshacharyulu P, Vaz A, Dey P, Batra SK. MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. J. Ovarian. Res. 2011;4:7–4. doi: 10.1186/1757-2215-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haridas D, Ponnusamy MP, Chugh S, Lakshmanan I, Seshacharyulu P, Batra SK. MUC16: molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014;10:4183–4199. doi: 10.1096/fj.14-257352. [DOI] [PubMed] [Google Scholar]
- 53.Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, Patankar MS. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theriault C, Pinard M, Comamala M, Migneault M, Beaudin J, Matte I, Boivin M, Piche A, Rancourt C. MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 2011;121:434–443. doi: 10.1016/j.ygyno.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, Wittel UA, Hollingsworth MA, Batra SK. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS. One. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakshmanan I, Ponnusamy MP, Das S, Chakraborty S, Haridas D, Mukhopadhyay P, Lele SM, Batra SK. MUC16 induced rapid G2/M transition via interactions with JAK2 for increased proliferation and anti-apoptosis in breast cancer cells. Oncogene. 2012;31:805–817. doi: 10.1038/onc.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci. Rep. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maher DM, Gupta BK, Nagata S, Jaggi M, Chauhan SC. Mucin 13: structure, function, and potential roles in cancer pathogenesis. Mol. Cancer Res. 2011;9:531–537. doi: 10.1158/1541-7786.MCR-10-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K, Kakehashi A, Wei M, Hirakawa K, Wanibuchi H. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int. J. Oncol. 2011;38:619–627. doi: 10.3892/ijo.2011.911. [DOI] [PubMed] [Google Scholar]
- 60.Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL, Jenkins MA, Haydon AM, Rosty C, English DR, Giles GG, McGuckin MA, Young JP, Buchanan DD. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod. Pathol. 2013;26:1642–1656. doi: 10.1038/modpathol.2013.101. [DOI] [PubMed] [Google Scholar]
- 61.Kawashima H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol. Pharm. Bull. 2012;35:1637–1641. doi: 10.1248/bpb.b12-00412. [DOI] [PubMed] [Google Scholar]
- 62.Pan S, Chen R, Tamura Y, Crispin DA, Lai LA, May DH, McIntosh MW, Goodlett DR, Brentnall TA. Quantitative glycoproteomics analysis reveals changes in N-glycosylation level associated with pancreatic ductal adenocarcinoma. J. Proteome. Res. 2014;13:1293–1306. doi: 10.1021/pr4010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 64.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robbe-Masselot C, Maes E, Rousset M, Michalski JC, Capon C. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj. J. 2009;26:397–413. doi: 10.1007/s10719-008-9186-9. [DOI] [PubMed] [Google Scholar]
- 66.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim. Biophys. Acta. 1999;1455:301–313. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 67.Campbell BJ, Finnie IA, Hounsell EF, Rhodes JM. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. J. Clin. Invest. 1995;95:571–576. doi: 10.1172/JCI117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein A, Roussel P. O-acetylation of sialic acids. Biochimie. 1998;80:49–57. doi: 10.1016/s0300-9084(98)80056-4. [DOI] [PubMed] [Google Scholar]
- 69.Corfield AP, Myerscough N, Warren BF, Durdey P, Paraskeva C, Schauer R. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconj. J. 1999;16:307–317. doi: 10.1023/a:1007026314792. [DOI] [PubMed] [Google Scholar]
- 70.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim. Biophys. Acta. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 71.van den Brule F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj. J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 72.Rabinovich GA, Rubinstein N, Toscano MA. Role of galectins in inflammatory and immunomodulatory processes. Biochim. Biophys. Acta. 2002;1572:274–284. doi: 10.1016/s0304-4165(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Q. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. 2009;69:6799–6806. doi: 10.1158/0008-5472.CAN-09-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS, Kuo PL. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin alpha6beta4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370–1381. doi: 10.1093/carcin/bgt040. [DOI] [PubMed] [Google Scholar]
- 76.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 78.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 79.Kim YJ, Borsig L, Han HL, Varki NM, Varki A. Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am. J. Pathol. 1999;155:461–472. doi: 10.1016/S0002-9440(10)65142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Invest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 2002;12:609–615. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 82.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 83.Nath D, Hartnell A, Happerfield L, Miles DW, Burchell J, Taylor-Papadimitriou J, Crocker PR. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98:213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanida S, Akita K, Ishida A, Mori Y, Toda M, Inoue M, Ohta M, Yashiro M, Sawada T, Hirakawa K, Nakada H. Binding of the sialic acid-binding lectin Siglec-9 to the membrane mucin MUC1 induces recruitment of beta-catenin and subsequent cell growth. J. Biol. Chem. 2013;288:31842–31852. doi: 10.1074/jbc.M113.471318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belisle JA, Horibata S, Jennifer GA, Petrie S, Kapur A, Andre S, Gabius HJ, Rancourt C, Connor J, Paulson JC, Patankar MS. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer. 2010;9:118–119. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Costello RT, Gastaut JA, Olive D. Tumor escape from immune surveillance. Arch. Immunol. Ther. Exp. (Warsz.) 1999;47:83–88. [PubMed] [Google Scholar]
- 87.Madsen CB, Lavrsen K, Steentoft C, Vester-Christensen MB, Clausen H, Wandall HH, Pedersen AE. Glycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killing. PLoS. One. 2013;8:e72413. doi: 10.1371/journal.pone.0072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Havrylov S, Redowicz MJ, Buchman VL. Emerging roles of Ruk/CIN85 in vesicle-mediated transport, adhesion, migration and malignancy. Traffic. 2010;11:721–731. doi: 10.1111/j.1600-0854.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 90.Cascio S, Farkas AM, Hughey RP, Finn OJ. Altered glycosylation of MUC1 influences its association with CIN85: the role of this novel complex in cancer cell invasion and migration. Oncotarget. 2013;4:1686–1697. doi: 10.18632/oncotarget.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK. Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin. Cancer Res. 2011;17:267–274. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, Yu LG. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer. 2010;9:154–166. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, Pastan I, Patankar MS. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kinlough CL, Poland PA, Gendler SJ, Mattila PE, Mo D, Weisz OA, Hughey RP. Core-glycosylated mucin-like repeats from MUC1 are an apical targeting signal. J. Biol. Chem. 2011;286:39072–39081. doi: 10.1074/jbc.M111.289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 98.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am. J. Physiol Cell Physiol. 2006;290:C1–C10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 99.Paszkiewicz-Gadek A, Porowska H, Lemancewicz D, Wolczynski S, Gindzienski A. The influence of N- and O-glycosylation inhibitors on the glycosylation profile of cellular membrane proteins and adhesive properties of carcinoma cell lines. Int. J. Mol. Med. 2006;17:669–674. [PubMed] [Google Scholar]
- 100.Engelmann K, Kinlough CL, Muller S, Razawi H, Baldus SE, Hughey RP, Hanisch FG. Transmembrane and secreted MUC1 probes show trafficking-dependent changes in O-glycan core profiles. Glycobiology. 2005;15:1111–1124. doi: 10.1093/glycob/cwi099. [DOI] [PubMed] [Google Scholar]
- 101.Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 102.Varki A. Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol. 1998;8:34–40. doi: 10.1016/s0962-8924(97)01198-7. [DOI] [PubMed] [Google Scholar]
- 103.Rini J, Esko J, Varki A. Glycosyltransferases and Glycan-processing Enzymes. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 5. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1921/ [PubMed] [Google Scholar]
- 104.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Kannagi R, Sakuma K, Miyazaki K, Lim KT, Yusa A, Yin J, Izawa M. Altered expression of glycan genes in cancers induced by epigenetic silencing and tumor hypoxia: clues in the ongoing search for new tumor markers. Cancer Sci. 2010;101:586–593. doi: 10.1111/j.1349-7006.2009.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maupin KA, Sinha A, Eugster E, Miller J, Ross J, Paulino V, Keshamouni VG, Tran N, Berens M, Webb C, Haab BB. Glycogene expression alterations associated with pancreatic cancer epithelial-mesenchymal transition in complementary model systems. PLoS. One. 2010;5:e13002. doi: 10.1371/journal.pone.0013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalthoff H, Kreiker C, Schmiegel WH, Greten H, Thiele HG. Characterization of CA 19-9 bearing mucins as physiological exocrine pancreatic secretion products. Cancer Res. 1986;46:3605–3607. [PubMed] [Google Scholar]
- 108.Miyazaki K, Ohmori K, Izawa M, Koike T, Kumamoto K, Furukawa K, Ando T, Kiso M, Yamaji T, Hashimoto Y, Suzuki A, Yoshida A, Takeuchi M, Kannagi R. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 109.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kawamura YI, Toyota M, Kawashima R, Hagiwara T, Suzuki H, Imai K, Shinomura Y, Tokino T, Kannagi R, Dohi T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology. 2008;135:142–151. doi: 10.1053/j.gastro.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 111.Groux-Degroote S, Wavelet C, Krzewinski-Recchi MA, Portier L, Mortuaire M, Mihalache A, Trinchera M, Delannoy P, Malagolini N, Chiricolo M, Dall’Olio F, Harduin-Lepers A. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014;53:442–449. doi: 10.1016/j.biocel.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 112.Malagolini N, Dall’Olio F, Di SG, Minni F, Marrano D, Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc alpha 2,3Gal beta-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466–6470. [PubMed] [Google Scholar]
- 113.Dohi T, Kawamura YI. Incomplete synthesis of the Sda/Cad blood group carbohydrate in gastrointestinal cancer. Biochim. Biophys. Acta. 2008;1780:467–471. doi: 10.1016/j.bbagen.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 114.Kawamura YI, Kawashima R, Fukunaga R, Hirai K, Toyama-Sorimachi N, Tokuhara M, Shimizu T, Dohi T. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005;65:6220–6227. doi: 10.1158/0008-5472.CAN-05-0639. [DOI] [PubMed] [Google Scholar]
- 115.Keita M, Wang ZQ, Pelletier JF, Bachvarova M, Plante M, Gregoire J, Renaud MC, Mes-Masson AM, Paquet ER, Bachvarov D. Global methylation profiling in serous ovarian cancer is indicative for distinct aberrant DNA methylation signatures associated with tumor aggressiveness and disease progression. Gynecol. Oncol. 2013;128:356–363. doi: 10.1016/j.ygyno.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 116.Wang ZQ, Bachvarova M, Morin C, Plante M, Gregoire J, Renaud MC, Sebastianelli A, Bachvarov D. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: possible implications in abnormal mucin O-glycosylation. Oncotarget. 2014;5:544–560. doi: 10.18632/oncotarget.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaziel-Sovran A, Segura MF, Di MR, Collins MK, Hanniford D, Vega-Saenz de ME, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tu L, Banfield DK. Localization of Golgi-resident glycosyltransferases. Cell Mol. Life Sci. 2010;67:29–41. doi: 10.1007/s00018-009-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pokrovskaya ID, Willett R, Smith RD, Morelle W, Kudlyk T, Lupashin VV. Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery. Glycobiology. 2011;21:1554–1569. doi: 10.1093/glycob/cwr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jackson CL. Mechanisms of transport through the Golgi complex. J. Cell Sci. 2009;122:443–452. doi: 10.1242/jcs.032581. [DOI] [PubMed] [Google Scholar]
- 122.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Landmark, editor. Mechanisms of cancer-associated glycosylation changes. Front Biosci. 2012;17:670–699. doi: 10.2741/3951. [DOI] [PubMed] [Google Scholar]
- 124.Freeze HH, Elbein AD. Glycosylation Precursors. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Chapter 4. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1929/ [PubMed] [Google Scholar]
- 125.Kumamoto K, Goto Y, Sekikawa K, Takenoshita S, Ishida N, Kawakita M, Kannagi R. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen-Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res. 2001;61:4620–4627. [PubMed] [Google Scholar]
- 126.Yusa A, Miyazaki K, Kimura N, Izawa M, Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70:4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]
- 127.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 128.Koike T, Kimura N, Miyazaki K, Yabuta T, Kumamoto K, Takenoshita S, Chen J, Kobayashi M, Hosokawa M, Taniguchi A, Kojima T, Ishida N, Kawakita M, Yamamoto H, Takematsu H, Suzuki A, Kozutsumi Y, Kannagi R. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng FL, Lin CH, Sato C, Kitajima K, Kannagi R. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66:2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 130.Banda K, Gregg CJ, Chow R, Varki NM, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. J. Biol. Chem. 2012;287:28852–28864. doi: 10.1074/jbc.M112.364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J. Biol. Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 132.Ota H, Nakayama J, Momose M, Hayama M, Akamatsu T, Katsuyama T, Graham DY, Genta RM. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998;433:419–426. doi: 10.1007/s004280050269. [DOI] [PubMed] [Google Scholar]
- 133.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 134.Campbell BJ, Yu LG, Rhodes JM. Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj. J. 2001;18:851–858. doi: 10.1023/a:1022240107040. [DOI] [PubMed] [Google Scholar]
- 135.Renkonen J, Tynninen O, Hayry P, Paavonen T, Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am. J. Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, Hansson GC. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel. Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 138.Wu YM, Nowack DD, Omenn GS, Haab BB. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J. Proteome. Res. 2009;8:1876–1886. doi: 10.1021/pr8008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kreisman LS, Cobb BA. Infection, inflammation and host carbohydrates: a Glyco-Evasion Hypothesis. Glycobiology. 2012;22:1019–1030. doi: 10.1093/glycob/cws070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barasch J, Kiss B, Prince A, Saiman L, Gruenert D, al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991;352:70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- 141.Kellokumpu S, Sormunen R, Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH. FEBS Lett. 2002;516:217–224. doi: 10.1016/s0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- 142.Rivinoja A, Kokkonen N, Kellokumpu I, Kellokumpu S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell Physiol. 2006;208:167–174. doi: 10.1002/jcp.20653. [DOI] [PubMed] [Google Scholar]
- 143.Chandrasekaran EV, Xue J, Xia J, Locke RD, Patil SA, Neelamegham S, Matta KL. Characterization of cancer associated mucin type O-glycans using the exchange sialylation properties of mammalian sialyltransferase ST3Gal-II. J. Proteome. Res. 2012;11:2609–2618. doi: 10.1021/pr201108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tuccillo FM, de LA, Palmieri C, Fiume G, Bonelli P, Borrelli A, Tassone P, Scala I, Buonaguro FM, Quinto I, Scala G. Aberrant glycosylation as biomarker for cancer: focus on CD43. Biomed. Res. Int. 2014;2014:742831. doi: 10.1155/2014/742831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peracaula R, Barrabes S, Sarrats A, Rudd PM, de LR. Altered glycosylation in tumours focused to cancer diagnosis. Dis. Markers. 2008;25:207–218. doi: 10.1155/2008/797629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochim. Biophys. Acta. 2012;1820:1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 147.Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, Fass L, Kaur J, Hu K, Shojaei H, Whelan RJ, Patankar MS. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol. Cancer. 2014;13:129. doi: 10.1186/1476-4598-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soletormos G, Nielsen D, Schioler V, Mouridsen H, Dombernowsky P. Monitoring different stages of breast cancer using tumour markers CA 15-3, CEA and TPA. Eur. J. Cancer. 2004;40:481–486. doi: 10.1016/j.ejca.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 149.Safi F, Schlosser W, Kolb G, Beger HG. Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J. Gastrointest. Surg. 1997;1:106–112. doi: 10.1016/s1091-255x(97)80097-2. [DOI] [PubMed] [Google Scholar]
- 150.Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis. Markers. 2000;16:105–110. doi: 10.1155/2000/595492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wahrenbrock MG, Varki A. Multiple hepatic receptors cooperate to eliminate secretory mucins aberrantly entering the bloodstream: are circulating cancer mucins the “tip of the iceberg”? Cancer Res. 2006;66:2433–2441. doi: 10.1158/0008-5472.CAN-05-3851. [DOI] [PubMed] [Google Scholar]
- 152.Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, Ragupathi G, Livingston PO, Hollingsworth MA, Taylor-Papadimitriou J, Burchell J, Clausen H. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pedersen JW, Blixt O, Bennett EP, Tarp MA, Dar I, Mandel U, Poulsen SS, Pedersen AE, Rasmussen S, Jess P, Clausen H, Wandall HH. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int. J. Cancer. 2011;128:1860–1871. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- 154.Pinto R, Carvalho AS, Conze T, Magalhaes A, Picco G, Burchell JM, Taylor-Papadimitriou J, Reis CA, Almeida R, Mandel U, Clausen H, Soderberg O, David L. Identification of new cancer biomarkers based on aberrant mucin glycoforms by in situ proximity ligation. J. Cell Mol. Med. 2012;16:1474–1484. doi: 10.1111/j.1582-4934.2011.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo Z, Wang Q. Recent development in carbohydrate-based cancer vaccines. Curr. Opin. Chem. Biol. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miles D, Papazisis K. Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin. Breast Cancer. 2003;3(Suppl 4):S134–S138. doi: 10.3816/cbc.2003.s.002. [DOI] [PubMed] [Google Scholar]
- 157.Ibrahim NK, Murray JL. Clinical development of the STn-KLH vaccine (Theratope) Clin. Breast Cancer. 2003;3(Suppl. 4):S139–S143. doi: 10.3816/cbc.2003.s.003. [DOI] [PubMed] [Google Scholar]
- 158.Parry AL, Clemson NA, Ellis J, Bernhard SS, Davis BG, Cameron NR. ‘Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013;135:9362–9365. doi: 10.1021/ja4046857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kaiser A, Gaidzik N, Westerlind U, Kowalczyk D, Hobel A, Schmitt E, Kunz H. A synthetic vaccine consisting of a tumor-associated sialyl-T(N)-MUC1 tandem-repeat glycopeptide and tetanus toxoid: induction of a strong and highly selective immune response. Angew. Chem. Int. Ed Engl. 2009;48:7551–7555. doi: 10.1002/anie.200902564. [DOI] [PubMed] [Google Scholar]
- 160.Westerlind U, Hobel A, Gaidzik N, Schmitt E, Kunz H. Synthetic vaccines consisting of tumor-associated MUC1 glycopeptide antigens and a T-cell epitope for the induction of a highly specific humoral immune response. Angew. Chem. Int. Ed Engl. 2008;47:7551–7556. doi: 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]
- 161.Cai H, Huang ZH, Shi L, Zhao YF, Kunz H, Li YM. Towards a fully synthetic MUC1-based anticancer vaccine: efficient conjugation of glycopeptides with mono-, di-, and tetravalent lipopeptides using click chemistry. Chemistry. 2011;17:6396–6406. doi: 10.1002/chem.201100217. [DOI] [PubMed] [Google Scholar]
- 162.Abdel-Aal AB, Lakshminarayanan V, Thompson P, Supekar N, Bradley JM, Wolfert MA, Cohen PA, Gendler SJ, Boons GJ. Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist. Chembiochem. 2014;15:1508–1513. doi: 10.1002/cbic.201402077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules. 2012;2:435–466. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hsu CC, Lin TW, Chang WW, Wu CY, Lo WH, Wang PH, Tsai YC. Soyasaponin-I-modified invasive behavior of cancer by changing cell surface sialic acids. Gynecol. Oncol. 2005;96:415–422. doi: 10.1016/j.ygyno.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 165.Chiang CH, Wang CH, Chang HC, More SV, Li WS, Hung WC. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J. Cell Physiol. 2010;223:492–499. doi: 10.1002/jcp.22068. [DOI] [PubMed] [Google Scholar]
- 166.Chang WW, Yu CY, Lin TW, Wang PH, Tsai YC. Soyasaponin I decreases the expression of alpha2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 2006;341:614–619. doi: 10.1016/j.bbrc.2005.12.216. [DOI] [PubMed] [Google Scholar]
- 167.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 168.Macauley MS, Arlian BM, Rillahan CD, Pang PC, Bortell N, Marcondes MC, Haslam SM, Dell A, Paulson JC. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J Biol. Chem. 2014;289:35149–35158. doi: 10.1074/jbc.M114.606517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, Haslam SM, Paulson JC. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012;8:661–668. doi: 10.1038/nchembio.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ernst B, Magnani JL. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marathe DD, Buffone A, Jr, Chandrasekaran EV, Xue J, Locke RD, Nasirikenari M, Lau JT, Matta KL, Neelamegham S. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood. 2010;115:1303–1312. doi: 10.1182/blood-2009-07-231480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, Schwientek T, Graham R, Taylor-Papadimitriou J, Hollingsworth MA, Burchell J, Clausen H. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]