Abstract
Neural progenitors are central players in the development of the brain neural circuitry. They not only produce the diverse neuronal and glial cell types in the brain, but also guide their migration in this process. Recent evidence indicates that neural progenitors also play a critical role in the development of the brain vascular network. At an early stage, neural progenitors have been found to facilitate the ingression of blood vessels from outside the neural tube, through VEGF and canonical Wnt signaling. Subsequently, neural progenitors directly communicate to endothelial cells to stabilize nascent brain vessels, in part through down-regulating Wnt pathway activity. Furthermore, neural progenitors promote nascent brain vessel integrity, through integrin αvβ8-dependent TGFβ signaling. In this review, we will discuss the evidence for, as well as questions that remain, regarding these novel roles of neural progenitors and the underlying mechanisms in their regulation of the nascent brain vascular network.
Keywords: neural progenitor, blood vessel stabilization, VEGF, Wnt/β-catenin, TGFβ, integrin αvβ8
1. Introduction
In the developing brain, neural progenitors refer to undifferentiated cells that eventually give rise to all the major cell types in the adult brain. These stem cells, called radial glia, derive from the early pseudostratified neuroepithelium (Kriegstein and Alvarez-Buylla, 2009), and are the founder for most mature neural cells, including neurons and glia, in the central nervous system (CNS) (Anthony et al., 2004). They also provide the scaffolding for migrating neurons during the formation of the layered cerebral cortex (Rakic, 1971). While it was clear from decades of research that radial glia play a pivotal role in neurogenesis, their impact on vascular development has only recently come under the spotlight (Ma et al., 2013). The vascular system delivers oxygen and nutrients to tissues throughout the body, and vessels are known to show distinct patterns based on the metabolic demands of specific tissues. This is especially prominent in the brain, an organ that consumes 10 times as much energy per unit volume as the rest of the body (Clarke and Sokoloff, 1999). Accordingly, the brain vasculature displays a highly complex and hierarchical pattern, with layer upon layers of organization (Blinder et al., 2013; Schaffer et al., 2006). This suggests intimate communication between blood vessel and neural cell types during brain vascular development. Indeed, recent findings have implicated neural progenitors as a major neural cell type in the regulation of several early steps of brain vessel development, including initial vessel ingression from outside the CNS, nascent vessel stabilization as well as vessel maturation and integrity (Arnold et al., 2014; Daneman et al., 2009; Haigh et al., 2003; James et al., 2009; Ma et al., 2013; McCarty et al., 2005; Proctor et al., 2005; Raab et al., 2004; Stenman et al., 2008). In the article, we will review the signaling mechanisms by which neural progenitors regulate these key steps of brain vascular network formation.
2. Regulation of vessel ingression by VEGF and Wnt signaling from neural progenitors
The first step in the development of CNS vasculature is the ingression of blood vessels into the neural tube. Originally it was thought that the neural tube contained angioblasts born in situ. However, grafting experiments using chick-quail chimeras showed that endothelial cells in the neural tube have an extraneural origin (Kurz et al., 1996). Studies over the past two decades showed that neural progenitors, the main cell type in the neural tube at the onset of vessel ingression, play a pivotal role in promoting vessel ingrowth. Vascular endothelial growth factor (VEGF) is a major mitogen for endothelial cells and regulates angiogenesis throughout the body (Carmeliet et al., 1996; Ferrara et al., 1996). VEGF is strongly induced by hypoxia via activation of the oxygen sensing hypoxia-inducible factor (HIF) (Semenza, 2012) and is found to generally co-localize with hypoxic regions in the embryo (Lee et al., 2001). In the early brain neuroepithelium, VEGF mRNA is strongly expressed in the ventricular zone, a region occupied by the neural progenitor cell body (Breier et al., 1992). This suggests that neural progenitors, driven by hypoxia-HIF activity and potentially other factors, may provide VEGF as a spatial cue for vessel ingression. Several groups have tested this idea using both genetic knockout and mis-expression approaches (Haigh et al., 2003; James et al., 2009; Raab et al., 2004). By taking advantage of VEGF-A conditional, as well as hypomorphic alleles, Haigh et al showed that CNS-specific loss of VEGF-A exerts a dosage-dependent effect on vessel development. In mice with CNS-specific deletion of one VEGF-A allele, they found a moderate decrease in vascular density in the postnatal brain. When a hypomorphic allele was introduced in the place of the wild type allele to further reduce VEGF dosage, this resulted in a drastic vascular deficiency in the cortex. These effects can be observed as early as embryonic day 10.5 (E10.5) with corresponding dosage effects on vessel branching (Haigh et al., 2003). Similarly, Raab et al generated mice with a complete loss of VEGF-A from the CNS, which resulted in smaller brains with reduced blood vessel density. Importantly, Raab et al found that at E10.5, large areas in the forebrain were avascular, indicating that VEGF is required for the invasion of blood vessels into the neural tube. Furthermore, they found that in the mid- and hindbrain, capillaries that have invaded stopped their radial growth and grew parallel to the ventricular zone (Raab et al., 2004). These results demonstrate that the spatial expression of VEGF in the embryonic brain is important for the invasion of capillary sprouts, and that local VEGF gradients provide positional information.
More recently, using mis-expression approaches, researchers have further illustrated the instructive role of VEGF-A in guiding vessel ingression (James et al., 2009). In the developing spinal cord, blood vessels enter the neural tube in a highly stereotypic manner, which suggests existence of local cues guiding vessel ingression. To analyze potential roles of the various VEGF-A isoforms, James et al optimized conditions for moderate and localized ectopic expression of VEGF 121, VEGF 165, or VEGF 189 in the neural tube. They found that ectopic blood vessel ingression was induced by the heparin-binding isoforms, VEGF165 and VEGF189. Indeed, supernumerary ingression points were consistently localized next to cells mis-expressing either of these isoforms. By contrast, ectopic expression of VEGF 121, which does not bind to heparin and is more diffusible in the extracellular space, did not induce ectopic ingression. These manipulations highlight the role played by specific VEGF isoforms in the process of vessel ingression.
Vessel ingression into the CNS is regulated not only by VEGF signaling, but also by CNS-specific pathways, especially canonical Wnt signaling, that emanate from neural progenitors (Fig. 1). Several Wnt ligands, especially Wnt7a and Wnt7b, are highly expressed in the neural tube during early development, and Wnt pathway activity is observed in CNS endothelial cells (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008). To examine whether neural progenitor-originated Wnt signaling regulates CNS angiogenesis, Stenman et al employed genetic approaches and found that conditional removal of both Wnt7a and Wnt7b from the neural tube resulted in severe hemorrhaging specific to the CNS (Stenman et al., 2008). These mutations also resulted in a severely reduced number of endothelial cells and pericytes in the neural tube. Consistent with the interpretation that Wnt signaling is directly received by endothelial cells, they also found that specific deletion of β-catenin, a key mediator of canonical Wnt signaling, from endothelial cells resulted in similar CNS-specific hemorrhaging as well as the absence of both endothelial cells and pericytes from the neuroepithelium. In contrast, removal of β-catenin from neural progenitors did not produce any obvious vascular phenotypes. These results thus revealed a specific role of neural progenitor-derived Wnt ligands in promoting vessel ingression into the CNS. Around the same time, Daneman et al also provided evidence for a critical role of Wnt/β-catenin signaling in CNS angiogenesis (Daneman et al., 2009). They found that the Wnt pathway was specifically activated in endothelial cells of the CNS during development, which correlated with neural progenitor expression of Wnt ligands and blood vessel expression of Wnt receptors. Importantly, through their experiments, Daneman et al showed that endothelial specific deletion of β-catenin resulted in major vascular defects in the CNS, but not in non-neural tissues including the liver, lung, skin, jaw and tail. Similarly, systematic delivery of a dominant negative Frizzled construct that interferes with Wnt signaling also resulted in vascular malformations in the forebrain, but not in non-neural tissues. Furthermore, they found that Wnt7a induced a strong effect on the migration of a mouse brain endothelial cell line in vitro. Taken together, these results illustrate a specific role of canonical Wnt signaling in promoting CNS angiogenesis.
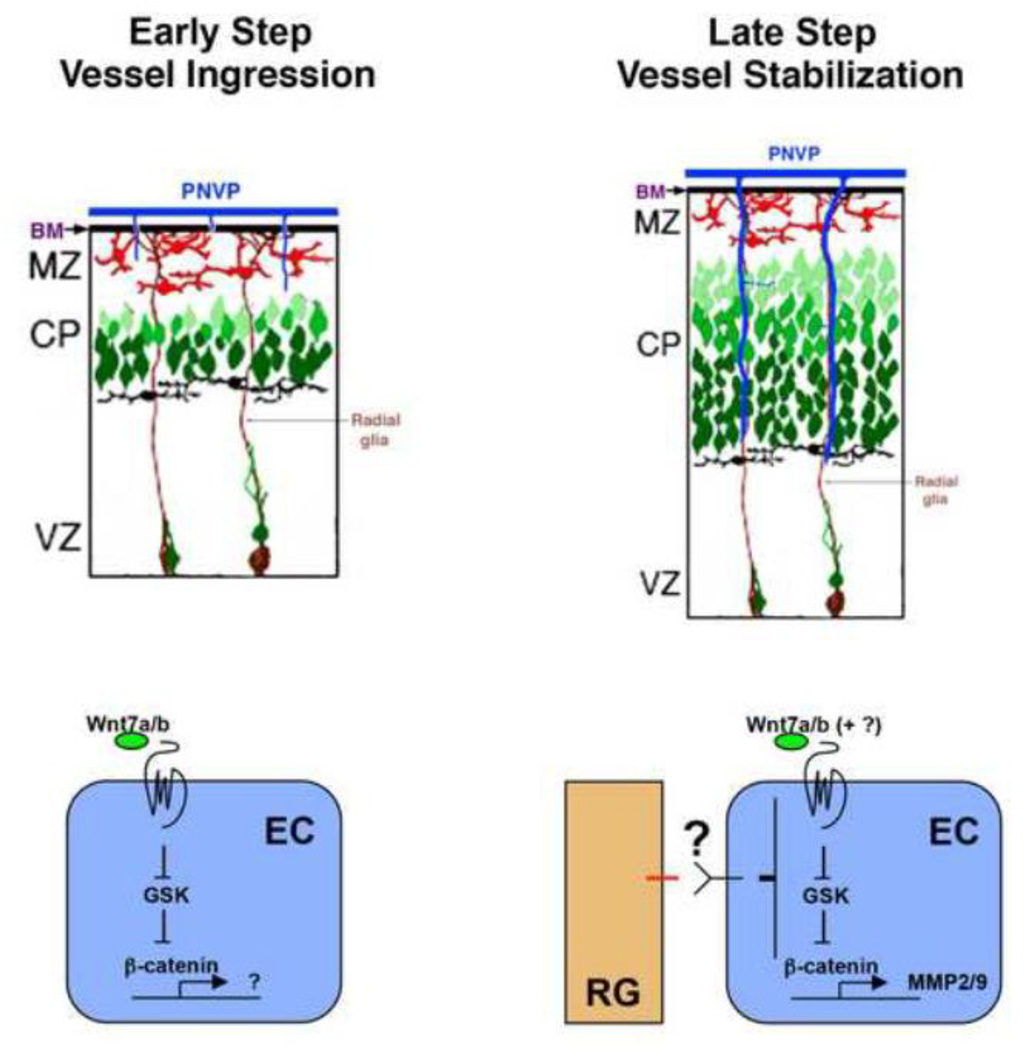
Figure 1. Neural progenitor (radial glial) regulation of vessel ingression and stabilization during vascular network formation in the developing brain.
At the onset of brain vascular network formation, Wnt7a/7b signaling from neural progenitors (radial glia, RG) is essential for activation of the canonical GSK/β-catenin pathway in endothelial cells (ECs) and facilitates EC migration and ingression from the perineural vascular plexus (PNVP) into the brain (early step). As brain vascular network begins to elaborate during late embryogenesis, neural progenitors send a second as yet unknown signal (large question mark) that shuts down canonical Wnt pathway activity and Wnt target gene expression, including that of matrix metalloproteinase 2 and 9 (MMP2/9), in ECs, allowing nascent vessels to become stabilized (late step). BM, basement membrane; MZ, marginal zone; CP, cortical plate; VZ, ventricular zone.
Interestingly, recent findings indicate that Wnt7a/b signaling in the brain requires the unique involvement of several unconventional cell surface components including GPR124, a G protein-coupled receptor (GPCR) of the adhesion GPCR subfamily. First noted for its high expression in the tumor vasculature, GPR124 is prominently expressed in brain endothelial cells during embryogenesis and, when mutated, results in severe brain hemorrhage and angiogenesis arrest (Cullen et al., 2011; Kuhnert et al., 2010). These phenotypic similarities to Wnt7a/b mutants prompted several groups to investigate a potential link. Indeed, GPR124 was found to potently and specifically stimulate Wnt7a/b-β-catenin signaling in cultured cells and to genetically interact with the canonical Wnt-β-catenin pathway in vivo (Posokhova et al., 2015; Vanhollebeke et al., 2015; Zhou and Nathans, 2014). Furthermore, by using a zebrafish model with live single-cell resolution, Vanhollebeke et al found that this GPR124-dependent Wnt7a/b-β-catenin pathway selectively controls tip cell function. These results have provided new insights into the regulation of brain angiogenesis by Wnt-β-catenin signaling.
3. Neural progenitors promote down-regulation of endothelial Wnt signaling and stabilization of nascent brain vessels
Blood vessels in the embryonic cortex, after initial ingression and rapid expansion, show an overall reduction in branching frequency despite a relatively constant net growth rate (Ma et al., 2013). This suggests potential involvement of vessel stabilization and/or pruning in cortical vascular patterning during late embryonic stages. A role for neural progenitors in this process was first revealed by chance, following the ablation of radial glia at this stage using a conditional orc3 allele, which resulted in brain vessel regression (Ma et al., 2013) (Fig. 1). orc3 encodes one of the core subunits of origin recognition complex (ORC), a protein complex essential for DNA replication and the cell cycle. Ma et al used a neural cell-specific nestin-cre to delete orc3 from cortical radial glia. Not surprisingly, they found that this resulted in the blockade of radial glial proliferation, leading to their depletion during late embryogenesis and consequently disrupted neurogenesis. To their surprise, they found that this also caused severe cortical hemorrhaging especially near the midline, an area with the most severe radial glial depletion. Analysis showed that blood vessels were nearly completely absent from the neonatal mutant cortex (Fig. 2). However, these observed vascular alterations do not appear to be secondary effects due to neurogenesis defects, since other mutations that similarly affect cortical neuron production and/or migration, as in orc3/nestin-cre mutants, do not produce these phenotypes. Moreover, deleting orc3 from radial glia using a different neural cell-specific hGFAP-cre also produced similar vascular phenotypes (Ma et al., 2012). Further analysis revealed that the cortical vascular network initially developed normally in the mutants, but after E16.5, vessel density and branching frequency began to decrease. Even after adjusting for increases in cortical size, vessel density in the mutants still decreased from E16.5 to E17.5 and from E17.5 to P0. Thus, not only does radial glial ablation result in stalled vessel growth, but it also leads to destabilization and consequent loss of vessels already formed. Thus, for the first time, these results implicate neural progenitors in the stabilization of nascent brain vascular network.
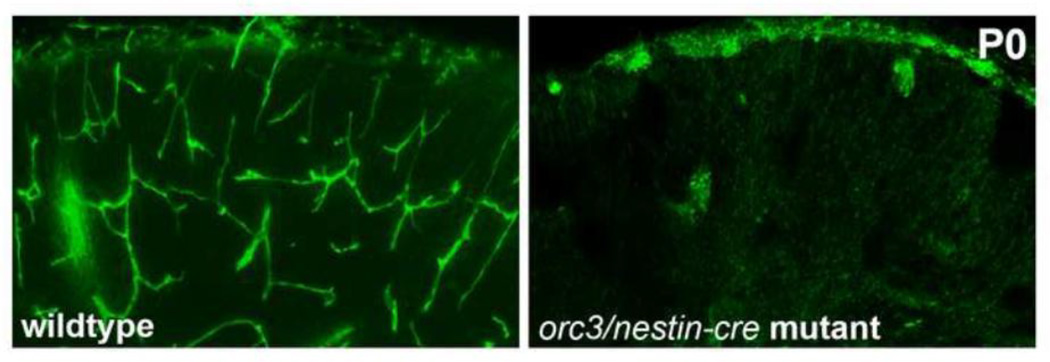
Figure 2. Radial glial ablation results in vessel loss in the developing mouse brain.
An elaborate vascular network (isolectin B4 staining, in green) is observed in wildtype cerebral cortex at the neonatal stage (P0). Following radial glial ablation during mid corticogenesis in the orc3/nestin-cre mutants, few vessels are observed in the neonatal brain. Analysis showed that this resulted from blood vessel regression during late embryogenesis.
To better understand the molecular mechanisms at play, Ma and colleagues focused on the Wnt pathway. As mentioned previously, Wnt signals provided by neural progenitors are critical to initial vessel ingression into the neural tube. Later, as vessel development continues in the brain during late embryogenesis, Wnt signaling becomes down-regulated (Liebner et al., 2008; Ma et al., 2013). Interestingly, Ma et al found that in orc3/nestin-cre mutants, Wnt signaling was not down-regulated, but remained high in brain endothelial cells. They found that the mutant endothelial cells exhibited increased proliferation and elevated expression of several genes regulated by Wnt signaling. The expression level of the BAT-lacZ Wnt pathway reporter was also substantially higher along mutant vessels, indicating abnormally high Wnt signaling in mutant endothelial cells. These authors further demonstrated that this ectopic Wnt signaling is at least in part responsible for cortical vessel regression. They showed that pharmacological activation of Wnt signaling during late embryonic corticogenesis resulted in vessel regression similar to that observed in orc3/nestin-cre mutants. This indicates that elevated endothelial Wnt signaling can lead to brain vessel destabilization and regression. They also showed that attenuating canonical Wnt signaling in orc3/nestin-cre mutants, by genetically removing one of the Wnt ligands involved, partially restored the vascular network and suppressed the severity of the hemorrhage. This indicates that the normal down-regulation of canonical Wnt signaling in cortical endothelial cells during development is critical for nascent brain vascular network stabilization, and neural progenitors play a pivotal role in this process. In addition, Ma et al provided some clues about why endothelial down-regulation of Wnt signaling may be important for vessel stabilization. They showed that elevated Wnt signaling is associated with increased vessel expression of matrix metalloproteinases as well as decreased vascular basement integrity. They further established that genetic inhibition of matrix metalloproteinase activity in orc3/nestin-cre mutants partially restored the brain vascular network and suppressed hemorrhaging. These results provide novel insights into the regulation of nascent brain vessel stabilization, a process that remains poorly understood at the molecular level.
Although the question of how neural progenitors down-regulate Wnt signaling remains open at the molecular level, Ma et al showed that this likely involves short distance interactions between radial glia and endothelial cells. Through three-dimensional confocal microscopy, these authors showed that in the embryonic cortex, radial glial processes physically interact with endothelial cells through surface areas not bound by pericytes. This suggests direct communication and coordination between these cell types during development. Employing an in vitro low-density co-culture assay, they demonstrated that endothelial cells in contact with neural progenitors overwhelmingly down-regulate Wnt signaling while those in isolation do not. This suggests that a short distance interaction is responsible for radial glial down-regulation of Wnt signaling in endothelial cells. At the molecular level, Wnt signaling is a well-studied pathway regulated by a multitude of mechanisms (Clevers and Nusse, 2012). For example, it has been shown that the Wnt pathway cross-talks with many other signaling pathways during development (e.g. Collu et al., 2014; Itasaki and Hoppler, 2010). Vertebrate Wnt target genes are also regulated by a variety of LEF/TCF isoforms, each of which possesses distinct transcriptional regulatory activities (Cadigan and Waterman, 2012). In addition, in different cell types, Wnt target genes appear to be influenced by distinct epigenetic modifications (Wohrle et al., 2007). Furthermore, Wnt pathway components and modulators can themselves be regulated at the epigenetic level (e.g., Jiang et al., 2008). These nodes of regulation provide many potential input points for radial glial down-regulation of endothelial Wnt signaling. Elucidation of the nature of the signal(s) involved as well as how it impinges on these nodes will further illuminate mechanisms of neurovascular crosstalk during brain vascular development.
4. Regulation of brain vessel maturation and integrity by integrin αvβ8-dependent TGFβ signaling from neural progenitors
Besides ingression and stabilization, neural progenitors also play a key role in regulating the integrity of nascent blood vessels in the developing brain. This function of neural progenitors was first suggested by the finding that genetic knockout of integrin αv resulted in severe intracerebral hemorrhaging in mutants that survived to the neonatal/postnatal stage (Bader et al., 1998; McCarty et al., 2002). In the embryonic cortex, αv expression is restricted to radial glial cells (Schmid and Anton, 2003). This suggests that αv function in neural progenitors is critical for regulating brain vessel integrity, likely including endothelial-endothelial as well as endothelial-pericyte cell-cell junctions and interactions. The subsequent observation of extensive intracerebral hemorrhaging in integrin β8 mutants further strengthened this interpretation, since β8 expression is also localized to periventricular (radial glial) cells in the brain (Zhu et al., 2002). Importantly, although αv subunits can normally form functional dimers with a number of β subunits, including β1, β3, β5, β6, and β8, only β8 mutants displayed vascular phenotypes similar to αv. This indicates that αv likely partners specifically with β8 in regulating brain vessel development. Curiously, despite distended vessel morphology, most other aspects of vessel development, including endothelial cell proliferation, migration, tube formation, sprouting and branching, appear normal in both αv and β8 mutant brains. Thus, integrin αvβ8 acts specifically to regulate vessel differentiation and integrity in the brain.
Cell type specific gene deletions have since further established the role of neural progenitors in regulating brain vessel integrity (McCarty et al., 2005; Proctor et al., 2005). By taking advantage of two different neural cell specific cre lines, hGFAP-cre and nestin-cre, McCarty et al showed that integrin αv is specifically required in neural cell types. Consistent with this conclusion, they also found that αv deletion using the endothelial cell-specific Tie2-cre line did not result in cerebral vascular defects (McCarty et al., 2005). Similarly, Proctor et al demonstrated that only deleting integrin β8 from neural cell types using nestin-cre resulted in cerebral hemorrhage, while deleting β8 from endothelial cells using Tie2-cre did not. Notably, deleting β8 from post-mitotic neurons using a nex-cre also failed to perturb brain vessel development (Proctor et al., 2005). Except for a very small number of immature astrocytes, radial glia and neurons are the only neural cell types in the brain throughout embryonic development (Kriegstein and Alvarez-Buylla, 2009). This therefore, specifically implicates neural progenitors as essential regulators of nascent brain vessel integrity.
Recent evidence indicates that neural progenitors likely regulate brain vessel integrity through αvβ8-dependent activation of transforming growth factor β (TGFβ) signaling (Fig. 3). TGFβ is a major family of extracellular signaling molecules involved in many developmental, physiological and pathological processes, including angiogenesis throughout the body (Jakobsson and van Meeteren, 2013; ten Dijke and Arthur, 2007). TGFβ ligands normally exist in a latent form, bound by peptides that include the latency-associated peptides (LAPs), which are themselves peptide fragments derived from the amino-terminal regions of full-length pro-TGFβ. Studies have found that integrin αvβ8 can activate TGFβ1 and TGFβ3 through binding to and then dissociating LAPs from the latent TGFβ complex, via a process dependent on membrane-bound metalloproteinases (Mu et al., 2002; Yang et al., 2007). In fact, double mutations in TGFβ1 and TGFβ3, consisting of a TGFβ3 null mutation in combination with a mutation in the LAP region of TGFβ1 that renders it incompatible for αvβ8 binding and activation, resulted in a cerebral hemorrhage phenotype very similar to αv and β8 mutations (Mu et al., 2008). This indicates that neural progenitor-expressed integrin αvβ8 likely regulates TGFβ activation to control vessel integrity. This conclusion is supported by the most recent finding that neural cell-specific β8 deletion results in a loss of active TGFβ1 from the brain, as well as a reduction in the endothelial activity of Smad3, a downstream mediator of TGFβ signaling (Arnold et al., 2014). It is also supported by previous findings from the adult brain and from other tissues. For example, adult neural stem cells from β8 null mutants have been found to exhibit a reduced capacity for activating TGFβ (Mobley et al., 2009). In the retina, deletion of integrin β8 from neuroglial progenitors also leads to a reduced activity of Smad3 in endothelial cells (Arnold et al., 2012). Furthermore, astrocytes, a cell type with significant similarity to neural progenitors, have also been found to activate TGFβ in vitro (Cambier et al., 2005; Hirota et al., 2011). Thus, these lines of evidence strongly indicate that neural progenitors regulate brain vessel integrity by controlling integrin αvβ8 and consequently TGFβ activation and signaling to the endothelium. Interestingly, recent findings showed that deletion of TGFβ receptors from brain neural precursors using a Foxg1-cre also resulted in intracerebral hemorrhage (Hellbach et al., 2014). This suggests a potentially more complex role of TGFβ signaling in regulating brain angiogenesis.
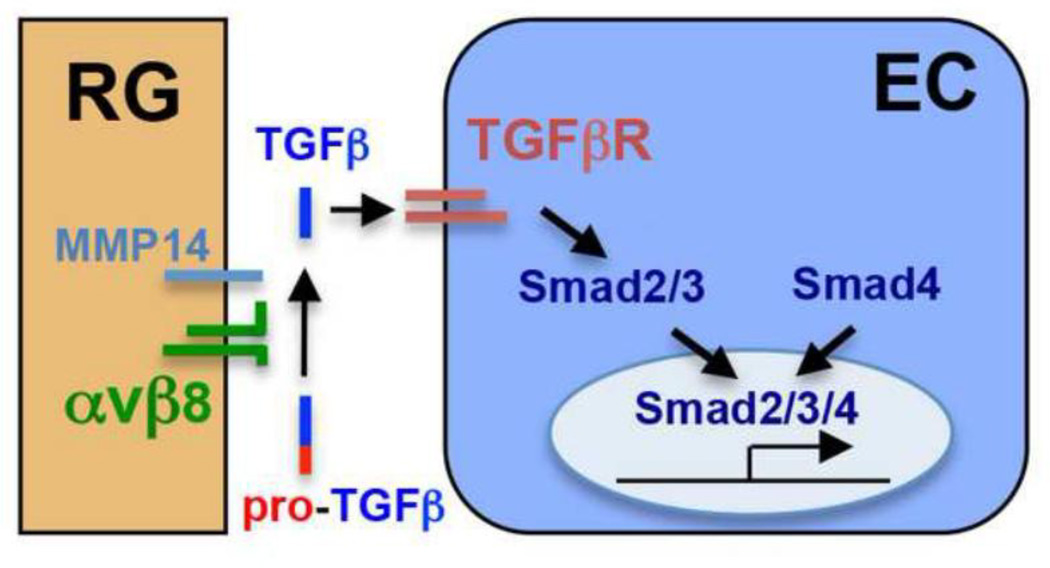
Figure 3. Neural progenitors regulate brain vessel maturation and integrity through integrin αvβ8-dependent activation of TGFβ signaling.
Integrin αvβ8 are specifically expressed by radial glial (RG) progenitors in the brain. They activate latent TGFβ ligands through binding to latency-associated peptides (LAPs) derived form the N-terminus of pro-TGFβ protein, and in a process depending on the activity of membrane bound metalloproteinases including MMP14. Once activated, TGFβ signals through receptors on endothelial cells (EC) and activates intracellular Smad2/3. This in turn leads to the formation of nuclear Smad2/3/4 complex and consequently the activation of genes promoting vessel maturation and integrity.
Unlike other family members, Smad4 is a common downstream mediator for both TGFβ and BMP (bone morphogenetic protein) signaling. Similar to αv and β8 mutants, conditional deletion of Smad4 from brain endothelial cells results in severe brain hemorrhaging (Li et al., 2011). However, Smad4 deficiency is additionally associated with defective mural cell (pericyte) coverage of the brain vasculature, a phenotype not observed in either αv or β8 mutants (Arnold et al., 2014; McCarty et al., 2002; Proctor et al., 2005). This suggests that Smad4 mediates more than αvβ8-dependent TGFβ signaling in brain endothelial cells. Moreover, Smad4 deletion from endothelial cells also results in impaired mural cell development outside the nervous system (Lan et al., 2007). This implicates TGFβ/BMP signaling in mural cell development throughout the vasculature of the body. Thus, while mural cell development appears to depend on a common TGFβ/BMP pathway conserved between neural and non-neural tissues, the differentiation and integrity of brain vessels are specifically regulated by αvβ8-dependent TGFβ signaling from neural progenitors.
5. Conclusion
Establishing the vascular network in the brain is an intricate and multifaceted process. Strong evidence indicates that neural progenitors play a central role throughout several steps of this process, including initial vessel ingression, nascent vessel stabilization, as well as vessel maturation and integrity. A complex interplay also begins to emerge between the molecular pathways that regulate these steps. For example, while canonical Wnt signaling is essential for initial vessel ingression into the CNS, its subsequent down-regulation by neural progenitors (via a yet to be identified pathway) appears to be critical for nascent vessel stabilization. Many questions still remain unanswered on how neural progenitors actually regulate these steps at the molecular level. For example, while Wnt signaling appears to be required specifically for CNS, but not non-CNS, angiogenesis, it remains unclear what specific gene expression programs are activated by Wnt signaling in endothelial cells to facilitate vessel ingression into the brain. It has also yet to be determined what molecular pathway(s) subsequently down-regulates Wnt signaling and how this contributes to vessel stabilization, and equally important, how vessel stabilization is temporally and spatially coordinated with that of vessel growth in this dynamic process. Similarly, although integrin αvβ8-dependent TGFβ signaling is crucial for CNS vessel integrity, it remains obscure what gene expression programs are involved and how they may regulate the various aspects of vascular structural and functional integrity. Increasing evidence indicates that vascular dysfunction, including compromised vessel integrity, destabilization, and regression, plays an important role in the pathogenesis of a large number of brain diseases in both children and adults (Lo et al., 2003; Lynch, 2009; Zlokovic, 2008). Elucidation of these questions will not only improve knowledge of normal brain development, but may also allow better understanding and improved treatment for these diseases.
Highlights.
VEGF and Wnt signaling from neural progenitors regulates brain vessel ingression
Neural progenitors stabilize new vessels by down-regulating Wnt/β-catenin signaling
Neural progenitors promote vessel integrity via integrin αvβ8 activation of TGFβ
Acknowledgement
This work was supported by an NIH grant NS076729-01A1 to Z.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arnold TD, Ferrero GM, Qiu H, Phan IT, Akhurst RJ, Huang EJ, Reichardt LF. Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. J Neurosci. 2012;32:1197–1206. doi: 10.1523/JNEUROSCI.5648-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama YS, Fuxe J, Akhurst R, Betsholtz C, Sheppard D, Reichardt LF. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development. 2014;141:4489–4499. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel G, Agranoff B, Albers RW, Fisher S, editors. Basic neurochemistry: molecular, cellular, and medical aspects. 6th edn. Philadelphia: Lippincott-Raven; 1999. pp. 637–669. [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014;71:3553–3567. doi: 10.1007/s00018-014-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Hellbach N, Weise SC, Vezzali R, Wahane SD, Heidrich S, Roidl D, Pruszak J, Esser JS, Vogel T. Neural deletion of Tgfbr2 impairs angiogenesis through an altered secretome. Hum Mol Genet. 2014;23:6177–6190. doi: 10.1093/hmg/ddu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Liu Q, Lee HS, Hossain MG, Lacy-Hulbert A, McCarty JH. The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development. 2011;138:5157–5166. doi: 10.1242/dev.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Hoppler S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev Dyn. 2010;239:16–33. doi: 10.1002/dvdy.22009. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, van Meeteren LA. Transforming growth factor beta family members in regulation of vascular function: in the light of vascular conditional knockouts. Exp Cell Res. 2013;319:1264–1270. doi: 10.1016/j.yexcr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tan J, Li J, Kivimae S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, Cheyette BN, Yu Q. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, Li W, Cheng X, Mao N, Yang X. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol. 2007;27:7683–7692. doi: 10.1128/MCB.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lynch JK. Epidemiology and classification of perinatal stroke. Semin Fetal Neonatal Med. 2009;14:245–249. doi: 10.1016/j.siny.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7:e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 2013;11:e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley AK, Tchaicha JH, Shin J, Hossain MG, McCarty JH. Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J Cell Sci. 2009;122:1842–1851. doi: 10.1242/jcs.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, Rubin JS, St Croix B. GPR124 functions as a WNT7-specific coactivator of canonical beta-catenin signaling. Cell Rep. 2015;10:123–130. doi: 10.1016/j.celrep.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971;33:471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Schaffer CB, Friedman B, Nishimura N, Schroeder LF, Tsai PS, Ebner FF, Lyden PD, Kleinfeld D. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 2006;4:e22. doi: 10.1371/journal.pbio.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. Elife. 2015;4 doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohrle S, Wallmen B, Hecht A. Differential control of Wnt target genes involves epigenetic mechanisms and selective promoter occupancy by T-cell factors. Mol Cell Biol. 2007;27:8164–8177. doi: 10.1128/MCB.00555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]