Abstract
Previous results have shown that Bone Morphogenetic Protein (BMP) signaling is essential for lens specification and differentiation. How BMP signals are regulated in the prospective lens ectoderm is not well defined. To address this issue we have modulated BMP activity in a chicken embryo pre-lens ectoderm explant assay, and also studied transgenic mice, in which the type I BMP receptors, Bmpr1a and Acvr1, are deleted from the prospective lens ectoderm. Our results show that chicken embryo pre-lens ectoderm cells express BMPs and require BMP signaling for lens specification in vitro, and that in vivo inhibition of BMP signals in the mouse prospective lens ectoderm interrupts lens placode formation and prevents lens invagination. Furthermore, our results provide evidence that BMP expression is negatively auto-regulated in the lens-forming ectoderm, decreasing when the tissue is exposed to exogenous BMPs and increasing when BMP signaling is prevented. In addition, eyes lacking BMP receptors in the prospective lens placode develop coloboma in the adjacent wild type optic cup. In these eyes, Bmp7 expression increases in the ventral optic cup and the normal dorsal-ventral gradient of BMP signaling in the optic cup is disrupted. Pax2 becomes undetectable and expression of Sfrp2 increases in the ventral optic cup, suggesting that increased BMP signaling alter their expression, resulting in failure to close the optic fissure. In summary, our results suggest that negative and positive auto-regulation of BMP expression is important to regulate early eye development.
Keywords: lens, specification, BMP, auto-regulation, coloboma, development
Introduction
Previous studies in Xenopus and chicken embryos identified four stages of lens formation: competence, lens-forming bias, specification and differentiation (Grainger et al., 1992; Grainger et al., 1997; Sullivan et al., 2004). With respect to cell specification, which is defined as the step whereby cells have received sufficient signals to acquire a specific cell identity if cultured outside of the embryo, studies in chicken embryos have suggested that lens cells are already specified at gastrula stages (Hamburger-Hamilton stage 4) (Bailey et al., 2006; Sjödal et al., 2007). How these cells maintain this early lens specification before the differentiation process begins at later stages is not completely known.
During early embryo development, prior to the formation of the neural tube, Bone Morphogenetic Protein (Bmp) 2 and 4 are expressed in the ectoderm at the anterior border of the neural plate in chicken (Chapman et al., 2002), the region where lens progenitors are situated (Bhattacharyya et al., 2004). These cells also contain phosphorylated (phospho-) Smad1, an indicator of BMP signaling (Faure et al., 2002). In the mouse, Bmp7 is present in the head surface ectoderm at the time of lens placode induction [embryonic day (E) 9.5], but gradually decreases in the lens placode (E10.0) and is eventually excluded from the lens vesicle (E10.5) (Dudley and Robertson, 1997). Bmp4 expression is observed already at E9.0 in the pre-lens surface ectoderm, but rapidly decreases around E9.5, and are completely absent in the lens placode (E10.0) (Furuta and Hogan, 1998; Wawersik et al., 1999). How this temporary expression pattern of Bmp4 and Bmp7 in the lens placodal region are regulated remains to be determined.
Ectodermal explants removed from the anterior border region at gastrula stages and cultured in isolation for 42-50 hours express proteins characteristic of the lens and olfactory placodes (Sjödal et al., 2007). However, when these explants are cultured in the presence of the BMP antagonist, Noggin, or a mixture of soluble BMP receptors, lens and olfactory placode differentiation is suppressed in favor of cells with the characteristics of the forebrain region (Sjödal et al., 2007). In contrast, blocking BMP signaling during the last 30-35 hours of culture resulted in the formation of cells with olfactory placode markers, but no lens markers (Sjödal et al., 2007). Lens progenitors in chicken embryos maintain both Bmp4 expression and enriched phospho-Smad1/5/8 at neural fold and neural tube stages (Pandit et al., 2011; Sjödal et al., 2007). Consistently, both in vitro and in vivo experiments have shown that maintenance of BMP activity during these stages is required for proper lens formation (Pandit et al., 2011; Sjödal et al., 2007). Furthermore, BMP activity has been shown to be crucial for primary lens fiber cell differentiation in mouse, chicken and zebrafish (Belecky-Adams et al., 2002; Faber et al., 2002; French et al., 2009; Rajagopal et al., 2009; Sjödal et al., 2007; Pandit et al., 2011). Specifically, BMP activity is required to induce L-Maf expression in the lens placode, one of the earliest lens placodal markers defining lens identity, whereas after L-Maf up-regulation the onset of δ-crystallin in lens fiber cells occurs independently of BMP signals (Pandit et al., 2011). Thus, lens placode progenitor cells express BMPs and prolonged exposure of the pre-lens ectoderm to endogenously-produced BMPs is required to promote lens cell identity.
Development of the lens is also closely tied to the formation of the optic cup. A combination of constrained growth and adhesion between the lens and optic vesicle initiates optic cup formation (Hendrix and Zwaan, 1974; Hendrix and Zwaan 1975; Zwaan and Webster, 1984; Huang et al., 2011). The ventral quadrant of the optic cup then invaginates to form the optic fissure. Incomplete closure of the optic fissure causes congenital eye defects including coloboma and microphthalmia, seen in humans and other species. Due in part to the juxtaposition of the optic cup and lens forming regions, abnormal BMP, Transforming Growth Factor (TGF-β), and Sonic hedgehog (Shh) signaling in the lens and retina have been shown to result in colobomatous defects (Zhang et al., 2013). Moreover, reduced BMP activity in the optic vesicle of both chicken and mouse results in failure to form the optic cup, and loss of or decreased levels of retina-specific markers (Pandit et al., 2015; Huang et al., 2015; Murali et al., 2005). However, the adaptive signaling and feedback mechanisms between the developing lens and optic vesicle are not well understood.
It is not known whether the prospective lens cells in the mouse express BMPs prior to reaching the eye region. However, by the time the prospective lens placode cells on the surface of the eye are contacted by the optic vesicle, they are already expressing Bmp4 and Bmp7 (Furuta and Hogan, 1998; Wawersik et al., 1999). This suggests that BMP expression may be a marker of avian and mammalian lens identity prior to lens differentiation. Here we observed that BMP expression is negatively auto-regulated in the chicken and mouse lens pre-placodal ectoderm. In addition, inhibition of BMP signals in pre- lens ectoderm inhibits both lens and optic vesicle development, in part by disrupting the dorsal-ventral BMP gradient in the optic vesicle. Thus, our results suggest that lens specification requires a balanced level of BMP activity, and indicate that auto-regulation of BMP expression is an important molecular function to regulate early lens development.
Materials and methods
Chicken and Mouse Embryos
White Leghorn chicken embryos (Strömbäcks Ägg, Umeå Sweden) were staged according to the protocol of Hamburger and Hamilton [HH, (Hamburger and Hamilton, 1951)].
Mice expressing Cre recombinase under the control of Pax6 enhancer/promoter (Le-Cre) was used (Ashery-Padan et al., 2000). Primers for genotyping mice carrying the Cre transgene or the floxed alleles (Acvr1, Bmpr1a) were used as previously described (Rajagopal et al., 2009). Homozygous floxed BMP receptor mice (Acvr1fx/fx and Bmpr1a fx/fx), one of which was Cre-positive, were mated to generate Cre-positive (conditional knockout, CKO) and Cre-negative offspring (wild type). Cre-positive animals were always mated to Cre-negative animals, assuring only one inherited copy of the Cre transgene.
Explant assay
Prospective lens (L) explants were isolated from HH stage 8 chicken embryos. Explants were cultured for 6 or 48 hours in serum-free OPTI-MEM (GIBCO) containing N2 supplement (Invitrogen) and fibronectin (Sigma), or on Millicell filters (0.4 μm pore size; Merck Millipore). Noggin conditioned medium was obtained from stably transfected or un-transfected Chinese hamster ovary (CHO) cells (Lamb et al., 1993) and cultured in CHO-S-SFM II media (GIBCO). Noggin conditioned media was used at an estimated concentration of 50 ng/ml. Human recombinant BMP4 and BMP7 (R&D Systems) were used at 35ng/ml and 500ng/ml, respectively.
For qRT-PCR analysis, 10 L explants were cultured for each experiment, and each experiment was done in triplicate. After culture, the L explants were washed briefly and gently in OptiMEM culture medium and then dissolved in 70 μl of the Qiagen RNeasy Microkit lysis buffer followed by 5min vortex. The lysate was stored at −20°C until qRT-PCR analysis.
For immunohistochemistry analysis, 6-8 L explants were cultured for each experiment, and each experiment was done in triplicate. Cultured L explants were fixed in 4% paraformaldehyde (PFA) at 4°C for 25 minutes, washed three times in PBS, thereafter freezed in Neg50 and stored at −80°C until use.
qRT-PCR analysis
Total RNA was extracted from each sample using a Qiagen RNeasy Microkit (Qiagen Technologies, Germantown, MD) and was reverse transcribed and amplified using the Ovation Pico WTA System V2 (NuGEN Technologies, San Carlos, CA). qRT-PCR amplification was performed using a SYBR Green JumpStart TaqReadyMix (Sigma-Aldrich, Saint Louis, MO) and an Eco Real-Time PCR System (Illumina, San Diego, CA) on 1.65 ng/μl of cDNA per sample. PCR primers were:
Gapdh – forward 5′CTAGGGAAGCAGGACCCTTT 3′, reverse 5′ GGAACAGAACTGGCCTCTCA 3′
Bmp2 – forward 5′ TTCGGTGAATTCCAAAATCC 3′, reverse 5′ CAGCCCTCCACAACCATATC 3′
Bmp4 – forward 5′ GCCACGCTCTCTATGTGGAT 3′, reverse 5′ GCAGTAAAACGCCTGGTAGC 3′
Bmp7 – forward 5′ GTGTTGGTGGTCACAGCATT 3′, reverse 5′ AACAAACAAACAATCCCCACA 3′
qRT-PCR: treatment groups (Noggin, BMP4/7) were compared with control data using a two-tailed Student's t-test with P < 0.05 for statistical significance. Results are plotted as mean ± standard deviation.
Microarray analysis
The prospective lens ectoderm was laser micro-dissected from three wild type and three BMPR conditional knockout embryos at E9.5 using a Leica LMD-6000 instrument. RNA was extracted from each sample using a Qiagen RNeasy Microkit (Qiagen Technologies, Germantown, MD) and reverse transcribed and amplified using a WT-Ovation Pico RNA amplification kit (NuGEN Technologies, San Carlos, CA). Amplified samples from each embryo were used to probe an Illumina Mouse-6 v.1.1 bead microarray (Illumina, San Diego, CA) and analyzed using Illumina Genome Studio software. The data were normalized using a cubic spline routine and statistical comparisons were made using the Illumina custom error model. Microarray data can be viewed from the GEO repository at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64005.
RNA-seq analysis
Prospective lens ectoderm RNA from three wild type and three BMPR CKO embryos was pooled, reverse transcribed and amplified using a NuGEN Ovation RNA-seq System V2 (NuGEN Technologies, San Carlos, CA). Amplified cDNA was fragmented with a Covaris sonicator at the Washington University Genome Technologies Access Center (GTAC) and used to create a sequencing library with the NuGEN Encore Rapid Library System. The library was quantified using a KAPA Biosystems kit and submitted for sequencing to the GTAC. Sequencing reads were aligned to the mouse genome and analyzed using the open source TOPHAT and CUFFLINKS software, respectively.
In situ hybridization
Embryos were collected at E9.5, oriented and frozen in OCT (Tissue-TEK Optimal Cutting Temperature compound; Sakura Finetek, Torrance CA). Frozen sections were fixed overnight in 4% PFA, washed and dehydrated in increasing concentrations of ethanol, dried from 100% ethanol and treated with proteinase-K according to the instructions included with the QuantiGene View probe kit (Affymetrix, Santa Clara, CA). Probes to Bmp4, Bmp7 and Shh were prepared by Affymetrix (Catalog number: VB1-13861, VB1-15142 and VB1-13062) and detected with an Affymetrix QuantiGene ViewRNA ISH Tissue 1-plex Assay Kit and a QuantiGene ViewRNA Chromogenic Signal Amplification Kit. Sections were imaged by fluorescence microscopy. The Sfrp2 probe were labeled with digoxygenin using a riboprobe synthesis kit (Roche Diagnostics, #11636090910, Indianapolis, IN) of a clone encoding mouse Sfrp2 (ATCC image clone #6488018) and reacted with tissue sections following instructions in the labeling kit.
Immunostaining
PFA-fixed, paraffin-embedded sections of wild type and BMPR CKO mouse embryos were reacted with rabbit antibodies to Pax2 (Life Technologies, #08-1483, Grand Island, NY, 1:200) and stained using a Vectastain Elite Rabbit IgG ABC kit (Vector Laboratories, Burlingame, CA), or a rabbit monoclonal anti-phospho-Smad1/5/8 antibody (Cell Signaling Technologies, #9511S, Danvers, MA,1:200) followed by staining with an Alexafluor488-labeled anti-rabbit secondary antibody (1:1,000, Life Technologies, Grand Island, NY). Stained sections were viewed on a Zeiss LSM510 confocal microscope.
PFA-fixed chick explants were cryostat-sectioned at 10μm on consecutive sections. Antibodies used were: anti-sheep δ-crystallin (1:1000; Beebe and Piatigorsky, 1981), anti-mouse HuC/D (1:200; Molecular Probes) and anti-rabbit Cytokeratin (1:1000; DAKO). Nuclei were stained using DAPI (Sigma).
Results
Auto-regulation of BMP expression in the chicken pre-lens ectoderm
The lens precursor tissue, the pre-placodal ectoderm adjacent to the neural plate, expresses Bmp2 and Bmp4, and this endogenous BMP signaling has been shown to be required for subsequent lens differentiation (Chapman et al., 2002; Sjödal et al., 2007). In the present study we tested whether auto-regulation of BMP expression might be a molecular key function during lens specification.
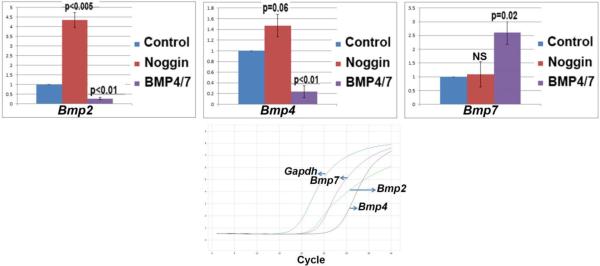
To evaluate whether auto-regulation of BMP expression play a role for early lens development, we dissected out pre-lens (L) explants from Hamburger-Hamilton stage 8 chicken embryos. L explants were cultured alone or in the presence of the BMP antagonist Noggin, or together with BMP4 and 7 for 6hr, and thereafter processed for qRT-PCR analyses. Control chicken embryo pre-lens ectoderm expressed Bmp2, 4 and 7 transcripts at baseline (Fig. 1). Treatment with the BMP antagonist, Noggin, significantly increased the levels of Bmp2 and marginally increased Bmp4, but did not affect Bmp7 levels (Fig. 1). Exposure of explants to BMP4 and 7 significantly lowered the levels of Bmp2 and Bmp4, but increased expression of Bmp7 transcripts (Fig. 1). These results indicate that the Bmp2 and Bmp4 are negatively regulated and that Bmp7 is positively regulated in response to BMP signaling in the chicken pre-lens ectoderm.
Fig. 1.
Results of qRT-PCR studies in which Hamburger-Hamilton stage 8 explants of pre-lens ectoderm were cultured in basal medium, or exposed to the BMP antagonist Noggin, or a mixture of BMP4 and BMP7. Expression levels relative to Gapdh are shown for Bmp2, 4 and 7 transcripts. The lower graph shows the basal expression levels of each of the BMP transcripts, relative to chicken Gapdh.
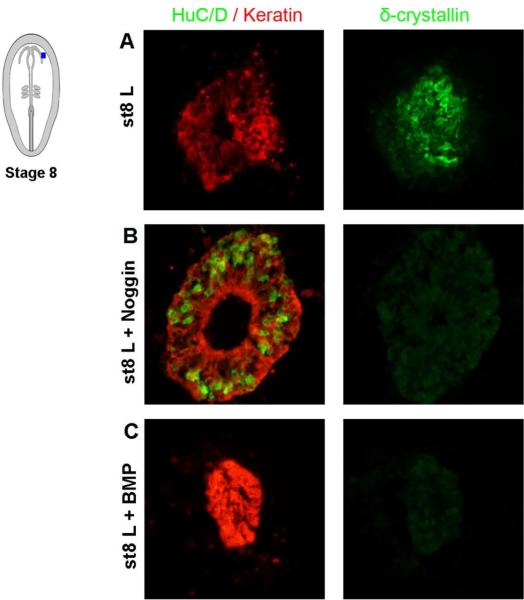
To further evaluate how modulations of the BMP pathway affect lens specification, we cultured stage 8 L explants for 48hr to allow lens fiber cell differentiation to occur. During these conditions, control L explants generated Keratin+ and δ-crystallin+ cells; characteristic of lens fiber cells (Fig. 2A), and no HuC/D+ neurons was observed after culture. The expression of the non-neural marker Keratin in all cells verified that the L explants did not contain any neural contamination from the prospective optic vesicle (Fig. 2A). Noggin blocked the generation of δ-crystallin+ lens fiber cells and under these conditions, HuC/D+ neurons and Keratin+ cells, which are characteristic of olfactory epithelial cells, were generated (Fig. 2B). This is in agreement with our previous publications showing that lens specification requires ongoing BMP activity (Sjödal et al., 2007; Pandit et al., 2011). In addition, ectopic BMP4/BMP7 inhibited the generation of δ-crystallin+ lens fiber cells and generated cells that expressed high levels of Keratin, but not HuC/D; characteristic of epidermal cells (Fig. 2C). This is consistent with findings that high levels of BMP signals promote epidermal specification and repress neural plate border derivatives (Sjödal et al., 2007; Patthey et al., 2009). Together these results provide further evidence that lens specification requires a balanced level of BMP activity, and suggest that auto-regulation of BMP expression is an important molecular function to regulate early lens development.
Fig. 2.
(A-C) Stage 8 L explants cultured for 48 hr and analyzed by immunohistochemistry. (A) Stage 8 L explants (n=15) cultured alone generated Keratin+ and δ-crystallin+ cells; characteristic of lens fiber cells. (B) In stage 8 L explants (n=15) cultured together with Noggin, the generation of lens cells was blocked, whereas HuC/D+/Keratin+ cells, characteristic of olfactory epithelial cells, were increased. (C) In stage 8 L explants (n=15) cultured together with BMP4 and BMP7, the generation of lens cells was blocked, whereas Keratin+ epidermal cells were generated.
Auto-regulation of BMP expression in the mouse lens placode and optic vesicle
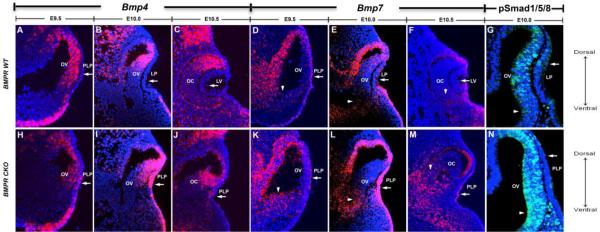
In mouse embryos, Bmp4 and Bmp7 transcripts are temporarily expressed in the head ectoderm during lens placode formation. Previous studies on Bmp4 and Bmp7 expression noted that as Bmp4 expression increased in the adjacent optic vesicle, Bmp4 transcripts decreased in the prospective lens placode, and both Bmp4 and Bmp7 were undetectable in the newly formed lens vesicle (Furuta and Hogan, 1998; Wawersik et al., 1999). To explore this observation in greater detail we examined the levels of Bmp2, 4 and 7 by microarray and RNA-sequencing (RNA-seq) analysis of prospective lens placode cells and using fluorescent in situ hybridization. In wild type E9.5 eyes, Bmp7 was expressed throughout the pre-lens ectoderm, and a few Bmp4+ cells were also detected (Figs. 3A,D). At E10 and E10.5, no Bmp4 expression was detected in the lens placode or lens vesicle (Figs. 3B,C), and the expression of Bmp7 gradually decreased in the lens placode/vesicle (Figs. 3E,F). At E10, enrichment of phospho-Smad1/5/8 indicated ongoing BMP activity in the lens placode (Fig. 3G). During E9.5-10.5, the developing optic vesicle expressed Bmp4 in a dorsal-ventral gradient (Figs. 3A-C), and consistently, phospho-Smad1/5/8 was enriched in a dorsal-ventral pattern at E10 (Fig. 3G). Bmp7 was expressed in a scattered pattern in the optic vesicle at E9.5 and E10, but not detectable at E10.5 (Figs. 3D-F). Thus, in wild type eyes, the expression of Bmp4 and Bmp7 decreased in the lens ectoderm in contact with the BMP-containing optic vesicle.
Fig. 3.
Fluorescent in situ hybridization showing the levels of Bmp4 and Bmp7 and immunofluorescent staining of phospho-Smad 1/5/8 (pSmad1/5/8) in wild type (WT) eyes (A-G) and in eyes in which the type I BMP receptors, Bmpr1a and Acvr1 were conditionally deleted in the prospective lens placode (BMPR CKO) (H-N). (A-C) In wild type eyes, Bmp4 expression was detected in the pre-lens ectoderm at E9.5 (A), but not at E10 (B) or 10.5 (C), and was restricted to the dorsal part of the optic vesicle and cup at E10 and E10.5 (B,C). (D-F) In wild type eyes, Bmp7 was expressed in the pre-lens ectoderm at E9.5 (D), decreased at E10 (E), but was not detected at E10.5 (F). Bmp7 expression was detected in a scattered pattern in the optic vesicle at E9.5 and E10, but not detected at E10.5. (H-J) In BMPR CKO eyes, Bmp4 expression was increased in both the presumptive lens ectoderm and optic vesicle. (K-M) In BMPR CKO eyes, Bmp7 expression was up-regulated at E10 (L) and maintained increased at E10.5 (M) in the presumptive lens ectoderm, and was expanded in the ventral optic vesicle (K-M). (G,N) Phospho-Smad1/5/8 enrichment is increased in the ventral optic vesicle at E10 in BMPR CKO eyes compared to wild type. Arrows indicate changes in levels of Bmp4, Bmp7 and pSmad1/5/8 in the lens region. Arrowheads indicate changes in level of Bmp7 and pSmad1/5/8 in the ventral optic vesicle. Abbreviations: BMPR, BMP receptor; CKO: conditional knockout; LP, lens placode; PLP, prospective lens placode; LV, Lens Vesicle; OV, optic vesicle; OC, optic cup.
Blocking BMP signaling by deletion of the type I BMP receptors, Bmpr1a and Acvr1 (BMPR CKO) in the lens-forming ectoderm increased Bmp2, 4 and 7 transcripts shown by microarray and RNA-seq analysis (Table 1). Table 1 includes data from microarray and RNA-seq analysis of transcript levels from the wild type and BMPR CKO lens placodal regions at E10.0. The microarrays did not detect Bmp2 transcripts, possibly because the single probe set on the array was not sufficiently sensitive. However, the RNA-seq data showed that the low level of Bmp2 transcripts in wild type lens ectoderm increased more than 80-fold after deletion of the type I BMP receptors (Table 1). Similarly, both Bmp4 and Bmp7 transcripts increased significantly in the microarray and/or RNA-seq data after BMP receptor deletion (Table 1). Fluorescent in situ hybridization confirmed the gene profiling results of increased Bmp4 and Bmp7 expression at E10 (Figs. 3I,L). In line with the deletion of the type I BMP receptors in the pre-lens ectoderm, the BMPR CKO presumptive lens ectoderm lacked BMP signaling, detected by phospho-Smad1/5/8 (Fig. 3N). In addition, we found increased expression of Bmp4 in the BMPR CKO presumptive lens ectoderm at E9.5 and E10 (Figs. 3H,I) and scattered expression at E10.5 (Fig. 3J). Moreover, an increase of Bmp7 expression was observed at E10, which persisted at E10.5 (Figs. 3K-M). Taken together, exposure to BMP signaling decreased the expression of BMP ligands and prevention of BMP signaling increased the expression BMP ligands in the murine prospective lens ectoderm, indicating a negative auto-regulation of BMP signaling.
Table 1.
| BMPR I WT and CKO microarray signal intensity | |||
|---|---|---|---|
| Transcript | WT | CKO | p-value |
| Bmp2 | ND | ND | |
| Bmp4 | 252 | 952 | <0.001 |
| Bmp7 | 154 | 539 | <0.001 |
| BMPR I WT and CKO RNA-Seq (FPKM) | |||
|---|---|---|---|
| Transcript | WT | CKO | p-value |
| Bmp2 | 0.43 | 36 | <0.0001 |
| Bmp4 | 23 | 131 | <0.001 |
| Bmp7 | 51 | 157 | 0.06 |
BMP transcript levels in the prospective lens placode of wild type (WT) and type I BMP receptor conditional knockouts (CKO), assayed by microarray and RNA-seq. ND, not detected; FPKM, fragments per kilobase per million mapped reads.
Besides the increase of BMP ligand expression in the presumptive lens ectoderm of the BMPR CKO eye, an increase of Bmp4 and Bmp7 expression and an enrichment of phospho-Smad1/5/8 along the dorsoventral axis were observed in the optic vesicle (Figs. 3H-N). Bmp4 expression was slightly elevated in the dorsal region of the optic cup, partially extending into the ventral region of the optic cup (Figs. 3H-J). The expression of Bmp7 was clearly elevated in the ventral optic cup of BMPR CKO eyes at all stages examined (Figs. 3L-N). Thus, the normal dorsal-ventral gradient of BMP signaling was clearly disrupted in the BMPR CKO optic vesicles These results provide evidence that disrupted BMP signaling in the lens ectoderm up-regulate BMP ligands in the presumptive lens placode, which in turn modulate BMP activity in the optic cup. Taken together, our data suggest that negative BMP autoregulation is important to regulate early eye development.
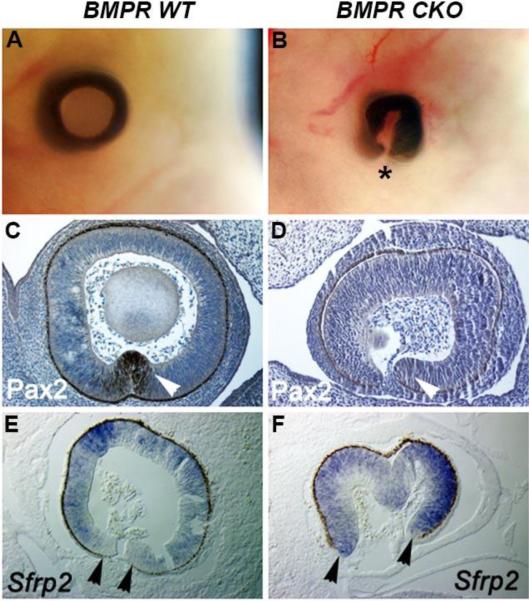
Coloboma formation in the BMPR CKO eyes
Our previous study showed that deletion of type I BMP receptors from the prospective lens ectoderm resulted in partial formation of the lens placode and failure of lens invagination (Rajagopal et al., 2009). Although the optic cup formed in the BMPR CKO eyes, we noted that the underlying wild type optic cup failed to close ventrally, forming a coloboma (Figs. 4A, B). To explore the etiology of the coloboma in BMPR CKO eyes, we analyzed the expression of Pax2 and Sfrp2, which are critical for optic fissure closure. At E12.5, the optic cups in BMPR CKO embryos had undetectable levels of Pax2 (Figs. 4C,D), which is required for closure of the optic fissure (Sanyanusin et al., 1995; Torres et al., 1996). They also showed increased expression of the Wnt antagonist, Sfrp2, at the margins of the optic fissure (Figs. 4E, F), which is consistent with previous studies showing that a proper balance of Wnt signaling is important for closure of the optic fissure (Liu and Nathans, 2008; Zhou et al., 2008). Thus, deletion of BMP signaling in the pre-lens ectoderm disrupts BMP activity in the optic cup, which leads to formation of coloboma.
Fig. 4.
Analysis of the optic cup of E12.5 wild type eyes and eyes in which the type I BMP receptors, Bmpr1a and Acvr1 were conditionally deleted in the prospective lens placode (BMPR CKO). (A, B) The extent of coloboma formation in whole embryo heads in the BMPR CKO embryos. Asterisk indicates ventral side of tissue where fissure has not closed in the CKOs. (C, D) Immunostaining (brown staining) for Pax2 was observed at the margins of the optic fissure in wild type eyes (white arrowhead), but undetectable in the BMPR CKO eyes (white arrowhead). (E, F) Sfrp2 transcripts (blue staining) were increased at the margins of the optic fissure in BMPR CKO eyes (black arrowheads).
It has been shown that inhibition of Shh signaling in the mouse optic vesicle extended the Bmp4 expression ventrally in the optic vesicle, decreased Pax2 expression and caused coloboma (Zhao et al., 2010). In BMPR CKO mice, Bmp7 expression and enrichment of phospho-Smad1/5/8, indicative of BMP activity, is increased in the ventral optic vesicle (Figs. 3K-N). To explore if elevated BMP signaling in the ventral optic vesicle alters Shh signaling reciprocally, we analyzed Shh expression in E9.5-E10.5 BMPR CKO mice. However, no change of Shh expression was detected in BMPR CKO eyes compared to wild type eyes (Figs. S1A-F), suggesting that in the ventral part of the retina increased BMP activity is not sufficient to inhibit Shh signaling.
Discussion
To our knowledge, negative auto-regulation of BMP expression, as detected in our studies in the developing lens, has not been reported during embryonic development. A recent study has, however, reported increased expression of BMPs after inhibition of BMP signaling in zebrafish retinal development (Holly et al., 2014), without discussing a possible negative feedback mechanism. Neither did the authors examine whether retinal exposure to BMPs caused a decrease in BMP expression in the developing zebrafish retinas, which otherwise would have represented a second case of negative BMP auto-regulation. On the other hand, positive auto-regulation of BMP signaling has been described previously, and assures the propagation of signals by “locking in” signaling through a pathway. Our studies now verify this mechanism and present an example of both Bmp4 and Bmp7 positive auto-regulation in the early forming optic vesicle. Another study has shown that Bmp7 positive auto-regulation is involved in patterning the sensory domains in the tongue, where its effects are limited by follistatin secreted by the adjacent mesoderm (Beites et al., 2009). Positive auto-regulation of Bmp2 expression has also been reported in osteocytes exposed to BMP2 (Ghosh-Choudhury et al., 2001). Dpp, the Drosophila ortholog of vertebrate Bmp2 and 4, positively regulates its expression during the progression of the morphogenetic furrow during patterning of the Drosophila retina (Chanut and Heberlein, 1997).
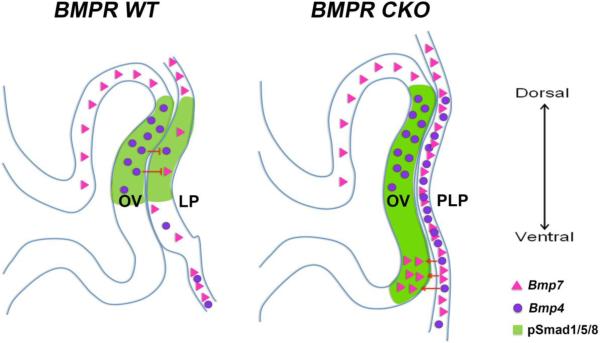
Negative auto-regulation of BMP expression maintains an intermediate and balanced level of gene expression and signaling, since increased signaling reduces BMP levels and decreased signaling increases BMP levels. We suggest that the maintenance of BMP expression in the pre-lens ectoderm may be a molecular key function for lens specification. This view is supported by the response of BMP signaling to inhibition by Noggin or by the deletion of BMP receptors, each of which increases BMP expression and block lens development (Fig. 5). Although we found that increased BMP signaling reduces Bmp2 and Bmp4 expression levels and that decreased BMP signaling increases Bmp2 and Bmp4 levels, we observed a slight discrepancy between the expression of Bmp7 in BMP inhibited chicken lens explants and mouse BMPR CKO presumptive lens ectoderm. The difference in results might be due to the different methodology approaches; in vivo assays in mouse versus in vitro cultures of only the prospective lens ectoderm in chicken. It is possible that in mouse, the increased levels of Bmp7 expression is caused by secondary signals from surrounding tissues, which would explain the unchanged levels of Bmp7 expression in the chicken assay. Another possibility is that the difference in Bmp7 expression is a temporal issue, in which an up-regulation of Bmp7 expression requires long-term inhibition of BMP signals. This view is supported by the slower increase of Bmp7 expression compared with the rapid increase of Bmp4 expression in the BMPR CKO presumptive lens ectoderm. Nevertheless, Bmp2 and Bmp4 transcripts were increased in both chicken and mouse after BMP inhibition. Conversely, exposure of the chicken pre-lens ectoderm to BMP4 and BMP7 lowers the expression of Bmp2 and Bmp4 in cultured explants. Similarly, exposure to BMP4 during normal lens induction in the mouse lowers the expression of Bmp4 and Bmp7 in the responding ectoderm. These results again suggest that auto-regulation of BMP expression maintains a moderate level of BMPs and BMP signaling from the time when cells are at the neural plate border until they begin the process of lens differentiation (Fig. 5).
Fig. 5.
Schematic image illustrating the negative and positive regulation of Bmp signaling/expression in wild type eyes and in eyes in which the type I BMP receptors, Bmpr1a and Acvr1 were conditionally deleted in the prospective lens placode. Abbreviations: LP, lens placode; PLP, prospective lens placode; OV, optic vesicle.
Our results show that lens specification requires balanced BMP activity, as either a reduction or an increase in BMP activity in prospective lens cells results in failure of lens specification, followed by a subsequent generation of olfactory placodal cells or epidermal cells, respectively. BMP signals have previously been shown to play important roles during lens formation. At the late gastrula stage in chicken, BMP activity induces lens and olfactory placodal cells in the anterior neural plate border, and sustained exposure to BMP signals promotes the generation of lens cells at the expense of olfactory placodal cells (Sjödal et al., 2007). Consistently, in mouse, targeted deletions of different components of the BMP pathway lead to disturbed lens formation (Beebe et al., 2004; Furuta and Hogan, 1998; Wawersik, 1999; Rajagopal et al 2009). If BMP auto-regulation establishes lens identity, it is possible that the formation of other cell types, such as olfactory placode cells from the anterior border ectoderm, requires inhibition of BMP auto-regulation. In agreement with this hypothesis, at neural folds stages Bmp4 and increased phospho-Smad1/5/8 are preferentially detected in the prospective lens ectoderm compared to the prospective olfactory placode domain (Sjödal et al., 2007). Moreover, unlike lens placode progenitors, the generation of olfactory placode cells becomes independent of BMP activity from the neural fold stages (Sjödal et al., 2007). Now, our studies extend this knowledge and provide evidence that negative auto-regulation of BMP expression is a key molecular mechanism that maintains a balanced BMP activity in the lens ectoderm, thereby regulating early lens specification.
The signaling pathway responsible for BMP auto-regulation in the mouse pre-lens ectoderm remains, however, unknown. Although phospho-Smad1 is detected in the ectoderm at the chicken neural plate border and in the mouse lens pre-placodal ectoderm (Belecky-Adams et al., 2002; Chapman et al., 2002; Rajagopal et al., 2009), our earlier studies showed that the formation of the lens by Bmp4 from the optic vesicle occurs in mice by a Smad-independent mechanism (Rajagopal et al., 2009). Moreover, although deletion of the type I BMP receptors prevented lens formation and caused coloboma, deletion of Smad1 and Smad5 or the co-Smad, Smad4 did not prevent lens formation and did not cause coloboma formation. Therefore, signaling from BMP receptors through an unknown, non-canonical pathway appears to negatively regulate BMP mRNA levels and promote lens formation in the mouse lens placode.
During early neural tube stages, the evagination of the optic vesicle brings it in close contact with the prospective lens ectoderm, which facilitates their interaction. The elevated level of Bmp7 transcripts in the ventral wild type optic vesicle of mice lacking type I BMP receptors in the ectoderm suggests that Bmp7 is positively auto-regulated in the optic vesicle by the increased level of BMPs produced by the ectoderm (Fig. 5). The possibility that increased BMPs from the surface ectoderm directly stimulate BMP signaling in the ventral optic cup is supported by our recent results in chicken, which show that lens-derived BMP signals maintain a Rax2-positive state in the optic vesicle and up-regulate neural retina identity (Pandit et al., 2015). However, a recent study suggests that after stage 13 in chicken, neural retina cells develop independently of signals from the lens ectoderm (Pandit et al., 2015). Consistent with this finding, surgical ablation of the lens placode after stage 13 resulted in intact optic vesicles that initiated neural retina differentiation (Hyer et al., 2003), whereas ablation of the lens placode prior to stage 13 resulted in failure of optic cup formation (Hyer et al., 1998). In addition, Pax6 lens-specific mutants, in which lens induction occurs but further lens development is arrested, exhibit normal differentiation of the neural retina (Ashery-Padan et al., 2000). These results support a model, in which BMPs from the surface ectoderm are crucial during a short time window for proper development of the optic vesicle.
Our study is the first one to show that BMP signaling in the lens ectoderm is required to enable closure of the optic fissure and prevent coloboma by establishing or maintaining the dorsal-ventral gradient of BMP signaling, promoting Pax2 expression and suppressing Sfrp2 expression in the ventral optic cup. This is in agreement with previous studies, which indicated that Pax2 is required for closure of the optic fissure (Morcillo et al., 2006; Sanyanusin et al., 1995; Viringipurampeer et al., 2012). Our results show that BMP signals from the lens are important for establishing or maintaining the normal dorsal-ventral gradient of BMP activity in the optic vesicle that is required to prevent coloboma. A recent study has also concluded that BMP signals emanating from the lens are crucial for the up-regulation of BMP activity in the optic vesicle (Pandit et al., 2015). Moreover, depletion of Smad7 in mice disrupted the dorsal-ventral gradient of BMP/Shh activity by an increase in Shh and decrease in Bmp7 expression, followed by various degrees of coloboma and microphthalmia (Zhang et al., 2013). Shh has been shown to inhibit BMP expression in the ventral retina (Zhao et al., 2010). In addition, our results provide evidence that the expression of Shh in the retina of BMPR CKO mice is unchanged, suggesting that increased BMP activity is not sufficient to inhibit Shh signaling in the ventral part of the retina.
In summary, this study provides the first direct evidence of negative auto-regulation of BMP expression in development and an additional instance of positive auto-regulation. The extent of negative auto-regulation of BMP signaling and its potential role in development remains to be determined. Together, our results suggest that negative and positive auto-regulation of BMP expression represents an important molecular mechanism that regulates early eye development.
Supplementary Material
Highlights.
BMP expression is negatively auto-regulated in the lens-forming ectoderm
Eyes lacking BMP receptors in the prospective lens placode develop coloboma
Loss of BMP activity in the lens disturb the BMP D-V gradient in the optic vesicle
Inhibition of BMP signals in pre- lens ectoderm inhibits lens development
Inhibition of BMP signals in pre- lens ectoderm disturb optic vesicle development
Acknowledgements
Research was supported by NIH grants EY04853 (DCB), NIH Core Grant EY02687 and an unrestricted grant to the Department of Ophthalmology and Visual Science from Research to Prevent Blindness, and by the Swedish Research Council, Ögonfonden and KMA foundation to L.G. B.F. acknowledges support from a Career Starter Award from the Knights Templar Eye Foundation. Partial support to the Genome Technologies Access Center for microarray and RNA-Seq analysis was provided by a NIH Clinical and Translational Science Award to Washington University [UL1TR000448].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens Specification Is the Ground State of All Sensory Placodes, from which FGF Promotes Olfactory Identity. Developmental Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Piatigorsky J. Translational regulation of delta-crystallin synthesis during lens development in the chicken embryo. Developmental Biology. 1981;84:96–101. doi: 10.1016/0012-1606(81)90374-2. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Beites CL, Hollenbeck PLW, Kim J, Lovell-Badge R, Lander AD, Calof AL. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development. 2009;136:2187–2197. doi: 10.1242/dev.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development (Cambridge, England) 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Developmental Biology. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Chanut F, Heberlein U. Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development. 1997;124:559–567. doi: 10.1242/dev.124.2.559. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Developmental Biology. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Dudley A, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Developmental Dynamics. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang R. BMP signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous Patterns of BMP Signaling during Early Chick Development. Developmental Biology. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- French CR, Erickson T, French DV, Pilgrim DB, Waskiewicz AJ. Gdf6a is required for the initiation of dorsal-ventral retinal patterning and lens development. Developmental Biology. 2009;333:37–47. doi: 10.1016/j.ydbio.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Ghosh Choudhury G, Harris MA, Wozney J, Mundy GR, Abboud SL, Harris SE. Autoregulation of Mouse BMP-2 Gene Transcription Is Directed by the Proximal Promoter Element. Biochemical and Biophysical Research Communications. 2001;286:101–108. doi: 10.1006/bbrc.2001.5351. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Henry JJ, Saha MS, Servetnick M. Recent progress on the mechanisms of embryonic lens formation. Eye. 1992;6:117–122. doi: 10.1038/eye.1992.26. [DOI] [PubMed] [Google Scholar]
- Grainger RM, Mannion JE, Cook TL, Jr., Zygar CA. Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev Genet. 1997;20:246–257. doi: 10.1002/(SICI)1520-6408(1997)20:3<246::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hendrix RW, Zwaan J. Changes in the glycoprotein concentration of the extracellular matrix between lens and optic vesicle associated with early lens differentiation. Differentiation. 1974;2:357–362. doi: 10.1111/j.1432-0436.1974.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Hendrix RW, Zwaan J. The matrix of the optic vesicle-presumptive lens interface during induction of the lens in the chicken embryo. J Embryol Morphol. 1975;33:1023–1049. [PubMed] [Google Scholar]
- Holly VL, Widen SA, Famulski JK, Waskiewicz AJ. Sfrp1a and Sfrp5 function as positive regulators of Wnt and BMP signaling during early retinal development. Developmental Biology. 2014;388:192–204. doi: 10.1016/j.ydbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Huang J, Rajagopal R, Liu Y, Dattilo LK, Shaham O, Ashery-Padan R, Beebe DC. The mechanism of lens placode formation: a case of matrix-mediated morphogenesis. Developmental Biology. 2011;355:32–42. doi: 10.1016/j.ydbio.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu Y, Oltean A, Beebe DC. BMP4 from the optic vesicle specifies murine retina formation. Developmental Biology. 2015;402:119–126. doi: 10.1016/j.ydbio.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–877. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Developmental Biology. 2003;259:351–363. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development. 2008;135:3567–3576. doi: 10.1242/dev.028076. [DOI] [PubMed] [Google Scholar]
- Morcillo J, Martinez-Morales JR, Trousse F, Fermin Y, Sowden JC, Bovolenta P. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006;133:3179–3190. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Pandit T, Jidigam VK, Gunhaga L. BMP-induced L-Maf regulates subsequent BMP-independent differentiation of primary lens fibre cells. Developmental Dynamics. 2011;240:1917–1928. doi: 10.1002/dvdy.22692. [DOI] [PubMed] [Google Scholar]
- Pandit T, Jidigam VK, Patthey C, Gunhaga L. Neural retina identity is specified by lens-derived BMP signals. Development. 2015;142:1850–1859. doi: 10.1242/dev.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73–83. doi: 10.1242/dev.025890. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Developmental biology. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont MEM, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Sjödal M, Edlund T, Gunhaga L. Time of Exposure to BMP Signals Plays a Key Role in the Specification of the Olfactory and Lens Placodes Ex Vivo. Developmental Cell. 2007;13:141–149. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Sullivan CH, Braunstein L, Hazard-Leonards RM, Holen AL, Samaha F, Stephens L, Grainger RM. A re-examination of lens induction in chicken embryos: in vitro studies of early tissue interactions. Int J Dev Biol. 2004;48:771–782. doi: 10.1387/ijdb.041894cs. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Developmental biology. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Viringipurampeer IA, Ferreira T, DeMaria S, Yoon JJ, Shan X, Moosajee M, Gregory-Evans K, Ngai J, Gregory-Evans CY. Pax2 regulates a fadd-dependent molecular switch that drives tissue fusion during eye development. Human Molecular Genetics. 2012;21:2357–2369. doi: 10.1093/hmg/dds056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Huang H, Cao P, Wang Z, Chen Y, Pan Y. Sma- and Mad-related protein 7 (Smad 7) is required for embryonic eye development in the mouse. J Biol Chem. 2013;288:10275–10285. doi: 10.1074/jbc.M112.416719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Saitsu H, Sun X, Shiota K, Ishibashi M. Sonic hedgehog is involved in formation of the ventral optic cup by limiting Bmp4 expression to the dorsal domain. Mechanisms of Development. 2010;127:62–72. doi: 10.1016/j.mod.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Zhou C-J, Molotkov A, Song L, Li Y, Pleasure DE, Pleasure SJ, Wang Y-Z. Ocular coloboma and dorsoventral neuroretinal patterning defects in Lrp6 mutant eyes. Developmental Dynamics. 2008;237:3681–3689. doi: 10.1002/dvdy.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan J, Webster EH. Histochemical analysis of extracellular matrix material during embryonic mouse lens morphogenesis in an aphakic strain of mice. Developmental Biology. 1984;104:380–389. doi: 10.1016/0012-1606(84)90093-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.