Abstract
Objective
The goal was to examine lipoprotein subclass responses to regular exercise as measured in 10 exercise interventions derived from six cohorts.
Methods
Nuclear magnetic resonance spectroscopy was used to quantify average particle size, total and subclass concentrations of very low-density lipoprotein, low-density lipoprotein, and high-density lipoprotein particles (VLDL-P, LDL-P, and HDL-P, respectively) before and after an exercise intervention in 1,555 adults from six studies, encompassing 10 distinct exercise programs: APOE (N=106), DREW (N=385), GERS (N=79), HERITAGE (N=715), STRRIDE I (N=168) and II (N=102). Random-effects meta-analyses were performed to evaluate the overall estimate of mean change across the unadjusted and adjusted mean change values from each exercise group.
Results
Meta-analysis of unadjusted data showed that regular exercise induced significant decreases in the concentration of large VLDL-P, small LDL-P, and medium HDL-P and mean VLDL-P size, with significant increases in the concentration of large LDL-P and large HDL-P and mean LDL-P size. These changes remained significant in meta-analysis with adjustment for age, sex, race, baseline body mass index, and baseline trait value.
Conclusions
Despite differences in exercise programs and study populations, regular exercise produced putatively beneficial changes in the lipoprotein subclass profile across 10 exercise interventions. Further research is needed to examine how exercise-induced changes in lipoprotein subclasses may be associated with (concomitant changes in) cardiovascular disease risk.
Keywords: exercise training, lipids, lipoprotein subfractions, NMR spectroscopy
Introduction
Physical inactivity is designated as a major target of intervention for therapeutic lifestyle change in the management of dyslipidemia,1 as regular exercise has been shown to be beneficial, on average, for the overall lipid and lipoprotein profile.2 For most adults, plasma levels of both high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) respond favorably to regular exercise in a reproducible manner, while total cholesterol and low-density lipoprotein cholesterol (LDL-C) concentrations are less responsive.3 However, there is large inter-individual variation in the magnitude of lipoprotein changes observed with regular exercise,4 ranging from large improvements to unfavorable changes.5 Furthermore, interpretation based solely on the cholesterol content of HDL and LDL particles (HDL-P and LDL-P) may not fully explain the positive adaptations associated with a physically active lifestyle.
Limitations in our ability to predict cardiovascular disease (CVD) risk have led to increased clinical interest in identifying novel risk markers and refining the measurement of traditional risk factors, such as LDL-C and HDL-C.6 Lipoproteins consist of a wide spectrum of heterogeneous particles that differ in size, density, composition, and function. Numerous studies have demonstrated an association between lipoprotein subfractions and CVD risk,7-13 particularly LDL14 and HDL15 subfractions. Overall, these results suggest that more refined analyses of lipoprotein subfractions will lead to further improvements in CVD risk evaluation and perhaps even to the identification of appropriate targets for therapeutic intervention in individual patients.9
A few exercise training studies have shown that regular endurance exercise has favorable effects on NMR-based lipoprotein subfraction traits.16-20 However, two of these studies are limited by their small sample sizes (e.g., N<4016, 18) and/or examination of specific populations (e.g., women with polycystic ovary syndrome16), with one intervention incorporating both caloric restriction and exercise.18 Given the limited sample sizes and differing inclusion/exclusion criteria, type of exercise programs, and algorithms used for determining lipoprotein subclasses of the available studies, we undertook a study to understand the magnitude and consistency of the effects of regular endurance exercise on the lipoprotein subclass profile by examining the response of NMR-based lipoprotein particle traits to regular exercise across six large, well-established exercise training studies. We hypothesized that regular exercise has beneficial effects on the overall lipoprotein subclass profile in previously sedentary adults.
Methods
The effects of standardized, regular endurance exercise on plasma lipoprotein particle traits, as determined by NMR spectroscopy, were examined across six exercise training studies with 10 distinct exercise programs, which are briefly described below. An overview of the six studies, including characteristics and description of the exercise training program for each is given in Table 1. More detailed information for each study can be found in the Supplemental Material.
Table 1.
Overview of the exercise training programs of the six studies.
| Study | N | Dose | Frequency | Intensity | Time | Mode | Duration |
|---|---|---|---|---|---|---|---|
| HERITAGE | 715 | ~12-14 KKW | 3×/wk | 55-75% VO2 max | 30-50 min/session | cycle | 20 weeks |
|
| |||||||
| DREW | 385 | 24 weeks | |||||
| Control | 87 | --- | --- | --- | --- | --- | |
| 4 KKW | 126 | 4 KKW | 3-4×/wk | 50% VO2 max | 72.2 (12.3) min/wk | cycle and treadmill |
|
| 8 KKW | 85 | 8 KKW | 3-4×/wk | 50% VO2 max | 135.8(19.5) min/wk | ||
| 12 KKW | 87 | 12 KKW | 3-4×/wk | 50% VO2 max | 191.7(33.7) min/wk | ||
|
| |||||||
| STRRIDE I | 168 | 35 weeks | |||||
| Control | 38 | --- | --- | --- | --- | --- | |
| MILD | 37 | 14 KKW | 3-4×/wk | 40-55% VO2 max | 170 min/wk | cycle, treadmill, elliptical |
|
| MOD | 47 | 14 KKW | 3-4×/wk | 65-80% VO2 max | 120 min/wk | ||
| HIGH | 46 | 23 KKW | 3-4×/wk | 65-80% VO2 max | 170 min/wk | ||
|
| |||||||
| STRRIDE II | 102 | 35 weeks | |||||
| MOD | 47 | 14 KKW | 3×/wk | 65-80% VO2 max | 130 min/wk | cycle, treadmill, elliptical |
|
| AT/RT (or MOD+RT) |
44 | >14 KKW | 3-5×/wk | AT: 130 min/wk + RT: 135-180 min/wk |
|||
| HIGH | 11 | 23 KKW | 3×/wk | 65-80% VO2 max | 170 min/wk | ||
|
| |||||||
| APOE | 106 | ~13-15 KKW | 4×/wk | 60-85% VO2 max | 40 min/session | treadmill, cycle, stair, ski, rowing |
24 weeks |
|
| |||||||
| GERS | 79 | ~10-12 KKW | 3×/wk | 55-70% VO2 max | 40 min/session; 45-60 min weekend walk |
treadmill, elliptical, rowing |
24 weeks |
KKW, kcal per kg of body weight per week.
Apolipoprotein E (APOE) Study
The APOE Study involved 106 (94% White) previously sedentary, normal to overweight (body mass index (BMI) <31 kg/m2) men and women (52%) aged 18-70 years from seven geographic sites that completed six months of progressive aerobic exercise training.20
Dose-Response to Exercise in Women (DREW) Study
The DREW Study was a randomized, dose-response exercise trial with sedentary, high-normal blood pressure, postmenopausal, overweight or obese women aged 45-75 years (N=385; 63% White) assigned to either a non-exercise control group (N=87) or to endurance exercise groups that expended 4 (N=126), 8 (N=85), or 12 (N=87) kcal per kg of body weight per week (KKW) in the form of aerobic exercise for a period of six months.21
HEalth, RIsk factors, exercise Training And GEnetics (HERITAGE) Family Study
The HERITAGE sample studied here included 715 Black (34%) and White men and women (56%) aged 17-65 years who completed a 20-week endurance exercise program consisting of aerobic exercise three days a week.22
Gene Exercise Research Study (GERS)
The GERS cohort included 79 sedentary, non-diabetic, non-smoking adults aged 50 to 71 years (83% White; 58% female, all post-menopausal) with no history of CVD but with high normal blood pressure or Stage 1 hypertension or at least one National Cholesterol Education Program lipid abnormality (cholesterol >200 mg/dL, HDL-C <40 mg/dL, TG >200 mg/dL) who completed six months of supervised endurance exercise.19
Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE)
The STRRIDE I23 and II24 studies were randomized, controlled, clinical studies that investigated the effects of the amount and intensity of exercise over eight months on CVD risk factors in 40-65 year old sedentary, overweight or class I obese (BMI 25-35 kg/m2) men and women with mild-to-moderate dyslipidemia (LDL-C 130-190 mg/dL and/or HDL-C ≤40 mg/dL for men or ≤45 mg/dL for women). In STRRIDE I, subjects (N=168; 80% White, 48% female) were assigned to one of four groups: 1) control (N=38), 2) MILD (14 KKW, N=37): low-amount, moderate-intensity exercise (40-55% VO2peak), 3) MOD (14 KKW, N=47): low-amount, vigorous-intensity (65-80% VO2peak), and 4) HIGH (23 KKW, N=46): high-amount, vigorous-intensity (65-80% VO2peak). Subjects in the control group maintained their normal diet and level of physical activity for six months. STRRIDE II subjects (N=102; 85% White, 55% female) assigned to one of the following endurance exercise groups were included in the present study: 1) MOD (14 KKW, N=47): low-amount, vigorous-intensity (65-80% VO2peak), 2) MOD plus resistance training (RT) (N=44): same as MOD plus RT 3 days/wk, 3 sets/day, 8-12 repetitions/set and 3) HIGH (23 KKW, N=11): high-amount, vigorous-intensity.
Lipoprotein Subclass and Particle Size Measurements
Comprehensive lipoprotein analysis was performed on fasting (12 hours) plasma samples collected before and after completion of exercise training (within 24 hours of last exercise session) in all studies by NMR spectroscopy at LipoScience, Inc (Raleigh, N.C.) using the LipoProfile-3 algorithm.25 Each measurement provides concentrations of large, medium, and small very low-density lipoprotein particles (VLDL-P) and HDL-P, large and small LDL-P, and intermediate-density lipoprotein particles (IDL-P), as well as weighted-average VLDL-P, LDL-P, and HDL-P sizes. The weighted average particle diameter for each lipoprotein is calculated as the sum of the lipoprotein subclass diameters multiplied by its relative mass percentage as estimated from the amplitude of its methyl NMR signal. Total VLDL-P, LDL-P, and HDL-P concentrations were calculated as the sum of their respective subclass concentrations (Note: IDL is grouped as a subclass of LDL).
The NMR analysis also provides estimates of the TG concentration of chylomicron and VLDL fractions (VLDL-TG; not part of primary meta-analysis). Changes in NMR-traits were calculated as post-training value minus baseline value.
Statistical Analysis
Since only two studies (DREW and STRRIDE I) included control groups, our primary analyses focused on the exercise groups only. As the exercise prescription and training programs differed between and within the studies, we analyzed each of the exercise groups independently. There were 12 distinct exercise programs across the six studies. However, since the MOD and HIGH programs of STRRIDE I and II were identical, we combined these exercise groups for the meta-analysis. Thus, 10 total exercise programs were examined in the meta-analysis.
Meta-Analysis
We did not perform a systematic literature-based meta-analysis. The included studies were part of an exisiting collaboration on the genetics of lipid response to exercise training. All studies met the following criteria: included adults (≥18 years) with available pre- and post-training NMR lipoprotein data, sample size ≥50, and the intervention was supervised, standardized, involved exercise only (e.g., no diet plus exercise), and lasted at least 12 weeks. Exercise response of the lipoprotein subclass traits was examined separately in each of the 10 exercise groups using two different methods. In Method 1 the individual raw estimates of mean change were used, i.e., the data were not adjusted for covariates. Method 2 utilized a linear model including adjustments for age, race, baseline BMI, baseline trait value, and sex (except in the all-female DREW exercise groups). Due to differences in the type of exercise intervention and populations of the included studies, random-effects meta-analysis were performed to quantify the overall mean change separately across the unadjusted and adjusted mean change values from the 10 exercise groups. The random-effects model takes two sources of variance into consideration: within-study error in the estimate of the effect size, and between-study variation in the true effect size. The Q-statistic and associated p-value were calculated to test the assumption of homogeneity in effect sizes. The I2 statistic was calculated to estimate the proportion of total observed variance attributable to between-study variation in effect size as opposed to random error.
Additionally, we performed secondary analyses that involved a separate meta-analysis of the DREW and STRRIDE I studies, as both included control groups. The analysis followed the same steps as described above, with the exception that net change from baseline to post-training for each outcome variable was calculated as the difference of the mean exercise-induced changes (post-training minus baseline) between each exercise group and their respective control group (exercise minus control).
Results
Baseline and response to exercise training characteristics, including mean values for the standard lipid profile and CVD risk factors, are listed for the entire cohort, for the 10 exercise groups, and by control group in each study in Supplemental Tables S1-S7. Table 2 shows the baseline values of the lipoprotein particle traits in each of the ten exercise groups, while Supplemental Table S8 shows these values for the control groups of DREW and STRRIDE I. Overall, 1,555 participants, (65% women, 26% Black) were included in the series of meta-analyses.
Table 2.
Baseline lipoprotein subfraction trait values in the ten exercise groups.
| Variable | DREW 4KKW (N=126) |
DREW 8KKW (N=85) |
DREW 12KKW (N=87) |
GERS (N=79) |
HER (N=715) |
STR I MILD (N=37) |
STR I & II MOD (N=94) |
APOE (N=106) |
STR II MOD+RT (N=44) |
STR I & II HIGH (N=57) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total VLDL-P | 48.4 (26.7) |
49.9 (26.2) |
53.4 (25.0) |
49.8 (25.4) |
45.4 (21.9) |
56.8 (35.0) |
64.3 (37.4) |
64.1 (44.8) |
65.3 (29.1) | 64.3 (33.9) |
| Large VLDL-P | 4.8 (3.6) | 4.6 (3.7) | 5.2 (4.8) | 7.3 (5.7) |
3.3 (3.1) | 8.1 (7.1) | 6.3 (6.4) | 7.2 (9.3) | 5.8 (6.9) | 7.2 (7.0) |
| Medium VLDL-P | 16.7 (12.1) |
17.5 (11.7) |
19.4 (12.4) |
21.0 (14.0) |
14.9 (11.2) |
23.8 (18.3) |
27.1 (21.4) |
31.1 (23.6) |
29.5 (20.1) | 29.7 (20.2) |
| Small VLDL-P | 26.9 (16.6) |
27.8 (16.4) |
28.7 (14.5) |
21.6 (16.7) |
27.3 (13.7) |
24.9 (21.1) |
30.8 (20.1) |
25.6 (23.0) |
30.7 (14.4) | 27.3 (17.2) |
| VLDL-P size | 51.9 (7.4) |
51.0 (6.5) |
50.5 (7.1) | 56.1 (8.8) |
48.8 (6.8) |
56.8 (10.1) |
51.3 (8.2) | 52.9 (7.5) |
49.8 (8.5) | 52.6 (8.0) |
| Total LDL-P | 1046 (243) |
1066 (255) |
1111 (295) |
1756 (469) |
978.6 (324) |
1566 (318) |
1456 (340) |
1751 (550) |
1304 (276) | 1565 (388) |
| IDL-P | 154.7 (95.2) |
147.6 (83.1) |
166.4 (88.9) |
378.2 (178) |
144.1 (85.5) |
289.0 (179) |
181.5 (124) |
386.5 (154) |
112.0 (70.1) |
254.5 (176) |
| Large LDL-P | 353.9 (188) |
337.2 (213) |
360.1 (179) |
456.8 (303) |
321.4 (176) |
566.6 (292) |
570.7 (289) |
380.0 (227) |
539.7 (275) |
541.1 (303) |
| Small LDL-P | 537.4 (224) |
581.7 (237) |
584.8 (280) |
920.5 (520) |
513.1 (288) |
709.9 (390) |
703.5 (374) |
801.9 (531) |
664.3 (302) |
779.1 (412) |
| LDL-P size | 20.9 (0.6) |
20.7 (0.5) |
20.8 (0.6) | 20.8 (0.9) |
20.9 (0.6) |
20.9 (0.8) |
20.8 (0.7) | 20.8 (0.7) |
20.7 (0.7) | 20.7 (0.7) |
| Total HDL-P | 35.0 (5.5) |
34.5 (6.2) |
34.2 (5.1) | 32.6 (5.8) |
29.6 (5.0) |
33.7 (6.9) |
31.9 (6.1) | 42.3 (7.8) |
33.4 (5.9) | 31.0 (5.8) |
| Large HDL-P | 5.5 (2.9) | 5.5 (2.8) | 5.1 (2.7) | 5.1 (2.5) |
4.6 (2.5) | 4.6 (2.4) | 4.2 (3.0) | 6.7 (4.0) | 4.2 (2.3) | 4.0 (2.5) |
| Medium HDL-P | 12.6 (6.0) |
12.3 (5.9) |
12.2 (5.3) | 14.6 (9.2) |
10.0 (4.7) |
14.5 (7.1) |
12.6 (6.2) | 17.7 (10.2) |
11.2 (6.5) | 11.6 (6.6) |
| Small HDL-P | 16.9 (5.7) |
16.7 (5.4) |
16.9 (5.1) | 12.9 (7.5) |
15.0 (4.6) |
14.5 (6.5) |
15.1 (5.7) | 17.3 (9.3) |
18.0 (5.5) | 15.4 (5.8) |
| HDL-P size | 9.2 (0.4) | 9.2 (0.4) | 9.1 (0.4) | 9.2 (0.4) |
9.1 (0.5) | 9.0 (0.4) | 8.9 (0.5) | 9.2 (0.5) | 8.9 (0.4) | 8.9 (0.4) |
Data shown as mean (SD). Units: VLDL-P and LDL-P concentrations are in nmol/L, HDL-P concentrations are in μmol/L, and particle size traits are in nm.
HER: HERITAGE, STR: STRRIDE.
Lipoprotein Subfraction Responses to Regular Exercise
NMR subfraction responses by individual exercise group
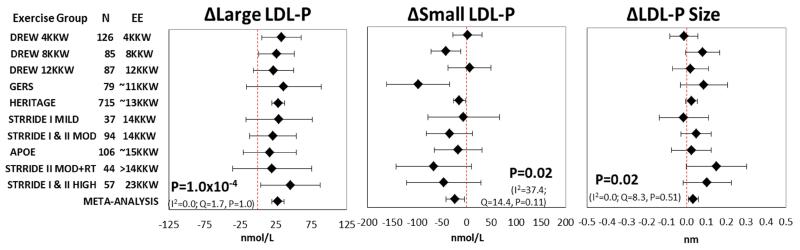
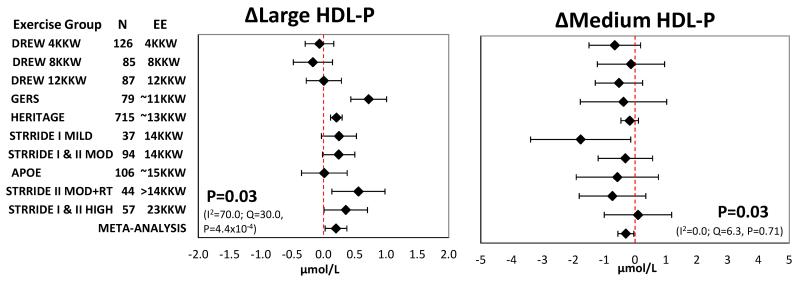
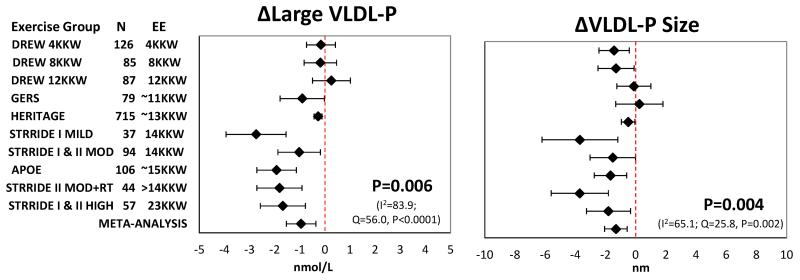
Mean change with 95% confidence intervals in the ten exercise groups including the summary estimate from meta-analysis for each NMR lipoprotein subclass trait from the unadjusted and adjusted methods are presented in Figures 1-3 and Supplemental Figures S1-S5. The most consistent exercise-training responses (i.e., direction of unadjusted raw values of mean change) were as follows: large LDL-P increased and medium HDL-P decreased in all ten exercise groups, while large VLDL-P decreased in nine of 10 groups. Similarly, the direction of change was consistent in eight of 10 groups for small LDL-P, large HDL-P, VLDL-P and LDL-P size. It should be noted that although trait responses were consistent across exercise groups for several traits, the responses were not necessarily statistically significant in all exercise groups.
Figure 1.
Adjusted meta-analysis results for the response of large LDL-P, small LDL-P, and LDL-P size to regular exercise. Adjusted mean responses with 95% confidence intervals across ten exercise groups including summary estimate from random-effects meta-analysis are shown. Mean values were adjusted for age, sex, race, baseline BMI, and baseline trait value. P-value and heterogeneity statistics for the meta-analysis summary estimate are shown within each graph. Exercise groups are listed in order from the lowest to highest weekly energy expended during the training program. EE, energy expenditure. KKW, kcal/kg body weight/week.
Figure 3.
Adjusted meta-analysis results for the response of large HDL-P and medium HDL-P to regular exercise. Adjusted mean responses with 95% confidence intervals across ten exercise groups including summary estimate from random-effects meta-analysis are shown. Mean values were adjusted for age, sex, race, baseline BMI, and baseline trait value. P-value and heterogeneity statistics for the meta-analysis summary estimate are shown within each graph. Exercise groups are listed in order from the lowest to highest weekly energy expended during the training program. P-value and heterogeneity statistics for meta-analysis shown within each graph. EE, energy expenditure. KKW, kcal/kg body weight/week.
Meta-analysis of NMR subfraction responses
A summary of the results for the unadjusted and adjusted meta-analyses can be found in Tables 3 and 4, respectively. Meta-analysis of unadjusted data showed that regular exercise induced significant decreases in the concentration of large VLDL-P, small LDL-P, and medium HDL-P and mean VLDL-P size, with significant increases in the concentration of large LDL-P and large HDL-P and mean LDL-P size (Supplemental Figures S1-S3). The exercise-induced changes in these seven lipoprotein subfraction traits remained significant after adjustment for age, sex, race, baseline BMI, and baseline trait value (Figures 1-3). The results were nearly identical when change in BMI was added as a covariate in the models (Supplemental Table S9).
Table 3.
Random-effects meta-analysis results for changes in lipoprotein subfraction traits in response to regular exercise across ten exercise groups in unadjusted models.
| Meta-analysis summary statistics | Heterogeneity statistics | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Overall mean change |
95% CI | P-value | Q | Q p-value |
I2 |
| Total VLDL-P, nmol/L | 1430 | −1.49 | −3.87 to 0.90 | 0.19 | 16.5 | 0.06 | 45.5 |
| Large VLDL-P, nmol/L | 1430 | −0.84 | −1.39 to −0.28 | 0.008 | 37.6 | 2.0×10−5 | 76.1 |
| Medium VLDL-P, nmol/L | 1429 | −0.75 | −2.25 to 0.75 | 0.28 | 20.6 | 0.01 | 56.4 |
| Small VLDL-P, nmol/L | 1430 | −0.003 | −1.46 to 1.46 | 0.99 | 12.7 | 0.17 | 29.3 |
| VLDL-P size, nm | 1332 | −1.42 | −2.26 to −0.58 | 0.004 | 26.4 | 0.002 | 66.0 |
|
| |||||||
| Total LDL-P, nmol/L | 1430 | −0.31 | −23.0 to 22.3 | 0.98 | 19.8 | 0.02 | 54.6 |
| IDL-P, nmol/L | 1430 | −0.81 | −13.0 to 11.4 | 0.88 | 22.4 | 0.008 | 59.9 |
| Large LDL-P, nmol/L | 1427 | 28.47 | 19.4 to 37.6 | 9.0×10−5 | 1.6 | 1.00 | 0.0 |
| Small LDL-P, nmol/L | 1429 | −21.77 | −38.5 to −5.10 | 0.03 | 14.5 | 0.11 | 37.8 |
| LDL-P size, nm | 1417 | 0.03 | 0.003 to 0.06 | 0.04 | 6.7 | 0.67 | 0.0 |
|
| |||||||
| Total HDL-P, μmol/L | 1430 | −0.15 | −0.59 to 0.29 | 0.46 | 28.5 | 0.0008 | 68.4 |
| Large HDL-P, μmol/L | 1430 | 0.21 | 0.04 to 0.37 | 0.02 | 25.9 | 0.002 | 65.2 |
| Medium HDL-P, μmol/L | 1429 | −0.32 | −0.60 to −0.04 | 0.03 | 4.9 | 0.84 | 0.0 |
| Small HDL-P, μmol/L | 1430 | −0.01 | −0.28 to 0.25 | 0.90 | 8.6 | 0.47 | 0.0 |
| HDL-P size, nm | 1430 | 0.008 | −0.02 to 0.04 | 0.53 | 17.7 | 0.04 | 49.2 |
The Q-statistic and associated p-value test the assumption of homogeneity in effect sizes. The I2 statistic estimates the proportion of total variation in exercise response that is due to heterogeneity between studies.
Table 4.
Random-effects meta-analysis results for changes in lipoprotein subfraction traits in response to regular exercise across ten exercise groups in adjusted models.
| Meta-analysis summary statistics | Heterogeneity statistics | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Overall mean change |
95% CI | P-value | Q | Q p-value |
I2 |
| Total VLDL-P, nmol/L | 1430 | −1.95 | −4.54 to 0.64 | 0.12 | 22.7 | 0.007 | 60.4 |
| Large VLDL-P, nmol/L | 1430 | −0.95 | −1.54 to −0.36 | 0.006 | 56.0 | 7.8×10−9 | 83.9 |
| Medium VLDL-P, nmol/L | 1429 | −1.12 | −2.77 to 0.53 | 0.16 | 29.9 | 0.0005 | 69.9 |
| Small VLDL-P, nmol/L | 1430 | −0.04 | −1.52 to 1.44 | 0.95 | 16.2 | 0.06 | 44.5 |
| VLDL-P size, nm | 1332 | −1.31 | −2.06 to −0.57 | 0.004 | 25.8 | 0.002 | 65.1 |
|
| |||||||
| Total LDL-P, nmol/L | 1430 | −0.53 | −20.1 to 19.0 | 0.95 | 16.4 | 0.06 | 45.3 |
| IDL-P, nmol/L | 1430 | −1.42 | −13.2 to 10.4 | 0.79 | 26.7 | 0.002 | 66.2 |
| Large LDL-P, nmol/L | 1427 | 28.20 | 19.7 to 36.7 | 0.0001 | 1.7 | 1.0 | 0.0 |
| Small LDL-P, nmol/L | 1429 | −23.27 | −41.9 to −4.67 | 0.02 | 14.4 | 0.11 | 37.4 |
| LDL-P size, nm | 1417 | 0.03 | 0.01 to 0.06 | 0.02 | 8.3 | 0.51 | 0.0 |
|
| |||||||
| Total HDL-P, μmol/L | 1430 | −0.15 | −0.60 to 0.30 | 0.46 | 31.5 | 0.0002 | 71.4 |
| Large HDL-P, μmol/L | 1430 | 0.20 | 0.03 to 0.37 | 0.02 | 30.0 | 0.0004 | 70.0 |
| Medium HDL-P, μmol/L | 1429 | −0.30 | −0.56 to −0.04 | 0.03 | 6.3 | 0.71 | 0.0 |
| Small HDL-P, μmol/L | 1430 | −0.04 | −0.28 to 0.20 | 0.73 | 8.6 | 0.47 | 0.0 |
| HDL-P size, nm | 1430 | 0.006 | −0.02 to 0.03 | 0.64 | 19.4 | 0.02 | 53.7 |
Overall estimated mean change values represent the weighted mean of the mean values within the ten exercise groups adjusted for age, sex, race, baseline BMI, and baseline trait value. The Q-statistic and associated p-value test the assumption of homogeneity in effect sizes The I2 statistic estimates the proportion of total variation in exercise response that is due to heterogeneity between studies.
Significant heterogeneity between studies was indicated for half of the analyzed lipoprotein traits using the Q statistic, while according to the I2 statistic heterogeneity was generally moderate to high (Tables 3 & 4). However, interpretation of a given degree of heterogeneity across studies differs according to whether the change values show the same direction of effect. For example, despite the adjusted meta-analysis of large VLDL-P having the largest I2 value of 83.9, the direction of effect was quite consistent, as nine of the 10 exercise groups exhibited decreases in large VLDL-P, with 7 of these being statistically significant at the individual group level (Figure 2).
Figure 2.
Adjusted meta-analysis results for the response of large VLDL-P and VLDL-P size to regular exercise. Adjusted mean responses with 95% confidence intervals across ten exercise groups including summary estimate from random-effects meta-analysis are shown. Mean values were adjusted for age, sex, race, baseline BMI, and baseline trait value. P-value and heterogeneity statistics for the meta-analysis summary estimate are shown within each graph. Exercise groups are listed in order from the lowest to highest weekly energy expended during the training program. EE, energy expenditure. KKW, kcal/kg body weight/week.
In exploratory analyses, we compared the response of NMR-based lipoprotein subfractions between each exercise group and control group in the two studies that included control groups: DREW and STRRIDE I (Supplemental Table S10 & Supplemental Figures S6-S11). In meta-analysis of unadjusted DREW and STRRIDE I data only, a nominal (P=0.05) decrease in total LDL-P concentration was observed, which was abolished after adjustment for covariates (Supplemental Figures S8-S9).
Last, VLDL-TG concentration decreased in nine of the 10 exercise groups. The overall estimate of decreased VLDL-TG concentration across the 10 exercise groups was significant in both the unadjusted (P=0.01) and adjusted (P=0.006) meta-analysis (Supplemental Figure S12).
Response of Traditional Lipid Profile to Regular Exercise
Of the six exercise training studies, four (APOE, DREW, GERS, HERITAGE) include data for the lipid profile (e.g., HDL-C, LDL-C, TG) measured using traditional methods, while STRRIDE I and II include NMR-based estimates of HDL-C and TG.
Traditional lipid responses by individual exercise group
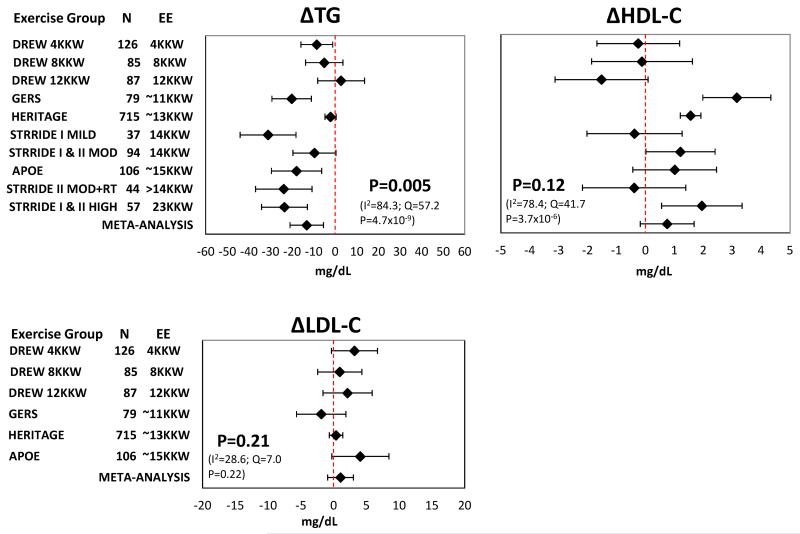
When examining the response to regular exercise in the individual exercise groups, five of the 10 exercise groups experienced mean increases in HDL-C, nine of 10 groups experienced decreases in TG, and five of six groups showed mean increases in LDL-C.
Meta-analysis of traditional lipid responses
Adjusted meta-analysis found that the exercise-induced decrease in TG across groups was significant (mean change −13.1 mg/dL, 95% CI: −5.3 to −20.9, P=0.005), while the overall mean exercise-induced increases in HDL-C and LDL-C did not reach significance (Figure 4). Unadjusted meta-analysis of the traditional lipid traits gave similar results (Supplemental Figure S13). More detailed analyses of changes in the standard lipid profile within each study, including comparisons between treatment groups, have been previously published.4, 17, 19, 24, 26-29
Figure 4.
Adjusted mean responses of TG, HDL-C, and LDL-C to regular exercise with 95% confidence intervals across ten exercise groups including summary estimate from random-effects meta-analysis. Mean values were adjusted for age, sex, race, baseline BMI, and baseline trait value. P-value and heterogeneity statistics for the meta-analysis summary estimate are shown within each graph. The TG and HDL-C values for the STRRIDE exercise groups were estimated from NMR spectroscopy. EE, energy expenditure. KKW, kcal/kg body weight/week.
Correlations between traditional and NMR lipid measures
The correlations between exercise-induced changes in the traditional and NMR-based lipid and lipoprotein profiles in the APOE, DREW, GERS, and HERITAGE studies can be found in Supplemental Table S11. Change in HDL-C was moderately correlated with change in large HDL-P (r = 0.33-0.56, P≤0.0003) and change in TG showed the strongest correlations with change in large VLDL-P (r = 0.30-0.82, P<0.0001) and VLDL-TG (r = 0.49-0.93, P<0.0001) across all four studies, while change in LDL-C was most strongly correlated with change in total LDL-P in APOE, DREW, and HERITAGE (r = 0.46-0.68, P<0.0001).
Discussion
We found that regular aerobic exercise led to beneficial changes in the lipoprotein subclass profile across ten exercise groups from six exercise training studies that differed in exercise program components and study populations. In a series of meta-analyses, we found increases in the concentrations of large HDL-P and large LDL-P and mean LDL-P size and decreases in the concentration of large VLDL-P, small LDL-P, and medium HDL-P and mean VLDL-P size in response to exercise training. Furthermore, these exercise-induced changes were independent of the age, sex, race, baseline BMI, delta BMI, and baseline trait values of the study populations.
Given the role of the liver in lipid and glucose metabolism, it represents an important target for the effects of exercise.30 The mechanisms underlying the beneficial effects of regular exercise on lipid metabolism have not been fully elucidated, but are thought to involve an increased clearance of plasma VLDL-TG31-33 and a reduced liver secretion of VLDL-TG.34 After being secreted by the liver into circulation, VLDL-P are subjected to the lipolytic action of lipoprotein lipase (LPL), which hydrolyzes VLDL-TG making VLDL-P smaller and denser,35 eventually forming large LDL-P as VLDL remnants and large HDL-P through the transfer of released surface components.36 These particles may be further delipidated by hepatic lipase (HL), producing smaller, denser LDL and HDL particles.36 Thus, exercise-induced increases in lipoprotein lipase (LPL) and reductions in hepatic lipase (HL) activities also contribute to the observed concomitant changes in lipoprotein particles.37 LPL activity and mass increases 8-22 hours post exercise, probably to replenish intramuscular TG consumed during exercise.35, 38-40 HL activity decreases for unclear reasons. For example, in HERITAGE (N=665) LPL activity increased 39% (+7.0 nmol/min/mL) and HL activity decreased 3% (−10.9 nmol/min/mL) after 20 weeks of exercise training (both P<0.0001).
Therefore, regular exercise likely decreases the secretion and clearance of VLDL-TG combined with increased catabolism of TG-rich lipoproteins leading to a reduced concentration of TG available for exchange with LDL and HDL particles and for hydrolysis by HL, along with a shift in the distribution of LDL and HDL particles towards larger ones. These exercise-induced changes in lipoprotein metabolism would increase the concentration of both large HDL-P and LDL-P and decrease the concentration of medium HDL-P, large VLDL-P and small LDL-P, as observed in the present study. As expected, these changes were paralleled by significant decreases in VLDL-TG and TG across the 10 exercise groups.
Although we found significant changes in the concentrations of large and medium HDL-P, overall we did not observe a significant change in average HDL-P size. This is likely due to the methodological limitations of the NMR particle size estimate. NMR lipoprotein particle sizes are mass-weighted, not particle number–weighted average diameters. Thus, reported diameters may often seem inconsistent with the relative numbers of large and small particle subclasses.25 Mean HDL sizes span a narrow range and the exercise-induced HDL subclass changes across the studies were modest, thus translating to only very small HDL size changes. In general, the expected HDL-P size increase was observed in the studies with the biggest increases in large HDL-P. However, when changes in large HDL-P were smaller, the variable changes in medium and small HDL-P particle concentrations contributed to obfuscating the HDL size findings.
The beneficial effects of exercise training likely result from a combination of the acute effects of the last session of exercise and the accumulation of previous exercise sessions. A single exercise session produces metabolic changes that last for a finite amount of time. Performing an additional bout of exercise (i.e., training) before the effect of the first/previous bout fully dissipates may result in even greater benefits (i.e., accumulated effects).41 In this case, the benefits of exercise training would be dependent on the last exercise session as well as the prior sessions completed within a given amount of time. However, it is difficult to quantify the interaction of the acute and chronic effects of exercise on plasma lipid and lipoprotein levels. There is strong evidence that a single exercise session can cause acute reductions in TG and increases in HDL-C levels.42 For example, it is well established that a single, prolonged bout of moderate intensity endurance exercise evokes a hypotriacylglycerolemic effect that occurs 12-24 hr after exercise and persists up to 72 hr post-exercise cessation.35, 42
How training status or chronic exercise may affect the response to acute exercise is unclear. It could be that the acute effects are prolonged or the effect is augmented in the trained state. Given the likely higher fitness level with training, it is difficult to distinguish between the effects of training per se versus the effects of a higher energy expenditure at the same relative intensity (which would lead to greater benefits, e.g. lower TG levels).35 Evidence from exercise cessation studies suggests that the lipid and lipoprotein levels in trained individuals are not due solely to the acute exercise effect.42 For example, a previous report from the STRRIDE cohort examined NMR-measured lipoprotein subfractions 5- and 15-days after exercise cessation (i.e., detraining period).28 The authors found that high-volume, vigorous intensity exercise training resulted in increases in HDL-C, large HDL-P, and HDL-P size at 24 hr after the last session that were sustained over 15 days of detraining.28 Additionally, low-volume, moderate intensity training produced reductions in large VLDL-P and VLDL-P size sustained at 5 days of detraining, while the reductions in VLDL-TG were sustained at 5 and 15 days of detraining.
There is still debate as to whether exercise-induced changes in the lipoprotein profile are independent of initial body weight and weight changes resulting from the exercise program. We found that improvements in the lipoprotein subfraction profile occurred with little to no weight loss across the 10 exercise groups (average weight loss ranged from 0.33 to 2.5 kg or 0.3% to 2.8%, see Supplemental Tables S1-S7). As Stevens and colleagues recommend that weight maintenance be defined as less than a 3% change in body weight,43 our findings can be considered independent of weight loss. Furthermore, significant changes in lipoprotein subclasses were present even after controlling for baseline and change in BMI, suggesting an independent effect of exercise training per se on the lipoprotein subclass profile. These results are in agreement with those in a previous report from the GERS cohort (N=100) which showed that the significant changes in HDL-P (small), LDL-P (total, medium, very small), and VLDL-P (small and large) concentration and HDL-P and VLDL-P size after six months of endurance exercise were independent of diet and baseline or change in body fat.19 These findings suggest that individuals can benefit from regular endurance exercise regardless of their body weight status and whether they experience exercise-induced weight loss or not.
Strengths and Limitations
The present study examined the response of lipoprotein subclass traits to regular exercise across populations of varying ethnicities, ages, and health characteristics. Our results are strengthened by the fact that the exercise response data were derived from six well-established, supervised and standardized exercise training studies with very high levels of compliance. Furthermore, the NMR measures were standardized and performed by the same commercial laboratory across all studies, thereby reducing measurement error in the phenotypes of interest. Our results identified consistent trends across several cohorts which individually might not have documented such associations.
Our study was limited by the fact that four of the six studies did not include control groups. Thus, we could only take into account trait changes after exercise training in comparison to baseline values for these studies. Although the dependent variables were all measured in the same way on the same scale, the amount, duration, and intensity of exercise varied across the six studies. We performed meta-analysis that statistically adjusted for multiple characteristics, but this does not fully account for the lack of controls. Heterogeneity between studies was moderate to high, which was expected given the diversity of interventions and study populations. This heterogeneity should reduce the overall effect, yet in both unadjusted and adjusted analyses, we found significant similarities in the lipoprotein particle changes with regular exercise.
Conclusions
Our study demonstrates that regular exercise reduced the concentration of large VLDL particles concomitant with a shift from smaller LDL and HDL particles to larger particles across a large, multi-ethnic population of men and women. These changes occurred with and without concomitant changes in the cholesterol content of the lipoproteins.
Supplementary Material
Highlights.
We examined lipoprotein subfraction responses to exercise across 10 interventions
Large VLDL, small LDL, and medium HDL particles decreased with exercise training
Large LDL and large HDL particles increased with exercise training
Acknowledgments
We would like to recognize the contribution of the late Dr Jack H. Wilmore (1938-2014) to the HERITAGE Family Study.
Funding Sources
This work was supported by multiple grants from the NIH: HL-66262 and the Life Fitness Company (DREW); AG-17474 and AG-15389 (GERS); HL-45670, HL-47323, HL-47317, HL-47327, HL-47321 (HERITAGE); HL-57354 (STRRIDE I and II); 8P20 GM-1033528 (COBRE center grant to M.A. Sarzynski); 1 U54 GM104940 (Louisiana Clinical and Translational Science Center pilot grant to M.A. Sarzynski). R. Landers-Ramos is supported by NIH Predoctoral Institutional Training Grant T32AG000268 (to J.M. Hagberg). A.S. Leon is partially supported by the Henry L. Taylor Professorship in Exercise Science and Health Enhancement. C. Bouchard is partially supported by the John W. Barton, Sr. Endowed Chair in Genetics and Nutrition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose.
References
- [1].National Cholesterol Education Program Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- [2].Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- [3].Durstine JL, Grandjean PW, Davis PG, et al. Blood Lipid and Lipoprotein Adaptations to Exercise: A Quantitative Analysis. Sports Medicine. 2001;31:1033–1062. doi: 10.2165/00007256-200131150-00002. [DOI] [PubMed] [Google Scholar]
- [4].Leon AS, Gaskill SE, Rice T, et al. Variability in the response of HDL cholesterol to exercise training in the HERITAGE Family Study. Int J Sports Med. 2002;23:1–9. doi: 10.1055/s-2002-19270. [DOI] [PubMed] [Google Scholar]
- [5].Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7:e37887. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].deGoma EM, Rader DJ. High-density lipoprotein particle number: a better measure to quantify high-density lipoprotein? J Am Coll Cardiol. 2012;60:517–520. doi: 10.1016/j.jacc.2012.03.058. [DOI] [PubMed] [Google Scholar]
- [7].Parish S, Offer A, Clarke R, et al. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- [8].Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- [9].Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–311. doi: 10.1097/MOL.0b013e32833b7756. [DOI] [PubMed] [Google Scholar]
- [10].Freedman DS, Otvos JD, Jeyarajah EJ, et al. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- [11].Kuller LH, Grandits G, Cohen JD, et al. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mackey RH, Kuller LH, Sutton-Tyrrell K, et al. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am J Cardiol. 2002;90:71i–76i. doi: 10.1016/s0002-9149(02)02636-x. [DOI] [PubMed] [Google Scholar]
- [13].Mora S, Otvos JD, Rifai N, et al. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ip S, Lichtenstein AH, Chung M, et al. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009;150:474–484. doi: 10.7326/0003-4819-150-7-200904070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- [16].Brown AJ, Setji TL, Sanders LL, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009;41:497–504. doi: 10.1249/MSS.0b013e31818c6c0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- [18].Shadid S, LaForge R, Otvos JD, et al. Treatment of obesity with diet/exercise versus pioglitazone has distinct effects on lipoprotein particle size. Atherosclerosis. 2006;188:370–376. doi: 10.1016/j.atherosclerosis.2005.10.038. [DOI] [PubMed] [Google Scholar]
- [19].Halverstadt A, Phares DA, Wilund KR, et al. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56:444–450. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- [20].Seip RL, Otvos J, Bilbie C, et al. The effect of apolipoprotein E genotype on serum lipoprotein particle response to exercise. Atherosclerosis. 2006;188:126–133. doi: 10.1016/j.atherosclerosis.2005.06.050. [DOI] [PubMed] [Google Scholar]
- [21].Morss GM, Jordan AN, Skinner JS, et al. Dose Response to Exercise in Women aged 45-75 yr (DREW): design and rationale. Med Sci Sports Exerc. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- [22].Bouchard C, Leon AS, Rao DC, et al. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc. 1995;27:721–729. [PubMed] [Google Scholar]
- [23].Kraus WE, Torgan CE, Duscha BD, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Med Sci Sports Exerc. 2001;33:1774–1784. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- [24].Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) Am J Cardiol. 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [26].Leon AS, Rice T, Mandel S, et al. Blood lipid response to 20 weeks of supervised exercise in a large biracial population: the HERITAGE Family Study. Metabolism. 2000;49:513–520. doi: 10.1016/s0026-0495(00)80018-9. [DOI] [PubMed] [Google Scholar]
- [27].Arsenault BJ, Cote M, Cartier A, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Slentz CA, Houmard JA, Johnson JL, et al. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2007;103:432–442. doi: 10.1152/japplphysiol.01314.2006. [DOI] [PubMed] [Google Scholar]
- [29].Thompson PD, Tsongalis GJ, Seip RL, et al. Apolipoprotein E genotype and changes in serum lipids and maximal oxygen uptake with exercise training. Metabolism. 2004;53:193–202. doi: 10.1016/j.metabol.2003.09.010. [DOI] [PubMed] [Google Scholar]
- [30].Hoene M, Weigert C. The stress response of the liver to physical exercise. Exerc Immunol Rev. 2010;16:163–183. [PubMed] [Google Scholar]
- [31].Annuzzi G, Jansson E, Kaijser L, et al. Increased removal rate of exogenous triglycerides after prolonged exercise in man: time course and effect of exercise duration. Metabolism. 1987;36:438–443. doi: 10.1016/0026-0495(87)90040-0. [DOI] [PubMed] [Google Scholar]
- [32].Sady SP, Cullinane EM, Saritelli A, et al. Elevated high-density lipoprotein cholesterol in endurance athletes is related to enhanced plasma triglyceride clearance. Metabolism. 1988;37:568–572. doi: 10.1016/0026-0495(88)90173-4. [DOI] [PubMed] [Google Scholar]
- [33].Tsekouras YE, Magkos F, Prentzas KI, et al. A single bout of whole-body resistance exercise augments basal VLDL-triacylglycerol removal from plasma in healthy untrained men. Clin Sci (Lond) 2009;116:147–156. doi: 10.1042/CS20080078. [DOI] [PubMed] [Google Scholar]
- [34].Tsekouras YE, Magkos F, Kellas Y, et al. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am J Physiol Endocrinol Metab. 2008;295:E851–858. doi: 10.1152/ajpendo.90545.2008. [DOI] [PubMed] [Google Scholar]
- [35].Magkos F. Basal very low-density lipoprotein metabolism in response to exercise: mechanisms of hypotriacylglycerolemia. Prog Lipid Res. 2009;48:171–190. doi: 10.1016/j.plipres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [36].Wang M, Briggs MR. HDL: the metabolism, function, and therapeutic importance. Chem Rev. 2004;104:119–137. doi: 10.1021/cr020466v. [DOI] [PubMed] [Google Scholar]
- [37].Church TS, Lavie CJ, Sarzynski MA, et al. Exercise and Lipids. In: Ballantyne CM, editor. Clinical Lipidology: A Companion to Braunwald’s Heart Disease. 2nd edition Saunders Elesevier; Philadelphia, PA: 2015. pp. 210–216. [Google Scholar]
- [38].Kantor MA, Cullinane EM, Sady SP, et al. Exercise acutely increases high density lipoprotein-cholesterol and lipoprotein lipase activity in trained and untrained men. Metabolism. 1987;36:188–192. doi: 10.1016/0026-0495(87)90016-3. [DOI] [PubMed] [Google Scholar]
- [39].Thompson PD. What do muscles have to do with lipoproteins? Circulation. 1990;81:1428–1430. doi: 10.1161/01.cir.81.4.1428. [DOI] [PubMed] [Google Scholar]
- [40].Seip RL, Semenkovich CF. Skeletal muscle lipoprotein lipase: molecular regulation and physiological effects in relation to exercise. Exerc Sport Sci Rev. 1998;26:191–218. [PubMed] [Google Scholar]
- [41].Wagganer JD, Robison CE, Ackerman TA, et al. Effects of exercise accumulation on plasma lipids and lipoproteins. Appl Physiol Nutr Metab. 2015;40:441–447. doi: 10.1139/apnm-2014-0321. [DOI] [PubMed] [Google Scholar]
- [42].Thompson PD, Crouse SF, Goodpaster B, et al. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–445. doi: 10.1097/00005768-200106001-00012. discussion S452-433. [DOI] [PubMed] [Google Scholar]
- [43].Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes. 2005;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.