Abstract
In our previous study we examined the functionality and stability of nicotinic acetylcholine receptors (nAChRs)-detergent complexes (nAChR-DCs) from affinity-purified Torpedo californica (Tc) using fluorescence recovery after photobleaching (FRAP) in Lipidic Cubic Phase (LCP) and planar lipid bilayer (PLB) recordings for phospholipid and cholesterol like detergents. In the present study we enhanced the functional characterization of nAChR-DCs by recording macroscopic ion channel currents in Xenopus oocytes using the two electrode voltage clamp (TEVC). The use of TEVC allows for the recording of macroscopic currents elicited by agonist activation of nAChR-DCs that assemble in the oocyte plasma membrane. Furthermore, we examined the stability of nAChR-DCs, which is obligatory for the nAChR crystallization, using a 30 day FRAP assay in LCP for each detergent. The present results indicate a marked difference in the fractional fluorescence recovery (ΔFFR) within the same detergent family during the 30 days period assayed. Within the cholesterol analogs family, sodium cholate and CHAPSO displayed a minimum ΔFFR and a mobile fraction (MF) over 80%. In contrast, CHAPS and BigCHAP showed a marked decay in both the mobile fraction and diffusion coefficient. nAChR-DCs containing phospholipid analog detergents with a alkylphosphocholine (FC) and Lysofoscholine (LFC) of 16 carbon chains (FC-16, LFC-16) were more effective in maintaining a mobile fraction of over 80% compared to their counterparts with shorter acyl chain (C12, C14). The significant differences in macroscopic current amplitudes, activation and desensitization rates among the different nAChR-DCs evaluated in the present study allows to dissect which detergents preserves both, agonist activation and ion channel function. Functionality assays using TEVC demonstrated that LFC16, LFC14 and cholate were the most effective detergents in preserving macroscopic ion channel function, however, the nAChR-cholate complex display a significant delay in the ACh-induce channel activation. In summary, these results suggest that the physical properties of the lipid analog detergents (headgroup and acyl chain length) are the most effective in maintaining both the stability and functionality of the nAChR in the detergent solubilized complex.
Keywords: Lipidic Cubic Phase, Detergents, nAChR, Two-electrode voltage clamp, Fluorescence Recovery after Photobleaching, Planar Lipid Bilayer
Graphical Abstract
Introduction
Integral membrane proteins (IMP), such as the nicotinic acetylcholine receptor (nAChR) from the ligand gated ion channels (LGIC), are known to mediate cell communication, regulation, and their role is essential for normal physiological functions. The nAChR from Torpedo californica (Tc) is a hetero-pentameric ion channel with a pseudosymetrical arrangement around a cation selective pore. The nAChRs have been extensively studied because of their implication in several disorders, such as Congenital Myasthenic Syndromes (CMS)1–4, Alzheimer’s disease (AD)5, Parkinson’s (PD) disease6, and other brain pathologies7.
Most of the nAChR structural and functional data available today has been obtain from detergent-solubilized receptors from organs of electric rays, such as Tc, Torpedo nobiliana (Tn) and Torpedo marmorata (Tm)8. Recently, studies of nAChRs structural composition have been done using expression systems such as bacteria and yeast, which are not ideal because they lack the proper machinery for posttranslational modifications, such as glycosylations, and often require the production of modified versions of the receptors which in most cases involve expressing either truncated or mutated receptors, or constructing chimeras that increase stability with unknown effects on the protein structure9. Studies performed by Corringer8 solved a high-resolution crystal structure of the homologous acetylcholine binding protein (AChBP) expressed in bacteria. AChBP has ~24% sequence identity with the nAChR and the structure determined to 3.3 Å resolution demonstrated similar pentameric symmetry. In 2007, a high-resolution X-ray structure of the mouse-α1 nAChR extracellular domain, which was expressed in yeast, was solved to 1.94 Å resolution10. In spite of all these structural studies, there is a lot of structural information that remains unknown about the transmembrane domains. Previous efforts used detergents to obtain a nAChR structure11, however, these studies failed to produce a high resolution structure. EM studies using nAChR-enriched membranes from the electric organ of Tm led to a complete three-dimensional structure at 4.0 Å resolution12,13, which showed limited structural insights as a result of the low atomic resolution. After several decades and a great deal of effort from independent laboratories the best approximation to a high-resolution structure of the nAChR has been a collection of fragmented structures from different species. A fundamental obstacle towards the achieving of high resolution structure of the nAChR is the preparation of milligram amounts of stable, homogeneous and functional nAChR-detergent complexes (nAChR-DCs). A key aspect in the preparation of suitable nAChR-DCs for structural studies is the preservation of the stability and functionality of the ion channel machinery.
There have been several unsuccessful efforts to obtain a nAChR high-resolution structure during the past three decades. Several research groups have reported crystallization conditions that yielded small protein crystals (~15 µm); however, no high-resolution X-ray diffractions for the nAChR have been reported11,14. Neither these nor any other groups ever succeeded in obtaining a high resolution structure of the nAChR. Over the course of the past two decades, no attempts have been made to bring back the possibility of making high quality nAChR crystals from natural source. It is important to note that the aforementioned nAChR crystallization studies11,14 used Octyl glucoside (OG) as the principal detergent. In a previous study, we demonstrated that OG decreased the stability and abolished the functional response of the nAChR15. Along that line, the nAChR purified in those studies was by no means in its optimal state for structural studies. It is important to note that in recent years there have not been any reports of further efforts to obtain high quality crystals of functional and intact receptor.
In the present study we examined the macroscopic ion channel behavior of the nAChR-DCs in xenopus oocytes and their stability using LCP-FRAP. Previously, we found that during solubilization, a detergent may selectively remove a native lipid specie(s) present in the native cell membrane that could be essential for protein function and/or stability15,16. In the present study we demonstrated that the LCP in combination with macroscopic functional assays in Xenopus oocytes can lead to high quality nAChR-DCs. For the first time we recorded macroscopic currents, as an indicator of the functional integrity of the detergent solubilized nAChR, prior to insertion into LCP and also evaluate the stability of detergent solubilized nAChR in LCP during a 30 day period. The stability of the nAChR-DCs was evaluated using LCP-FRAP approaches to estimate nAChR mobile fraction and diffusion coefficient that had been correlated with receptor stability and/or aggregation. Overall, the present study demonstrates that lipid-analog detergents that resemble native environment of nAChR induce a functional stabilization of the nAChR-DCs. Using these approaches we have prepared highly functional and stable nAChR-DCs that could be the excellent candidates for future structural studies of the nAChR and other related proteins.
Materials and Methods
Crude Membrane Isolation, Detergent Solubilization and Receptor Purification
Crude membrane isolation, detergent solubilization and receptor purification were carried out as described in Asmar-Rovira et al. (2008)15. This study examined the ion channel function and LCP mobility of the affinity-purified T. californica nAChR for each of the following detergents: n-dodecylphosphocholine (FC-12), n-tetradecylphosphocholine (FC-14), n-hexadecylphosphocholine (FC-16) and 1-dodecanoyl-sn-glycero-3-phosphocholine (LFC-12) 1-tetradecanoyl-sn-glycero-3-phosphocholine (LFC-14), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (LFC-16), from the phospholipid-analog or FC family, as well as CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO), (N,N′-bis-[3-D-gluconamidopropyl] cholamide) (Big CHAP) and sodium cholate from the cholesterol-analog family. All detergents were obtained from Anatrace (Maumee, OH).
Injection of oocytes with crude or nAChR detergent complex
This protocol is a modified version of the protocol used by the Miledi and Morales group17–19. Briefly, oocytes were obtained from Xenopus leavis in the V or VI developmental stage and microinjected with 50 nL of 6 mg/mL of crude membrane or 3 mg/mL of 1.5 fold critical micellar concentration nAChR detergent complex, affinity purified from Tc. Subsequently, the oocytes were incubated at 18°C for 16–36 hours in ND-96 solution containing in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 2.5 Na-pyruvate supplemented with gentamicin (50 mg/mL), tetracycline (50 mg/mL) and theophyline (0.5 mM); and adjusted to a pH of 7.6 with NaOH.
Two electrode voltage clamp experiments (TEVC) on oocytes
For the TEVC experiments we used a modified version of previous protocols published by our group20–22. Briefly, Membrane current recordings were performed at room temperature (21–25°C) 16–36 hr after injection, longer incubation times were tested, but oocyte viability was greatly reduced for some detergents, therefore we choose a timeframe that was favorable for all the detergents present in this study. Oocytes were placed in a 200 µL chamber that was continuously perfused with 5 mL/min of a calcium-depleted OR-2 (containing in mM: 82 NaCl, 2.5 KCl, 1 MgCl2, 5 HEPES; and adjusted to a pH of 7.6 with NaOH) to elude the activation of an endogenous Ca2+ dependent chloride current. The macroscopic currents were induced by a 5 seconds application of a non-saturating concentration of Acetylcholine (100 µM, to make sure that we are not underestimating the amplitudes because of desensitized receptors) through a computer control 8 channel perfusion system (VC-8, Warner Instruments, Hamden, CT) connected to a 8-1 perfusion mini manifold, at a holding potential of −70 mV using a Gene Clamp 500B amplifier (Axon Instruments, Foster City, CA). The electrodes were filled with a solution of 3 M KCl and the resistances were calculated to average 1.3 mΩ. Macroscopic currents were filtered at 100 Hz and digitized at 1000 Hz using a Digidata 1440A interface (Axon Instruments, Foster City, CA) and acquired using the Clampex 10.2, (pCLAMP 10.2 software, Molecular Devices) running on a Microsoft Windows-based computer.
LCP fractional recovery after photobleaching, mobile fraction and diffusion coefficient analysis
For the FRAP experiments, 50µl of previously purified nAChR at approximately ~2.0 mg/mL was incubated with α-BTx labeled with Alexa-fluor 488 in a 1:1 ratio respectively for 2 hours at 4°C, the sample was not exposed to light to limit the damage to the fluorophore. The monoolein (1-oleoyl-rac-glycerol, Nu Chek) was melted in area of low humidity and low light place to avoid lipid damage. The monoolein and the nAChRα-BTx complex solution were mixed in a 3/2 ratio until clear appearance using two syringe (Hamilton Gas tight, 250 µl) with a syringe coupler (Rigaku Reagents, Asymetric Mixer Union). The resultant LCP was deposited on a 75 mm x 25 mm slide and washed with 1.5 mL of detergent buffer solution three times to remove non-bound α-BTx. The washed LCP was recovered with the syringe. Using an automatic dispenser (Rigaku reagent, Wizard Cubic LCP Kit) 600 nl of LCP, were transferred into 4 of the 7 mm diameter punched holes that were made in a 75 mm x 25 mm slide with 50 µm thick transfer tape (3M, 9482PC). A cover slip was pressed with a rubber roll against the slide, until the LCP was flattened. It is imperative to mention that this protocol was performed under a high humidity environment, since a long-term exposure of the LCP to ambience can compromise the LCP integrity. This protocol is similar to the protocol described by Caffrey and Cherezov on 200923, with minor modifications. To collect FRAP data, five pre-bleach images were used to establish baseline fluorescence, the laser is triggered to bleach at 75% laser power, immediately followed by a sequence of 500 images scanning at 2.6% power, with 600 ms laser scanning delay. All images were obtained and processed by LSM 510 Meta ZEN program. All experiments were made at room temperature (21–23°C). This protocol was repeated 7 times for a 30 day period, using a 5 day interval.
FRAP data analysis
The fluorescence intensity of each image was integrated within a 14.0 µm diameter circular region of interest (ROI1). Averaged integrated intensity of another 14.0 µm circular region of interest (ROI2), positioned near the bleached ROI1, was used to correct for photobleaching from irradiation during the image sequence acquisition. Fluorescence intensity was corrected by dividing the value of the integrated intensity ROI1 in the bleach spot by the average integrated intensity of the ROI2. As described by Cherezov 200824, fractional fluorescence recovery curves F(t) were calculated using the following Equation 1.
| (1) |
f(t) is the corrected fluorescence intensity of the bleached spot, f0 is the corrected fluorescence intensity of the bleached spot in the 600 ms after bleaching, and f∞ is the average of corrected fluorescence intensity in the five pre-bleached images. Fractional Mobility values where obtained by calculating the average of the last 50 values of F(t). The fractional fluorescence recovery curves were fitted with a one dimensional equation (one-phase exponential plot, Graph Pad Prism). The Diffusion coefficient value was calculated using Equation 2 as described by Cherezov 200824 and Pucadyil 200625.
| (2) |
Results
Functional Characterization of Tc nAChR
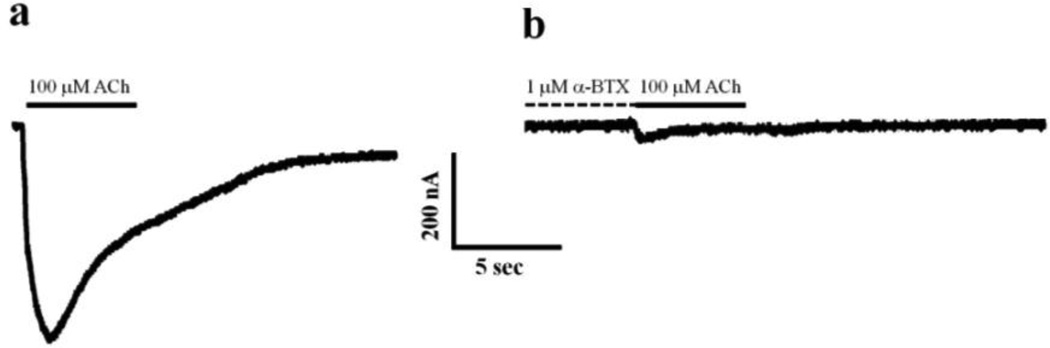
In order to access the functionality of the Tc nAChRs rich membrane, crude membranes extract or nAChR-DC were injected into Xenopus laevis Oocytes and the macroscopic ACh evoked currents were measured using the TEVC as described in Methods section. A 5 sec. application of 100 µM ACh in an oocyte injected with Tc nAChRs crude membrane resulted in a mean amplitude of −274 nA response (−274 ± 36 nA; n=10)(Fig. 1a); a 5 sec pre-application of 1 µM α-BTX abolished this response completely (only 1 of 5 oocytes gave a measurable response of −17 nA following α-BTX pre-application)(Fig. 1b). Suggesting that the ACh evoked response seen in these oocytes is coming from functional Tc nAChRs present in the crude membrane preparation.
FIGURE 1.
Macroscopic ion channel functional assay of crude membrane extracts from Tc nAChRs. Crude membranes extracted from Tc were microinjected into Xenopus laevis oocytes and macroscopic ACh evoked currents were measured at −70 mV using TEVC. a Representative response to a 5 sec application of 100 µM (represented by a bar) of oocytes injected with crude membrane extracts. b Representative response to a 5 sec application of 100 µM ACh (represented by a bar) following a 5 sec pre-application of 1 µM α-BTX.
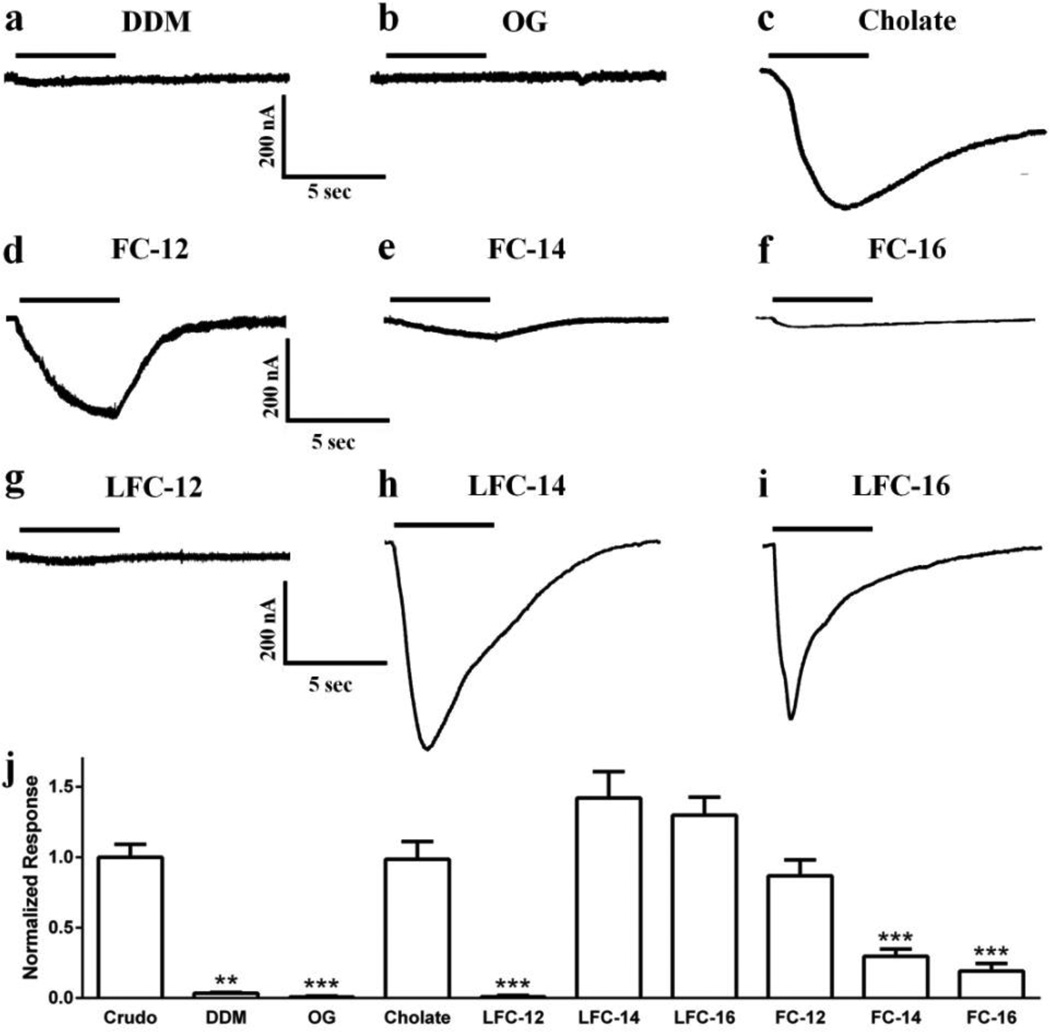
Furthermore, when we solubilized the Tc membranes with different detergents and purified the receptor using affinity columns, results are consistent with our previously published data using PLB, in which non-lipid detergent solubilization did not produce a stable and functional nAChR15,16. This is evident when we examined the mean amplitude of ACh evoked responses of nAChR’s solubilized using detergents such as DDM (−12 ± 2 nA, n=4)(Fig. 2a), and OG (only 1 of 4 oocytes gave a measurable response of −10 nA)(Fig. 2b); indicating that these detergents are poor at maintaining the function of these channels. It should be noted that identical solubilization protocol with sodium cholate did result in ACh evoked responses (−338 ± 43 nA, n=13)(Fig. 2c), this detergent has previously been reported to support Tc nAChR function26. Moreover, nAChR purification using a phosphatidylcholine analogue detergent family foscholine (FC), shows that detergent structural features and the physicochemical properties are vital to maintaining proper receptor function. When we compare the mean amplitude of ACh evoked currents for FC-12 (−177 ± 23 nA, n=7)(Fig. 2d) with both FC-14 (−60 ± 10 nA, n=3)(Fig. 2e) and FC-16 (−26 ± 10 nA, n=9)(Fig. 2f), we observe that a small change in the length of the side chain of the detergent can result in a major change in nAChR function. Interestingly, the finding that the mean amplitude of ACh evoked responses in oocytes injected with nAChRs purified depends on the side chain of the phospholipid analog detergent used, also applies to the lysofoscholine (LFC) family. However, the effects were opposite to those seen with FC detergents, with the shorter side chain LFC-12 displaying the smallest mean amplitudes (only 1 of 4 oocytes gave a measurable response of −10 nA)(Fig. 2g) than the longer side chain, LFC-14 (−488 ± 64 nA, n=4)(Fig. 2h) and LFC-16 (−446 ± 44 nA, n=5)(Fig. 2i). These results suggest that the longer acyl chain containing detergents in this family (LFC-14 and LFC-16) are able to support channel function better than the shorter side chain. Due to the high variability in the amplitude of the responses17–19 and in order to compare the ability of each detergent at maintaining the nAChRs function, responses for the nAChR-DCs were normalized to the respective crude membranes used and pool together (Fig. 2j). Our findings are consistent with published data showing that sodium cholate is able to maintain the function of nAChRs26 at a level similar to the function seen prior to detergent extraction. Furthermore we found that detergents such as LFC-14, LFC-16 and FC-12 are able to sustain functionality, indicating that these detergents might be better over detergents such as LFC-12, FC-14 and FC16 that cannot sustain the same levels of function.
FIGURE 2.
Macroscopic ion channel functional assays of detergent solubilized and affinity purified nAChR-DCs. Responses were evoked by a 5 second application of 100 µM ACh (represented by bars) at −70 mV on oocytes injected with detergent solubilized purified nAChR-DCs. The following detergents were used a DDM; b OG; c sodium cholate; d FC-12; e FC-14; f FC-16; g LFC-12; h LFC-14 and I LFC-16. j Responses for all detergents were normalized to the respective crude membranes used for solubilization plotted as mean ± SEM and compared using an unpaired t-test in Graph Pad Prism 6; ***p<0.0001; **p<0.001.
Assessment of nAChR stability on a lipidic matrix
As mentioned before FRAP can be interpret as a stability measurement for transmembrane proteins embedded on lipid matrixes. Our strategy involve the calculation of mobile fraction (eq. 2) and diffusion coefficient (eq. 3) from fractional recovery curves (eq. 1) using lipid analog detergents classified as cholesterol analogs (Fig. 3a,b,c,d) and phospholipid analogs (Fig. 4a,b,c and Fig. 5a,b,c). This strategy was previously validated by Cherezov et al 200824, and our group on Padilla et al 201116, were we look at nAChR-DCs stability for a period of 5 days. However, in order to establish the feasibility of protein mobility and nucleation which is obligatory for the nAChR-DCs crystallization, a 30 day FRAP assay was carried out for each detergent.
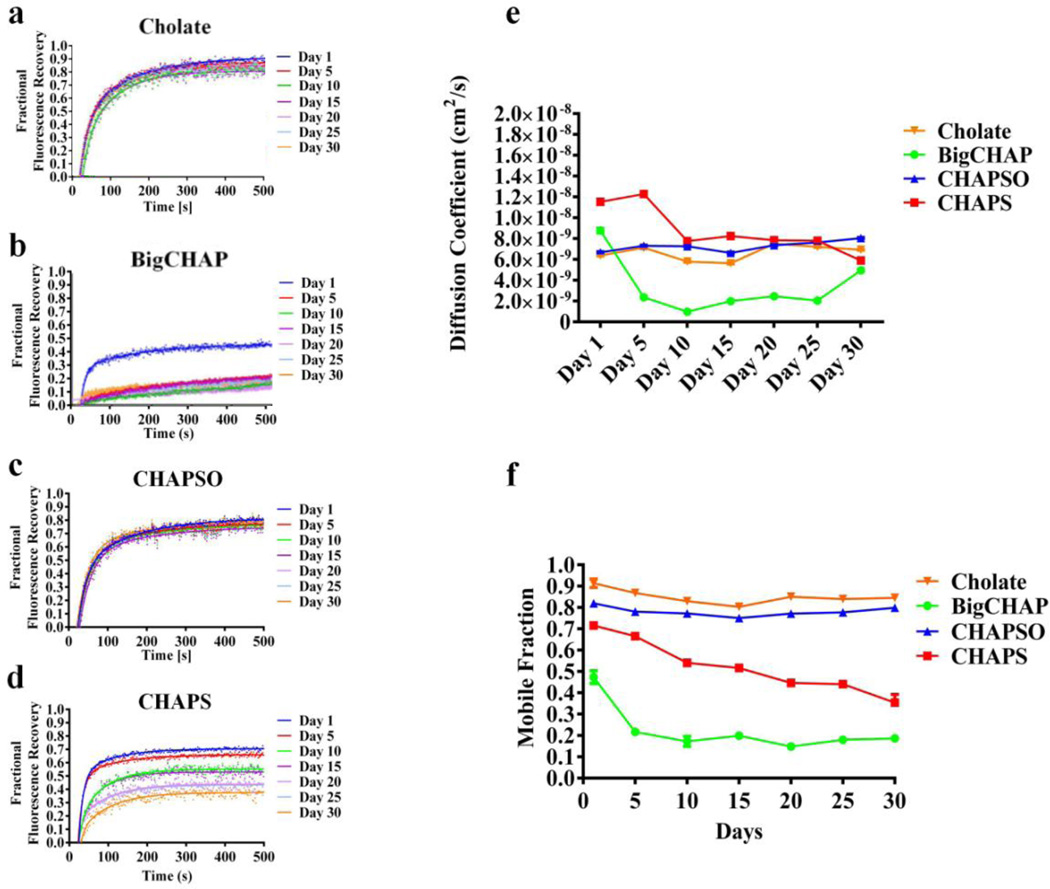
FIGURE 3.
Cholesterol analog detergents LCP-FRAP stability assay. Fractional fluorescence recovery and diffusion coefficient of each affinity purified nAChR using cholesterol analogue detergents. FRAP experiments of a sodium cholate, b BigChap and c CHAPSO and d CHAPS were recorded every five days for thirty days. All fluorescence recovery experiments were performed in triplicates, averaging five recoveries on different areas of the lipidic matrix with the nAChR incorporated. The fractional recovery was calculated using equation 1 for each fractional recovery of the triplicates. The mobile fraction e was obtained by averaging the last twenty points of the fractional recovery obtained in a,b,c. The diffusion coefficient f was calculated using equation 2
FIGURE 4.
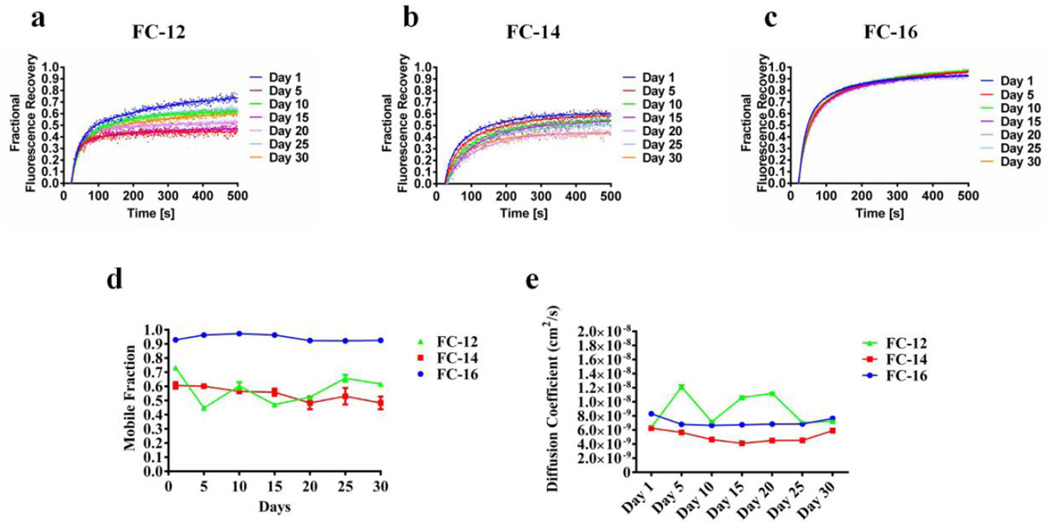
Phospholipid analog detergents lipidic matrix stability, LCP-FRAP assay. Fractional fluorescence recovery and diffusion coefficient of each affinity purified nAChR using phospholipid analog detergents of the Fos choline (FC) family. FRAP experiments of a FC-12, b FC-14 and c FC-16 were recorded every five days for thirty days. All fluorescence recovery experiments were performed in triplicates, averaging five recoveries on different areas of the lipidic matrix with the nAChR incorporated. The fractional recovery was calculated using equation 1 for each fractional recovery of the triplicates. The mobile fraction d was obtained by averaging the last twenty points of the fractional recovery obtained in a,b,c. The diffusion coefficient e was calculated using equation 2
FIGURE 5.
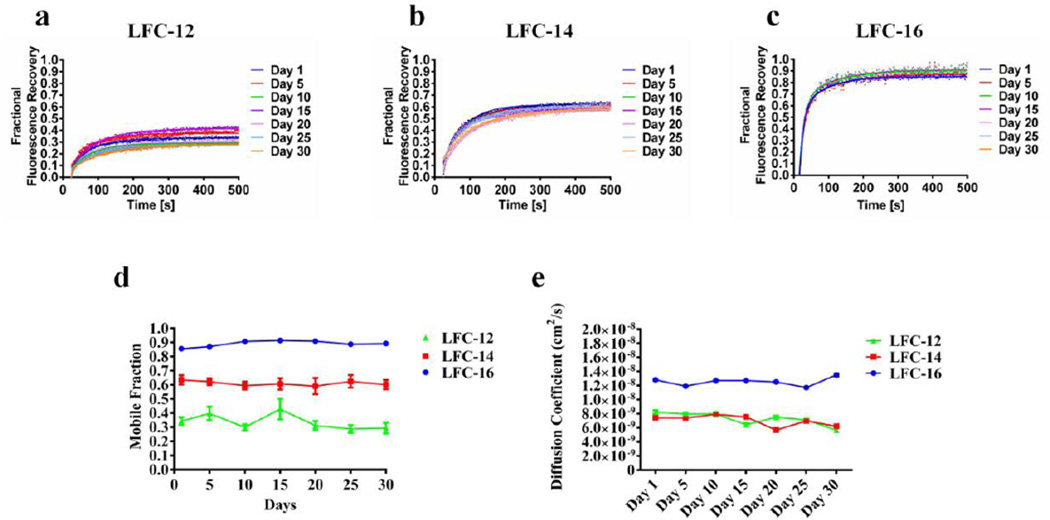
Phospholipid analog detergents lipidic matrix stability, LCP-FRAP assay. Fractional fluorescence recovery and diffusion coefficient of each affinity purified nAChR using phospholipid analog detergents of the LysoFos choline (LFC) family. FRAP experiments of a LFC-12, b LFC-14 and c LFC-16 were recorded every five days for thirty days. All fluorescence recovery experiments were performed in triplicates, averaging five recoveries on different areas of the lipidic matrix with the nAChR incorporated. The fractional recovery was calculated using equation 1 for each fractional recovery of the triplicates. The mobile fraction d was obtained by averaging the last twenty points of the fractional recovery obtained in a,b,c. The diffusion coefficient e was calculated using equation 2
Cholesterol analogs diffusion
Mobility curves of cholesterol analogs detergents fits consistently with a single component diffusion equation (Fig. 3). All Cholesterol analogue detergents showed no considerable diffusion differences during the 30 day period; all showed 10−9 cm2/s diffusion coefficients and most sustain the same diffusion coefficient values during the 30 day period. However, both BigCHAP and CHAPS deviate from this trend (Fig. 3e). Big CHAP diffusion displayed an evident decay from ~8.0×10−9 cm2/sec to ~2.0×10−9 cm2/sec during the first 5 day period. Moreover, during the first 5 days CHAPS showed the highest diffusion coefficient of ~1.2×10−8 cm2/sec, before a constant decay to a lower ~7.5×10−9 cm2/sec. On the other hand, CHAPSO and sodium cholate showed a constant diffusion coefficient of an average of 7.5×10−9 cm2/sec during the 30-day period, with no considerable differences. These values are consistent with previous studies where the diffusion coefficients for transmembrane proteins on lipidic matrixes were analyzed16,24.
Phospholipid analogs diffusion
All phospholipid analog detergents showed a good fit with a single component diffusion equation. For the Foscholine family there is no substantial changes for FC-14 and FC-16 during the 30-day period. Both detergents showed a monthly average of 6.0×10−9 cm2/sec and 8.0×10−9 cm2/sec respectively. Nevertheless, FC-12 showed an alternating pattern that contrast the behavior of the longer acyl chains −14 and −16. During the 30 day period the diffusion coefficient of FC-12 fluctuates between ~6.0×10−9 cm2/sec and ~1.2×10−9 cm2/sec. In contrast the lysofoscholine family (LFC types) shows no major changes on the diffusion coefficient during the 30-day period. LFC-12 and LFC-14 show similar diffusion coefficient values with a monthly average of 7.25×10−9 cm2/s and 7.01×10−9 cm2/sec respectively. On the other hand LFC-16 showed a considerable difference on the diffusion coefficient value with a monthly average of 1.3×10−8 cm2/sec, a ~2 fold increment compared to LFC-12 and −14; which makes LFC-16 producing the fastest population of nAChR-DCs mobile on the lipidic matrix.
Phospholipid analog mobile fraction
According to our results FC-14 and FC-16 did not show a noteworthy change on the mobile fraction during the 30 day period, where FC-14 presented the lowest MF 54% and FC-16 the highest MF 94% (Fig. 4d). In contrast, FC-12 showed an alternating behavior with values from 70% to 40% during the 30-day period. The phospholipid analog detergents, LFC types, showed no substantial changes among the 30-day period, although the LFC-12 displayed similar alternating behavior of FC-12; the fluctuations goes from the 30% to 40%. In addition an interesting pattern were observed for the LFC types, according to the present data the population of mobile nAChR-DCs augment with the length of the detergent acyl chain. The LFC family showed average mobile fraction during the study period of 34%, 61% and 90%, for LFC-12, LFC-14 and LFC-16 respectively. Considering those results a linear increment of the mobile fraction is observed as function of the detergent acyl chain length.
Discussion
The manner by which detergents affect lipid composition, functionality, and stability of solubilized membrane proteins is a poorly understood aspect of the structural biology of membrane proteins. For the past 3 decades, the selection of detergents for membrane protein solubilization and/or crystallization has been an empiric experiment. In order to identify the best detergents for future structural studies on nAChRs we have developed a strategy that allowed us to simultaneously assess the function and stability of nAChR detergent complexes. Previous publications from our laboratory examined the functionality of the detergent solubilized and affinity-purified nAChRs from Tc prior to LCP preparation using PLB16. Here, we introduced the use of TEVC as a more efficient approach to assess the functional properties of the Tc nAChRs crude membranes and purified nAChR-DCs. This method allows for the acquisition of macroscopic ion channel currents from nAChRs that incorporate in the plasma membrane after a direct injection of nAChR-DC into the oocyte. The injection of oocytes with crude membranes and measure ACh evoked responses using TEVC that were sensitive to pre-treatment with α-BTX (Fig. 1) suggesting that responses seen in these oocytes is coming from functional Tc nAChRs present in the crude membrane preparation. Is important to mention that we have not been able to perform the same experiments using PLB, since synthetic membranes seems to be too unstable to incorporate crude membranes. In addition, as shown previously by Marsal et al 199517 the activity of the nAChR crude membrane injected into oocytes produces qualitatively similar responses to those generated by the injection of a cocktail of mRNA coding for the nAChR20–22,27–34. These results validate the functional assays of injecting nAChR-DCs into Xenopus oocytes.
We found that the detergent used to extract and form complexes with the Tc nAChRs can completely alter the functionality of the receptor, for example when we compare the activation of the nAChRs from crude membranes with sodium cholate extracted nAChRs we found that there was a significant reduction in the apparent speed at which the channel is able to open (10%-90% rise times = 4.49 ± 0.65 sec, n=13 versus 1.23 ± 0.36 sec, n=9 for crude membranes; p =0.0008). Similarly, the apparent desensitization rate (estimated from the half time (t1/2) of the macroscopic current time-decay) displayed a significant reduction for sodium cholate extracted (t1/2= 11.64 ± 1.65 sec, n=13 versus 5.59 ± 1.65 sec, n=9 for crude membranes; p =0.009). Unfortunately, injection with 1.5 fold critical micellar concentrations of CHAPS, Big CHAPS or CHAPSO reduced oocytes viability and these detergents were not tested with this protocol. However, we have published PLB data showing a decreased function for both CHAPS and CHAPSO16; and no function for Big CHAPS15, implying that this detergents are inferior to the phospholipid analog detergents at maintaining the function of Tc nAChRs.
Both FC-14 and FC-16 displayed a significant reduction in current amplitude, which indicates that they are not able to support proper channel function. Furthermore, when we look at responses from nAChRs extracted using FC-12, even though current amplitude is not significantly affected, there is a significant reduction in the apparent rate of activation (10%-90% rise time = 3.57 ± 0.52 sec, n=5 versus 1.23 ± 0.36 sec, n=9 for crude membranes; p =0.007). These results suggest that the ion channel structure in the nAChR-FC-12DC display a distorted conformation.
As shown in Figure 2, FC-12 displays normal current amplitude with a remarkable reduction in the activation rate, whereas LFC-12 does not support proper channel function. In contrast, LFC-16 was the only detergent that was able to support the function of nAChR with not only the same level of current amplitudes, but also kinetics when compared to crude membrane preparations. These results underscore the advantage of this approach as a way to recognize the detergents that retain the native properties of the nAChRs.
Our data suggest that the longer acyl chain containing detergents in this family (LFC-14 and LFC-16) are able to support channel function better than the shorter side chain. Interestingly, we found that both of these detergents (LFC-14 and LFC-16) maintain the apparent activation kinetics (10%-90% rise times = 1.23 ± 0.36 sec, n=9; 2.74 ± 0.68 sec, n=4; 1.26 ± 0.21 s, n=5; crude membranes, LFC-14 and LFC-16 respectively); and desensitization profile of the Tc nAChR (t1/2= 5.59 ± 1.65 sec, n=9; 7.73 ± 0.72 sec, n=4; 6.11 ± 0.29 sec n=5; crude membranes, LFC-14 and LFC-16 respectively). The present data illustrate the importance of assessing the functionality of the receptor after detergent extraction, since minor changes in the structure of the detergent appear to be sufficient to produce enormous effects on the functional behavior of the nAChR-DCs (see Figure 3 in Padilla-Morales et al.35). Results from both techniques, PLB15,16 and TEVC, consistently showed that lipid analog detergents (such as sodium cholate, FC-12, LFC-14 and LFC-16) are better than non-lipid analog detergents (DDM and OG)(Fig. 2) at maintaining the function of the nAChRs. The same techniques demonstrated that non-lipid analog detergents are not able to sustain a favorable environment for nAChRs function which is consistent with previous reports using PLB only, that showed that these detergents are not able to stabilize nAChRs resulting in the loss of functionality15,16. Furthermore, the present results show that not all lipid analogue detergents are able to sustain the functional activity of the nAChR- DCs. Even within phospholipid detergent families, such as foscholine and lysofoscholine, we found that small differences in the length of the detergent acyl chain are critical and can cause remarkable changes in nAChR function. One possible explanation for these phenomena is that lipid analogue detergents are able to replace some of the native lipids and interact either transiently or remain bound to the nAChR after reaching the plasma membrane. If a detergent remains bounds to the nAChR after assembled in the plasma membrane, is very likely that specific interactions between the detergent and the nAChR might affect specific ion channel properties such as activation and desensitization kinetics that will in turn affect the amplitude and decay-time of the currents. This might explain the differences observed when we compared the foscholine and lysofoscholine families.
We use confocal laser scanning microscopy in order to measure the fluorescence recovery after photobleaching (FRAP) of the different preparations of detergents solubilized nAChR labeled with Alexa α-BTX in LCP. This approach has been validated by Cherezov et al., 200824 (see: http://www.jove.com/video/2501/crystallization-of-membrane-proteins-in-lipidic-mesophases) and Padilla-Morales et al., 201116 and appears to be a very good technique for the determination of diffusion coefficient of our affinity-purified nAChR using different detergents.
At least 48 membrane protein structure from bacterial as well as mammals have been obtained using LCP technology according to the Protein Data Bank (http://www.pdb.org). The success of LCP technology lies on the monoolein amphiphilic properties featuring a polar head-group and non-polar hydrocarbon chain. However, the LCP should confer membrane protein to freely diffuse within the three dimensional cubic phase structure formed by a single connected lipid bilayer24. In order to access the stability of the nAChR in LCP we quantify the diffusion of affinity purified nAChR solubilized with cholesterol-analog detergents for a period of 30-days (Fig 3).
Interestingly we found that for the cholesterol-analog detergents only CHAPSO and sodium cholate are able to maintain a high percentage of receptors mobile during the 30-days testing period. The difference in fractional fluorescence recovery (ΔFFR) over the 30-days period for CHAPSO was of 0.5 compared to sodium cholate ΔFFR of 0.09. In other hand, CHAPS and BigCHAP showed instability, decaying mobile fraction and diffusion coefficients and presents a ΔFFR of 0.32 and 0.22 respectively. Is important to mention that CHAPS was considered an excellent detergent choice for the purification and reconstitution of Tc nAChRs36. Moreover, both detergents were shown by our group to reduce functionality of the nAChR16. One possible explanation for the reduced mobility and function was described in Asmar-Rovira et al., 200815, were we found that the amount of residual lipids on purified nAChR is 2 fold greater for a detergent such as BigCHAP versus sodium cholate. Interestingly, we have shown that sodium cholate is able to maintain both mobility and function of the nAChR37, suggesting that this excess of residual lipids might be responsible for changes to the biophysical properties of the LCP resulting in changes to the nAChRs.
Although Cholate and CHAPSO were able to sustain nAChR stability with more than 70% of the nAChR population mobile, a highly desirable biophysical property when in meso crystallization is used, they produce a non-desirable aggregation state16, and in the case of CHAPSO it is not able to sustain the functionality of nAChRs. Albeit sodium cholate is able to sustain the functionality of nAChRs, in previous crystallization trials in our laboratory we found that this detergent crystallizes under acid conditions (data not shown). Although these cholesterol-analogue detergents are highly homologous, they have different functional groups (see Table I) and consequently different physicochemical characteristics which are likely to provide dissimilar forms of interactions with the protein nAChR and the LCP.
Table I.
Chemical and physical properties of the lipid and non lipid-analog detergents
| Name | CMC (mM) |
Aggregation Number |
Solubilization concentration (mM) |
Affinity wash concentration CMC (mM) |
column Structure buffer 1.5 x |
|---|---|---|---|---|---|
| Sodium Cholate | 14 | ~2–4.8 | 46.4 (2%) | 14.25 | |
| BIG CHAPS | ~2.9 | ~10 | 45.6 (4%) | 4.35 | |
| CHAPS | ~8 | 10 | 32.5 (2%) | 12 | |
| CHAPSO | ~8 | 11 | 32.5 (2%) | 12 | |
| FC-12 | 1.5 | 54 | 28.4 (1%) | 2.25 | |
| FC-14 | 0.12 | 108 | 26.35 (1%) | 0.18 | |
| FC-16 | 0.013 | 178 | 24.53 (1%) | 0.0195 | |
| LFC-12 | 0.32 | n/a | 0.48 | ||
| LFC-14 | 0.036 | n/a | 0.054 | ||
| LFC-16 | 0.0032 | n/a | 20.17 (1%) | 0.0048 | |
| DDM | ~0.17 | ~78–149 | 19.6 (1%) | 0.255 |
The FRAP curves for the nAChR-αBTX-Alexa-488 complex in LCP (Fig. 4) for the FC detergent family showed a maximum fractional fluorescence recovery near 90% with a ΔFFR of less than 0.07 only for the sixteen saturated carbon chain (FC-16). The fourteen carbon FC-14 presents a more disperse FRAP curves with a maximum at 62% of fluorescence recovery ΔFFR of 0.16. The twelve carbon version of FC showed a more unstable MFs pattern with a dispersed curve ranging from of 79% on day 1 to 42% on day 5 and finally increasing again to 60% on day 30. This behavior could be explain in terms of the short chain of FC-12 and even FC14 with a relative small head-group that enable these detergents to increase the number of molecules that can interact with the hydrophobic surface of the transmembrane domain of the nAChR. In this form the alky chain form a belt-like around the hydrophobic nAChR residues and expose the polar head-group to aqueous environment. This could help to stabilize the nAChR and even more increase its incorporation into lipid bilayer, but decrease the interaction of the nAChR detergent complex with the LCP integral monoolein molecule. This behavior was observed for the FC-12 in terms of the response evoked by ACh on oocyte injected with affinity purified nAChR-detergent complex. Increasing the chain length in the FC family decrease or eliminate the activity of the nAChR. These LCP-FRAP results suggest that small structural differences between different detergents can cause a remarkable difference in the stability of the protein detergent complex (see Figure 2 in Padilla-Morales et al.35). It is important to notice that FC-12 shows varieties in terms of diffusion coefficient between days changing its values from 10−8 to 10−9. This behavior can explain the difference between the first day of FRAP in this report compared to Padilla et al., 2011. FC-12 detergent is very efficient during the solubilization process and is able to produce high amount of purified protein, however some protein tend to aggregate in its presence38. These nAChR-FC-12 complex aggregates have been previously confirmed in our laboratory15. The FRAP behavior for the phospholipid like detergents as the LFC family is more consistent (Fig. 5). Increasing the hydrophobic chain length of the LFC detergent linearly improves the fractional fluorescence recovery for 30-days period assayed, but also reduced the variability between days. LFC-16 produced a maximum recovery of almost 90% with a ΔFFR of 0.08. The two carbon difference of LFC-14 attained the maximum fractional fluorescence recovery at 64% with a ΔFFR of 0.06. LFC-12 only presents a maximum recovery at 44% with a ΔFFR of 0.13. The functional assays showed a difference between stability and function regarding the length of the acyl chain (Fig. 2). In the LFC family of detergents, we observed that the longer acyl chains are able to sustain the highest levels of functionality, with LFC-14 and LFC-16 displaying macroscopic responses similar to the crude membranes suggesting that the conformation of the channel appears to be almost intact. In contrast, the shorter acyl chain LFC-12 produced a dramatic reduction in ion the nAChR channel function. In contrast, in the FC family only the shorter FC-12 is able to sustain unusual ion channel function (lacking fast activation) and longer acyl chain containing FC-14 and FC-16 showed a significant reduction in function. If the hydrophobic part of an amphiphilic molecule is the only parameter that dictates the physicochemical factors that govern the interaction of this with a hydrophobic region of another molecule, one might expect that LFC-12 could support the same activity of FC-12, and that is not the case for this particular detergent. The protein-detergent interaction greatly depends on the amphiphilic nature of the detergent, in other words, the size, electric charge and polarity of the headgroup as well as the magnitude, flexibility and shape of the hydrophobic part of the molecule38–40. The differences between the LFCs and FCs in our results could be attributed to the esterified sn-1-acyl glycerol as well as the phosphocholine part of the LFCs head-group. LFCs head-group provides greater stability compared to the FCs; it should not pass unnoticed that LFCs head-group are more likely to mimic the stabilizing interactions that the native like phosphatidylcholine contribute to the nAChR, as previously observed41. The LCP-FRAP experiments demonstrate the C12 acyl chain produce the least stable nAChR-DCs for both detergent families, being the LFC12 the least stable nAChR-DCs (MF<0.5) than the FC12 (MF<0.7). Based on these results and previous observations41 we suggest that FC12 has a reduced affinity for the nAChR due to the lack of head-group, therefore, this detergent could be partially washed from the nAChR-DCs before reaching the plasma membrane. The presence of a head-group in LFC-12 could increase the affinity of this detergent for the nAChR leading to more unstable nAChR-DCs with less possibility of removing the C12 acyl chain before reaching the plasma membrane.
Once the nAChR-DCs interact with the bilayer-like environment, the complexes, are able to move freely through the entire network42,43. The diffusion depends on different factors that dictate the mixability of the nAChR-DCs. During the mixing the protein-detergent complexes (which includes also residual native lipids) and monoolein melts and dissolves forming the cubic Pn3m monoolein. The process of mixing depends on the similarity of the lipids components (detergent-monoolein) and the capacity of the Pn3m to overcome the lipid-protein hydrophobic mismatch44,45. Integral proteins are characterized by a specific hydrophobic length along with a native membrane lipid bilayer thickness46, that provides a unique environment for stability and functionality. The solubilization of membrane proteins with detergents and reconstitution into other lipid environments can induce an energetically unfavorable hydrophobic mismatch that could affect the native protein conformation and consequently its stability and functional activity. Taking in consideration these requirements is not surprising that LFC-16 glycerol phosphocholine headgroup moiety confer superior lipid-lipid interactions and mixability with the Pn3m monoolein than FC-12. In addition, different independent studies with membrane protein such as ion-channel, ion pumps and sugar transporters demonstrated the requirement of acyl chain length to obtain maximum activity44,47–49. Interestingly, the acyl chain length for optimal functionality of these membrane protein range from 16–18 carbon chain.
Overall the present study demonstrate that he nAChR-LFC16 complex is an excellent candidate to be use in LCP crystallization trails but also to assess new technologies developed for membrane protein crystallization. Another fundamental aspect that remains to be elucidated is the possibility that the structural basis for the functionality and stability of some of these nAChR-DCs is merely determined by their lipid composition.
Supplementary Material
Highlights.
Detergents affect the functional characteristics of nAChRs.
Detergents affect the stability of nAChRs in LCP.
Testing detergent effects on nAChR functionality and stability is key for crystallization.
Acknowledgment
Funding Sources
This research was supported by the National Institutes of Health NIGMS grants 1R01GM098343 (JALD and O.Q), 1P20GM103642 (S.T. and JALD), the RISE Program (2R25GM061151-5A1 to M. D-V and O. Q) and MARC Program (5T34GM007821-31 to O. Q). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- nAChR-DCs
nicotinic acetylcholine receptors detergent complexes
- Tc
Torpedo californica
- Tm
Torpedo Marmorata
- PLB
Planar lipid bilayer
- TEVC
two electrode voltage clamp
- LCP
Lipidic Cubic Phase
- FC
Foscholine family 12–16 carbon chain
- LFC
Lysofoscholine family 12–16 carbon chain
- MF
mobile fraction
- AChBP
acetylcholine binding protein
- α-BTx
alpha bungarotoxin
- F(t)
fractional fluorescence recovery curves
- FRAP
Fluorescence Recovery after Photobleaching
- ΔFFR
fractional fluorescence recovery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
References
- 1.Beeson D. Synaptic dysfunction in congenital myasthenic syndromes. Ann. N. Y. Acad. Sci. 2012;1275:63–69. doi: 10.1111/nyas.12000. [DOI] [PubMed] [Google Scholar]
- 2.Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr. Opin. Pharmacol. 2005;5:308–321. doi: 10.1016/j.coph.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Müller JS, Mihaylova V, Abicht A, Lochmüller H. Congenital myasthenic syndromes: spotlight on genetic defects of neuromuscular transmission. Expert Rev. Mol. Med. 2007;9:1–20. doi: 10.1017/S1462399407000427. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez Cruz PM, Sewry C, Beeson D, Jayawant S, Squier W, McWilliam R, Palace J. Congenital myopathies with secondary neuromuscular transmission defects; a case report and review of the literature. Neuromuscul. Disord. NMD. 2014;24:1103–1110. doi: 10.1016/j.nmd.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 5.DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer’s disease: a review. J. Alzheimers Dis. JAD. 2010;19:761–780. doi: 10.3233/JAD-2010-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson’s disease. Biochem. Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gahring LC, Persiyanov K, Rogers SW. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol. Aging. 2005;26:973–980. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Corringer P-J, Baaden M, Bocquet N, Delarue M, Dufresne V, Nury H, Prevost M, Van Renterghem C. Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues. J. Physiol. 2010;588:565–572. doi: 10.1113/jphysiol.2009.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L. In pursuit of the high-resolution structure of nicotinic acetylcholine receptors. J. Physiol. 2010;588:557–564. doi: 10.1113/jphysiol.2009.184085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellisanti CD, Yao Y, Stroud JC, Wang Z-Z, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat. Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- 11.Hertling-Jaweed S, Bandini G, Müller-Fahrnow A, Dommes V, Hucho F. Rapid preparation of the nicotinic acetylcholine receptor for crystallization in detergent solution. FEBS Lett. 1988;241:29–32. doi: 10.1016/0014-5793(88)81024-x. [DOI] [PubMed] [Google Scholar]
- 12.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 14.Mitra AK, McCarthy MP, Stroud RM. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22 A by low dose electron microscopy and x-ray diffraction to 12.5 A. J. Cell Biol. 1989;109:755–774. doi: 10.1083/jcb.109.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asmar-Rovira GA, Asseo-García AM, Quesada O, Hanson MA, Cheng A, Nogueras C, Lasalde-Dominicci JA, Stevens RC. Biophysical and ion channel functional characterization of the Torpedo californica nicotinic acetylcholine receptor in varying detergent-lipid environments. J. Membr. Biol. 2008;223:13–26. doi: 10.1007/s00232-008-9107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padilla-Morales LF, Morales-Perez CL, De La Cruz-Rivera PC, Asmar-Rovira G, Baez-Pagan CA, Quesada O, Lasalde-Dominicci JA. Effects of Lipid-Analog Detergent Solubilization on the Functionality and Lipidic Cubic Phase Mobility of the Torpedo californica Nicotinic Acetylcholine Receptor. J. Membr. Biol. 2011;243:47–58. doi: 10.1007/s00232-011-9392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsal J, Tigyi G, Miledi R. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5224–5228. doi: 10.1073/pnas.92.11.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales A, Aleu J, Ivorra I, Ferragut JA, Gonzalez-Ros JM, Miledi R. Incorporation of reconstituted acetylcholine receptors from Torpedo into the Xenopus oocyte membrane. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8468–8472. doi: 10.1073/pnas.92.18.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivorra I, Fernández A, Gal B, Aleu J, González-Ros JM, Ferragut JA, Morales A. Protein Orientation Affects the Efficiency of Functional Protein Transplantation into the Xenopus Oocyte Membrane. J. Membr. Biol. 2002;185:117–127. doi: 10.1007/s00232-001-0118-x. [DOI] [PubMed] [Google Scholar]
- 20.Santiago J, Guzmán GR, Torruellas K, Rojas LV, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis in the TM3 domain of the Torpedo californica acetylcholine receptor beta subunit reveals an alpha-helical structure. Biochemistry (Mosc.) 2004;43:10064–10070. doi: 10.1021/bi0362368. [DOI] [PubMed] [Google Scholar]
- 21.Caballero-Rivera D, Cruz-Nieves OA, Oyola-Cintrón J, Torres-Nunez DA, Otero-Cruz JD, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis reveals distortions in the helical structure of the δM4 transmembrane domain of the Torpedo californica nicotinic acetylcholine receptor. Channels Austin Tex. 2012;6:1111–123. doi: 10.4161/chan.19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Acevedo A, Melendez M, Asseo AM, Biaggi N, Rojas LV, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis of the gammaM4 transmembrane domain of the acetylcholine receptor from Torpedo californica. J. Biol. Chem. 2004;279:42250–42257. doi: 10.1074/jbc.M405132200. [DOI] [PubMed] [Google Scholar]
- 23.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherezov V, Liu J, Griffith M, Hanson MA, Stevens RC. LCP-FRAP Assay for Pre-Screening Membrane Proteins for in Meso Crystallization. Cryst. Growth Des. 2008;8:4307–4315. doi: 10.1021/cg800778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pucadyil TJ, Chattopadhyay A. Confocal fluorescence recovery after photobleaching of green fluorescent protein in solution. J. Fluoresc. 2006;16:87–94. doi: 10.1007/s10895-005-0019-y. [DOI] [PubMed] [Google Scholar]
- 26.Nelson N, Anholt R, Lindstrom J, Montal M. Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc. Natl. Acad. Sci. U. S. A. 1980;77:3057–3061. doi: 10.1073/pnas.77.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero-Rivera D, Cruz-Nieves OA, Oyola-Cintrón J, Torres-Núñez DA, Otero-Cruz JD, Lasalde-Dominicci JA. Fourier transform coupled tryptophan scanning mutagenesis identifies a bending point on the lipid-exposed δM3 transmembrane domain of the Torpedo californica nicotinic acetylcholine receptor. Channels Austin Tex. 2011;5:345–356. doi: 10.4161/chan.5.4.17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz-Martín A, Mercado JL, Rojas LV, McNamee MG, Lasalde-Dominicci JA. Tryptophan substitutions at lipid-exposed positions of the gamma M3 transmembrane domain increase the macroscopic ionic current response of the Torpedo californica nicotinic acetylcholine receptor. J. Membr. Biol. 2001;183:61–70. doi: 10.1007/s00232-001-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzmán GR, Santiago J, Ricardo A, Martí-Arbona R, Rojas LV, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis in the alphaM3 transmembrane domain of the Torpedo californica acetylcholine receptor: functional and structural implications. Biochemistry (Mosc.) 2003;42:12243–12250. doi: 10.1021/bi034764d. [DOI] [PubMed] [Google Scholar]
- 30.Lasalde JA, Tamamizu S, Butler DH, Vibat CR, Hung B, McNamee MG. Tryptophan substitutions at the lipid-exposed transmembrane segment M4 of Torpedo californica acetylcholine receptor govern channel gating. Biochemistry (Mosc.) 1996;35:14139–14148. doi: 10.1021/bi961583l. [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Li L, Lasalde J, Rojas L, McNamee M, Ortiz-Miranda SI, Pappone P. Mutations in the M4 domain of Torpedo californica acetylcholine receptor dramatically alter ion channel function. Biophys. J. 1994;66:646–653. doi: 10.1016/s0006-3495(94)80838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz-Miranda SI, Lasalde JA, Pappone PA, McNamee MG. Mutations in the M4 domain of the Torpedo californica nicotinic acetylcholine receptor alter channel opening and closing. J. Membr. Biol. 1997;158:17–30. doi: 10.1007/s002329900240. [DOI] [PubMed] [Google Scholar]
- 33.Santiago J, Guzmàn GR, Rojas LV, Marti R, Asmar-Rovira GA, Santana LF, McNamee M, Lasalde-Dominicci JA. Probing the effects of membrane cholesterol in the Torpedo californica acetylcholine receptor and the novel lipid-exposed mutation alpha C418W in Xenopus oocytes. J. Biol. Chem. 2001;276:46523–46532. doi: 10.1074/jbc.M104563200. [DOI] [PubMed] [Google Scholar]
- 34.Tamamizu S, Guzmán GR, Santiago J, Rojas LV, McNamee MG, Lasalde-Dominicci JA. Functional effects of periodic tryptophan substitutions in the alpha M4 transmembrane domain of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry (Mosc.) 2000;39:4666–4673. doi: 10.1021/bi992835w. [DOI] [PubMed] [Google Scholar]
- 35.Padilla-Morales LF, Colón-Sáez JO, González-Nieves JE, Quesada-González O, Lasalde-Dominicci JA. Effects of changing the lipid analogue detergent headgroup on the Assessment of the Functionality and Stability of Detergent Purified nAChR from Torpedo using Lipidic Matrixes and Macroscopic Electrophysiology. BBA Biomembranes. 2015 doi: 10.1016/j.bbamem.2015.10.002. "Submitted". [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schürholz T, Kehne J, Gieselmann A, Neumann E. Functional reconstitution of the nicotinic acetylcholine receptor by CHAPS dialysis depends on the concentrations of salt, lipid, and protein. Biochemistry (Mosc.) 1992;31:5067–5077. doi: 10.1021/bi00136a020. [DOI] [PubMed] [Google Scholar]
- 37.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry (Mosc.) 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 38.Matar-Merheb R, Rhimi M, Leydier A, Huché F, Galián C, Desuzinges-Mandon E, Ficheux D, Flot D, Aghajari N, Kahn R. Structuring detergents for extracting and stabilizing functional membrane proteins. PloS One. 2011;6:e18036. doi: 10.1371/journal.pone.0018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Privé GG. Detergents for the stabilization and crystallization of membrane proteins. Methods. 2007;41:388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Sunshine C, McNamee MG. Lipid modulation of nicotinic acetylcholine receptor function: the role of neutral and negatively charged lipids. Biochim. Biophys. Acta. 1992;1108:240–246. doi: 10.1016/0005-2736(92)90031-g. [DOI] [PubMed] [Google Scholar]
- 42.Nollert P, Qiu H, Caffrey M, Rosenbusch JP, Landau EM. Molecular mechanism for the crystallization of bacteriorhodopsin in lipidic cubic phases. FEBS Lett. 2001;504:179–186. doi: 10.1016/s0014-5793(01)02747-8. [DOI] [PubMed] [Google Scholar]
- 43.Wallace E, Dranow D, Laible PD, Christensen J, Nollert P. Monoolein Lipid Phases as Incorporation and Enrichment Materials for Membrane Protein Crystallization. PLoS ONE. 2011;6:e24488. doi: 10.1371/journal.pone.0024488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen MO, Mouritsen OG. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta. 2004;1666:205–226. doi: 10.1016/j.bbamem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Marsh D. Energetics of Hydrophobic Matching in Lipid-Protein Interactions. Biophys. J. 2008;94:3996–4013. doi: 10.1529/biophysj.107.121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanford C. The hydrophobic effect: formation of micelles and biological membranes. New York: John Wiley & Sons Inc; 1973. [Google Scholar]
- 47.Cornelius F. Modulation of Na, K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry (Mosc.) 2001;40:8842–8851. doi: 10.1021/bi010541g. [DOI] [PubMed] [Google Scholar]
- 48.Dumas F, Tocanne JF, Leblanc G, Lebrun MC. Consequences of hydrophobic mismatch between lipids and melibiose permease on melibiose transport. Biochemistry (Mosc.) 2000;39:4846–4854. doi: 10.1021/bi992634s. [DOI] [PubMed] [Google Scholar]
- 49.Lee AG. How lipids and proteins interact in a membrane: a molecular approach. Mol. Biosyst. 2005;1:203–212. doi: 10.1039/b504527d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.