Abstract
Lung cancer remains the leading cause of cancer mortality in men and women in the U.S. and worldwide. About 90% of lung cancer cases are caused by smoking and the use of tobacco products. However, other factors such as radon gas, asbestos, air pollution exposures, and chronic infections can contribute to lung carcinogenesis. In addition, multiple inherited and acquired mechanisms of susceptibility to lung cancer have been proposed. Lung cancer is divided into two broad histologic classes, which grow and spread differently: small-cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC). Treatment options for lung cancer include surgery, radiation therapy, chemotherapy, and targeted therapy. Therapeutic-modalities recommendations depend on several factors, including the type and stage of cancer. Despite the improvements in diagnosis and therapy made during the past 25 years, the prognosis for patients with lung cancer is still unsatisfactory. The responses to current standard therapies are poor except for the most localized cancers. However, a better understanding of the biology pertinent to these challenging malignancies, might lead to the development of more efficacious and perhaps more specific drugs. The purpose of this review is to summarize the recent developments in lung cancer biology and its therapeutic strategies, and discuss the latest treatment advances including therapies currently under clinical investigation.
Keywords: Lung cancer, Non-Small Cell Lung, Small-Cell Lung Carcinomas, Mesothelioma, Therapies, Treatments, Surgery, targeted therapies, Immunotherapies
1. Introduction
Lung cancer, a highly invasive, rapidly metastasizing and prevalent cancer, is the top killer cancer in both men and women in the United States of America (USA). During 2014, an estimated 224,210 new cases and 159,260 deaths for lung cancer were predicted in the USA [1]. It causes more deaths per year than the next four leading causes of cancer (Colon/rectal, breast, pancreas, and prostate) death combined in the United States. Its incidence and mortality patterns are consistently associated with 20 or more years of smoking history. The individual susceptibility to tobacco-induced lung cancer may be dependent on competitive gene–enzyme interactions that affect activation or detoxification of procarcinogens and levels of DNA adduct formation as well as determined by the integrity of endogenous mechanisms for repairing lesions in DNA. Lung cancer is highly heterogeneous that can arise in many different sites in the bronchial tree, therefore presenting highly variable symptoms and signs depending on its anatomic location. 70% of patients diagnosed with lung cancer present with advanced stage disease (stage III or IV) (Figure.1).
Figure 1.
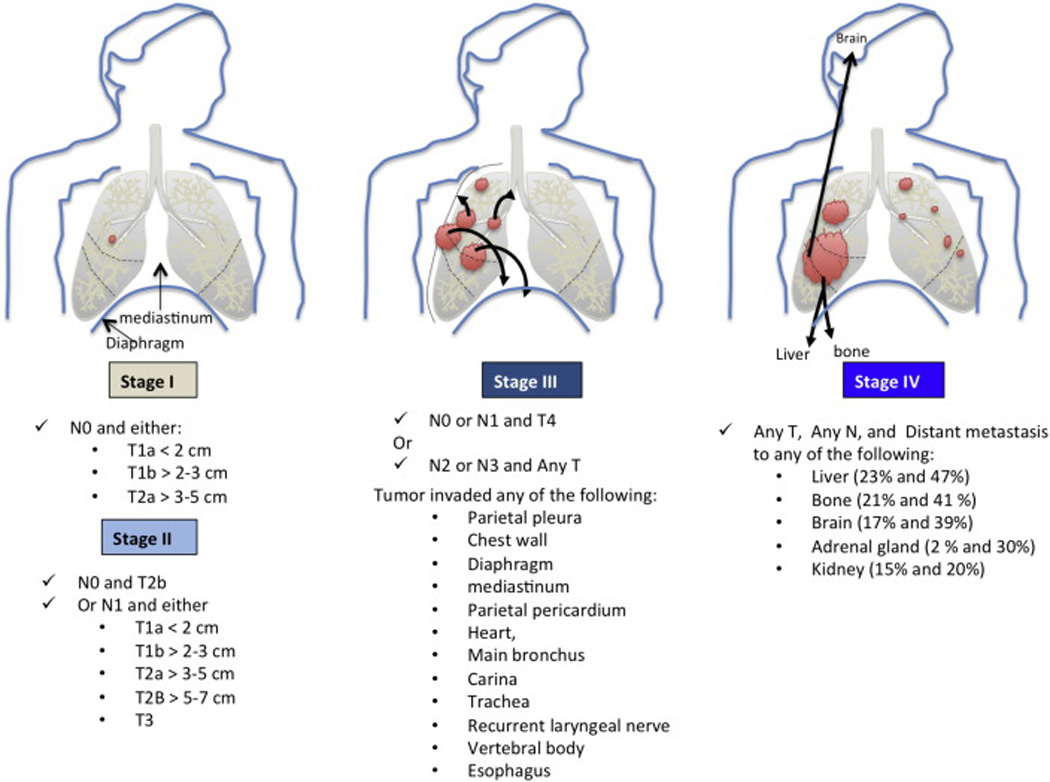
Schematic illustration of the Non-Small Lung Cancer (NSCLC) staging. Of note, Squamous cell lung carcinomas arise in the epithelial cells of main and lobar Bronchi (not shown), whereas Adenocarcinomas originate in the peripheral lung tissue and arise in the epithelial cells of segmental bronchi. For stage IV NSCLC cancers, the incidence of distant metastasis to the extrathoracic organs is depicted. For each organ, the percentages represent the incidence of metastasis for squamous cell lung carcinomas and adenocarcinomas, respectively.
Squamous cell lung cancers (SQCLC) represent about 25%–30% of all lung cancers and tend to arise in the main bronchi and advance to the carina (Table 1). Adenocarcinomas (AdenoCA) account for approximately 40% of all lung cancers and consist of tumors arising in peripheral bronchi. AdenoCAs advance by producing lobar atelectasis and pneumonitis. Bronchioloalveolar cancers (BAC), now reclassified into adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), arise in alveoli and spread through the interalveolar connections. AIS and MIA describe patients with very good disease-free survival after complete resection (5-year rate nears 100%) [2,3]. Small cell lung cancers (SCLC) derived from the hormonal cells of the lung, are the most dedifferentiated cancers and tend to be central mediastinal tumors. SCLCs comprise 10%–15% of all lung cancers, and are extremely aggressive disseminating rapidly into submucosal lymphatic vessels and regional lymph nodes, and almost always present without a bronchial invasion. Large cell anaplastic carcinomas (LCAC), also termed NSCLC not otherwise specified (NOS), are more proximal in location and locally tend to invade the mediastinum and its structures early. NSCLC-NOS comprises about 10% of all NSCLC. and behaves similarly to small cell cancers with a rapid fatal spread. Pancoast cancer arises in superior sulcus and advances by local invasion into juxta-opposed structures. All lung cancer types can become multifocal in the lobe they arise in (T3), or spread into the lung of origin (T4), or spread to the contralateral lung (M1) (Figure.1) [3]. The compression of mediastinal structures is associated invariably with advanced lymph node involvement, which can lead variously to esophageal compression and difficulty in swallowing, venous compression and congestion associated with collateral circulation, or tracheal compression. Signs of metastatic disease involving such remote sites as the liver, brain, or bone are seen before any knowledge of a primary lung lesion.
Table 1.
Types of Lung Cancer.
| Lung Cancer Type | % of All Lung Cancer |
Anatomic Location |
|---|---|---|
| Squamous cell lung cancers (SQCLC) | 25–30% | Arise in main bronchi and advance to the carina |
| Adenocarcinomas (AdenoCA) | 40% | Arise in peripheral bronchi |
| Large cell anaplastic carcinomas (LCAC) | 10% | Tumors lack the classic glandular or squamous morphology |
| Small cell lung cancers (SCLC) | 10–15% | Derive from the hormonal Cells Disseminate into submucosal lymphatic vessels and regional lymph nodes almost without a bronchial invasion |
2. International Staging System for Lung Cancer
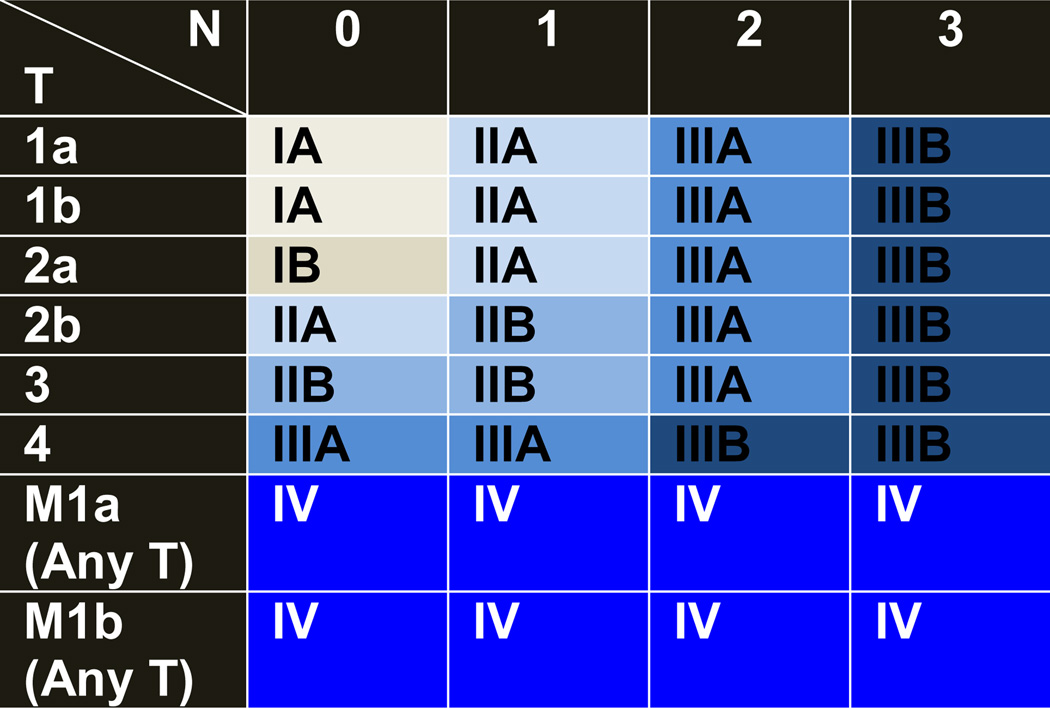
Cancer staging is a critical step in the diagnosis process, and its objectives are multifarious including 1) Helping the clinician to recommend a treatment plan; 2) Giving some indication of prognosis; 3) Aiding in the evaluation of the results of treatment; 4) Facilitating the exchange of information between treatment centers; 5) Contributing to the continuing investigation of human cancer. The international TNM-based staging system describes the anatomical extent of the disease (Table 3). The T category describes the size and extent of the primary tumor. The N category describes the extent of involvement of regional lymph nodes. The M category describes the presence or absence of distant metastatic spread. The addition of numbers to these categories describes the extent of the cancer. All possible combinations of the T, N, and M categories are then used to create TNM subsets (Table 2). TNM subsets with similar prognoses are then combined into stage groupings. NSCLC stages range from one to four (I through IV). The lower the stage, the less the cancer has spread. SCLC is defined using two stages: Limited (confined to the hemithorax of origin, the mediastinum, or the supraclavicular lymph nodes) and extensive (spread beyond the supraclavicular areas) [4].
Table 3.
TNM-based staging system.
| Category | Description |
|---|---|
| T | Size and extent of the primary tumor |
| N | Extent of involvement of regional lymph nodes |
| M | Presence or absence of distant metastatic spread |
| Numbers (0–3) | Extent of the cancer |
| cTNM | Pretreatment, Clinical stage |
| pTNM | Postsurgical stage |
| ycTNM | Post-preoperative therapy stage |
| ypTNM | Postsurgical stage with preoperative therapy |
Table 2.
TNM stage grouping for NSCLC. TNM stand for the size and location of the Tumor, the location of cancer in the lymph Nodes and where the cancer has spread (Metastases).
T = Primary tumor: T1a (Tumor size ≤2 cm), T1b (>2–3 cm): T2a (>3–5 cm), T2b (>5–7 cm); T3 (>7 cm) and/or (Multiple tumor nodules in the same lobe); T4 (Multiple tumor nodules (of any size) in the same lung but a different lobe).
N0 = No regional lymph node metastasis; N1 = Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension; N2 = Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s); N3 = Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s).
M = Distant metastasis: M1a (Malignant pleural or pericardial effusions and/or Separate tumor nodules in the contralateral lung); M2b (Distant metastasis in extrathoracic organs).
The term stage, without further classification, relates to the pretreatment, clinical stage or cTNM [3,5–7]. cTNM is derived using the evidence available from clinical history and examination, blood tests, imaging, endoscopic examination, biopsy material, surgical examination, and any other test considered necessary prior to making a decision as to the appropriate treatment in any individual. If this decision leads to surgical treatment, then additional information becomes available at surgery and by pathological examination allowing a more accurate assessment of disease indicated by the pathological, postsurgical stage or pTNM. pTNM does not replace the cTNM, which should remain as a record in the patient’s notes. If the patient undergoes preoperative “induction” therapy, usually with either or both chemotherapy and radiotherapy, then a reassessment is made after this treatment, prior to a final decision on surgical treatment [3,5–7]. The evidence available from this process is used to create the ycTNM, and after surgical treatment in these circumstances, the postsurgical pathological extent of disease is described as ypTNM. At various points in the patient’s journey, events may allow or demand a reassessment of disease extent. An rTNM may be established if relapse occurs after a disease-free interval. An aTNM may be formulated if the disease is first discovered at an autopsy. In each case, previous assessments of TNM are retained in the patient records [3,5–7].
3. Lung cancer biology
Lung cancer cells have defects in the regulatory circuits that govern normal cell proliferation and homeostasis. The transformation from a normal to malignant lung cancer phenotype is thought to arise in a multistep fashion, through a series of genetic and epigenetic alterations, ultimately evolving into invasive cancer by clonal expansion [8,9]. Following the development of the primary cancer, continued accumulation of genetic and epigenetic abnormalities, acquired during clonal expansion, influences the processes of invasion, metastasis, and resistance to cancer therapy [8,9]. The identification and characterization of these molecular changes are of critical importance for improving disease prevention, early detection, and treatment. The knowledge of both a patient’s tumor characteristics and genetics will significantly advance the personalized prognosis and ideal treatment selection for each patient.
3.1. Genomic alterations
Recently, the Cancer Genome Atlas Research Network reported the molecular profiling of 230 lung adenocarcinomas. High somatic mutation rates were seen from the whole-exome sequencing (mean 8.87 mutations per megabase of DNA). Eighteen genes were identified to be significantly mutated both in the abovementioned 230 AdenoCA cases as well as in 182 AdenoCA tumors previously analyzed in similar fashion. Genomic alterations include point mutations (missense and nonsense mutations, frameshift and slicing site alterations), rearrangement (transversions and transitions) as well as somatic copy number alterations [10]. The alterations of kinase protein levels or activities have also been studied. Using RNA-seq analysis in 7000 tumors (20 solid cancer types), novel and recurrent kinase gene fusion events were identified. In lung adenocarcinomas (513 samples), fusion events were found in ROS1, RET, PRKCB, NTRK, MET and ALK genes and were found in PRKCB, PRKCA, PKN1, FGR, FGFR1, FGFR2 an FGFR3 genes in lung squamous cell carcinomas (492 samples) (Table 5) [11]. These findings may have significant clinical impact and new therapeutic approaches could be developed targeting these alterations. Another large systematic genomic study reclassified 12 tumor types into 11 subtypes based on the sequencing data from 3527 tumor cases (DNA copy number, DNA methylation, mRNA expression, microRNA expression, protein expression and somatic point mutation). Somatic mutations such as KEAP1 and STK11 are preferentially mutated in LUAD-enriched tumors group, containing most of the lung adenocarcinoma cases, while CDKN2A, NOTCH1, MLL2 and NFE2L2 were found mutated preferentially in squamous-like tumors group encompassing most of the lung squamous cell carcinoma cases. Squamous-like tumors also showed frequent MYC amplification and loss of CDKN2A, RB1 and TP53. The reclassification generated new prognostic information that could be used to guide therapeutic decision [12].
Table 5.
Kinase fusion events identified in AdenoCA and SQCLC [12]
|
Lung Adenocarcinoma |
||
| Gene name | Number of cases altered |
Alteration |
| ALK | 5/513 | Known fusion |
| MET | 1/513 | New fusion |
| NTRK2 | 2/513 | Known kinase-novel indication |
| PRKCB | 1/513 | New fusion |
| RET | 2/513 | Known fusion |
| ROS1 | 8/513 | Known fusion |
|
Lung Squamous Cell Carcinoma |
||
| Gene name |
Number of cases altered |
Alteration |
| FGFR1 | 1/492 | Known fusion |
| FGFR2 | 1/492 | Known fusion |
| FGFR3 | 3/492 | Known fusion |
| FGR | 1/492 | New fusion |
| PKN1 | 1/492 | New fusion |
| PRKCA | 2/492 | Known kinase-novel indication |
| PRKCB | 1/492 | New fusion |
3.2. Molecular pathology of lung cancer
Several targetable genetic alterations have been identified in lung cancer [13] (Table 6), including 1) Activating mutations in a number of proto-oncogenes including KRAS, EGFR, BRAF, PI3K, MEK and HER2. Noteworthy, EGFR (Epidermal growth factor receptor) plays a critical role in regulating normal cell proliferation, apoptosis, and other cellular functions. Approximately 10% of NSCLC patients in the US and 35% in East Asia have tumor associated EGFR mutations [14–16]. 2) Structural rearrangements in ALK, ROS1 and possibly RET. 3) Amplification of proto-oncogenes such as MET in adenocarcinomas, FGFR1 and DDR2 in squamous cell lung carcinomas. 4) Oncogenic gene overexpression by microRNAs (miRNAs). 5) Inactivation of Tumor Suppressor Genes (TSG), including TP53, RB1, CDKN2A, FHIT, RASSF1A, and PTEN. 6) Enhanced telomerase activity, which contributes to cellular immortality by maintaining telomere length through de novo synthesis of telomeres and elongation of existing telomeres (100% of SCLCs and 80% to 85% of NSCLCs). The hTERT gene is amplified in 57% of NSCLCs.
Table 6.
Oncogenes and tumor suppressor genes altered in NSCLC [14].
| ONCOGENE | CANCER TYPE | Pathway |
| AKT1, AKT2, AKT3 | AdenoCA (rare), SQCLC (20%, AKT3: 16%) | PI3K |
| ALK | AdenoCA (3–13%) | RTK |
| BRAF | AdenoCA (6%), SQCLC (4%) | RAF |
| CCNE1 | AdenoCA (12%) | RB1/CDK |
| DDR2 | SQCLC (3–8%) | RTK |
| EGFR | AdenoCA (40–50%), SQCLC (7%) | RTK |
| ERBB2 | AdenoCA (7–14%) | RTK |
| ERBB3 | SQCLC (2%) | RTK |
| FGFR1 | AdenoCA (1–3%), SQCLC (22%), SCLC (6%) | RTK |
| HRAS | SQCLC (3%) | RAS |
| IGF1R | SCLC (95%) | RTK |
| KRAS | AdenoCA (30%), SQCLC (5%) | RAS |
| MDM2 | AdenoCA (20%) | TP53 |
| MET | AdenoCA (25%) | RTK |
| MLL | SCLC (10%) | Epigenetic regulation |
| MYC, MYCN, MYCL | AdenoCA (31%), SQCLC (rare), SCLC (16%) | Transcriptional regulators |
| NKX2.1/TTF1 | AdenoCA (20%) | Developmental pathways |
| NRAS | AdenoCA (<1%), SQCLC (<1%) | RAS |
| NRF2 | SQCLC (19%) | Oxidative stress response |
| PIK3CA | AdenoCA (rare), SQCLC (16%) | PI3K |
| RET | AdenoCA (1–2 %) | RTK |
| ROS | AdenoCA (1.5%) | RTK |
| SOX2 | SQCLC (21%) | Developmental pathways |
| TP63 | SQCLC (16%) | Developmental pathways |
| TUMOR SUPPRESSOR GENE | CANCER TYPE | Pathway |
| PTEN | AdenoCA (rare), SQCLC (8%) | PI3K |
| ARID1A | AdenoCA (8%) | Epigenetic Regulation |
| ASCL4 | SQCLC (3%) | Developmental pathways |
| CDKNA2/p16INK 4 | AdenoCA (>20%), SQCLC (72%) | RB1/CK |
| CEBBP | SCLC (9%) | Epigenetic Regulation |
| CUL3 | SQCLC (7%) | Oxidative stress response |
| EP300 | SCLC (9%) | Epigenetic Regulation |
| KEAP1 | AdenoCA (11%), SQCLC (12%) | Oxidative stress response |
| LKB1 | AdenoCA (15–30%), SQCLC (2%) | LKB1/AMPK |
| MLL2 | SQCLC (19%) | Epigenetic Regulation |
| NF1 | AdenoCA (8–10%), SQCLC (11%) | RAS |
| NOTCH | SQCLC (13%) | Developmental pathways |
| RASA1 | SQCLC (4%) | RAS |
| RB1 | AdenoCA (rare), SQCLC (7%), SCLC (100%) | RB1/CDK |
| SETD2 | AdenoCA (5%) | Epigenetic Regulation |
| SMARCA4 | AdenoCA (10%) | Epigenetic Regulation |
| TP53 | AdenoCA (70%), SQCLC (80%), SCLC (70%) | TP53 |
| TSC1, TSC2 | SQCLC (6%) | PI3K |
Remarkably, scores of the aforementioned aberrations correlate with patient’s smoking history as well as with racial and gender differences, which suggest a possible role of the host’s genetic makeup as key determinants in lung carcinogenesis [8,9].
3.3. Clinical applications
Tremendous work has been conducted to translate the acquired information of these genetic anomalies into improvement of patient care in the clinic including early detection and treatment and prognosis prediction:
Discovery of biomarkers for early detection of primary and recurrent disease: Currently, the diagnosis of lung cancer is primarily based on symptoms and lung cancer detection often occurs when curative intervention (i.e., surgery) is no longer possible. The five-year survival rate in early-stage, operable NSCLC is approximately 50%–70%, but drops to 2%–5% for patients whose cancers have spread distantly [17]. Numerous potential early lung cancer detection biomarkers, have been investigated. However, there are still no biomarkers for detection of lung cancer in clinical use due to the lack of either or both a robust sensitivity and specificity or a functional relevance of these biomarkers to lung carcinogenesis.
-
Development of novel therapies: EGFR- and ALK- targeted therapies are currently approved for lung cancer. Angiogenesis inhibitors (i.e., Bevacizumab) are also available for treatment of lung cancer. These targeted therapies are a promising effective way to personalize treatment of lung cancer. However, resistance to these treatments often develops and side effects can be an issue. Therefore, the clinical challenge is to determine for each patient the most effective combination therapy that may provide optimal treatment with minimum side effects.
Platinum-based regimens are standard of care in advanced lung cancer. However, their clinical effectiveness is limited by cumulative haemato- and neuro-toxicities highlighting the need for alternative treatment strategies. ERCC1 functions as a key enzyme in nucleotide excision repair (NER). Low ERCC1 expression correlates with increased sensitivity to platinum-based therapy and high ERCC1 expression correlates with better overall prognosis in NSCLC [18,19]. Nearly 50% of NSCLC patients have low levels of ERCC1, and therefore could benefit from alternative therapies exploiting this tumor ERCC1 deficiency [19]. RRM1 is the regulatory subunit of ribonucleotide reductase essential for the deoxyribonucleotides (dNTP) synthesis.
RRM1 is the main target for the antimetabolite drug gemcitabine, which is an underpinning cancer therapy in the treatment of many malignancies including lung cancer. Gemcitabine directly binds to RRM1 and irreversibly inactivates ribonucleotide reductase [20–28]. High RRM1 levels are associated with tumor resistance and low RRM1 levels with tumor sensitivity to gemcitabine treatment [21,23,25–28].
Recent studies have suggested that low levels of the heparan sulfate 6-O-endosulfatase (SULF2) through methylation in NSCLC may be predictive of better survival and increase sensitivity to topoisomerase-1 inhibitors (TPI) [29]. SULF2 is overexpressed in many tumors including lung adenocarcinomas and lung squamous carcinomas to remove critical sulfation modifications from sulfated heparin sulfate proteoglycans (HSPGs) and thus release growth factors essential for tumor growth [30–32]. It was established that SULF2 methylation via induction of high expression of Ubiquitin-Like Modifier (ISG15) sensitizes lung cancer cells to TPIs via suppression of ubiquition and proteasomal degradation [29].
A number of new potentially targetable alterations were identified in NSCLC including FGFR1 amplification and DDR1 mutation found in squamous cell lung carcinomas. These alterations might be important prognostic and predictive factors for patient’s response to treatments with FGFR inhibitors or DDR1 inhibitors (e.g., Dasatinib) [33,34].
Discovery of prognostic and predictive biomarkers: The prognostic and/or predictive value of an extensive panel of molecular markers has been tested in early and advanced stage lung cancer. In advanced NSCLC, positive EGFR mutation (10–15% of NSCLC) or ALK rearrangement (ALK-EML4 fusion) status (5–7% of NSCLC) was shown to be predictive for a significant clinical benefit from EGFR tyrosine kinase inhibitors (TKIs) [35,36], or ALK TKI (crizotinib) [37–40], respectively. ALK-EML4 fusion positivity in NSCLC seems to be a prognosticator of poor response to EGFR TKI therapy [41–44] as EGFR mutations and EML4-ALK fusions occur almost exclusively in adenocarcinomas. Nevertheless, EML4-ALK appears to be mutually exclusive to that of EGFR or KRAS mutations in NSCLC and is more common in never or former light smokers [45].
3.4. Tumor microenvironment
The tumor microenvironment and the complex interactions of its various cell types and their released signaling molecules are an emerging hallmark of cancer [46]. It consists of stromal cells, cancer-associated fibroblasts, stem cells and a comprehensive set of immune cells recruited into tumors. The tumor microenvironment is altered to suppress host immune responses, foster tumor growth, and help cancer cells evade immune surveillance [47]. The tumor-associated immune cells include tumor-associated macrophages (TAM), dendritic cell (DC) subsets, cytotoxic and regulatory T-cells (CTLs and Tregs), natural killer (NK) cells, and myeloid-derived suppressor cells (MDSC). The amounts of different immune cell subsets in the tumor microenvironment can vary considerably among patients and may be used as a predictor of treatment outcome and survival in certain cancers [48–50]. The altered tumor microenvironment is established by the cancer cells through the loss of MHC class I molecules, the loss of antigen variants, and the active secretion of several growth factors, such as vascular endothelial growth factor (VEGF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) [51]. The immune cells present in the tumor microenvironment are functionally impaired, and the newly infiltrating immune cells become alternatively activated, resulting in a perturbed phenotype [51]..
Myeloid cells including myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells (DC), can act as regulatory cells in the tumor microenvironment. MDSCs exert their pro-tumor effects through the inhibition of T-cell proliferation and activation by increasing levels of NO synthase and arginase-1 [52] [51,53,54], releasing IL-10 and overproducing reactive oxygen species (ROS) [55]. In the peripheral blood of advanced stage NSCLC patients, increased levels of MSDC were detected compared to healthy controls and were associated with lower levels of CD8+ T-cells [56].
Tumor-associated macrophages (TAM) show a higher frequency of the pro-tumor M2-phenotype, which accumulate in the tumor stroma and correlate with poor patient outcome and decreased OS [57]. These alternatively activated macrophages show impaired ability to present antigen and appropriate co-stimulation to T-cells [51]. In contrast, macrophages of the M1 phenotype accumulate intratumorally and show antitumor functions through expression of HLA-DR, inducible nitric oxide synthase (iNOS) and TNF-α [58]. Furthermore, a high intratumoral density of CD68+ macrophages correlates with increased survival in NSCLC [59].
DCs are the most important antigen-presenting cells at the border of the innate and adaptive immune system. However, in the tumor microenvironment, DCs are often in an immature state that fail to prime T-cells efficiently due to low expression levels of co-stimulatory molecules such as CD80 and CD86 and a weak antigen presenting capacity [60,61]. Peripheral blood lymphocytes can also be used as a prognostic marker in NSCLC: 1) the total blood count of lymphocytes was shown to be associated with a lower hazard ratio for death [57,62]; 2) a neutrophil to lymphocyte ratio (NLR) >3.81 was identified in the same study as a predictor of survival in patients with Stage I NSCLC; 3)Treg cells were also detected at elevated levels in the peripheral blood of NSCLC patients compared to healthy controls [63,64].
In early-stage NSCLC, a tertiary lymphoid structure is detected in the tumor stroma that contains mature and follicular DC, CD4+ T-cells, and CD20+ B-cells. This tertiary lymphoid structure is defined as Tumor-induced Bronchus Associated Lymphoid Tissues (Ti-BALT) [48,65]. A small retrospective study demonstrated that the density of mature DC (DC-LAMP +) homing in the Ti-BALT correlates with disease-free survival, disease-specific survival, and OS in early-stage NSCLC patients [48].
Chronic inflammation can also play a significant role in the tumor environment through the release of reactive oxygen and nitrogen species, as well as TNF-α. Consequently, chronic inflammation can facilitate tumor growth via activation of NF-κB and the subsequent suppression of adaptive immune responses [48]. NSCLC tumors often show hypoxic areas, which leads to the release of pro-angiogenic factors such as VEGF, thereby increasing tumor angiogenesis [66,67].
3.5. Racial and ethnic diversity in lung cancer
There is considerable variation in cancer incidence as well as death rates among different racial and ethnic groups [68]. Although the cause of this racial and ethnic disparity in cancer risk and outcomes remains controversial [69], there is a growing consensus that the interaction of genetic and environmental factors including diet is at least partially responsible for the ethnic differences in cancer risk and outcome [70].
Regarding lung cancer, women have lower incidence and death rates than men. African-American men have the highest incidence and death rate in the United States, followed by White, American Indian or Alaskan Natives, Asian American or Pacific Islanders, and Hispanic/Latino men. In women, the highest rates are in white women, followed by American Indian or Alaskan Natives and African Americans, Asian American or Pacific Islanders, and Hispanic/Latino groups [68]. Moreover, clinical trials have shown that Asian ethnicity is an independent favorable prognostic factor for OS in NSCLC patients regardless of their smoking history. The frequency of the activated EGFR mutations is higher in East Asian patients as compared with Caucasian patients (30 vs. 7 %, respectively). Numerous studies showed that EGFR mutation-positive patients of Asian origin have better efficacy outcomes with first-line EGFR tyrosine kinase inhibitors (TKI), especially patients with adenocarcinoma histology and never smokers [71]. In contrast, prevalence of K-ras mutations is lower in Asian patients (<10 vs. 18 %) [71]. Deciphering these racial disparities requires the identification of risk factors for lung cancer in multiracial, multiethnic groups such as genetic polymorphisms and gene-environment interactions. Moreover, inclusion of minority groups in lung cancer screening and clinical trials may be advantageous in reducing these disparities.
Environmental factors/geography as well as socioeconomic status may also affect lung cancer susceptibility, treatment outcome, and survival rates [72]. Access to treatment and adherence to treatment regimen [73] appear to be an enabling factor for racial disparities in lung cancer. It has been shown that White and African-American patients with early-stage NSCLC who were eligible and received surgical resection had comparable survival rates. In contrast, African-Americans who did not undergo surgery (due to un-insurance, limited access to health care, fear to diagnosis or beliefs) had the lowest survival rate [73]
4. Treatment of Non-Small-Cell Lung Cancer
In this section, the standard and emerging treatments for early stage, advanced, and recurrent NSCLC, as well as brain metastasis will be discussed (Table 7). The various drugs and corresponding targets mentioned in this section are summarized in (Table 8).
Table 7.
Treatment options for NSCLC.
| Stage | Treatment options |
|---|---|
| I, II-resectable | Surgery |
| I,II-unresectable | Radiotherapy |
| II-resectable | Surgery followed by chemotherapy (adjuvant) |
| IIIA-resectable | Surgery followed by chemotherapy |
| IIIA-resectable | Chemotherapy (neoadjuvant) followed by surgery |
| IIIA-unresectable | Sequential or combined chemoradiation |
| IIIA with bulky primary tumors | Radical surgery |
| IIIB | Chemotherapy or radiation |
| IIIB | Chemoradiation |
| IV with PS of 0 or 1 | Chemotherapy (a combination of two cytotoxic drugs, platinum-based) |
| IV with PS of 0 or 1, with no squamous carcinoma histology, brain metastasis, significant cardiovascular disease | Bevacizumab (VEGF antibody) + first line doublet combination chemotherapy |
| IV with PS of 2 | Chemotherapy (single cytotoxic drug) |
| NSCLC with EGFR mutations | EGFR targeted therapy (erlotinib and gefitinib) |
| III, IV (first-line platinum-based therapy failed with acceptable PS) | Docetaxel, pemetrexed, erlotinib and gefitinib |
| Recurrent NSCLC | External palliative radiation therapy, Cytotoxic chemotherapy, EGFR inhibitors (with or without EGFR mutations), EML4-ALK inhibitor (with EML-ALK translocations), Surgical resection (isolated cerebral metastases), Laser therapy or interstitial radiation therapy (endobronchial lesions), Stereotactic radiation surgery |
| Brain Metastases | Whole Brain Radiotherapy (WBRT), Surgery, Stereotactic Radiosurgery (SRS) with and without WBRT, Systemic Therapy and Radiosensitization |
Table 8.
Drugs and corresponding targets.
| Drug | Target | Type |
|---|---|---|
| Aflibercept (AVE0005) | VEGF | Humanize VEGFR-trap |
| AMG 655/Conatumumab | TRAIL-R2 | Monoclonal antibody |
| Apomab | DR5/TRAIL-R2/TNFRSF10B | Monoclonal antibody |
| Axitinib | PDGFR | Tyrosine kinase inhibitor |
| Bendamustine | DNA (SSB and DSB) | Alkylating agent |
| Bevacizumab | VEGF | Monoclonal antibody |
| Carboplatin | DNA | Small molecule inhibitor |
| Cediranib | PDGFR | Tyrosine kinase inhibitor |
| Cediranib | VEGF | Small molecule inhibitor |
| CI-994 | Histone deactylases (HDACs) | Small molecule inhibitor |
| Cisplatin | DNA | Small molecule inhibitor |
| Cixutumumab | IGF-IR | Monoclonal antibody |
| Dalotuzumab | IGF-IR | Monoclonal antibody |
| Docetaxel | Tubulin | Small molecule inhibitor |
| Entinostat | Histone deactylases (HDACs) | Small molecule inhibitor |
| Erlotinib | EGFR | Tyrosine kinase inhibitor |
| Etoposide | Topoisomerase II | Small molecule inhibitor |
| Figitumumab | IGF-IR | Monoclonal antibody |
| Gefitinib | EGFR | Tyrosine kinase inhibitor |
| Ifosfamide | DNA | Alkylating agent |
| Iniparib | PARP-1 | Small molecule inhibitor |
| Ipilimumab | CTLA-4 | Monoclonal antibody |
| Irinotecan | Topoisomerase I | Small molecule inhibitor |
| Linifinib | PDGFR | Tyrosine kinase inhibitor |
| Mapatumumab | DR4/TRAIL-R1 | Monoclonal antibody |
| Medl-4736 | PD-L1 | Monoclonal antibody |
| Motesanib | PDGFR | Tyrosine kinase inhibitor |
| MPDL-3280A | PD-L1 | Monoclonal antibody |
| Nintedanib | VEGFR-1/2/3, PDGFR-α/β, FGFR-1/2/3, Flt-3, Src family | Multiple target Tyrosine kinase inhibitor |
| nivolumab | PD-1 | Monoclonal antibody |
| Olaparib | PARP-1 | Small molecule inhibitor |
| Paclitaxel | Tubulin | Small molecule inhibitor |
| Panobinostat | Histone deactylases (HDACs) | Small molecule inhibitor |
| Pazopanib | VEGFR-1/2/3, PDGFR-α/β, and FGFR-1 and 3 | Multiple target Tyrosine kinase inhibitor |
| Pemetrexed | Thymidylate Synthase | Small molecule inhibitor |
| Pembrolizumab/MK-3475 | PD-1 | Monoclonal antibody |
| Pivanex | Histone deactylases (HDACs) | Small molecule inhibitor |
| Romidepsin (Depsipeptide, FK228) | Histone deactylases (HDACs) | Small molecule inhibitor |
| Sorafenib | VEGFR-2/3, PDGFR-β, c-Kit, Raf, and Flt-3 | Multiple target Tyrosine kinase inhibitor |
| Sunitinib | VEGFR-1/2/3, PDGFR-α/β, c-Kit, Flt-3, and RET | Multiple target Tyrosine kinase inhibitor |
| Talactoferrin | Immune-modulatory function | Glycoprotein, recombinant |
| Topotecan | Topoisomerase I | Small molecule inhibitor |
| Tremelimumab | CTLA-4 | Monoclonal antibody |
| Veliparib | PARP-1 | Small molecule inhibitor |
| Vinblastine | Tubulin | Small molecule inhibitor |
| Vinorelbine | Tubulin | Small molecule inhibitor |
| Vorinostat | Histone deactylases (HDACs) | Small molecule inhibitor |
| YM155/Sepantronium bromide | Survivin | Small molecule inhibitor |
4.1. Treatment of early stage (stage I and Stage II) Non- Small-Cell Lung Cancer
The primary treatment for resectable and operable early stage disease (Stage I and II) is surgery [74] which provides the best option for long-term survival [75]. Five-year survival rates after surgical resection are 60%–80% for stage I NSCLC and 30%–50% for stage II NSCLC patients [76]. For patients refusing surgical resection or with unresectable tumors, primary radiotherapy can be used such as stereotactic body radiotherapy (SBRT) for high-risk patients or unresectable tumors [72]. However, post-surgery radiotherapy is not recommended for stage I and II patients [72]. To date, adjuvant platinum-based chemotherapy was shown to be beneficial for stage II NSCLC patients [77] and is the recommended treatment strategy for completely resected patients [72]. Conversely, a clear benefit has so far not been proven for adjuvant chemotherapy in stage I NSCLC patients [78].
4.2. Treatment of stage III Non- Small-Cell Lung Cancer
More than 70 % of NSCLC patients are diagnosed in advanced stages or metastatic disease [2] (stages III and IV). Stage III NSCLC is a heterogeneous disease, and varies from resectable tumors with microscopic metastases to lymph nodes to unresectable, bulky disease involving multiple nodal locations. The 5-year OS rate varies between 10% to 15% for stage IIIA-N2 disease and 2% to 5% for stage IIIA bulky disease with mediastinal involvement. In this heterogeneous population of stage III NSCLC patients, the treatment strategies, including radiotherapy, chemotherapy, and surgical resection are determined by the tumor location and whether it is resectable.
The standard treatment consists of surgery followed by chemotherapy for patients with resectable stage IIIA NSCLC. It has been shown that the adjuvant chemotherapy significantly prolonged OS rate in clinical studies [79–83] and that adjuvant radiation therapy can improve control of resected stage IIIA-N2 disease [84]. Meta-analyses of numerous clinical studies showed that neoadjuvant chemotherapy provides a modest 5% to 6% improvement in survival at five years [85].
For unresectable stage IIIA patients, standard treatment may include either a sequential or concurrent combination of chemotherapy and radiation therapy (chemoradiation), and external radiation therapy for patients who cannot be treated with combined therapy. Meta-analyses of multiple randomized clinical studies showed that platinum based chemoradiation therapy provides a significant 10% reduction in the risk of death when compared with radiation therapy alone [86–88]. Several clinical investigations showed that the radical surgery in Stage IIIA patients with bulky primary tumors may provide up to 50% increase in the 5-year survival rate as compared to patients with incomplete resection [89–91].
Stage IIIB NSCLC represents about 17.6 % of all lung cancers [92] with a 5-year survival rate of 3% to 7% [93]. The options and sequence of treatments for stage IIIB NSCLC are determined based on the site of tumor involvement and the patient’s performance status (PS) (Table 4). Generally, patients with stage IIIB NSCLC do not benefit from surgery alone. The standard therapy for these patients consists of either a sequential combination of chemotherapy or external radiation therapy. As palliative treatment, Stage IIIB NSCLC may receive external radiation therapy alone to relieve pain and other symptoms to improve the quality of life.
Table 4.
Eastern Cooperative Oncology Group (ECOG) Performance Status. The ECOG scale and criteria are used to assess how a patient's disease is progressing, assess how the disease affects the daily living abilities of the patient, and determine appropriate treatment and prognosis.
| Grade | ECOG |
|---|---|
| 0 | Fully active, able to carry on all pre-disease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care, confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair |
| 5 | Dead |
4.3. Treatment of Stage IV Non-Small Cell Lung Cancer
Stage IV NSCLC accounts for 40% of the newly diagnosed NSCLC patients. The choice of treatment for stage IV NSCLC patients depends on many factors including, comorbidity, PS, histology, and molecular genetic features of the cancer [94]. Standard treatment options for stage IV NSCLC disease may include palliative external radiation therapy, combination chemotherapy, combination chemotherapy and targeted therapy, and any Laser therapy or internal endoscopic radiation therapy as needed. Similar to radiation therapy, surgery could also be used in some cases to alleviate disease-related symptoms.
4.3.1. Chemotherapy
For NSCLC, Chemotherapy is usually well tolerated by patients with PS 0 and 1 but rarely effective in patients with a PS 3 and 4 where palliative care is preferred. Use of chemotherapy is controversial in PS 2 NSCLC patients, which represent nearly 40% of advanced stage NSCLC patients. Chemotherapy is recommended only for PS 2 patients who are reasonably fit, and awake for more than 50 % of the day.
i. First-line Chemotherapy
The median OS is 4.5 months when no chemotherapy is given to advanced metastatic NSCLC or after failure of all treatments. Use of chemotherapy improves the 1-year OS rate from 10%–20% up to 30%–50% [74]. A combination of two cytotoxic drugs is the recommended first-line therapy for Stage IV NSCLC patients with a PS of 0 or 1. Platinum (Cisplatin or carboplatin)-based combination therapies yield better response and OS rates than the non-platinum combination therapies. First line platinum-based chemotherapeutics may include doublets of cisplatin or carboplatin given in combination with taxanes (paclitaxel, docetaxel, or vinorelbine), antimetabolites (gemcitabine or pemetrexed), or vinca alkaloids (vinblastine) with comparable activity [95]. Use of single cytotoxic chemotherapy is preferred in stage IV patients with a PS of 2 due to their greater risk of toxicity and drug intolerance, comparing to patients with a PS 0 to 1.
ii. First-line combination chemotherapy with targeted therapy
Currently, the addition of bevacizumab, an antibody targeting VEGF, to first-line doublet combination chemotherapy, is supported for the treatment of stage IV NSCLC patients with exception (squamous carcinoma histology, brain metastasis, significant cardiovascular disease or a PS greater than 1) due to fatal bleeding concerns. The combination of bevacizumab with carboplatin and paclitaxel doublet appeared to be superior to the combination with cisplatin and gemcitabine.
4.3.2. EGFR tyrosine kinase inhibitors (first line)
The first of the approved targeted drugs for NSCLC patients are agents that specifically block the EGFR) such as tyrosine kinase inhibitor (TKI) Erlotinib (Tarceva) and gefitinib (Iressa). Mutations of EGFRs can lead to abnormal activation of this receptor triggering uncontrolled cell growth, which may account for several subsets of cancers including NSCLC.
Evidence from several randomized clinical trials demonstrated that use of single-agent gefitinib as a first-line therapy might be recommended for patients with activating EGFR mutations, particularly for patients who have contraindications to platinum therapy. Conversely, cytotoxic chemotherapy is preferred if EGFR mutation status is negative or unknown. Three large controlled and randomized trials showed that gefitinib or erlotinib are better than platinum combination chemotherapy as first-line treatment for stage IIIB or IV lung adenocarcinomas in nonsmokers or former light smokers in East Asia [35,36,96,97].. Data from these trials demonstrated that gefitinib or erlotinib improved PFS but not OS and have favorable toxicity profiles compared with combination chemotherapy of patients with chemotherapy-naïve and EGFR mutations adenocarcinoma. Similar benefits of erlotinib versus platinum-based chemotherapy as first-line were reported in one European large randomized clinical trial (PFS: 9.7 vs. 5.2 months, respectively) [98]. Neither erlotinib nor gefitinib is recommended for use in combination with cytotoxic chemotherapy as first-line therapy
4.3.3. Maintenance therapy following first-line chemotherapy
Maintenance therapy is the treatment continuation until disease progression of a cancer that has not advanced following the first-line therapy. The primary goal is to improve cancer-related symptoms, and, hopefully, improve survival time beyond that provided by the first-line therapy. It has lately gained great interest in the treatment of advanced NSCLC (stage IIIB and stage IV) [99]. To date, pemetrexed (Alimta), and erlotinib (Tarceva) are the two medications that have been approved by the FDA as maintenance therapy for advanced lung cancer. Evidence from two randomized controlled clinical trials showed a statistically significant improvement of PFS (4.1 to 4.3 months vs. 2.6 to 2.8 months), with the addition of pemetrexed as maintenance therapy following standard first-line platinum-based combination chemotherapy [100–103]. Remarkably, pemetrexed maintenance therapy appears to be effective only in patients with adenocarcinoma and large cell carcinoma as well as in patients with EGFR mutations in their tumors, but not in patients with squamous cell lung carcinoma.
Data from a large randomized controlled clinical trial showed improved survival (both PFS and OS) with erlotinib maintenance treatment following platinum-based chemotherapy in NSCLC patients without progressive disease (PFS: 12.3 weeks vs. 11.1 weeks) [104]. Similar to pemetrexed, erlotinib maintenance therapy improved outcome primarily in non-squamous cell lung carcinoma NSCLC patients. Patients with activating EGFR mutations in their tumors showed greatest PFS benefit comparing to patients with wild type EGFR who also experienced improvement in their median PFS. This trial also demonstrated that KRAS mutation status was a significant, negative prognostic factor for maintenance erlotinib-induced PFS [105]. Moreover, never-smoking women with better PS seems to derive the utmost survival advantage from maintenance erlotinib therapy.
4.3.4. Second- and third-line therapies in the treatment of advanced NSCLC
Docetaxel (Taxotere), pemetrexed, erlotinib, and gefitinib, are currently approved as second-line therapy for patients with advanced NSCLC who have failed first-line platinum-based therapy and have an acceptable PS.
Evidence from several randomized clinical trials and meta-analyses [106–108] showed that docetaxel in the second-line setting leads to better survival and quality of life (QoL) when compared to best supportive care [107] or to single agent ifosfamide or vinorelbine [109]. Pemetrexed yielded similar clinical response comparing to docetaxel (a median survival of about 8 months, one-year survival of 30%, and a response rate of 10%) [110] with better toxicity profile that may benefit older patients with a PS of 3 [111]. Pemetrexed also provided better outcome in lung adenocarcinoma patients, whereas docetaxel treatment was more effective in lung squamous cell carcinoma patients [112]. Erlotinib related response was more common in women with adenocarcinoma, never-smokers, or east-Asians, which is correlated with more frequent EGFR activating mutations [113]. To date, erlotinib is approved and may be recommended as second- or third-line therapy for patients with a PS of 0 to 3 who have not received prior erlotinib or gefitinib.
Because of the scarcity of data on cytotoxic chemotherapy as third-line therapy, there are no recommendations for or against using a cytotoxic chemotherapy in the third-line setting. However, Phase III clinical trials of docetaxel, erlotinib, gefitinib, and pemetrexed allowed patients to continue chemotherapy, as tolerated, until disease progression.
4.4. Standard Treatment Options for Recurrent NSCLC
Recurrent or relapsed NSCLC is a cancer that has progressed or returned following an initial treatment with surgery, radiation therapy, and/or chemotherapy. The cancer may return in the lung, brain, or other parts of the body. For NSCLC patients who have never been treated with chemotherapy, the treatment plan is similar to that of Stage IV NSCLC. For those patients who have already been treated with chemotherapy, standard treatment options may include: 1) External palliative radiation therapy, which achieves palliation of symptoms from a localized tumor mass [114], to relieve pain and other symptoms and improve the quality of life; 2) Cytotoxic chemotherapy [107,110,115]; 3) EGFR inhibitors (TKIs) in patients with or without EGFR mutations.; 4) EML4-ALK inhibitor (Crizotinib) in patients with EML-ALK translocations.[116,117]; 5) Surgical resection of isolated cerebral metastases (for selected patients who have a very small amount of cancer that has spread to the brain) [118]; 6) Laser therapy or interstitial radiation therapy using an endoscope (for endobronchial lesions) [119]. 7) Stereotactic radiation surgery (for selected patients who cannot have surgery) [120,121].
4.4.1. Cytotoxic Chemotherapy for Recurrent NSCLC
Evidence from clinical studies showed that use of cytotoxic chemotherapy and targeted therapy may achieve objective responses, albeit with small improvement in survival for patients with recurrent NSCLC [122]. In some trials, platinum based chemotherapy has also been shown to achieve palliation of symptoms, which occurred more often than the objective response in patients with good PS [123,124]. Treatment options for NSCLC patients whose cancer has recurred after platinum-based chemotherapy may include either new cytotoxic chemotherapy such as docetaxel [107,114] and pemetrexed [110], or a targeted therapy such as erlotinib [113], gefitinib [115], and crizotinib for cancers with EML4-ALK translocations [116,117]. Patients with squamous lung carcinomas benefit more from docetaxel, whereas those with non-squamous NSCLC appeared to benefit more from pemetrexed [125].
4.4.2. EGFR Inhibitors for Recurrent NSCLC
A large randomized phase III trial comparing gefitinib to placebo in recurrent NSCLC patients suggested that gefitinib might be a valid treatment for recurrent NSCLC patients with improved survival compared to placebo in never-smokers (median survival 8.9 mo vs. 6.1 mo), and Asian patients (median survival 9.5 mo vs. 5.5 mo) [126], In two large randomized, placebo controlled trials, erlotinib has also been shown to improve survival and quality of life in patients with recurrent NSCLC after first-line or second-line chemotherapy compared to placebo [113,127]. Moreover, erlotinib treatment also induced a greater improvement in patients’ symptoms, such as cough, pain, and difficulty in breathing, compared to placebo [113]. Conversely, erlotinib did not improve survival when compared to standard second-line chemotherapy with docetaxel or pemetrexed [128], in recurrent NSCLC patients after a first-line platinum combination therapy.
4.4.3. ALK/MET Inhibitors for Recurrent NSCLC
Translocations of EML4 and ALK occur on the short arm of chromosome 2, and the fusion of EML4 and ALK (normally a dormant gene) results in a constitutive activation of the ALK kinase. The EML4-ALK fusion oncogene has been identified in approximately 4% in the NSCLC population, and is generally found in individuals who do not typically respond to EGFR TKI therapy [40,45]. EGFR and EML4-ALK mutations appear to be mutually exclusive with exceptions. Tumors harboring the EML4-ALK fusion oncogene are sensitive to crizotinib, a selective, ATP-competitive ALK and MET/HGF dual TKI, which is FDA approved for the treatment of patients with locally advanced or metastatic ALK positive-NSCLC [117]. Crizotinib (XALKORI@, Pfizer) is currently approved in Switzerland for treatment of patients with previously treated ALK-positive advanced NSCLC, and in the USA for treatment of patients with locally advanced or metastatic, ALK-positive NSCLC.
Crizotinib therapy has shown improvement in survival of patients with advanced, ALK-positive NSCLC compared to standard therapies for advanced NSCLC [117] [129]. Similar to the kinase inhibitors already used in clinic, such as imatinib and EGFR inhibitors, resistance to crizotinib frequently develops in patients’ tumors [130]. These tumors might either acquire additional ALK kinase domain mutations (i.e., L1196M, C1156Y mutations) that alter drug sensitivity [131], or other ALK alterations, including amplification, gain in copy number, and loss of ALK genomic rearrangement [132]. Furthermore, signaling through other kinases, such as EGFR, might compensate for ALK inhibition, thereby mediating resistance to ALK inhibitors [132]. Mutation in the KRAS gene was also shown to play a role in resistance to crizotinib and around 8 % of ALK-positive NSCLC patients were shown to harbor either a KRAS or EGFR mutation in addition [130].
4.5. Treatment of Second Primary Tumor
A second primary cancer is a separate cancer arising in a patient who had another cancer in the past. Second or higher order primary tumors account for about 6 to 10% of all cancer diagnoses, and are the fifth most commonly diagnosed cancer in Western countries. The risk of developing a second primary cancer may increase with the use of cancer therapies, such as chemotherapy and radiation therapy. However, it is crucially important to remember that this cancer therapy-related risk is minimal when compared to the benefits of treating the original primary cancer. Patients with lung cancer are at high risk of developing second primary lung cancers. However, it may be difficult to accurately determine whether the new tumor is a secondary primary cancer or a metastasis from the original cancer. Studies have shown that in the majority of lung cancer patients the new lesion is a second primary tumor. When the original primary tumor has been surgically removed, surgical resection of second primary tumors may achieve a 5-year survival rate of 60%, with a comparable expected operative morbidity and mortality to the primary surgery. Tumors 2 cm or smaller are associated with significant positive long-term prognostic factors for survival and freedom from recurrence following resection of a the second primary cancer [133–135]
4.6. Treatment of Brain Metastases
Brain metastases are a common problem in lung cancer patients and a significant cause of morbidity and mortality. Brain metastases are found in about 80% of SCLC and 30% NSCLC at two years from diagnosis [136,137]. Among the various histologies of NSCLC, the incidence of brain metastases in patients with adenocarcinoma and large cell carcinoma is greater than in patients with squamous cell carcinoma [138,139]. The median survival for untreated lung cancer patients with brain metastases is 4 to 7 weeks [140–142]. The treatment may be for relief of symptoms or therapeutic strategies. Treatment options for lung cancer patients with brain metastases may include Whole Brain Radiotherapy (WBRT), surgical resection, Stereotactic Radiosurgery (SRS), Systemic therapy and Radiosensitization, or a combination of these various treatment modalities.
a) Whole Brain Radiotherapy (WBRT)
WBRT is the standard of care for cerebral metastasis in lung cancer patients. Several randomized trails have assessed numerous WBRT dose and fractionation schedules but showed no significant difference in either survival times, or symptomatic response rates and duration. Nevertheless, the results of these trials have suggested better palliative effects from the more prolonged schedules and the choice of dose fractionation schedule should be based on patients’ prognosis [143]. Additionally, a systematic imaging study of dose response based on tumor size and histology, following WBRT (30 Gy in 10 fractions) [144], showed an improved response rate for smaller tumors without necrosis. The complete response rate was 37% for SCLC, 25% for squamous cell carcinoma, and 14% for non-breast adenocarcinoma.
b) Surgery
Resection of a single brain metastasis combined with WBRT is a standard treatment option of brain metastases [134,145]. A prospective randomized study [134], demonstrated superiority of surgical removal of the brain tumor followed by radiotherapy over needle biopsy and radiotherapy, with lower recurrence rates at the site of the original metastasis (20% vs. 52%,), and a significantly longer time to recurrence of the original brain metastasis (median >59 weeks vs. 21, p < 0.0001). Moreover, the median survival with surgery and adjuvant WBRT was much longer than with WBRT alone (40 weeks vs. 15 weeks, p < 0.01). Patient’s functional independence (KPS score of ≥70) was also preserved much longer with combined surgery and WBRT than with radiation alone (median: 38 weeks vs. 8 weeks, p < 0.005).
In patients with multiple brain metastases, surgery is typically limited to the resection of the dominant, symptomatic lesion. Various studies have shown that surgery combined with adjuvant WBRT or stereotactic radiosurgery (SRS) has similar survival outcome in patients with multiple lesions compared with patients with single brain metastasis or a single lesion [146–148]. About 50% of patients treated with resection and postoperative radiation therapy develop recurrence in the brain [118]. Few patients with recurrent brain metastasis and good PS, but without progressive metastases outside of the brain, may be treated with surgery or stereotactic radiation surgery [118,120]. However, most patients with recurrent brain metastasis may be treated with additional radiation therapy, albeit with a limited palliative benefit [149].
c) Stereotactic Radiosurgery (SRS) with and without WBRT
Stereotactic radiosurgery (SRS) is a form of non-invasive radiation therapy that focuses high-power energy on a precisely defined small target (e.g. the center of the tumor). The suggested mechanisms of SRS-induced tumor killing are radiation-induced DNA damage, endothelial cell apoptosis, microvascular dysfunction, and induction of a T-cell response against the tumor [150–152]. Because of the generally small size and well-defined margin of brain metastases at presentation [153,154], SRS may be an effective alternative to surgery for up to four small brain metastases (up to 4 cm in size) [154].
Several studies showed that SRS might achieve better prognosis and prolonged survival in lung cancer patients with good PS, no systemic disease, and longer survival time from the diagnosis of primary disease [155–160]. The addition of SRS to WBRT could be beneficial for patients who are not eligible for surgery due to tumor location in the brain or other medical contradictions.. A large randomized controlled Phase III trial study, showed that the local recurrence at one year decreased significantly with the combination of WBRT and SRS (18 vs. 29%, p = 0.01) [161]. A planned sub-analysis, in patients with a single brain metastasis revealed an improved median survival (6.5 vs. 4.9 months; p = 0.039) and improved quality of life with WBRT and SRS.[161–163]. The addition of adjuvant WBRT to SRS yielded a significant increase in the average time to deterioration in patients with one to four brain metastases (16.5 months vs. 7.6 months, p = 0.05) although no survival advantage was observed [164].
d) Systemic Therapy and Radiosensitization
In 30–70% of patients with a single brain metastasis, lung cancer is the primary disease [165]. Generally, most chemotherapeutic agents are unable to cross the blood brain barrier reach the CNS. However, the endothelium leakiness of the tumor vessels, which may disrupt the blood-brain barrier, is well documented in human cancer, particularly in case of macroscopic metastases or relapsed disease. In keeping, several small phase II studies demonstrated that chemotherapy alone yields response rates of brain metastases of 43%–100% and 0%–38% for metastases from SCLC and NSCLC, respectively [165]. However, combining chemotherapies (thalidomide, teniposide, topotecan, paclitaxel, and cisplatin) to WBRT did not demonstrate survival benefit although some showed enhanced response rates [166–175].
Radiosensitizing agents, such as motexafin gadolinium (Xcytrin) and efaproxyn (efaproxiral or RSR-13), may increase oxygen levels in the tumor and therefore enhance its sensitivity to radiation therapy. However, initial trials showed that the addition of radiosensitizers to WBRT may improve response rate and time to progression (TTP), but not survival. Overall, the evidence to date does not support the clinical use of chemotherapy or radiosensitizers in conjunction with WBRT in the treatment of brain metastases.
4.7. Role of Angiogenesis Inhibitors in NSCLC
Angiogenic pathways provide an important target in NSCLC treatment since they foster tumor growth through the development of new blood vessels. The complex process of angiogenesis is regulated by pro-angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), as well as angiopoietins [176]. Currently, only the monoclonal antibody bevacizumab, targeting circulating VEGF, is approved for first-line treatment of advanced NSCLC in combination with platinum-base chemotherapy [176]. Several anti-angiogenic agents are under clinical investigation, including sorafenib and sunitinib.
A randomized phase III trial (ECOG 4599) assessing the efficacy of bevacizumab in combination with first-line platinum-based chemotherapy (carboplatin/paclitaxel) showed significantly improved response rates (35 % vs. 15 %, P<0.001), PFS (6.2 vs. 4.5 months, P<0,001), and overall survival (OS) (12.3 vs. 10.3 months, P=0.003) in the antibody group, compared to chemotherapy alone [177]. Further analysis revealed that baseline tumor cavitation is the most significant risk factor for the fatal side effect after bevacizumab therapy [178]. The trial also suggested that patients with adenocarcinoma histology might benefit more from the treatment with bevacizumab [179].
However, resistance to treatment with anti-VEGF agents is a challenge and occurs in all patients eventually. This resistance might, at least partially, be caused by up-regulation of compensatory angiogenic pathways, e.g. through PDGF or FGF signaling [176]. Multi-targeted anti-angiogenesis therapies therefore represent an interesting treatment strategy for NSCLC patients. In keeping, the multiple tyrosine kinase inhibitor (TKI) sorafenib which targets VEGFR-2/3, PDGFR-β, c-Kit, Raf, and Flt-3, produced promising response rates in several phase I and II studies [176]. Although sorafenib showed activity as single agent in NSCLC patients, it did not show an added improvement when combined with the carboplatin, paclitaxel and EGFR TKI (erlotinib) [176]. Interestingly, Sorafenib treatment resulted in an increased disease control rates in NSCLC patients with K-Ras mutations [176]. Several phase II/III studies for sorafenib in NSCLC are still ongoing [176].
Another multiple TKI, sunitinib, targeting VEGFR-1/2/3, PDGFR-α/β, c-Kit, Flt-3, and RET, have also been evaluated in NSCLC patients [176]. Similar to sorafenib, sunitinib demonstrated singe-agent activity in pretreated NSCLC patients but did not show promising results when combined with paclitaxel/carboplatin alone, with bevacizumab or with erlotinib in two phase II trials [180,181]. Moreover, several other agents inhibiting VEGFR in combination with PDGFR are currently in clinical development, including cediranib, axitinib, motesanib, and linifinib [176].
Conversely, the angiogenesis inhibitors have not proven to increase the efficacy of standard platinum-based chemotherapy in advanced NSCLC possibly due to lower doses required to reduce toxicities [176]. In addition to dual-targeted therapies, several agents with activity against three angiogenic pathways (VEGFR, PDGFR, and FGFR) are currently under clinical investigation. An example of the aforementioned inhibitors is the small molecule inhibitor nintedanib which targets VEGFR-1/2/3, PDGFR-α/β, FGFR-1/2/3, as well as Flt-3 and Src family members [182]. Nintedanib achieved disease stabilization in 46 % of the patients, with a median PFS of 6.9 weeks and median OS of 21.9 weeks, in stage IIIB/IV advanced NSCLC patients [183]. Pazopanib is another interesting multi TKI, targeting VEGFR-1/2/3, PDGFR-α/β, and FGFR-1 and 3 [184]. In a phase II study treating naïve stage I/II resectable NSCLC patients, pazopanib showed significant activity as single agent. 86 % of the pazopanib treated patients had a reduction in tumor volume, with two patients achieving a reduction of over 50 %. Pazopanib is currently under investigation in advanced NSCLC [176].
4.8. Other Molecular Targeted Agents, under Clinical Evaluation for NSCLC Treatment
a) Talactoferrin
The glycoprotein lactoferrin was first described as an iron-binding protein in breast milk and shows immune-modulatory functions [185,186]. The human recombinant lactoferrin, talactoferrin, is given orally, and is able to recruit immature dendritic cells (DC) into the gut-associated lymphoid tissue, where cross-presentation of tumor antigens and subsequent DC maturation can occur [186]. Preclinical data also showed an increase in splenic NK cell activity and inhibition of NSCLC tumor growth with talactoferrin [187]. A double blind, placebo controlled phase II study using talactoferrin as monotherapy showed improved OS (median 6.1 vs, 3.7 months after a follow-up of 15.2 months). When combining with chemotherapy (carboplatin and paclitaxel) in treating advanced stage IIIB/IV NSCLC patients, talactoferrin also showed a promising trend for disease-control rates in the intention-to-treat and evaluable population [188].
b) Insulin-like Growth Factor Inhibitors
The insulin-like growth factor system (IGF system) comprises two receptors: Insulin-like growth factor 1 receptor (IGF-IR) and IGF-IIR with their respective ligands: Insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) and six high-affinity IGF binding proteins (IGFBP) that function as carrier proteins for these ligands. IGF 1 and 2 are involved in the regulation of the development and growth of somatic tissues, as well as carbohydrate metabolism [189,190]. The IGF signaling pathway promotes cell growth by stimulating cell proliferation and differentiation. Additionally, IGF-IR, but not IGF-IIR, signaling inhibits apoptosis [191]. These ligands bind to the extracellular domain of the IGF receptor 1 (IGF-1R), which is expressed on many normal human cells [192,193], and overexpressed in many cancers, including lung cancer. Furthermore, increased IGF-1 levels, and decreased IGFBP-3/4 level correlate with a higher risk of lung cancer [190,194].
A number of inhibitors for the IGF signaling pathway have been developed, including monoclonal antibodies and small-molecule TKIs, which target the intracellular domain of the receptor [190]. Figitumumab, a monoclonal antibody against IGF-1R, showed significantly increased overall response rates compared to chemotherapy alone (54 % vs. 42 %, P<0.0001) in a phase II trial in previously untreated locally advanced or metastatic NSCLC patients, Squamous tumors showed a response rate of 78 % and PFS (12-week) of 89 % [195]. However, two phase III trials that used Figitumumab in combination with chemotherapy in non-adenocarcinoma NSCLC were closed due to severe lethal adverse events and unmet primary endpoint [190,196,197]. Two other monoclonal antibodies against IGF ligands, cixutumumab and dalotuzumab, as well as small molecule TKIs are currently under clinical investigation [190].
c) Histone Deacetylase Inhibitors
Histones are nuclear structural enzymes and, as part of the chromatin, are involved in nucleosomal DNA organization and gene regulation. Conformational changes in DNA structure are regulated by histone acetylation and deacetylation, a mechanism that is often affected in tumor cells [198]. Histone deactylases (HDACs) are involved in chromatin condensation and repression of gene expression and are frequently overexpressed in many cancers [190]. Contrary to genetic mutations, the epigenetic modifications induced by HDACs are reversible, and therefore, HDACs are an attractive target for cancer therapy [198]. Various HDAC inhibitors have been developed and shown to modulate the acetylation status of several important cellular proteins involved in tumor cell growth and proliferation, including p53, HSP90, STAT3, subunits of NFκ-B and α-tubulin [190,199,200]. Moreover, the HDAC inhibitors can also modify the cell cycle and lower the apoptotic threshold [190,199,200]. Many HDAC inhibitors showed anticancer activity in cell culture and animal models of carcinogenesis. Two of these HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA, Vorinostat) and Romidepsin (Depsipeptide, FK228), have already been FDA approved for the treatment of cutaneous T-cell lymphoma (CTCL). The addition of vorinistat to chemotherapy was able to improve the response rate compared to the placebo addition to chemotherapy (34 vs. 12.5 %, P=0.48) in treating advanced stage IIIB/IV NSCLC patients [201,202]. However, a phase III trial of vorinostat in combination with carboplatin and paclitaxel was stopped due to increased adverse effects, and a lack of efficacy in the vorinostat group [203]. A number of other HDAC inhibitors including entinostat, pivanex, cI-994, panobinostat, and romidepsin, are currently under clinical investigation for treatment of NSCLC [204].
d) Pro-Apopototic Agents
Apoptosis has long been known as a hallmark of cancer, and cancer cells exploit both upregulation of antiapoptotic as well as downregulation of pro-apoptotic mechanisms [46,205]. Novel pro-apoptotic drugs are currently being investigated for the treatment of NSCLC. To date, both Mapatumumab, a high-affinity monoclonal antibody against the death receptor DR4/TRAIL-R1, and a pro-apoptotic agent apomab did not show clinical benefit as monotherapy or in combining with chemotherapies (carboplatin and paclitaxel) in clinical trials [206,207] [208] [190,209]. A number of other new pro-apoptotic agents, such as Conatumumab (targeting DR1) and YM155 (targeting survivin), are currently under clinical investigation for the treatment of NSCLC and have shown synergistic effects in combination with chemotherapy [190,210,211].
4.9. Immunotherapy
a) Immune Checkpoint Inhibitors
1) CTLA4
Tumors ascribe certain immune-checkpoint pathways as a chief mechanism of immune resistance, particularly against T cells that are specific for tumor antigens. Several of these immune checkpoints are initiated by ligand–receptor interactions, and thus are amenable to inhibition by antibodies or modulated by recombinant forms of ligands or receptors [186]. Two monoclonal antibodies, ipilimumab and tremelimumab, have been used successfully in NSCLC against the cytotoxic T-lymphocyte-associated antigen (CTLA-4), an inhibitory T-cell co-receptor found on activated T-cells and regulatory T-cell subsets [186,212]. A multicenter double-blind phase II trial showed that the combination of ipilimumab and chemotherapy (carboplatin or paclitaxel) significantly improved the immune-related PFS in advanced stage IIB/IV NSCLC patients with squamous cell carcinoma (without prior chemotherapy) [186,213]. Tremelimumab has also been tested in a randomized, phase II trial as maintenance after first-line chemotherapy, compared to best supportive care. However, the results of this trial showed no improvement in PFS [214].
2) PD-1 and PD-L1
The immune-checkpoint receptor, programmed death-1 (PD-1), is a promising target, for stimulation of antitumor immune responses by the patient's own immune system. Unlike CTLA4, the main role of PD-1 is to control the activity of T cells in peripheral tissues at the time of an inflammatory response to infection and to limit autoimmunity [215–222]. This translates into a major immune resistance mechanism within the tumor microenvironment [223–225]. Another interesting immunotherapeutic option is the direct targeting of PD-1 ligands (PD-L1), B7-H1/PD-L1 and B7-DC/PD-L2. It has been shown that B7-H1/PD-L1 is selectively upregulated in many human cancers including lung cancer [223,226]. An encouraging phase I study showed clinical activity of PD-L1 blocking agents in NSCLC [223,226].
A dose-escalation study testing a monoclonal antibody against PD-1 (MDX-1106) in the treatment of refractory-metastatic solid tumors (melanoma, renal cell cancer, colon cancer, NSCLC), showed objective responses in five of 49 NSCLC patients [222]. This result highlights the potential activity of anti-PD-1 against a non-immunogenic tumor [226]. Another study assessed the safety and antitumor activity of the anti-PD-1 monoclonal antibody BMS-936558 or nivolumab in patients with advanced tumors (NSCLC, melanoma, prostate, renal cell, and colon cancer) [227]. Durable, objective responses (partial and complete) were observed in 18 % of NSCLC patients. Significantly, the objective responses to anti-PD-1 therapy and clinical benefit correlated with PD-L1 expression by tumor cells (P=0.025 and 0.005, respectively). Although the expression of PD-L1 by infiltrating immune cells did not significantly correlate with objective response (P=0.14), marked correlation with clinical benefit was reported (P=0.038). The toxic side effects were milder with PD-1 inhibition compared to CTLA-4 inhibition, thus underlining the importance of targeting immune checkpoint-pathways with better benefit-to-toxicity ratios [186,226].
Several clinical trials are currently investigating immune checkpoint inhibitors, such as anti-CTLA-4 (nivolumab) and anti-PD-1 (ipilimumab), as monotherapy or in combination with chemotherapy in NSCLC [228]. Recently, results from two phase III trials (CheckMate-017 and 057) showed an OS benefit with Nivolumab compared to docetaxel in both nonsquamous and squamous NSCLC. In the phase III CheckMate-017 trial [229], there was a 41% OS improvement with nivolumab compared to docetaxel in the squamous setting. In the phase III CheckMate-057 trial [230], the OS benefit with nivolumab was 27% in patients with nonsquamous NSCLC. Based on data from CheckMate-017 trial, Nivolumab is now approved by the FDA in squamous NSCLC.
Recent publications evaluated expression of PD-1 and PD-L1 in NSCLC. Patients with KRAS mutations were shown to express higher levels of PD-1 compared to patients with wild type KRAS, whereas increased levels of PD-L1 were detected in patients with EGFR mutations or ALK translocations [231]. Interestingly, the clinical profile of PD-1 expressing patients included male smokers with adenocarcinoma more frequently, whereas PD-L1 was more frequently expressed in female non-smokers with adenocarcinoma. Both PD-1 and PD-L1 were recently shown to be upregulated through activation of EGFR, thereby leading to immune evasion [232,233]. Furthermore, patients with EGFR mutations and increased expression of PD-L1 showed a higher response rate to treatment with the EGFR-TKIs gefitinib or erlotinib, compared to PD-L1 negative patients, which resulted in longer TTP (11.7 vs. 5.7 months, P<0.0001) and OS (21.9 vs. 12.5 months, P=0.09) [231]. Taken together, these recent studies suggest that combination of EGFR TKIs with PD-1 inhibitors might be beneficial in treatment of NSCLC [231,232].
Another study analyzed the mechanism of combining immunotherapy with immune checkpoint inhibition in a B16 tumor mouse model. A TLR agonist enhanced GM-vaccine (TEGVAX) was shown to induce anti-tumor immune responses in vivo, which was associated with IFN-y dependent upregulation of PD-L1 in the tumor microenvironment. Combined treatment with TEGVAX and PD-1 inhibition led to regression of established tumors, whereas PD-1 inhibition alone did not induce anti-tumor immune responses [234]. Other antibodies against PD-1, such as pembrolizumab (MK-3475), MPDL3280A, and MEDI4736 are currently being investigated and have shown promising results in Phase 1 clinical trials [235]. A recent study used whole-exome sequencing to investigate the genomic determinants of response in two independent cohorts of NSCLC treated with this therapy [236]. This study revealed that a higher nonsynonymous mutation burden in tumors was associated with improved ORR, durable clinical benefit, and PFS. Pembrolizumab clinical efficacy also correlated with the molecular smoking signature, higher neoantigen burden, and DNA repair and replication pathway mutations (e.g., mutations in POLD1, POLE, MSH2, Rad51, Rad17, DNA-PK) [236]. Remarkably, pembrolizumab–induced neoantigen-specific T cell reactivity was also observed in the peripheral blood, thus, suggesting possible blood-based assays to monitor response during anti–PD-1 therapy.
b) Vaccine Therapy for NSCLC
Vaccination against pathogens is one of the most important developments in modern medicine and saves millions of lives each year. For advanced NSCLC patients, median OS is about one year, and only 3.5 % survive five years after diagnosis, despite the addition of new therapies to standard chemotherapy [186]. Therefore, vaccinations for solid tumors, either preventive (for tumors related to infections such as human papilloma virus-associated cervical cancer [237]) or therapeutic (breaking tolerance and achieving long lasting response in tumors such as ipilimumab (anti-CTLA-4) in advanced melanomas [226,238]), have long been seen as the ultimate treatment option for cancer patients.
As many other cancers, NSCLC belongs to the non-immunogenic tumors and therefore the identification of tumor specific immunogenic antigens for vaccine therapy presents a major challenge. The most promising results of vaccines for NSCLC patients have been observed in the adjuvant setting and in locally advanced NSCLC [190]. A Summary of the various vaccine therapy evaluated in NSCLC is shown in Table 9.
Table 9.
Types of vaccine therapy for NSCLC.
| BEC2/BCG | glycosphingolipid GD3 vaccine |
Combines a monoclonal antibody that mimics the glycosphingolipid GD3 with the adjuvant bacillus Calmette-Guerin |
|---|---|---|
| Belagenpumatucel-L | Allogeneic vaccine | Four irradiated lung cancer cell lines and an antisense plasmid against TGF-beta |
| CimaVax EGF | EGF vaccine | Cyclophosphamide and EGF |
| CRS-207 | Mesothelin vaccine | Genetically-engineered Listeria monocytogenes |
| INGN-225 | p53 vaccine | Produced from patients’ autologous peripheral blood mononuclear cells (PBMCs) |
| L-BLP25/emepepimut-S | MUC-1 vaccine | Synthetic peptide derived from the mucin 1 |
| MAGE-A3 | Melanoma-associated antigen A3 vaccine | Tumor specific antigen Mage-A3 |
| PRAME | PRAME vaccine | Recombinant PRAME protein combined with the AS15 Adjuvant System |
| TG4010 | MVA-MUC1-IL2 vaccine | Modified vaccinia ankara encoding human MUC-1 antigen and interleukin-2 |
A. MUC1
Mucin-1 (MUC1) is a glycoprotein present on normal epithelial tissue and in various cancers, including NSCLC [66,239]. A mutated MUC1protein overexpressed in cancer cells shows aberrant glycosylation pattern that is antigenically different from wild-type protein expressed on normal epithelial cells [66]. L-BLP25 is a synthetic vaccine against the core peptide of MUC1 combining the peptide with cyclophosphamide as an adjuvant [186,240]. Recently updated data from a phase IIB randomized study treating stage IIIB/IV NSCLC patients with L-BLP25 showed significant improvement in the vaccine group comparing to the supportive group (3-year survival rates: 31 vs. 17 % [241]).
The efficacy of TG4010, a recombinant vaccinia virus that combines the human MUC1 and interleukin-2 coding sequences [66], in combination with cisplatin and vinorelbine or as monotherapy has been investigated in a randomized phase II study for advanced NSCLC patients [242,243]. A subgroup with a detectable CD8+ T-cell response was able to generate an immune response against MUC1 and had longer median survival [243]. Data from another phase II study comparing the vaccine plus chemotherapy with chemotherapy alone in advanced NSCLC patients with confirmed MUC1 expression showed that higher numbers of activated NK cells might suppress DC and effector T-cells and result in decreased median OS rates as well as increased adverse effects. [244] [243].
B. EGF
CimaVax EGF is a new vaccine that is being developed for NSCLC treatment. This vaccine is made up of a low dose cyclophosphamide and EGF. It works as an immunoadjuvant to reduce the inhibition of Tsuppressor cells and to stimulate the production of anti-EGF antibodies that may inhibit EGF binding EGFR on cancer cells, and consequently decrease cancer cells growth [66,243,245,246].
A phase I study showed that the production of anti-EGF antibodies and serum EGF levels after use of the EGF-based vaccine, correlate with increased survival rates in NSCLC patients [247]. A randomized phase II trial comparing the CimaVax vaccine to best supportive care in stage IIIB/IV patients [248] showed minimal toxicity and good antigen responses in 51.4% of the patients although the median OS did not improve [66]. Longer median survival rates were seen in good antigen responders than in poor antigen responders (11.7 vs. 3.6 months) [243]. In addition, longer median OS rates were seen in patients with EGF levels below 168 pg/ml (13 vs. 5.6 months) and under 60 years (11.57 vs. 5.33 months, P=0.0124) [243]. The vaccine has been approved for clinical trial development in the US stage IIIB/IV patients [243].
C. Melanoma-associated antigen (MAGE)
Melanoma-associated antigen A3 vaccine (MAGE-A3) uses a tumor specific antigen, which is expressed in 35 % of NSCLC, most frequently in squamous cell carcinomas [186]. It ranges from 16 % in stage IA to 48 % in stage IIIB and may be associated with poor prognosis [66,249,250]. The MAGE-A3 vaccine, initially developed for metastatic melanoma patients, showed a positive sign of activity after 28 months in a phase II adjuvant therapy study in early stage NSCLC [251]. Prior to treatment, the NSCLC tumors were analyzed by gene expression profiling to identify a gene signature that correlates with the clinical activity of the vaccine [252]. The identified signature of genes related to the immune system overlapped with the gene set detected in the melanoma trial. Reduction in the relative recurrence risk after treatment with the MAGE-A3 vaccine showed a 2-fold increase for tumors containing the gene signature (57%), compared to the non-selected population (25%). This result underlines the value of the stratification of patient subpopulations and suggests that gene profiling for a certain tumor microenvironment or immune cells might have a predictive value in cancer immunotherapy [66,251–253].
D. TGF beta
The allogeneic vaccine belagenpumatucel-L combines four irradiated lung cancer cell lines (two adenocarcinoma, 1 squamous cell and one large cell carcinoma) with an antisense plasmid against TGF-b (transforming growth factor beta) [186], a poor prognostic factor in NSCLC. TGF-b2 suppresses dendritic cells, NK cells, and activated cytotoxic T lymphocytes and thereby may help tumors to escape immunosurveillance [186]. The use of four tumor cell lines in the belagenpumatucel-L vaccine increases the number of tumor antigens and the suppression of TGF-b expression through the antisense plasmid removes a major source of immune suppression at the injection site [186]. Preclinical data showed that the inhibition of TGF-beta2 could help to break the tolerance and to increase the immunogenicity of tumor vaccines [66,254,255]. Patients who received a high dose of the vaccine showed a significantly improved OS compared to low dose group in a randomized, dose-variable phase II trial with 75 NSCLC patients (stages II-IV) [255] [186].
E. PRAME
Another important tumor antigen for vaccine therapy is PRAME (preferentially expressed antigen of melanoma), which has recently been shown to contribute to carcinogenesis in NSCLC [256]. As the name suggests, it was first detected in a melanoma patient [67], and is expressed in a variety of tumors [66]. PRAME seems to function via the suppression of the retinoic acid receptor (RAR), a signaling pathway which regulates cell death and cell cycle [66,257].. Overexpression of PRAME might be used by tumor cells to escape suppressive RAR signaling, thereby fostering tumor-progression [66]. Clinical data suggests that poor clinical outcome of some patient subpopulations correlates with PRAME expression in neuroblastoma [258] and breast cancer [259–261].
A new PRAME vaccine combining the purified recombinant PRAME protein with an adjuvant (liposomal preparation with the AS15 adjuvant system) has been developed. A currently ongoing phase II study (PEARL study) is evaluating the efficacy of the PRAME vaccine in resected NSCLC. This study enrolled patients radically resected for NSCLC, stages IA (T1b), IB, II or IIIA, and whose tumors exhibit PRAME expression. The primary endpoint of the study is disease-free survival (DFS) [257,262].
5. Small-cell lung cancer
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung carcinomas [4,263]. The highest risk-factor for development of SCLC is smoking, and the decrease in percentage of smokers and amount of cigarettes smoked per person in the US might explain the recent decrease in SCLC incidence rates [263]. 30% of SCLC patients are diagnosed with limited-stage disease (LS-SCLC), where the cancer is confined to the hemithorax, the mediastinum, or the supraclavicular lymph nodes. 70% of SCLC patients are diagnosed with extensive-stage disease (ES-SCLC), with tumors spreading beyond the supraclavicular areas [4,263]. Although SCLC is initially more responsive to chemotherapy and radiation therapy than all other lung cancer types, it is very difficult to cure, due to its aggressive growth and its wide dissemination at the time of diagnosis.
As for other lung cancers, the treatment options for SCLC patients are determined by histology, stage, and general health and comorbidities of the patient. For patients with LS-SCLC, standard treatment options include platinum based chemotherapy and radiation therapy, combination chemotherapy alone, surgery followed by chemotherapy or chemoradiation therapy, and prophylactic cranial irradiation [264]. For patients with ES-SCLC patients, current treatment recommendations include combination chemotherapy, radiation therapy and prophylactic cranial irradiation [264]. For recurrent SCLC, the treatment options are chemotherapy and palliative therapy. Although chemotherapy and radiotherapy can lead to strong initial responses in SCLC, disease recurrence is common. Notwithstanding the improvements in diagnosis and therapy made during the past two decades, the current prognosis for patients with SCLC remains substandard. Untreated SCLC is the most aggressive of all type of lung cancers, with a median survival from diagnosis of only 2 to 4 months. The 2 years DFS, following treatment of SCLC patients, remains a dismal 10% [265]. Moreover, the OS at 5 years of all population of SCLC patients is merely 5% to 10% [4,265–267], with a better prognosis for patients with LS-SCLC (5-year survivals of 14%) than for patients with ES-SCLC [4,266,268,269]. In patients who showed complete response to chemoradiation, prophylactic cranial radiation may avert brain metastases recurrence, and thus, increase patients’ survival [270,271].
Although surgery or chemotherapy alone can improve survival in patients with LS-SCLC, a greater improvement in long-term survival has been shown with combination therapy [269,272]. Particularly combining chemotherapy with thoracic radiation therapy (TRT) increases OS by 5% compared to chemotherapy alone [4,273–276]. Although median survival of 6 to 12 months may be achieved in patients with ES-SCLC with the currently available therapy, long-term DFS is rare in these patients [4,275,276].
Targeted therapies in SCLC
a) VEGF inhibitors
Inhibition of circulating VEGF with bevacizumab has been studied in ES-SCLC. Chemotherapy naïve patients treated with cisplatin, irinotecan, and bevacizumab, showed an ORR of 75 %, a median OS of 11.6 months, and a median PFS of 7.0 months [277]. A study investigating cisplatin, etoposide, and bevacizumab in previously untreated ES-SCLC patients showed that a higher baseline level of vascular cell adhesion molecule (VCAM) was associated with a higher risk of progression or death, compared to lower levels of VCAM, but no other biomarkers could be correlated with treatment outcome [278].
Other VEGF inhibitors, including the multi-kinase inhibitors sorafenib, sunitinib, and cediranib, are currently under clinical investigations in SCLC. [279] [264]. Aflibercept (AVE0005) is a fully humanized protein that contains immunoglobulin domains from the two VEGF receptors VEGFR1/2, fused to the constant region of IgG1. These soluble receptors work as a VEGFR-trap and inhibit binding of VEGF to its common receptors. Aflibercept is currently investigated in combination with topotecan in previously treated ES-SCLC [264].
b) EGFR inhibitors
Mutation of the EGFR is less frequent in SCLC compared to NSCLC, and only around 4% of patients were shown to harbor the mutation [280]. In a phase II study, patients were stratified according to chemosensitive or chemo-refractory relapsed SCLC and treated with gefitinib. However, the abovementioned study could not demonstrate a gefitinib benefit for SCLC patients [281].
c) Bendamustine
This cytotoxic agent causes DNA breaks through its alkylating activity. Compared with other alkylating agents, bendamustine causes more extensive and durable DNA single- and double-strand breaks [264]. Combining bendamustine with carboplatin in treating ES-SCLC [282], the ORR was 72.7 %, with a median TTP of 5.2 months and median survival time of 8.3 months.. As a single agent in second- and third-line setting in patients with relapsed/refractory SCLC, Bendamustine is well tolerated and effective agent [283].
d) Immunotherapy
To date, only few studies have evaluated immunotherapy in the treatment of SCLC [243]. The BEC2/BCG vaccine combines a monoclonal antibody that mimics the glycosphingolipid GD3, which is selectively expressed in SCLC, with the adjuvant bacillus Calmette-Guerin [243]. The BEC2/BCG vaccine has been demonstrated to develop antibodies against GD3 in Melanoma patients. In an early clinical study, although only 30% of the SCLC patients (5/15, ES/LS-SCLC=8/7) developed measureable anti-GD3 antibodies, the median OS was 20.5 months (relapse-free survival was longer in patients who developed measurable anti-GD3 antibodies) [243,284,285]..
Another novel vaccine (INGN-225) targeting p53 protein was developed, and is currently under investigation for treatment of SCLC [243]. The INGN-225 vaccine is produced from patients’ autologous peripheral blood mononuclear cells (PBMCs). The autologous PBMCs are cultured in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) prior to incubation with a viral construct containing wild-type p53 (adenovirus Ad.p53). In a preclinical study, dendritic cells transfected with Ad.p53 were able to induce cytotoxic T lymphocytes following vaccination [243]. In a recent phase I/II study.14 of the 54 enrolled ES-SCLC patients demonstrated a positive immune response and showed increased median survival (12.6 vs. 8.2 months, P=0.131) [243,286]. Among those responsive patients, 78.6% responded to second-line chemotherapy treatment.
6. Palliative Care for Patients with Lung Cancer
A high percentage of lung cancer patients suffers from substantial symptom burden, including fatigue, loss of appetite and weight loss, as well as dyspnea, hemoptysis, and chest pain [287]. Better quality of life, lower depressive symptoms, higher median survival and fewer needs on aggressive end-of-life care were observed in patients receiving early palliative care combined with standard oncologic care compared to standard oncologic alone for metastatic NSCLC.[287]. Megestrol acetate (MA), an appetite stimulant, is one of the supporting treatments that have shown success in treating cancer-related anorexia (CRA) and improve quality of life. Combining MA with olanzapine (OLN) for the treatment of CRA in 80 patients with either advanced gastrointestinal cancer or advanced lung cancer patients (stages III and IV) resulted in a significant improvement of mean symptom scores as measured by the MD Anderson Symptom Inventory (MDASI) [287] suggesting that combination MA and OLN may be an effective palliative therapy for patients with CRA [288]..
7. Mesothelioma
Malignant Mesothelioma (MM) is an extremely aggressive tumor affecting about 3,000 new patients in the United States annually [289]. The incidence of the disease in the US is expected to rise steadily and peak with about 85,000 new MM cases over the course of the next 20 years [290,291]. MM arises from the surface serosal cells of the pleura and, less frequently, from the peritoneum. Exposure to asbestos is a wellestablished cause, with occupational exposure being documented in 70–80% of MM patients [292–294]. MM is sub-typed into three forms according to the histological morphology: epithelial, sarcomatoid, and biphasic. Diffuse MM comprises about 75% of mesotheliomas diagnosed [295].
Treatment of MM with surgery, chemotherapy, or radiation therapy is rarely curative. Clinical trials of single modality treatment with extrapleural pneumonectomy or pleurectomy, chemotherapy or radiation therapy, have not shown significant improvement in survival compared with supportive treatment. Median survival has ranged from 10 to17 months [296]. Sugarbaker et al. reported a 15% 5-year survival with multimodal therapy and a 25% 5-year survival in patients who underwent complete surgical resection [297]. Recent trials of new-generation platinum- and pemetrexed based regimens have reported encouraging results. In particular, a phase II trial of pemetrexed plus cisplatin for MM reported a median survival of 12 months compared with nine months after treatment with cisplatin alone [298]. Despite these promising results, long-term survival with currently available treatment is rare. Therefore, novel meaningful therapies for MM are urgently needed. Recent advances have shown that tumors carrying activating mutations in some cellular proto-oncogenes are particularly sensitive to targeted therapies directed against the mutant proteins [14,16,299–307].
Role of immunotherapy in malignant mesothelioma
A number of immunotherapy strategies have been tested in mesothelioma patients, with varying degrees of success.
a) Mesothelin
Mesothelin is an immunogenic glycoprotein specifically overexpressed in malignant mesothelioma, NSCLC, ovarian, and pancreatic cancers [186]. CRS-207, is a genetically-engineered, double-deleted bactria Listeria monocytogenes strain expressing the human mesothelin [186]. Phagocytic cells, such as macrophages and dendritic cells, take up CRS-207, and mesothelin is subsequently expressed and processed through the MHC I presentation pathway. This process is predicted to activate T cells in order to attack mesothelin-positive mesothelioma cells. In preclinical studies, CRS-207 was shown to elicit anti-mesothelin cell-mediated immunity [186]. In a phase I dose-escalation study treating patients with advanced mesothelin expression and treatmentrefractory cancers, with CRS-207, mesothelin-specific T-cell responses were in one of five patients with mesothelioma, with 15 months or more survival after the first dose [186]. Patients who received sequential CRS-207 treatment with prior immunotherapy or subsequent local radiation therapy benefited the most. CRS-207 is currently investigated in combination with first-line chemotherapy in mesothelioma patients.
b) WT1 analogue peptide vaccine
The transcription factor Wilms’ tumor suppressor gene 1 (WT1) is frequently overexpressed in mesothelioma and other solid and hematopoietic tumors [186]. Recently, a multivalent WT1 peptide analog vaccine was developed [186]. A CD-4+ T-cell proliferation to WT1-specific peptides was seen in six of nine patients, and a CD8+ T-cell response was detected in six of six HLA-A0201 patients in an early study investigating the WT1 peptide analog vaccine in MM and NSCLC patients. Stimulated T-cells also showed cytotoxicity against WT1 positive cells [308]. A subsequent randomized phase II study is currently investigating the adjuvant WT1 analog peptide vaccine in MM patients who have completed combined modality therapy [309].
c) Dendritic cell vaccine
Dendritic cells (DCs) are the most potent antigen-producing cells, capable of sensitizing T cells to both new and recall antigens. DC-based cancer immunotherapy is aimed at using these cells to prime specific antitumor immunity through the generation of effector cells that attack and kill tumors. Dendritic cells can be matured using a standard cytokine cocktail and pulsed with autologous tumor cell lysates, which presents a source of tumor antigens for immunotherapy [186].
A phase I study has shown that the DC vaccine was well tolerated in newly diagnosed MM patients, who experienced a partial response or stable disease after previous combination chemotherapy [310]. A significant humoral response to keyhole limpet hemocyanin, a marker for immune response, was detected in serum samples from all patients. Nine patients had successful lymphocyte activation according to increased levels of granzyme B expressing CD3 and CD8 T-cells. However, no correlation between the clinical responses and the humoral or cellular immune responses was observed [186].
Another small Phase I/II study evaluated the feasibility, safety, immunogenicity, and clinical efficacy of consolidation treatment with autologous DC transfected with mRNA encoding the malignant mesothelioma-associated WT1 antigen [311]. Ten patients with unresectable MM and non-progressive disease after platinum/pemetrexed-based chemotherapy underwent leukapheresis: Isolating CD14+ monocytes to produce mature DC, followed by electroporation of WT1 mRNA and biweekly intradermal vaccinations [311].. DC vaccination was well tolerated; no systemic toxicity was recorded. The 6-, 12- and 18-month survival rates were 100%, 90%, and 75%, respectively. In vivo evidence of vaccine-elicited immunity to the DC vaccine administered was obtained in nine of the ten enrolled patients. The OS data suggest that adjuvant DC-based immunotherapy may provide a clinical benefit for patients with MM [311].
8. The future of thoracic malignancies
Despite the intensive research and development of several new targeted agents and immunotherapies, survival rates for lung cancer and mesothelioma patients remain dismal. More studies are still needed to identify the underlying genetic alterations and predispositions affecting clinical outcome. Early detection and treatment of these cancers may help dramatically in the improvement of patients’ survival. Over 60% of lung cancer patients are in fact diagnosed at late stages of the disease, where current treatment modalities are unlikely to be effective. The 5-year OS rate is less than 10 % in patients with advanced disease, and greater than 70 % in patients with stage 1 disease. Reliable biomarkers are greatly needed to predict sensitivity to each therapeutic modality in thoracic malignancies that could support optimal selection of treatment on individual patient basis as well as for early detection of lung cancer that could improve its prognosis.
Acknowledgments
Funding
This work was supported in part by The Doctors Cancer Foundation grant A118560, the American Cancer Society grant IRG-97-150-13, and The NIH/NCI U01 grant NIH Grants 5U01CA168878.
Abbreviations
- SCLC
Small-Cell Lung Carcinomas
- NSCLC
Non-Small Cell Lung Carcinomas
- MM
Malignant Mesothelioma
- AdenoCA
Adenocarcinomas
- SQCLC
Squamous Cell Lung Cancers
- LCAC
Large Cell Anaplastic Carcinomas
- OS
Overall Survival
- PFS
Progression-Free Survival
- TTP
Time to Progression
- ORR
Objective Response Rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests:
None.
References
- 1.American-Cancer-Society. Cancer facts & figures 2014. Atlanta: American Cancer Society. 2014 [Google Scholar]
- 2.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31(8):992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 3.Rubin P, Hansen JT. Tnm staging atlas with oncoanatomy. Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 4.Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, Payne D, Kostashuk EC, Evans WK, Dixon P, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The national cancer institute of canada clinical trials group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11(2):336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Giroux DJ, Chansky K, Crowley J, Asamura H, Brambilla E, Jett J, Kennedy C, Rami-Porta R, Rusch VW, Goldstraw P. The iaslc lung cancer staging project: Proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the tnm classification for lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3(11):1213–1223. doi: 10.1097/JTO.0b013e31818b06e3. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho L, Cardoso E, Nunes H, Baptista V, Gomes A, Couceiro P. the iaslc lung cancer staging project. Comparing the current 6(th) tnm edition with the proposed 7(th) edition. Revista portuguesa de pneumologia. 2009;15(1):67–76. doi: 10.1016/s0873-2159(15)30110-0. [DOI] [PubMed] [Google Scholar]
- 7.Tsim S, O'Dowd CA, Milroy R, Davidson S. Staging of non-small cell lung cancer (nsclc): A review. Respir Med. 2010;104(12):1767–1774. doi: 10.1016/j.rmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 9.Pass HI, Carbone DP, Johnson dH, Minna JD. Principles & practice of lung cancer: The official reference text of the iaslc. Wolters Kluwere: Lippincott williams and Wilkins; 2010. [Google Scholar]
- 10.Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nature communications. 2014;5(4846) doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MD, Niu B, McLellan MD, Uzunangelov V, Zhang J, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158(4):929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shtivelman E, Hensing T, Simon GR, Dennis PA, Otterson GA, Bueno R, Salgia R. Molecular pathways and therapeutic targets in lung cancer. Oncotarget. 2014;5(6):1392–1433. doi: 10.18632/oncotarget.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, et al. Egf receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, et al. Egfr mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science (New York, NY. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 17.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging C, Participating I. The iaslc lung cancer staging project: Proposals for the revision of the tnm stage groupings in the forthcoming (seventh) edition of the tnm classification of malignant tumours. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 18.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, et al. DNA repair by ercc1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. The New England journal of medicine. 2006;355(10):983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 19.Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, Cardenal F, Sanchez JM, Gumerlock PH, Taron M, Sanchez JJ, et al. Low ercc1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(7):2286–2291. [PubMed] [Google Scholar]
- 20.Zhang Y, Li X, Chen Z, Bepler G. Ubiquitination and degradation of ribonucleotide reductase m1 by the polycomb group proteins rnf2 and bmi1 and cellular response to gemcitabine. PLoS One. 2014;9(3):e91186. doi: 10.1371/journal.pone.0091186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds C, Obasaju C, Schell MJ, Li X, Zheng Z, Boulware D, Caton JR, Demarco LC, O'Rourke MA, Shaw Wright G, Boehm KA, et al. Randomized phase iii trial of gemcitabine-based chemotherapy with in situ rrm1 and ercc1 protein levels for response prediction in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(34):5808–5815. doi: 10.1200/JCO.2009.21.9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez MA, Cerqueira NM, Fernandes PA, Ramos MJ. Ribonucleotide reductase: A mechanistic portrait of substrate analogues inhibitors. Curr Med Chem. 2010;17(26):2854–2872. doi: 10.2174/092986710792065054. [DOI] [PubMed] [Google Scholar]
- 23.Nakahira S, Nakamori S, Tsujie M, Takahashi Y, Okami J, Yoshioka S, Yamasaki M, Marubashi S, Takemasa I, Miyamoto A, Takeda Y, et al. Involvement of ribonucleotide reductase m1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. International journal of cancer Journal international du cancer. 2007;120(6):1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 24.Mao SS, Holler TP, Yu GX, Bollinger JM, Jr, Booker S, Johnston MI, Stubbe J. A model for the role of multiple cysteine residues involved in ribonucleotide reduction: Amazing and still confusing. Biochemistry. 1992;31(40):9733–9743. doi: 10.1021/bi00155a029. [DOI] [PubMed] [Google Scholar]
- 25.Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64(11):3761–3766. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Zhou J, Zhang Y, Bepler G. Modulation of the ribonucleotide reductase m1-gemcitabine interaction in vivo by n-ethylmaleimide. Biochem Biophys Res Commun. 2011;413(2):383–388. doi: 10.1016/j.bbrc.2011.08.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman AM, Eijk PP, Ruiz van Haperen VW, Smid K, Veerman G, Hubeek I, van den Ijssel P, Ylstra B, Peters GJ. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit m1 as the major determinant. Cancer Res. 2005;65(20):9510–9516. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 28.Bepler G, Kusmartseva I, Sharma S, Gautam A, Cantor A, Sharma A, Simon G. Rrm1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(29):4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 29.Tessema M, Yingling C, Thomaa C. Sulf2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-i inhibitors via induction of isg15. Oncogene. 2011;12(1 – 10) doi: 10.1038/onc.2011.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen SD, Lemjabbar-Alaoui H. Sulf-2: An extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010;14(9):935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. The Journal of biological chemistry. 2002;277(51):49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemjabbar-Alaoui H, van Zante A, Singer MS, Xue Q, Wang YQ, Tsay D, He B, Jablons DM, Rosen SD. Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene. 2010;29(5):635–646. doi: 10.1038/onc.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, Sharifnia T, Chande A, Tanaka KE, Stransky N, Greulich H, Gray NS, et al. Inhibitor-sensitive fgfr1 amplification in human non-small cell lung cancer. PLoS One. 2011;6(6):e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated egfr. The New England journal of medicine. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 36.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 37.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, Choi HG, Kim J, Chiang D, Thomas R, Lee J, et al. Eml4-alk fusion gene and efficacy of an alk kinase inhibitor in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(13):4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosse YP, Wood A, Maris JM. Inhibition of alk signaling for cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(18):5609–5614. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of eml4-alk non-small cell lung cancer. Eur J Cancer. 2010;46(10):1773–1780. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M University of Hong Kong Lung Cancer Study G. The eml4-alk fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type egfr and kras. Cancer. 2009;115(8):1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 41.Kulig K, Wang Y, Iyer S, Yang P. Predictive and prognostic value of alk gene rearrangement in non-small cell lung cancer. Epidemiol. 2014;4:146. [Google Scholar]
- 42.Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, Wampfler J, Jatoi A, Deschamps C, Marks R, Fortner C, Stoddard S, et al. Worse disease-free survival in never-smokers with alk+ lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7(1):90–97. doi: 10.1097/JTO.0b013e31823c5c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JK, Park HS, Kim DW, Kulig K, Kim TM, Lee SH, Jeon YK, Chung DH, Heo DS, Kim WH, Bang YJ. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer. 2012;118(14):3579–3586. doi: 10.1002/cncr.26668. [DOI] [PubMed] [Google Scholar]
- 44.Kim HR, Shim HS, Chung JH, Lee YJ, Hong YK, Rha SY, Kim SH, Ha SJ, Kim SK, Chung KY, Soo R, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor egfr or kras mutations or alk rearrangement. Cancer. 2012;118(3):729–739. doi: 10.1002/cncr.26311. [DOI] [PubMed] [Google Scholar]
- 45.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S, McDermott U, Settleman J, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor eml4-alk. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(26):4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 48.Sautes-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, Dieu-Nosjean MC. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30(1):13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 49.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 50.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, et al. Effector memory t cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 51.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72(13):3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated cd8+ t cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175(7):4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bronte V. Myeloid-derived suppressor cells in inflammation: Uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39(10):2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 54.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit t-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, et al. Population alterations of l-arginase- and inducible nitric oxide synthase-expressed cd11b+/cd14(−)/cd15+/cd33+ myeloid-derived suppressor cells and cd8+ t lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, Rusch VW, Travis WD, Adusumilli PS. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17(16):5247–5256. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 58.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within nsclc tumour islets are predominantly of a cytotoxic m1 phenotype associated with extended survival. Eur Respir J. 2009;33(1):118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 59.Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23(35):8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 60.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 61.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: A dangerous liaison. Immunol Invest. 2006;35(3–4):459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 63.Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, Pak PS, Elashoff D, Reckamp K, Zhang L, Fishbein MC, et al. Pge(2) contributes to tgf-beta induced t regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2(4):356–367. [PMC free article] [PubMed] [Google Scholar]
- 64.De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, Catalano G. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. 2000;117(2):365–373. doi: 10.1378/chest.117.2.365. [DOI] [PubMed] [Google Scholar]
- 65.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 66.De Pas T, Giovannini M, Rescigno M, Catania C, Toffalorio F, Spitaleri G, Delmonte A, Barberis M, Spaggiari L, Solli P, Veronesi G, et al. Vaccines in non-small cell lung cancer: Rationale, combination strategies and update on clinical trials. Critical reviews in oncology/hematology. 2012;83(3):432–443. doi: 10.1016/j.critrevonc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20(3):156–163. doi: 10.1016/j.semradonc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 69.Olden K, White SL. Health-related disparities: Influence of environmental factors. The Medical clinics of North America. 2005;89(4):721–738. doi: 10.1016/j.mcna.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Brawley OW. Lung cancer and race: Equal treatment yields equal outcome among equal patients, but there is no equal treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(3):332–333. doi: 10.1200/JCO.2005.03.7077. [DOI] [PubMed] [Google Scholar]
- 71.Zhou W, Christiani DC. East meets west: Ethnic differences in epidemiology and clinical behaviors of lung cancer between east asians and caucasians. Chin J Cancer. 2011;30(5):287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage i and ii non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 73.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. The New England journal of medicine. 1999;341(16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 74.da Cunha Santos G, Shepherd FA, Tsao MS. Egfr mutations and lung cancer. Annu Rev Pathol. 2011;6(49–69) doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 75.Sangha R, Price J, Butts CA. Adjuvant therapy in non-small cell lung cancer: Current and future directions. Oncologist. 2010;15(8):862–872. doi: 10.1634/theoncologist.2009-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanoue LT, Detterbeck FC. New tnm classification for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9(4):413–423. doi: 10.1586/era.09.11. [DOI] [PubMed] [Google Scholar]
- 77.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the lace collaborative group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 78.Group NM-aC, Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, Souhami RL, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet. 2010;375(9722):1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, et al. Vinorelbine plus cisplatin vs. Observation in resected non-small-cell lung cancer. The New England journal of medicine. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 80.Scagliotti GV, Fossati R, Torri V, Crino L, Giaccone G, Silvano G, Martelli M, Clerici M, Cognetti F, Tonato M Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer-Lung Cancer Cooperative Group I. Randomized study of adjuvant chemotherapy for completely resected stage i, ii, or iiia non-small-cell lung cancer. J Natl Cancer Inst. 2003;95(19):1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 81.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: Reappraisal with a meta-analysis of randomized controlled trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(19):3860–3867. doi: 10.1200/JCO.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 82.Edell ES, Cortese DA. Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest. 1992;102(5):1319–1322. doi: 10.1378/chest.102.5.1319. [DOI] [PubMed] [Google Scholar]
- 83.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J International Adjuvant Lung Cancer Trial Collaborative G. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. The New England journal of medicine. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 84.Group PM-aT. Postoperative radiotherapy for non-small cell lung cancer. The Cochrane database of systematic reviews. 2005;(2):CD002142. doi: 10.1002/14651858.CD002142.pub2. [DOI] [PubMed] [Google Scholar]
- 85.Gilligan D, Nicolson M, Smith I, Groen H, Dalesio O, Goldstraw P, Hatton M, Hopwood P, Manegold C, Schramel F, Smit H, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the mrc lu22/nvalt 2/eortc 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369(9577):1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 86.Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small cell lung cancer collaborative group. Bmj. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 87.Auperin A, Le Pechoux C, Pignon JP, Koning C, Jeremic B, Clamon G, Einhorn L, Ball D, Trovo MG, Groen HJ, Bonner JA, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (nsclc): A meta-analysis of individual data from 1764 patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2006;17(3):473–483. doi: 10.1093/annonc/mdj117. [DOI] [PubMed] [Google Scholar]
- 88.Rowell NP, O'Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. The Cochrane database of systematic reviews. 2004;(4):CD002140. doi: 10.1002/14651858.CD002140.pub2. [DOI] [PubMed] [Google Scholar]
- 89.Doddoli C, D'Journo B, Le Pimpec-Barthes F, Dujon A, Foucault C, Thomas P, Riquet M. Lung cancer invading the chest wall: A plea for en-bloc resection but the need for new treatment strategies. The Annals of thoracic surgery. 2005;80(6):2032–2040. doi: 10.1016/j.athoracsur.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 90.Facciolo F, Cardillo G, Lopergolo M, Pallone G, Sera F, Martelli M. Chest wall invasion in non-small cell lung carcinoma: A rationale for en bloc resection. The Journal of thoracic and cardiovascular surgery. 2001;121(4):649–656. doi: 10.1067/mtc.2001.112826. [DOI] [PubMed] [Google Scholar]
- 91.Matsuoka H, Nishio W, Okada M, Sakamoto T, Yoshimura M, Tsubota N. Resection of chest wall invasion in patients with non-small cell lung cancer. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;26(6):1200–1204. doi: 10.1016/j.ejcts.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 92.Wisnivesky JP, Yankelevitz D, Henschke CI. Stage of lung cancer in relation to its size: Part 2. Evidence. Chest. 2005;127(4):1136–1139. doi: 10.1378/chest.127.4.1136. [DOI] [PubMed] [Google Scholar]
- 93.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 94.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. American journal of clinical oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 95.Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, Paschold E, Iannotti NO, Dakhil S, Gorton S, Pautret V, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: Results of the randomized multicenter phase iii trial bms099. J Clin Oncol. 2010;28(6):911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 96.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced egfr mutation-positive non-small-cell lung cancer (optimal, ctong-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 97.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 98.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced egfr mutation-positive non-small-cell lung cancer (eurtac): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 99.Gerber DE, Schiller JH. Maintenance chemotherapy for advanced non-small-cell lung cancer: New life for an old idea. J Clin Oncol. 2013;31(8):1009–1020. doi: 10.1200/JCO.2012.43.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Plessen C, Bergman B, Andresen O, Bremnes RM, Sundstrom S, Gilleryd M, Stephens R, Vilsvik J, Aasebo U, Sorenson S. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer. 2006;95(8):966–973. doi: 10.1038/sj.bjc.6603383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, Unger P, Lee J, Lee JH, Tynan M, Moore M, et al. Phase iii trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage iiib/iv non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(5):1335–1343. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]
- 102.Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (paramount): A double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 103.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 104.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, Esteban E, Molinier O, Brugger W, Melezinek I, Klingelschmitt G, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 105.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, Trunzer K, et al. Prospective molecular marker analyses of egfr and kras from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(31):4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 106.Bria E, Cuppone F, Ciccarese M, Nistico C, Facciolo F, Milella M, Izzo F, Terzoli E, Cognetti F, Giannarelli D. Weekly docetaxel as second line chemotherapy for advanced non-small-cell lung cancer: Meta-analysis of randomized trials. Cancer treatment reviews. 2006;32(8):583–587. doi: 10.1016/j.ctrv.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(10):2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 108.Di Maio M, Perrone F, Chiodini P, Gallo C, Camps C, Schuette W, Quoix E, Tsai CM, Gridelli C. Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(11):1377–1382. doi: 10.1200/JCO.2006.09.8251. [DOI] [PubMed] [Google Scholar]
- 109.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, et al. Randomized phase iii trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The tax 320 non-small cell lung cancer study group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(12):2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 110.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(9):1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 111.Weiss GJ, Langer C, Rosell R, Hanna N, Shepherd F, Einhorn LH, Nguyen B, Paul S, McAndrews P, Bunn PA, Jr, Kelly K. Elderly patients benefit from second-line cytotoxic chemotherapy: A subset analysis of a randomized phase iii trial of pemetrexed compared with docetaxel in patients with previously treated advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(27):4405–4411. doi: 10.1200/JCO.2006.06.7835. [DOI] [PubMed] [Google Scholar]
- 112.Peterson P, Park K, Fossella F, Gatzemeier U, John W, Scagliotti G. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase iii trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (nsclc): P2-328. Journal of Thoracic Oncology. 2007;2(8) [Google Scholar]
- 113.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, et al. Erlotinib in previously treated non-small-cell lung cancer. The New England journal of medicine. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 114.Sundstrom S, Bremnes R, Aasebo U, Aamdal S, Hatlevoll R, Brunsvig P, Johannessen DC, Klepp O, Fayers PM, Kaasa S. Hypofractionated palliative radiotherapy (17 gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: A national phase iii trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(5):801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 115.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (interest): A randomised phase iii trial. Lancet. 2008;372(9652):1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 116.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, Salgia R, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring alk gene rearrangement: A retrospective analysis. Lancet Oncol. 2011;12(11):1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arbit E, Wronski M, Burt M, Galicich JH. The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer. 1995;76(5):765–773. doi: 10.1002/1097-0142(19950901)76:5<765::aid-cncr2820760509>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 119.Miller JI, Jr, Phillips TW. Neodymium:Yag laser and brachytherapy in the management of inoperable bronchogenic carcinoma. The Annals of thoracic surgery. 1990;50(2):190–195. doi: 10.1016/0003-4975(90)90731-k. discussion 195–196. [DOI] [PubMed] [Google Scholar]
- 120.Loeffler JS, Kooy HM, Wen PY, Fine HA, Cheng CW, Mannarino EG, Tsai JS, Alexander E., 3rd The treatment of recurrent brain metastases with stereotactic radiosurgery. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990;8(4):576–582. doi: 10.1200/JCO.1990.8.4.576. [DOI] [PubMed] [Google Scholar]
- 121.Alexander E, 3rd, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, Kooy HM, Loeffler JS. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87(1):34–40. doi: 10.1093/jnci/87.1.34. [DOI] [PubMed] [Google Scholar]
- 122.Souquet PJ, Chauvin F, Boissel JP, Cellerino R, Cormier Y, Ganz PA, Kaasa S, Pater JL, Quoix E, Rapp E, et al. Polychemotherapy in advanced non small cell lung cancer: A meta-analysis. Lancet. 1993;342(8862):19–21. doi: 10.1016/0140-6736(93)91882-m. [DOI] [PubMed] [Google Scholar]
- 123.Ellis PA, Smith IE, Hardy JR, Nicolson MC, Talbot DC, Ashley SE, Priest K. Symptom relief with mvp (mitomycin c, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. Br J Cancer. 1995;71(2):366–370. doi: 10.1038/bjc.1995.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bleehen NM, Girling DJ, Machin D, Stephens RJ. A randomised trial of three or six courses of etoposide cyclophosphamide methotrexate and vincristine or six courses of etoposide and ifosfamide in small cell lung cancer (sclc). Ii: Quality of life. Medical research council lung cancer working party. Br J Cancer. 1993;68(6):1157–1166. doi: 10.1038/bjc.1993.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, Simms L, Shepherd FA. The differential efficacy of pemetrexed according to nsclc histology: A review of two phase iii studies. Oncologist. 2009;14(3):253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 126.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (iressa survival evaluation in lung cancer) Lancet. 2005;366(9496):1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 127.Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, Ayoub J, Lago S, de Albuquerque Ribeiro R, Gerogianni A, Cyjon A, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the national cancer institute of canada clinical trials group study br.21. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(24):3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 128.Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK, Klughammer B, Gonzalez EE. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (titan): A randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13(3):300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 129.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, et al. Crizotinib versus chemotherapy in advanced alk-positive lung cancer. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 130.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ, Heasley LE, Franklin WA, Varella-Garcia M, et al. Mechanisms of resistance to crizotinib in patients with alk gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, Yatabe Y, Takeuchi K, Hamada T, Haruta H, Ishikawa Y, et al. Eml4-alk mutations in lung cancer that confer resistance to alk inhibitors. The New England journal of medicine. 2010;363(18):1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 132.La Madrid AM, Campbell N, Smith S, Cohn SL, Salgia R. Targeting alk: A promising strategy for the treatment of non-small cell lung cancer, non-hodgkin's lymphoma, and neuroblastoma. Target Oncol. 2012;7(3):199–210. doi: 10.1007/s11523-012-0227-8. [DOI] [PubMed] [Google Scholar]
- 133.Salerno TA, Munro DD, Blundell PE, Chiu RC. Second primary bronchogenic carcinoma: Life-table analysis of surgical treatment. The Annals of thoracic surgery. 1979;27(1):3–6. doi: 10.1016/s0003-4975(10)62961-x. [DOI] [PubMed] [Google Scholar]
- 134.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. The New England journal of medicine. 1990;322(8):494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 135.Hamaji M, Allen MS, Cassivi SD, Deschamps C, Nichols FC, Wigle DA, Shen KR. Surgical treatment of metachronous second primary lung cancer after complete resection of non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2013;145(3):683–690. doi: 10.1016/j.jtcvs.2012.12.051. discussion 690-681. [DOI] [PubMed] [Google Scholar]
- 136.Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. Journal of neurooncology. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 137.Kelly K, Bunn PA., Jr Is it time to reevaluate our approach to the treatment of brain metastases in patients with non-small cell lung cancer? Lung Cancer. 1998;20(2):85–91. doi: 10.1016/s0169-5002(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 138.Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1(3):205–210. doi: 10.1016/s1556-0864(15)31569-0. [DOI] [PubMed] [Google Scholar]
- 139.Cox JD, Yesner RA. Adenocarcinoma of the lung: Recent results from the veterans administration lung group. The American review of respiratory disease. 1979;120(5):1025–1029. doi: 10.1164/arrd.1979.120.5.1025. [DOI] [PubMed] [Google Scholar]
- 140.Chang DB, Yang PC, Luh KT, Kuo SH, Hong RL, Lee LN. Late survival of non-small cell lung cancer patients with brain metastases. Influence of treatment. Chest. 1992;101(5):1293–1297. doi: 10.1378/chest.101.5.1293. [DOI] [PubMed] [Google Scholar]
- 141.Richards P, Mc KW. Intracranial metastases. Br Med J. 1963;1(5322):15–18. doi: 10.1136/bmj.1.5322.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lang EF, Slater J. Metastatic brain tumors. Results of surgical and nonsurgical treatment. The Surgical clinics of North America. 1964;44(865–872) doi: 10.1016/s0039-6109(16)37308-x. [DOI] [PubMed] [Google Scholar]
- 143.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 144.Nieder C, Berberich W, Schnabel K. Tumor-related prognostic factors for remission of brain metastases after radiotherapy. International journal of radiation oncology, biology, physics. 1997;39(1):25–30. doi: 10.1016/s0360-3016(97)00154-5. [DOI] [PubMed] [Google Scholar]
- 145.Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. International journal of radiation oncology, biology, physics. 1994;29(4):711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 146.Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: Review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56(5):1021–1034. discussion 1021–1034. [PubMed] [Google Scholar]
- 147.Stark AM, Tscheslog H, Buhl R, Held-Feindt J, Mehdorn HM. Surgical treatment for brain metastases: Prognostic factors and survival in 177 patients. Neurosurgical review. 2005;28(2):115–119. doi: 10.1007/s10143-004-0364-3. [DOI] [PubMed] [Google Scholar]
- 148.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79(2):210–216. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 149.Hazuka MB, Kinzie JJ. Brain metastases: Results and effects of re-irradiation. International journal of radiation oncology, biology, physics. 1988;15(2):433–437. doi: 10.1016/s0360-3016(98)90026-8. [DOI] [PubMed] [Google Scholar]
- 150.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 151.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, et al. Therapeutic effects of ablative radiation on local tumor require cd8+ t cells: Changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 153.Suh JH. Stereotactic radiosurgery for the management of brain metastases. The New England journal of medicine. 2010;362(12):1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 154.Suh JH, Chao ST, Vogelbaum MA. Management of brain metastases. Current neurology and neuroscience reports. 2009;9(3):223–230. doi: 10.1007/s11910-009-0033-6. [DOI] [PubMed] [Google Scholar]
- 155.Zabel A, Milker-Zabel S, Thilmann C, Zuna I, Rhein B, Wannenmacher M, Debus J. Treatment of brain metastases in patients with non-small cell lung cancer (nsclc) by stereotactic linac-based radiosurgery: Prognostic factors. Lung Cancer. 2002;37(1):87–94. doi: 10.1016/s0169-5002(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 156.Kim YS, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for patients with nonsmall cell lung carcinoma metastatic to the brain. Cancer. 1997;80(11):2075–2083. doi: 10.1002/(sici)1097-0142(19971201)80:11<2075::aid-cncr6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 157.Williams J, Enger C, Wharam M, Tsai D, Brem H. Stereotactic radiosurgery for brain metastases: Comparison of lung carcinoma vs. Non-lung tumors. Journal of neuro-oncology. 1998;37(1):79–85. doi: 10.1023/a:1005958215384. [DOI] [PubMed] [Google Scholar]
- 158.Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: Long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97(6):1276–1281. doi: 10.3171/jns.2002.97.6.1276. [DOI] [PubMed] [Google Scholar]
- 159.Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: Outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247–254. doi: 10.3171/jns.2005.102.s_supplement.0247. [DOI] [PubMed] [Google Scholar]
- 160.Mariya Y, Sekizawa G, Matsuoka Y, Seki H, Sugawara T. Outcome of stereotactic radiosurgery for patients with non-small cell lung cancer metastatic to the brain. J Radiat Res. 2010;51(3):333–342. doi: 10.1269/jrr.90130. [DOI] [PubMed] [Google Scholar]
- 161.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase iii results of the rtog 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 162.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. International journal of radiation oncology, biology, physics. 1999;45(2):427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 163.Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers. 2010;2(4):2100–2137. doi: 10.3390/cancers2042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. Jama. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 165.Schuette W. Treatment of brain metastases from lung cancer: Chemotherapy. Lung Cancer. 2004;45(Suppl 2):S253–S257. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- 166.Komarnicky LT, Phillips TL, Martz K, Asbell S, Isaacson S, Urtasun R. A randomized phase iii protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (rtog-7916) International journal of radiation oncology, biology, physics. 1991;20(1):53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 167.Phillips TL, Scott CB, Leibel SA, Rotman M, Weigensberg IJ. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: Report of rtog trial 89-05. International journal of radiation oncology, biology, physics. 1995;33(2):339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 168.Ushio Y, Arita N, Hayakawa T, Mogami H, Hasegawa H, Bitoh S, Oku Y, Ikeda H, Kanai N, Kanoh M, et al. Chemotherapy of brain metastases from lung carcinoma: A controlled randomized study. Neurosurgery. 1991;28(2):201–205. doi: 10.1097/00006123-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 169.Robinet G, Thomas P, Breton JL, Lena H, Gouva S, Dabouis G, Bennouna J, Souquet PJ, Balmes P, Thiberville L, Fournel P, et al. Results of a phase iii study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe francais de pneumo-cancerologie (gfpc) protocol 95-1. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2001;12(1):59–67. doi: 10.1023/a:1008338312647. [DOI] [PubMed] [Google Scholar]
- 170.Neuhaus T, Ko Y, Muller RP, Grabenbauer GG, Hedde JP, Schueller H, Kocher M, Stier S, Fietkau R. A phase iii trial of topotecan and whole brain radiation therapy for patients with cns-metastases due to lung cancer. Br J Cancer. 2009;100(2):291–297. doi: 10.1038/sj.bjc.6604835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guerrieri M, Wong K, Ryan G, Millward M, Quong G, Ball DL. A randomised phase iii study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer. 2004;46(1):107–111. doi: 10.1016/j.lungcan.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 172.Mehta MP, Shapiro WR, Phan SC, Gervais R, Carrie C, Chabot P, Patchell RA, Glantz MJ, Recht L, Langer C, Sur RK, et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: Results of a phase iii trial. International journal of radiation oncology, biology, physics. 2009;73(4):1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 173.Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, Senzer N, Chang EL, Boyd AP, Cagnoni PJ, Shaw E. Phase iii study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(1):106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 174.Verger E, Gil M, Yaya R, Vinolas N, Villa S, Pujol T, Quinto L, Graus F. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: A phase ii randomized trial. International journal of radiation oncology, biology, physics. 2005;61(1):185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 175.Antonadou D, Paraskevaidis M, Sarris G, Coliarakis N, Economou I, Karageorgis P, Throuvalas N. Phase ii randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(17):3644–3650. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 176.Ellis PM, Al-Saleh K. Multitargeted anti-angiogenic agents and nsclc: Clinical update and future directions. Crit Rev Oncol Hematol. 2012;84(1):47–58. doi: 10.1016/j.critrevonc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 177.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 178.Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, Wang LI, Johnson DH. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J Clin Oncol. 2009;27(9):1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sandler A, Yi J, Dahlberg S, Kolb MM, Wang L, Hambleton J, Schiller J, Johnson DH. Treatment outcomes by tumor histology in eastern cooperative group study e4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1416–1423. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 180.Socinski MA, Scappaticci FA, Samant M, Kolb MM, Kozloff MF. Safety and efficacy of combining sunitinib with bevacizumab + paclitaxel/carboplatin in non-small cell lung cancer. J Thorac Oncol. 2010;5(3):354–360. doi: 10.1097/JTO.0b013e3181c7307e. [DOI] [PubMed] [Google Scholar]
- 181.Groen HJMSM, Grossi F, et al. Randomized phase ii study of sunitinib (su) plus erlotinib (e) for the treatment of advanced/metastatic nonsmall cell lung cancer (nsclc) Annals of Oncology. 2010;5(354–360) [Google Scholar]
- 182.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, et al. Bibf 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 183.Reck M, Kaiser R, Eschbach C, Stefanic M, Love J, Gatzemeier U, Stopfer P, von Pawel J. A phase ii double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor bibf 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol. 2011;22(6):1374–1381. doi: 10.1093/annonc/mdq618. [DOI] [PubMed] [Google Scholar]
- 184.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 185.Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, Giovarelli M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008;22(8):2747–2757. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- 186.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13(7):e301–e310. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Varadhachary A, Wolf JS, Petrak K, O'Malley BW, Jr, Spadaro M, Curcio C, Forni G, Pericle F. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer. 2004;111(3):398–403. doi: 10.1002/ijc.20271. [DOI] [PubMed] [Google Scholar]
- 188.Digumarti R, Wang Y, Raman G, Doval DC, Advani SH, Julka PK, Parikh PM, Patil S, Nag S, Madhavan J, Bapna A, et al. A randomized, double-blind, placebo-controlled, phase ii study of oral talactoferrin in combination with carboplatin and paclitaxel in previously untreated locally advanced or metastatic non-small cell lung cancer. J Thorac Oncol. 2011;6(6):1098–1103. doi: 10.1097/JTO.0b013e3182156250. [DOI] [PubMed] [Google Scholar]
- 189.Maki RG. Small is beautiful: Insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28(33):4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Capelletto E, Novello S. Emerging new agents for the management of patients with non-small cell lung cancer. Drugs. 2012;72(Suppl 1):37–52. doi: 10.2165/1163028-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 191.Vincent AM, Feldman EL. Control of cell survival by igf signaling pathways. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2002;12(4):193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 192.Neal JW, Sequist LV. Exciting new targets in lung cancer therapy: Alk, igf-1r, hdac, and hh. Curr Treat Options Oncol. 2010;11(1–2):36–44. doi: 10.1007/s11864-010-0120-6. [DOI] [PubMed] [Google Scholar]
- 193.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 194.Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67(3):257–274. doi: 10.1016/j.lungcan.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 195.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ, Blumenschein G, Jr, Johnson FM, et al. Phase ii study of the anti-insulin-like growth factor type 1 receptor antibody cp-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27(15):2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 196.Jassem JLC, Karp DD, et al. Randomized, open label, phase iii trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (nsclc) J Clin Oncol. 2010;28(7500) doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pfizer discontinues a phase 3 study of figitumumab in previously treated patients with advanced non-small cell lung cancer [media release] 2010 [Google Scholar]
- 198.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 199.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 200.Schrump DS. Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: Mechanisms and potential clinical implications. Clin Cancer Res. 2009;15(12):3947–3957. doi: 10.1158/1078-0432.CCR-08-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA. Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer. 2010;126(3):743–755. doi: 10.1002/ijc.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ramalingam SS, Maitland ML, Frankel P, Argiris AE, Koczywas M, Gitlitz B, Thomas S, Espinoza-Delgado I, Vokes EE, Gandara DR, Belani CP. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(1):56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Belani CRS, Kalemkerian G, et al. Randomised, double-blind, phase ii-iii study of first line paclitaxel (p) plus carboplatin (c) in combination with vorinostat or placebo in patients with advanced non-small cell lung cancer (nsclc) Eur J Cancer. 2009;(Suppl. 7)(2):507. abst 500–9007. [Google Scholar]
- 204.Zhang W, Peyton M, Xie Y, Soh J, Minna JD, Gazdar AF, Frenkel EP. Histone deacetylase inhibitor romidepsin enhances anti-tumor effect of erlotinib in non-small cell lung cancer (nsclc) cell lines. J Thorac Oncol. 2009;4(2):161–166. doi: 10.1097/JTO.0b013e318194fae7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Haura EB, Cress WD, Chellappan S, Zheng Z, Bepler G. Antiapoptotic signaling pathways in non-small-cell lung cancer: Biology and therapeutic strategies. Clin Lung Cancer. 2004;6(2):113–122. doi: 10.3816/CLC.2004.n.025. [DOI] [PubMed] [Google Scholar]
- 206.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, et al. Phase i pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25(11):1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 207.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the trail receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61(1):82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 208.von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL, Camidge DR. Phase ii trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (trail-r1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Clinical lung cancer. 2014;15(3):188–196 e182. doi: 10.1016/j.cllc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 209.Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, Schwall R, et al. Cooperation of the agonistic dr5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(23):7733–7740. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- 210.Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E, Spencer S, Carter CA, Guha U, Khozin S, Poondru S, et al. A phase i/ii study of sepantronium bromide (ym155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(10):2601–2606. doi: 10.1093/annonc/mdt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paz-Ares L, Balint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, Galimi F, Haddad V, Sabin T, Hei YJ, Pan Y, et al. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8(3):329–337. doi: 10.1097/JTO.0b013e31827ce554. [DOI] [PubMed] [Google Scholar]
- 212.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by cd28 and ctla-4. Nat Rev Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 213.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage iiib/iv non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase ii study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 214.Zatloukal PHD, Park K, et al. Randomized phase ii clinical trial comparing tremelimumab (cp-675,206) with best-supportive care (bsc) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (nsclc) J Clin Oncol. 2009;27 (abstract 8071). [Google Scholar]
- 215.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of pd-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, et al. Engagement of the pd-1 immunoinhibitory receptor by a novel b7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of pd-l1 mediates peripheral t cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in pd-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 219.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the pd-1 gene encoding an itim motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 220.Okazaki T, Honjo T. Pd-1 and pd-1 ligands: From discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 221.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(677–704) doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, et al. Phase i study of single-agent anti-programmed death-1 (mdx-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, et al. Tumor-associated b7-h1 promotes t-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 224.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. Pd-l1/b7h-1 inhibits the effector phase of tumor rejection by t cell receptor (tcr) transgenic cd8+ t cells. Cancer Res. 2004;64(3):1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 225.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29(36):4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in nsclc: Latest evidence and clinical potential. Therapeutic advances in medical oncology. 2015;7(2):85–96. doi: 10.1177/1758834014567470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Spigel DR, Reckamp KL, Rizvi NA, et al. A phase iii study (checkmate 017) of nivolumab (nivo; anti-programmed death-1 [pd-1]) vs docetaxel (doc) in previously treated advanced or metastatic squamous (sq) cell non-small cell lung cancer (nsclc) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(suppl) abstr 8009. [Google Scholar]
- 230.Paz-Ares LG, Horn L, Borghaei H, et al. Phase iii, randomized trial (checkmate 057) of nivolumab (nivo) versus docetaxel (doc) in advanced non-squamous cell (non-sq) non-small cell lung cancer (nsclc) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(suppl) abstr LBA109. [Google Scholar]
- 231.D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E, Chella A, et al. Pd-1 and pd-l1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, et al. Activation of the pd-1 pathway contributes to immune escape in egfr-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin C, Chen X, Li M, Liu J, Qi X, Yang W, Zhang H, Cai Z, Dai Y, Ouyang X. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015 doi: 10.1016/j.cllc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 234.Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that pd1 blockade cooperates with cancer vaccine tegvax to elicit regression of established tumors. Cancer Res. 2014;74(15):4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee SM, Chow LQ. A new addition to the pd-1 checkpoint inhibitors for non-small cell lung cancer-the anti-pdl1 antibody-medi4736. Translational lung cancer research. 2014;3(6):408–410. doi: 10.3978/j.issn.2218-6751.2014.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, et al. Cancer immunology. Mutational landscape determines sensitivity to pd-1 blockade in non-small cell lung cancer. Science (New York, NY. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, Alvarez FB, Bautista OM, Jansen KU, Barr E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: A randomized controlled trial. Obstet Gynecol. 2006;107(1):18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 238.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987;47(20):5476–5482. [PubMed] [Google Scholar]
- 240.Sangha R, Butts C. L-blp25: A peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13(15 Pt 2):s4652–s4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 241.Butts C, Maksymiuk A, Goss G, Soulieres D, Marshall E, Cormier Y, Ellis PM, Price A, Sawhney R, Beier F, Falk M, et al. Updated survival analysis in patients with stage iiib or iv non-small-cell lung cancer receiving blp25 liposome vaccine (l-blp25): Phase iib randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137(9):1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, Krzakowski M, Hess D, Tartour E, Chenard MP, Limacher JM, et al. A phase ii study of tg4010 (mva-muc1-il2) in association with chemotherapy in patients with stage iii/iv non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3(7):735–744. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 243.Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: A review of novel immunotherapy trials for the treatment of lung cancer. Cancer control : journal of the Moffitt Cancer Center. 2013;20(1):22–31. doi: 10.1177/107327481302000105. [DOI] [PubMed] [Google Scholar]
- 244.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, Debieuvre D, et al. Therapeutic vaccination with tg4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2b trial. Lancet Oncol. 2011;12(12):1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 245.Gonzalez G, Crombet T, Catala M, Mirabal V, Hernandez JC, Gonzalez Y, Marinello P, Guillen G, Lage A. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: Report of a pilot clinical trial. Ann Oncol. 1998;9(4):431–435. doi: 10.1023/a:1008261031034. [DOI] [PubMed] [Google Scholar]
- 246.Rodriguez PC, Rodriguez G, Gonzalez G, Lage A. Clinical development and perspectives of cimavax egf, cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev. 2010;12(1):17–23. doi: 10.37757/MR2010.V12.N1.4. [DOI] [PubMed] [Google Scholar]
- 247.Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupale IL, Perez LM, Marinello GG, Rodriguez RP, Davila AL. Treatment of nsclc patients with an egf-based cancer vaccine: Report of a phase i trial. Cancer Biol Ther. 2006;5(2):145–149. doi: 10.4161/cbt.5.2.2334. [DOI] [PubMed] [Google Scholar]
- 248.Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, Catala Ferrer M, Bravo I, Mendoza del Pino M, Abreu Abreu D, Acosta Brooks S, Rives R, del Castillo Carrillo C, Gonzalez Duenas M, et al. Phase ii randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–1458. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 249.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, Altorki NK. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 250.Sienel W, Varwerk C, Linder A, Kaiser D, Teschner M, Delire M, Stamatis G, Passlick B. Melanoma associated antigen (mage)-a3 expression in stages i and ii non-small cell lung cancer: Results of a multi-center study. Eur J Cardiothorac Surg. 2004;25(1):131–134. doi: 10.1016/j.ejcts.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 251.Vansteenkiste JZM, Linder A, et al. Phase ii randomized study of mage-a3 immunotherapy as adjuvant therapy in stage ib/ii non-small cell lung cancer (nsclc): 44 month follow-up, humoral and cellular immune response data. J Thorac Oncol. 2008;3:55–56. abstract 1480. [Google Scholar]
- 252.Vansteenkiste JZM, Dahabreh IJ, et al. Association of gene expression signature and clinical efficacy of mage-a3 antigen-specific cancer immunotheraputic (asci) as adjuvant therapy in resected stage ib/ii non-small cell lung cancer (nsclc) J Clin Oncol. 2008;26 (abstr 7501) [Google Scholar]
- 253.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: A potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 254.Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: Depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136(10):3916–3920. [PubMed] [Google Scholar]
- 255.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, et al. Phase ii study of belagenpumatucel-l, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 256.Bankovic J, Stojsic J, Jovanovic D, Andjelkovic T, Milinkovic V, Ruzdijic S, Tanic N. Identification of genes associated with non-small-cell lung cancer promotion and progression. Lung Cancer. 2010;67(2):151–159. doi: 10.1016/j.lungcan.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 257.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen prame is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122(6):835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 258.Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen prame is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004;10(13):4307–4313. doi: 10.1158/1078-0432.CCR-03-0813. [DOI] [PubMed] [Google Scholar]
- 259.Bogaerts J, Cardoso F, Buyse M, Braga S, Loi S, Harrison JA, Bines J, Mook S, Decker N, Ravdin P, Therasse P, et al. Gene signature evaluation as a prognostic tool: Challenges in the design of the mindact trial. Nat Clin Pract Oncol. 2006;3(10):540–551. doi: 10.1038/ncponc0591. [DOI] [PubMed] [Google Scholar]
- 260.Doolan P, Clynes M, Kennedy S, Mehta JP, Crown J, O'Driscoll L. Prevalence and prognostic and predictive relevance of prame in breast cancer. Breast Cancer Res Treat. 2008;109(2):359–365. doi: 10.1007/s10549-007-9643-3. [DOI] [PubMed] [Google Scholar]
- 261.Epping MT, Hart AA, Glas AM, Krijgsman O, Bernards R. Prame expression and clinical outcome of breast cancer. Br J Cancer. 2008;99(3):398–403. doi: 10.1038/sj.bjc.6604494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, Jose-Eneriz ES, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, et al. Epigenetic regulation of prame gene in chronic myeloid leukemia. Leuk Res. 2007;31(11):1521–1528. doi: 10.1016/j.leukres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 263.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the united states over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 264.Joshi M, Ayoola A, Belani CP. Small-cell lung cancer: An update on targeted therapies. Adv Exp Med Biol. 2013;779(385–404) doi: 10.1007/978-1-4614-6176-0_18. [DOI] [PubMed] [Google Scholar]
- 265.Johnson BE, Grayson J, Makuch RW, Linnoila RI, Anderson MJ, Cohen MH, Glatstein E, Minna JD, Ihde DC. Ten-year survival of patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990;8(3):396–401. doi: 10.1200/JCO.1990.8.3.396. [DOI] [PubMed] [Google Scholar]
- 266.Fry WA, Menck HR, Winchester DP. The national cancer data base report on lung cancer. Cancer. 1996;77(9):1947–1955. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 267.Lassen U, Osterlind K, Hansen M, Dombernowsky P, Bergman B, Hansen HH. Long-term survival in small-cell lung cancer: Posttreatment characteristics in patients surviving 5 to 18+ years--an analysis of 1,714 consecutive patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13(5):1215–1220. doi: 10.1200/JCO.1995.13.5.1215. [DOI] [PubMed] [Google Scholar]
- 268.Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. The New England journal of medicine. 1999;340(4):265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 269.Janne PA, Freidlin B, Saxman S, Johnson DH, Livingston RB, Shepherd FA, Johnson BE. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in north america. Cancer. 2002;95(7):1528–1538. doi: 10.1002/cncr.10841. [DOI] [PubMed] [Google Scholar]
- 270.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. The New England journal of medicine. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 271.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S. Prophylactic cranial irradiation in extensive small-cell lung cancer. The New England journal of medicine. 2007;357(7):664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 272.Videtic GM, Stitt LW, Dar AR, Kocha WI, Tomiak AT, Truong PT, Vincent MD, Yu EW. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(8):1544–1549. doi: 10.1200/JCO.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 273.Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. The New England journal of medicine. 1992;327(23):1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 274.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10(6):890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 275.Perry MC, Eaton WL, Propert KJ, Ware JH, Zimmer B, Chahinian AP, Skarin A, Carey RW, Kreisman H, Faulkner C, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. The New England journal of medicine. 1987;316(15):912–918. doi: 10.1056/NEJM198704093161504. [DOI] [PubMed] [Google Scholar]
- 276.Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, et al. Phase iii study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the japan clinical oncology group study 9104. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(14):3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 277.Ready NE, Dudek AZ, Pang HH, Hodgson LD, Graziano SL, Green MR, Vokes EE. Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: Calgb 30306, a phase ii study. J Clin Oncol. 2011;29(33):4436–4441. doi: 10.1200/JCO.2011.35.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Horn L, Dahlberg SE, Sandler AB, Dowlati A, Moore DF, Murren JR, Schiller JH. Phase ii study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern cooperative oncology group study e3501. J Clin Oncol. 2009;27(35):6006–6011. doi: 10.1200/JCO.2009.23.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gitlitz BJ, Moon J, Glisson BS, Reimers HJ, Bury MJ, Floyd JD, Schulz TK, Sundaram PK, Ho C, Gandara DR. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: A southwest oncology group (swog 0435) phase ii trial. J Thorac Oncol. 2010;5(11):1835–1840. doi: 10.1097/JTO.0b013e3181f0bd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tatematsu A, Shimizu J, Murakami Y, Horio Y, Nakamura S, Hida T, Mitsudomi T, Yatabe Y. Epidermal growth factor receptor mutations in small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(19):6092–6096. doi: 10.1158/1078-0432.CCR-08-0332. [DOI] [PubMed] [Google Scholar]
- 281.Moore AM, Einhorn LH, Estes D, Govindan R, Axelson J, Vinson J, Breen TE, Yu M, Hanna NH. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: A hoosier oncology group phase ii trial. Lung Cancer. 2006;52(1):93–97. doi: 10.1016/j.lungcan.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 282.Koster W, Heider A, Niederle N, Wilke H, Stamatis G, Fischer JR, Koch JA, Stahl M. Phase ii trial with carboplatin and bendamustine in patients with extensive stage small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007;2(4):312–316. doi: 10.1097/01.JTO.0000263714.46449.4c. [DOI] [PubMed] [Google Scholar]
- 283.Venepalli N, Carbine D, Johnson D, Keedy V, Pao W, Sandler A, Horn L. A phase ii study of second-line bendamustine in relapsed or refractory small cell lung cancer (sclc) J Clin Oncol 29: 2011 (suppl; abstr e17505) 2011 [Google Scholar]
- 284.Grant SC, Kris MG, Houghton AN, Chapman PB. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody bec2 plus bacillus calmette-guerin. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(6):1319–1323. [PubMed] [Google Scholar]
- 285.Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, Thiberville L, D'Addario G, Coens C, Rome LS, Zatloukal P, et al. Phase iii study of adjuvant vaccination with bec2/bacille calmette-guerin in responding patients with limited-disease small-cell lung cancer (european organisation for research and treatment of cancer 08971-08971b; silva study) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(28):6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 286.Chiappori AA, Soliman H, Janssen WE, Antonia SJ, Gabrilovich DI. Ingn-225: A dendritic cell-based p53 vaccine (ad.P53-dc) in small cell lung cancer: Observed association between immune response and enhanced chemotherapy effect. Expert opinion on biological therapy. 2010;10(6):983–991. doi: 10.1517/14712598.2010.484801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Davis MP. The emerging role of palliative medicine in the treatment of lung cancer patients. Cleveland Clinic journal of medicine. 2012;79(Electronic Suppl 1):eS51–eS55. doi: 10.3949/ccjm.79.s2.11. [DOI] [PubMed] [Google Scholar]
- 288.Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: A randomized trial. Support Care Cancer. 2010;18(8):951–956. doi: 10.1007/s00520-009-0739-7. [DOI] [PubMed] [Google Scholar]
- 289.Grondin SC, Sugarbaker DJ. Malignant mesothelioma of the pleural space. Oncology (Williston Park) 1999;13(7):919–926. discussion 926, 931-912. [PubMed] [Google Scholar]
- 290.Musk AW, de Klerk NH. Epidemiology of malignant mesothelioma in australia. Lung cancer. 2004;45(Suppl 1):S21–S23. doi: 10.1016/j.lungcan.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 291.Robinson BW, Lake RA. Advances in malignant mesothelioma. The New England journal of medicine. 2005;353(15):1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 292.Stahel RA, Weder W, Lievens Y, Felip E. Malignant pleural mesothelioma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v126–v128. doi: 10.1093/annonc/mdq173. [DOI] [PubMed] [Google Scholar]
- 293.Pass H, Vogelzang N, Hahn S, et al. Benign and malignant mesothelioma. B. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 8th ed. Philadelphia: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 294.Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma 2008. Current opinion in pulmonary medicine. 2008;14(4):303–309. doi: 10.1097/MCP.0b013e328302851d. [DOI] [PubMed] [Google Scholar]
- 295.Bridda A, Padoan I, Mencarelli R, Frego M. Peritoneal mesothelioma: A review. MedGenMed. 2007;9(2):32. [PMC free article] [PubMed] [Google Scholar]
- 296.Borasio P, Berruti A, Bille A, Lausi P, Levra MG, Giardino R, Ardissone F. Malignant pleural mesothelioma: Clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33(2):307–313. doi: 10.1016/j.ejcts.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 297.Sugarbaker DJ. Multimodality management of malignant pleural mesothelioma: Introduction. Semin Thorac Cardiovasc Surg. 2009;21(2):95–96. doi: 10.1053/j.semtcvs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 298.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, et al. Phase iii study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 299.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9(2–3):147–157. doi: 10.1007/s11864-008-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, et al. Discovery of a selective inhibitor of oncogenic b-raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Singer CF, Kostler WJ, Hudelist G. Predicting the efficacy of trastuzumab-based therapy in breast cancer: Current standards and future strategies. Biochim Biophys Acta. 2008;1786(2):105–113. doi: 10.1016/j.bbcan.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 302.Sanford M, Scott LJ. Gefitinib: A review of its use in the treatment of locally advanced/metastatic non-small cell lung cancer. Drugs. 2009;69(16):2303–2328. doi: 10.2165/10489100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 303.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, Kumar R, Rusnak DW, Takle AK, Wilson DM, Hugger E, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic braf by the kinase inhibitor sb-590885. Cancer Res. 2006;66(23):11100–11105. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 304.Hitoshi Y, Lin N, Payan DG, Markovtsov V. The current status and the future of jak2 inhibitors for the treatment of myeloproliferative diseases. Int J Hematol. 2010;91(2):189–200. doi: 10.1007/s12185-010-0531-y. [DOI] [PubMed] [Google Scholar]
- 305.Gustin JP, Cosgrove DP, Park BH. The pik3ca gene as a mutated target for cancer therapy. Curr Cancer Drug Targets. 2008;8(8):733–740. doi: 10.2174/156800908786733504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Giles FJ, O'Dwyer M, Swords R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2009;23(10):1698–1707. doi: 10.1038/leu.2009.111. [DOI] [PubMed] [Google Scholar]
- 307.Giles FJ, DeAngelo DJ, Baccarani M, Deininger M, Guilhot F, Hughes T, Mauro M, Radich J, Ottmann O, Cortes J. Optimizing outcomes for patients with advanced disease in chronic myelogenous leukemia. Semin Oncol. 2008;35(1) Suppl 1:S1–S17. doi: 10.1053/j.seminoncol.2007.12.002. quiz S18-20. [DOI] [PubMed] [Google Scholar]
- 308.Krug LM, Dao T, Brown AB, Maslak P, Travis W, Bekele S, Korontsvit T, Zakhaleva V, Wolchok J, Yuan J, Li H, et al. Wt1 peptide vaccinations induce cd4 and cd8 t cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer immunology, immunotherapy : CII. 2010;59(10):1467–1479. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Krug L, Tsao A, Kass S, Rusch V, Travis WD, Panageas K, Adusumili P, Kris M, Maslak P, Scheinberg D. Randomized, double-blinded, phase ii trial of a wt1 peptide vaccine as adjuvant therapy in patients with malignant pleural mesothelioma (mpm) J Clin Oncol 29: 2011 (suppl; abstr TPS139) 2011 [Google Scholar]
- 310.Hegmans JP, Veltman JD, Lambers ME, de Vries IJ, Figdor CG, Hendriks RW, Hoogsteden HC, Lambrecht BN, Aerts JG. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010;181(12):1383–1390. doi: 10.1164/rccm.200909-1465OC. [DOI] [PubMed] [Google Scholar]
- 311.Berneman Z, Germonpre P, Huizing M, Van de Velde A, Nijs G, Stein B, Van Tendeloo V, Lion E, Smits E, Anguille S. Dendritic cell vaccination in malignant pleural mesothelioma: A phase i/ii study. J Clin Oncol 32:5s, 2014 (suppl; abstr 7583) 2014 [Google Scholar]