Abstract
Background
Valid and precise measures of androgen concentrations are needed for etiologic studies of hormonally-related cancers. We developed a high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with two sample preparations to measure 11 androgens, including adrenal and gonadal androgenic precursors and their 5α-reduced metabolites.
Methods
Androgen levels were measured in serum from 20 healthy volunteers (5 men, 10 premenopausal women, 5 postmenopausal women). Two blinded, randomized aliquots per individual were assayed in each of three batches. A fourth batch of samples was measured at an external laboratory using comparable methodology to measure 9 of the 11 androgens. Coefficients of variation (CV) and intraclass correlation coefficients (ICC) were calculated from the individual components of variance. Comparability of 9 androgens across laboratories was assessed using Spearman ranked correlations, Deming regression and bias plots.
Results
The laboratory CVs were <5% and ICCs were uniformly high (>95%) for all androgens measured across sex/menopausal status groups. Spearman ranked correlations for 9 hormones measured in the comparison laboratory were high (>0.85), suggesting good agreement.
Conclusion
Our high-performance LC-MS/MS assays of 11 androgens, including adrenal and gonadal androgenic precursors and their 5α-reduced metabolites demonstrated excellent laboratory reproducibility, and good comparability with an established method that measured 9 of the 11 hormones tested. The serum androgen metabolite assays are suitable for use in epidemiologic research.
Keywords: reproducibility, serum androgen assay, liquid chromatography tandem mass spectrometry, metabolites, endogenous hormones
1. INTRODUCTION
Sex steroid hormones are hypothesized to play key roles in the development of many cancers (e.g. breast, endometrial, ovarian, prostate, and testicular). Androgens are hypothesized to be positively associated with prostate cancer and inversely associated with testicular cancer [1-3]. In the study of female tumors, attention has shifted to androgens in recent years as associations with estrogens have been increasingly well-delineated [4]. Prospective studies have linked circulating androgen levels to the risk of postmenopausal breast, endometrial, and ovarian cancers; however, these studies have often focused on a small number of androgens, typically testosterone and androstenedione, as existing assays have required large amounts of serum [5-8]. At present, it is unclear whether these androgens adequately capture underlying androgenic activity or reflect local tissue production of bioactive androgens [9,10], and for women, whether they simply reflect a reservoir of precursor substrates for estrogens.
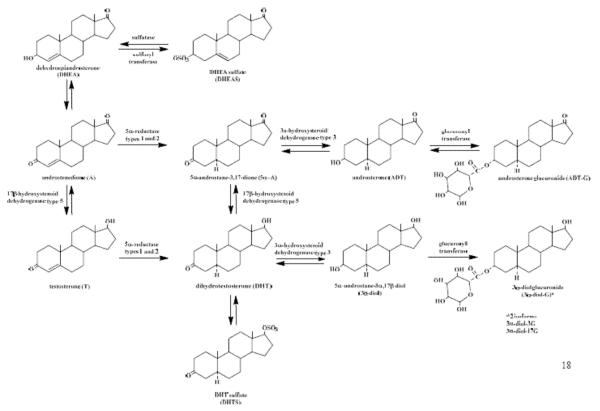
In both men and women, metabolism of androgens and their precursors occurs in peripheral target tissues [11], and the derived 5α-reduced metabolites (Figure 1), particularly dihydrotestosterone (DHT), are the most potent local bioactive agents. However, serum DHT does not adequately reflect increased 5α-reductase activity [12], probably due to its high affinity to sex hormone binding globulin (SHBG), therefore studies of prostate cancer as well as hirsutism in women have used androsterone glucuronide (ADT-G), a distal metabolite of DHT, as a marker of 5α-reductase/peripheral androgen activity [13,14]. Recent studies suggest circulating levels of ADT-G and androstanediol glucuronide (found as two isomers: 5α-androstane-3α,17β diol-3-glucuronide (3α-diol-3G) and 5α-androstane-3α,17β diol-17-glucuronide (3α-diol-17G)) together are a better indicator of total androgenicity than their precursors alone [9,11]. Another potentially important androgen conjugate is dihydrotestosterone sulfate (DHTS), which may form an inactive reservoir for the highly potent DHT. As such, all of the 5α-reduced metabolites are important and require mass spectrometry assay methodology for accurate and reliable measurements. Further, efforts to capture a panel of androgens including the adrenal androgens, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS), and the adrenal and gonadal androgens, androstenedione (A) and testosterone (T), as well as their 5α-reduced metabolites, are needed to clarify the role of androgens in cancer etiology. We have developed a stable isotope dilution high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method that requires 0.6 mL of serum and two sample preparations to measure 11 androgens, including the principal androgens secreted by the adrenals and gonads, as well as their 5α-reduced metabolites. To demonstrate that our assays are suitable to assess the role of endogenous androgens in epidemiologic studies of complex disease, the purpose of this report is three-fold: 1) to evaluate laboratory variability of the assays based on a formal reproducibility study in which blinded, randomized serum samples from men, premenopausal women, and postmenopausal women were assayed over several weeks; 2) to provide a comparison of hormone measurements using the current LC-MS/MS method with an established assay from a laboratory that measures 9 of the 11 androgens using either gas chromatography-mass spectrometry (GC-MS) or LC-MS/MS; and 3) to assess the interindividual variability of androgen metabolite levels by sex and menopausal status.
Figure 1.
Formation of 5α-reduced androgens from adrenal androgenic precursors, DHEA and DHEAS. The current assays measure 11 of these 12 metabolites, the only metabolite not measured is 5α-androstane-3α,17β diol (3α-diol) due to very low abundance in serum.
2. MATERIALS AND METHODS
2.1. Study Design
The 11 hormones measured in this study include: 1) adrenal androgens: DHEA and DHEAS; 2) adrenal and gonadal androgens: A and T; and 3) 5α-reduced peripheral androgen metabolites: 5α-androstane-3,17-dione (androstanedione or 5α-A), DHT, androsterone (ADT), DHTS, 3α-diol-3G, 3α-diol-17G, and ADT-G (Figure 1).
Hormone levels were measured in serum provided by 20 healthy volunteers who were recruited from the Research Donor Program administered by the Frederick National Laboratory for Cancer Research’s Occupational Health Services in Frederick, MD under an approved protocol. Donors included 5 men ages 24 to 48 years, 10 premenopausal women ages 27 to 38 years, and 5 naturally postmenopausal women ages 54 to 65 years. Donors were identified only by code, and the specimens were further de-identified using laboratory accession numbers. Donors reporting use of hormone supplements, oral contraceptives, or menopausal hormone therapy within 3 months of blood draw were ineligible. Because the purpose of this study was to assess the performance of the assays, we did not select donors based on other characteristics suspected of modifying androgen profiles, such as age, race, medication use, anthropometry, or thyroid status.
For each donor, 8 aliquots of 0.6 ml serum were prepared, with 2 aliquots randomly placed within each of 4 batches. The Laboratory of Proteomics and Analytical Technologies, Cancer Research Technology Program at the Frederick National Laboratory for Cancer Research received 3 batches of 40 serum samples and assayed one batch at the start of each of 3 consecutive weeks. Each batch contained two aliquots per subject for the 20 subjects as described above; the 40 samples per batch were placed randomly without regard to sex or menopausal status and labelled based on batch and position to mask sample identity. All samples were stored at −80°C until assayed. A total of 6 measurements were obtained for each subject in the reproducibility study. All steps of the assay procedures, including enrichment and extraction, were done separately for each aliquot. A fourth batch of 40 blinded, randomly distributed samples was sent to the Pharmacogenomics laboratory at the research center of CHU de Québec that measured 9 of the 11 androgens using GC-MS or LC-MS/MS. The assays were developed by Dr. Alain Bélanger’s group at Laval University [15,16]. The purpose of these measurements was to provide an external comparison for our androgen measurements, as there is no current standard for these assays.
Several sources of variability in serum hormones can be assessed in this study, including differences among subjects, measurement variability over batches for a given subject, variability among duplicate aliquots in a given batch, as well as across laboratory variability in serum hormone measurements. Of note, the current study did not measure variability over time for a given subject, as serum samples were collected from individuals at only one time point.
2.2. Laboratory Methods
2.2.1. Assay procedures
Our assay procedures were designed to distinguish serum unconjugated and conjugated androgens (Supplemental Figure 1). The steps for measuring unconjugated serum androgens included extraction with hexane, derivatization with hydroxylamine, and quantitation using stable isotope dilution LC-MS/MS. Unconjugated serum androgens were oxime-labelled to improve their ionization efficiency and thus the sensitivity of the assay. Briefly, 20 μL internal standard solution and 0.5 mL of freshly prepared phosphate buffered normal saline solution was added to 0.4 mL human serum. The sample underwent inverse extraction with 5 mL hexane.
After extraction the organic solvent was transferred to a clean glass tube and evaporated to dryness at 60°C under a stream of nitrogen gas. The dried sample was then derivatized using 100 μL of 0.1 M hydroxylamine solution in methanol with 5% (v/v) glacial acetic acid [17]. After derivatization, the sample was dried and reconstituted with 80 μL 50% methanol/water and analyzed by stable isotope dilution LC-MS/MS in positive ion mode using a Thermo TSQ™ Vantage triple quadrapole mass spectrometer (Thermo Scientific, San Jose, CA) coupled with a Prominence UFLC system (Shimadzu Scientific Instruments, San Jose CA), and quantified using a Xcaliber™ Quan Browser (Thermo Scientific). During oxime derivatization of ketosteroids, two geometric stereoisomers can be formed from a single ketosteroid. To avoid undesirable twin peaks for a single steroid, the chromatography conditions described in our manuscript ensure each unconjugated androgen is eluted as a single oxime derivatized ketosteroid peak.
Given the excellent sensitivity and lower background noise obtained for androgen sulfate and glucuronide conjugates during the negative ionization, conjugated androgen metabolites were quantified in their latent form directly without hydrolysis or derivatization, employing a one-step liquid-liquid extraction followed by quantitation using stable isotope dilution LC-MS/MS. Briefly, 0.4 mL acetone containing stable isotopically-labeled standards was added to 0.2 mL human serum. Following vortexing and centrifugation, 500 μL supernatant was transferred to a clean glass vial for lyophilization. The dried sample was reconstituted in 80 μL 20% methanol/water and analyzed by stable isotope dilution LC-MS/MS in negative ion mode using the same instrument as described above.
Calibration standards were prepared in double charcoal-stripped human serum (Bioreclamation Inc., Westbury NY) and assayed in duplicate (concentration ranges are provided in online supplement). The double charcoal-stripped human serum was tested before use to ensure that no detectable levels of targeted androgens remained. Calibration curves for each androgen were constructed by plotting peak area ratios (androgen/stable isotope labeled androgen) obtained from calibration standards versus the amount of androgen and fitting these data using linear regression with 1/X weighting. The amount of each androgen in the serum sample was then interpolated using this linear function. Based on their similarity of structures and retention times, d5-DHEA was employed as internal standard for DHEA; 13C3-A for A; 13C3-T for T and 5α-A; d3-DHT for DHT; and d4-ADT for ADT. For the conjugated androgens, d5-DHEAS was employed as internal standard for DHEAS; d3-DHTS for DHTS; d3-3α-diol-17G for 3α-diol-3G and 3α-diol-17G; and d4-ADT-G for ADT-G. Additional details of our analytic methods are described in the online supplement.
The methods for the quantification of androgens at Laval University have been described in detail elsewhere [15,16,18]. Briefly, serum levels of DHEA, A, T, DHT, and ADT were measured with gas chromotagraphy-MS/MS (GC-MS/MS) after derivatization by either pentafluorobenzoylchloride (DHEA, T, DHT) or pentafluorobenzylhydroxylamine (A); serum levels of DHEAS, ADT-G, 3α-diol-3G, and 3α-diol-17G were measured with LC-MS/MS after C18 solid phase extraction. The primary difference in the two assays is that the Laval University method quantifies the conjugated androgens using GC-MS/MS, while the current assay quantifies both conjugated and unconjugated androgens using LC-MS/MS. Further, the current assay also quantifies two additional androgens: 5α-A and DHTS.
2.2.2. Assay validation
The calibration curves for the unconjugated and conjugated androgens measured in this study were linear over 500-1000 fold concentration range with linear regression correlation coefficients greater than 0.998. The limit of quantitation (LOQ) in the present assay was defined as the lowest concentration of an androgen in a sample that could be determined with acceptable precision and accuracy [19] defined as intra- and inter-batch CV within 15% and measured analyte values within 85% to 115% of known target values under the condition of the described method. The LOQ for the unconjugated androgens and conjugated androgens are as follows: 0.01 ng/mL for A and T; 0.05 ng/mL for DHEA, DHT, and ADT; 0.1 ng/mL for 5α-A; 0.05 ng/mL for DHEAS and DHTS; 0.1 ng/mL for 3α-diol-3G, 3α-diol-17G, and ADT-G.
For the purposes of this reproducibility study, assay accuracy was measured as the percent recovery of the known added amount of unconjugated and conjugated androgens in spiked samples [19] (results are reported in the online supplement). High specificity of our assays was achieved through selective reaction monitoring coupled with high resolution chromatography.
2.3. Statistical Analysis
To quantify reproducibility of the androgen assays, estimates of the variability among subjects , assay variability among batches for a given subject , and assay variability associated with different aliquots in the same batch for the same subject (σ2) were obtained using a nested component of variance analysis (SAS procedure VARCOMP). Hormone values were log-transformed to stabilize variances. With ytjk = μ + ai + bj(t) + εk(ij), where ai, bj(t) and εk(ij) are independent variables, each with mean 0 and corresponding variances , , and σ2. Using the variance estimates we computed the total, intra- and inter-batch coefficients of variation (CV) for each assay. We also computed the intraclass correlation coefficient for each sex/menopausal status group. Paired t-tests and Spearman’s rank correlation coefficients were used to compare results from the 9 hormones measured in both laboratories. To further evaluate the agreement between the methods we conducted Deming regression and generated Bland-Altman plots to graphically provide an assessment of bias. Interindividual differences in the distribution of androgen measurements across sex/menopausal status groups were compared using box plots. The analyses for this paper were generated using SAS/STAT software, Version 9.3 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA). Bland-Altman plots were generated using the MedCalc Statistical Software version 15.4 (Ostend, Belgium).
3. RESULTS
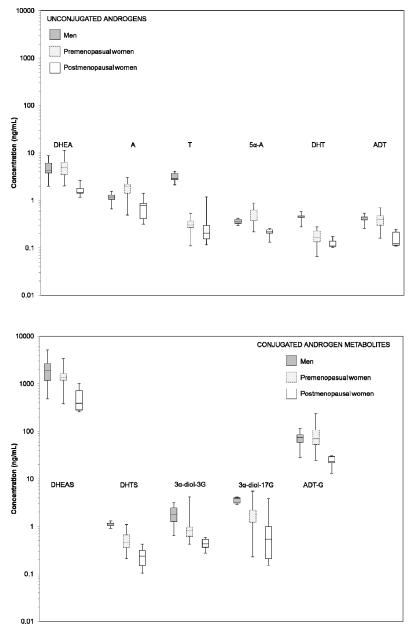
The serum levels of the principal androgens and their metabolites measured by LC-MS/MS in 20 research donor samples are shown in Table 1. The ranges of serum androgen concentrations, by sex/menopausal status group, are shown as box plots in Figure 2. The concentrations of serum androgens were similar for the follicular and luteal phases of premenopausal women and are presented as combined values. As expected, DHEA, DHT, and ADT were found to largely circulate as their sulfated or glucuronidated metabolites: DHEAS, DHTS, and ADT-G, respectively. DHEAS, ADT-G, DHEA, A, 3α-diol-3G and 3α-diol-17G were the most abundant androgens in the circulation of both men and women. Testosterone levels were more than 10 times higher in men compared with women. Concentrations of the adrenal precursors, DHEA and DHEAS, were highest in men, 16% and 29% lower, respectively, in premenopausal women, and considerably lower among postmenopausal women. Across every androgen measured, the average concentration in postmenopausal women was the lowest of the three groups.
Table 1.
Geometric mean concentration (ng/mL), range*, and rank order of 11 serum androgens by sex/menopausal status group.
| Men | Premenopausal women | Postmenopausal women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean |
(Min-Max) | Rank | Geometric Mean |
(Min-Max) | Rank | Geometric Mean |
(Min-Max) | Rank | |
|
|
|||||||||
| Dehydroepiandrosterone (DHEA) | 4.47 | (2.00-8.77) | 3 | 3.75 | (2.03-10.19) | 3 | 1.65 | (1.18-2.67) | 3 |
| Androstenedione (A) | 1.11 | (0.67-1.56) | 7 | 1.24 | (0.50-2.48) | 4 | 0.67 | (0.32-1.42) | 4 |
| Testosterone (T) | 3.04 | (2.13-4.11) | 5 | 0.26 | (0.11-0.50) | 10 | 0.27 | (0.12-1.19) | 7 |
| Androstanedione (5α-A) | 0.36 | (0.29-0.42) | 11 | 0.38 | (0.22-0.76) | 8 | 0.21 | (0.13-0.26) | 9 |
| Dihydrotestosterone (DHT) | 0.44 | (0.27-0.58) | 9 | 0.13 | (0.07-0.25) | 11 | 0.12 | (0.10-0.17) | 11 |
| Androsterone (ADT) | 0.40 | (0.26-0.54) | 10 | 0.30 | (0.16-0.61) | 9 | 0.15 | (0.11-0.25) | 10 |
| Dehydroepiandrosterone-sulfate (DHEAS) | 1719 | (483-5198) | 1 | 1221 | (376-3299) | 1 | 461 | (258-1023) | 1 |
| Dihydrotestosterone-sulfate (DHTS) | 1.10 | (0.91-1.29) | 8 | 0.40 | (0.21-0.99) | 7 | 0.22 | (0.10-0.42) | 8 |
| 5α-androstane 3α, 17β diol-3-glucuronide (3α-diol-3G) | 1.61 | (0.60-3.16) | 6 | 0.76 | (0.42-3.96) | 6 | 0.42 | (0.27-0.59) | 6 |
| 5α-androstane 3α, 17β diol-17-glucuronide (3α-diol-17G) | 3.58 | (2.90-4.12) | 4 | 1.17 | (0.20-4.93) | 5 | 0.58 | (0.15-3.82) | 5 |
| Androsterone-glucuronide (ADT-G) | 65.7 | (27.7-116.2) | 2 | 60.3 | (24.4-218.5) | 2 | 22.6 | (13.2-30.3) | 2 |
Androgen levels for all samples were above the lower limit of quantitation (0.01 ng/mL for A and T; 0.05 ng/ml for DHEA, DHT, ADT, DHEAS, and DHTS; and 0.1 ng/mL for 5α-A, 3α-diol-3G, 3α-diol-17G, and ADT-G).
Figure 2.
Concentration of serum androgens by sex/menopausal status.
Y-axis androgen concentration (ng/mL) plotted on a logarithmic scale. Box represents 25th and 75th percentile with center line at median value of distribution. Whiskers represent minimum and maximum concentration.
The reproducibility measures for each sex/menopausal status group are presented in Table 2. Laboratory variability for each hormone across sex/menopausal status groups was very similar. The overall laboratory CVs were <5% (within batch and between batch CVs were also <5% (results not shown)). Further, the ICCs were uniformly high (>95%) for all hormones measured across sex/menopausal status groups.
Table 2.
Reproducibility of liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to measure principal serum androgens and their metabolites by sex/menopausal status group.
| Men | Premenopausal women | Postmenopausal women | ||||
|---|---|---|---|---|---|---|
| CV (%) | ICC (%) | CV (%) | ICC (%) | CV (%) | ICC (%) | |
|
|
||||||
| Dehydroepiandrosterone (DHEA) | 0.70 | 99.98 | 0.59 | 99.99 | 0.95 | 99.90 |
| Androstenedione (A) | 1.10 | 99.87 | 0.98 | 99.96 | 1.61 | 99.93 |
| Testosterone (T) | 0.62 | 99.94 | 1.89 | 99.78 | 3.50 | 99.84 |
| Androstanedione (5α-A) | 2.01 | 97.69 | 2.12 | 99.72 | 3.51 | 97.70 |
| Dihydrotestosterone (DHT) | 1.66 | 99.59 | 1.93 | 99.80 | 2.20 | 98.84 |
| Androsterone (ADT) | 2.02 | 99.41 | 2.00 | 99.73 | 2.48 | 99.55 |
| Dehydroepiandrosterone sulfate (DHEAS) | 0.58 | 99.99 | 0.69 | 99.98 | 0.91 | 99.98 |
| Dihydrotestosterone sulfate (DHTS) | 1.45 | 98.63 | 2.29 | 99.82 | 1.98 | 99.87 |
| 5α-androstane-3α,17β diol-3-glucuronide (3α-diol-3G) | 0.92 | 99.98 | 1.26 | 99.97 | 0.80 | 99.93 |
| 5α-androstane-3α,17β diol-17-glucuronide (3α-diol-17G) | 0.71 | 99.79 | 1.06 | 99.99 | 1.49 | 99.99 |
| Androsterone glucuronide (ADT-G) | 1.11 | 99.96 | 1.49 | 99.95 | 1.51 | 99.79 |
Spearman’s rank correlation, paired t-tests, Deming regression intercept and slope estimates, and Bland-Altman estimates of bias comparing the 9 hormones measured in both laboratories are presented in Table 3. The absolute levels for T and A were virtually identical across laboratories [geometric mean of samples measured in current laboratory and comparison laboratory: T = 0.49 ng/ml and 0.49 ng/mL, respectively; A = 1.03 ng/mL and 1.00 ng/mL, respectively]. For the remaining 7 hormones measured, the geometric means of the absolute levels were significantly different across laboratories, however, ranked correlations for all 9 hormones measured were high (>0.85), suggesting good agreement. Deming regression showed no deviation from an intercept of 0 and slope of 1 for ADT and DHEAS. For the remaining androgens compared there were statistically significant systematic (intercept) and/or proportional (slope) differences in measurement across the two laboratories; however, the overall magnitude of the statistically significant differences was small (<1.2). The Bland-Altman estimates and plots (Supplemental Figure 2) suggest no statistically significant bias for 8 of the 9 hormones measured. The current laboratory method measured ADT-G significantly higher than the comparison laboratory (Bland-Altman bias = 0.61 (0.09, 1.13)).
Table 3.
Comparison of 9 serum androgens (ng/mL) across two laboratories.
| GM comparison |
GM current |
Paired t-test p-value |
Spearman’s correlation coefficient |
Deming intercept (95% CI) |
Deming slope (95% CI) |
Bland-Altman bias (95% CI) |
|
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Dehydroepiandrosterone (DHEA)* | 2.82 | 3.19 | <0.01 | 0.99 | 0.19 (0.13, 0.25) | 0.94 (0.90, 0.98) | 0.12 (−0.02, 0.27) |
| Androstenedione (A)* | 1.00 | 1.03 | 0.34 | 0.95 | 0.03 (−0.03, 0.09) | 0.87 (0.78, 0.96) | 0.03 (−0.26, 0.32) |
| Testosterone (T)* | 0.49 | 0.49 | 0.84 | 0.94 | −0.11 (−0.17, −0.06) | 0.86 (0.83, 0.90) | −0.02 (−0.51, 0.47) |
| Dihydrotestosterone (DHT)* | 0.09 | 0.17 | <0.01 | 0.86 | −0.23 (−0.50, 0.03) | 0.61 (0.48, 0.74) | 0.72 (−0.25, 1.69) |
| Androsterone (ADT)* | 0.19 | 0.27 | <0.01 | 0.88 | 0.00 (−0.28, 0.29) | 0.77 (0.60, 0.95) | 0.38 (−0.21, 0.97) |
| Dehydroepiandrosterone sulfate (DHEAS) | 945 | 1043 | <0.01 | 0.96 | 0.20 (−0.16, 0.56) | 0.98 (0.94, 1.03) | 0.10 (−0.12, 0.31) |
| 5α-androstane- 3α,17β diol-3-glucuronide (3α-diol-3G) | 0.99 | 0.79 | 0.01 | 0.88 | −0.22 (−0.40, −0.05) | 1.17 (0.86, 1.49) | −0.22 (−0.89, 0.45) |
| 5α-androstane-3α,17β diol-17-glucuronide (3α-diol-17G) | 1.21 | 1.30 | <0.01 | 0.96 | 0.27 (0.13, 0.41) | 1.10 (0.92, 1.28) | 0.27 (−0.30, 0.84) |
| Androsterone glucuronide (ADT-G) | 26.2 | 48.2 | <0.01 | 0.92 | 0.91 (0.49, 1.33) | 0.91 (0.77, 1.04) | 0.61 (0.09, 1.13) |
GM = Geometric mean
All androgens were natural log-transformed to achieve a normal distribution
Androgen measured via GC-MS at comparison laboratory, all others measured via LC-MS/MS.
4. DISCUSSION
Laboratory results were highly reproducible with overall CVs of <5% for each of 11 hormones measured. ICCs for all hormones measured were high, >95%, demonstrating that interindividual variability in measurements was much greater than within laboratory variation for all hormones measured. Further, the substantial interindividual variability of circulating principal androgens as well as their 5α-reduced metabolites measured in serum from men, premenopausal women, and postmenopausal women, suggests that these assays are suitable to assess the role of endogenous androgens in a number of conditions, including cancer, that are more common with aging in both men and women when androgen concentrations are at their lowest [20,21].
Although absolute values were not identical, these findings compare favorably (Spearman’s rank correlation, >0.85) with those obtained in the external laboratory in which 9 of the 11 hormones were measured via GC-MS or LC-MS/MS. Differences in the absolute values across laboratories could be due to different sources of androgen reference compounds employed in the laboratories, as well as potential lot-to-lot variability of the calibrators. The complexity of human sera with possible co-eluting species during chromatography could also contribute to the observed differences in absolute values. However, given the generally small magnitude (Deming slopes and intercepts <1.2) of differences combined with the high ranked correlations of measurements across the two laboratories the quantile categorization of androgen levels employed in epidemiologic studies using either laboratory should be comparable.
Several limitations should be noted. Given that our results are based on a small number of participants (n=20), it is possible that the variance estimates may not adequately represent the underlying population values and provide imprecise estimates of the relevant statistics.
However, the general agreement of results between the laboratories, as well as the reproducibility and ICC findings for this novel method across sex/menopausal status groups, especially among postmenopausal women where concentrations are lowest, is notable. Further, the small number of subjects in each sex/menopausal status group limits our ability to explore hormone profiles with clinical, demographic, and anthropometric characteristics and argues for the need to further evaluate these associations in large population-based studies. We did not measure androgens among individuals with exceptionally low levels; as such the current assays may not be sensitive enough to measure androgen levels in select cancer patients or individuals on androgen deprivation therapy. However, studies have shown that only certain levels of androgens decrease in men taking the type 2 5α-reductase inhibitor Finasteride (e.g. DHT decreases from an average of 0.5 ng/mL to 0.1 ng/mL over the course of treatment), and our assay limit of detection (e.g. for DHT = 0.05 ng/mL) is smaller than the lowest detected levels in those men [14]. Finally, the use of single blood draw from research donors does not address intra-individual variation in androgen profiles.
A notable strength of our study is the development of well validated LC-MS/MS assays that measure a profile of clinically relevant principal androgens and their metabolites in a total serum volume of 0.6 ml. A serious deficiency of conventional radioimmunoassays and direct immunoassays of steroid hormones is their limitation in measuring multiple steroids in a single aliquot of serum. The enormous progress made in developing MS assays for quantifying patterns of steroid hormone metabolites can be seen in the methodology established previously by Xu et al. [22,23]. This group developed a stable isotope dilution LC-electrospray ionization-MS/MS assay that can measure concurrently a total of 15 estrogens (estriol and estrone and their metabolites) in 0.5 ml of serum or urine with high validity. Thus, the measurement of 15 estrogens can be used concurrently with the present MS assays developed to measure 11 androgens. This methodology will be especially important in epidemiologic studies on effects of principal estrogens and androgens, together with their metabolites, in complex diseases. Many challenges will be encountered in the rapidly growing field of metabolomics, but the results are likely to provide a valuable contribution to understanding different disease states [24].
Another strength of the study is that the five conjugated steroids in the profile were quantified as entire molecules, without requiring prior hydrolysis. It is well recognized that conjugated steroid assay methods requiring a hydrolysis procedure to split sulfates and glucuronides are not only cumbersome and time-consuming but also lack precision due to variability in the efficiency of the hydrolysis procedure.
In conclusion, the findings of our study including excellent laboratory reproducibility and sensitivity of the androgen LC-MS/MS assays, comparability with an established technique, and substantial interindividual variation in androgen concentrations, suggest that the serum assays are suitable for use in epidemiologic research.
Supplementary Material
Highlights.
The role of androgen metabolites in cancer etiology remains largely unexplored.
LC-MS/MS methods measured 11 androgens and androgen metabolites in serum.
Excellent reproducibility and comparability with an established method.
Assays are suitable to measure endogenous androgens in epidemiologic studies.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Department of Health and Human Services.
The authors gratefully acknowledge Norma Diaz-Mayoral, Gretchen Gierach, Barbara Fuhrman, and Cher Dallal for assistance with sample acquisition.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rajpert-De Meyts E, Skakkebaek NE. The possible role of sex hormones in the development of testicular cancer. Eur Urol. 1993;23:54–9. doi: 10.1159/000474570. [DOI] [PubMed] [Google Scholar]
- [2].Hsing AW. Hormones and prostate cancer: what's next? Epidemiol Rev. 2001;23:42–58. doi: 10.1093/oxfordjournals.epirev.a000795. [DOI] [PubMed] [Google Scholar]
- [3].Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–53. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [4].Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2014 doi: 10.1016/j.steroids.2014.12.013. [DOI] [PubMed] [Google Scholar]
- [5].Allen NE, Key TJ, Dossus L, Rinaldi S, Cust A, Lukanova A, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2008;15:485–97. doi: 10.1677/ERC-07-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rinaldi S, Dossus L, Lukanova A, Peeters PH, Allen NE, Key T, et al. Endogenous androgens and risk of epithelial ovarian cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2007;16:23–9. doi: 10.1158/1055-9965.EPI-06-0755. [DOI] [PubMed] [Google Scholar]
- [7].Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer. 2006;118:2832–9. doi: 10.1002/ijc.21730. [DOI] [PubMed] [Google Scholar]
- [8].Danforth KN, Eliassen AH, Tworoger SS, Missmer SA, Barbieri RL, Rosner BA, et al. The association of plasma androgen levels with breast, ovarian and endometrial cancer risk factors among postmenopausal women. Int J Cancer. 2010;126:199–207. doi: 10.1002/ijc.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99:182–8. doi: 10.1016/j.jsbmb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [10].Hsing AW, Chu LW, Stanczyk FZ. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol Biomarkers Prev. 2008;17:2525–30. doi: 10.1158/1055-9965.EPI-08-0448. [DOI] [PubMed] [Google Scholar]
- [11].Labrie F. Intracrinology in action: Importance of extragonadal sex steroid biosynthesis and inactivation in peripheral tissues in both women and men. J Steroid Biochem Mol Biol. 2015;145:131–2. doi: 10.1016/j.jsbmb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- [12].Stanczyk FZ, Bretsky P. Biosynthesis, transport and metabolism of steroid hormones. In: Henderson BE, Ponder B, Ross RK, editors. Hormones, Genes and Cancer. Oxford University Press; Oxford: 2003. pp. 12–34. [Google Scholar]
- [13].Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, Henderson BE. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992;339:887–9. doi: 10.1016/0140-6736(92)90927-u. [DOI] [PubMed] [Google Scholar]
- [14].Stanczyk FZ, Azen CG, Pike MC. Effect of finasteride on serum levels of androstenedione, testosterone and their 5 alpha-reduced metabolites in men at risk for prostate cancer. J Steroid Biochem Mol Biol. 2013;138:10–16. doi: 10.1016/j.jsbmb.2013.02.015. [DOI] [PubMed] [Google Scholar]
- [15].Audet-Walsh E, Lepine J, Gregoire J, Plante M, Caron P, Tetu B, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocrinol Metab. 2011;96:E330–E339. doi: 10.1210/jc.2010-2050. [DOI] [PubMed] [Google Scholar]
- [16].Levesque E, Laverdiere I, Lacombe L, Caron P, Rouleau M, Turcotte V, et al. Importance of 5alpha-reductase gene polymorphisms on circulating and intraprostatic androgens in prostate cancer. Clin Cancer Res. 2014;20:576–84. doi: 10.1158/1078-0432.CCR-13-1100. [DOI] [PubMed] [Google Scholar]
- [17].Lunn G, Hellwig LC. Handbook of derivatization reaction for HPLC. John Wiley and Sons, Inc.; New Jersey: 1998. [Google Scholar]
- [18].Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, et al. Metabolism of DHEA in postmenopausal women following percutaneous administraiton. J Steroid Biochem Mol Biol. 2007;103:178–88. doi: 10.1016/j.jsbmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- [19].Swartz ME, Krull IS. Analytic method validation and development, a primer. Marcel Dekker, Inc.; New York: 1997. [Google Scholar]
- [20].Labrie F, Belanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab. 1997;82:2403–9. doi: 10.1210/jcem.82.8.4161. [DOI] [PubMed] [Google Scholar]
- [21].Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- [22].Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromotography-mass spectrometry. Anal Chem. 2005;77:6646–54. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- [23].Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RD. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromotography-tandem mass spectrometry. Anal Chem. 2007;79:7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- [24].Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010;121:491–495. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.