Abstract
Background & Aims
The functional lumen imaging probe (FLIP) could improve characterization of achalasia subtypes by detecting non-occlusive esophageal contractions not observed with standard manometry. We aimed to evaluate for esophageal contractions during volumetric distention in patients with achalasia using FLIP topography.
Methods
Fifty one treatment-naïve patients with achalasia, defined and sub-classified by high-resolution esophageal pressure topography, and 10 asymptomatic individuals (controls) were evaluated with the FLIP during endoscopy. During stepwise distension, simultaneous intra-bag pressures and 16 channels of cross-sectional areas were measured; data were exported to software that generated FLIP topography plots. Esophageal contractility was identified by noting periods of reduced luminal diameter. Esophageal contractions were further characterized by propagation direction, repetitiveness, and based on whether they were occluding or non-occluding.
Results
Esophageal contractility was detected in all 10 controls: 8/10 had repetitive, antegrade, contractions and 9/10 had occluding contractions. Contractility was detected in 27% (4/15) of patients with type I achalasia and 65% (18/26, including 9 with occluding contractions) of patients with type II achalasia. Contractility was detected in all 10 patients with type III achalasia; 8 of these patients had a pattern of contractility not observed in controls (repetitive, retrograde contractions).
Conclusions
Esophageal contractility not observed with manometry can be detected in patients with achalasia using FLIP topography. The presence and patterns of contractility detected with FLIP topography may represent variations in pathophysiology, such as mechanisms of pan-esophageal pressurization in patients with type II achalasia. These findings could have implications for additional sub-classification to supplement prediction of the achalasia disease course.
Keywords: Esophagus, motility, peristalsis, EndoFLIP
Introduction
Achalasia is a primary esophageal motor disorder characterized by abnormal deglutitive lower esophageal sphincter (LES) relaxation and absent peristalsis and is traditionally defined by evaluation with esophageal manometry.1 Evaluation with high-resolution manometry (HRM) and esophageal pressure topography (EPT) have allowed further sub-classification of achalasia based on the pressurization patterns observed in the esophageal body.2 Type I (classic) achalasia is characterized by absent contractility, type II achalasia by pan-esophageal pressurization, and type III (spastic) achalasia by spastic (reduced latency) contractions.2, 3 Sub-classification of achalasia has demonstrated clinical utility in predicting symptomatic response to treatment (such as pneumatic dilation and Heller myotomy).2, 4–7
Though absent peristalsis is a defining feature of achalasia, multiple studies have reported return of peristalsis following treatment of achalasia.8–10 Recovery of peristalsis has been attributed to removal of the distal esophagogastric junction (EGJ) obstruction, similar to animal models with imposed esophageal ligature or humans with gastric lap bands.11–13 However, an alternative hypothesis is that esophageal dilatation, common in achalasia, impedes contact of the esophageal wall with the manometry catheter to induce a measurable pressure signal and subsequent detection of esophageal contractions and peristalsis. Additionally, pan-esophageal pressurization, the defining feature of type II achalasia, may obscure manometric detection of esophageal contractions. Longitudinal muscle has been implicated as the causal mechanism of common cavity pressurization and esophageal emptying in type II achalasia based primarily on an elegant ultrasound study of seven patients with type II achalasia.14 However, we have observed contractile activity of the esophagus during endoscopy in some patients with type I and II achalasia during endoscopy and suspect that this may be an additional mechanism of pan-esophageal pressurization.
The functional lumen imaging probe (FLIP) may offer a unique method to evaluate esophageal contractility in achalasia. The FLIP is a commercially available device (EndoFLIP, Crospon, Inc. Galway, Ireland) that utilizes multiple, closely-spaced impedance planimetry channels located within a distensible bag to simultaneously measure luminal diameters and intra-bag pressure during controlled, volumetric distension.15 Though the FLIP has primarily been used in achalasia for evaluation of EGJ distensibility,16, 17 we recently described a novel methodology to assess contractility in the esophageal body of asymptomatic controls using the FLIP: FLIP topography.18 By positioning the FLIP catheter to span the distal esophagus and incorporating novel software programs to interpolate the multiple luminal diameter measurements, a FLIP topography plot can be generated to visualize esophageal body contractility with concurrent assessment of EGJ distensibility.19
Because the FLIP catheter bag distends within the esophageal lumen and can detect contractions by sequenced changes in luminal diameters, we hypothesized that application of FLIP topography may offer a method to detect non-occlusive esophageal contractions in patients with achalasia. The aim of this study was to utilize FLIP topography in patients with achalasia to define patterns of esophageal contractions as they relate to pre-treatment, HRM/EPT-defined subtypes.
Methods
Subjects
Fifty one patients with achalasia (ages 19 – 82, 21 female) without previous pneumatic dilation or esophageal myotomy that had FLIP completed during endoscopy were included. Five patients had received previous botulinum toxin injection (at 3/3, 5/5, 5/4, 62/62, and 196/196 months prior to FLIP/HRM, respectively). Patients presented to the Esophageal Center of Northwestern for evaluation of dysphagia, non-cardiac chest pain and/or regurgitation from November 2012 to June 2015. Achalasia was diagnosed and sub-classified by HRM (4.2-mm outer diameter, solid-state assembly with 36 circumferential pressure sensors spaced 1 cm apart; Medtronic Inc, Shoreview, MN, USA) of ten supine, 5-ml water swallows. Using Manoview analysis software version 3.0 (Medtronic), EPT was analyzed by measurement of the integrated relaxation pressure (IRP), distal latency, and esophageal body pressurization patterns.3 All patients had a median IRP > 15 mmHg. Type I (classic) achalasia (n = 15) was defined by absent contractility in 100% of swallows. Type II achalasia (N=26) was defined by pan-esophageal pressurization at an isobaric contour of 30 mmHg in > 20% of swallows. Type III (spastic) achalasia (N = 10) was defined by ≥20% of premature swallows (i.e. distal latency < 4.5s).
Ten asymptomatic, healthy volunteers (ages 20–49; 6 female) were included as a control group. These subjects have been previously described.18, 20 None of the subjects had a history of malignancy or gastrointestinal surgery or endoscopic evidence of hiatal hernia, esophagitis, stricture, and/or mucosal changes suggestive of eosinophilic esophagitis. Control subjects did not undergo manometry as part of this study protocol. Informed consent was obtained from each subject.
The study protocol was approved by the Northwestern University Institutional Review Board.
Functional lumen imaging probe system and study protocol
The FLIP assembly consisted of a 240-cm long, 3-mm outer diameter catheter with an infinitely compliant bag (up to a distension volume of 60 mL) mounted on the distal 18 cm of the catheter. The bag, tapered at both ends to assume a 16-cm long cylindrical shape in the center that formed the impedance planimetry segment, housed 17 ring electrodes spaced 1 cm apart and a solid-state pressure transducer positioned at the distal end to provide simultaneous measurement of 16 channels of cross-sectional area (CSA) and intra-bag pressure. The impedance planimetry segment had a minimum-to-maximum range of measureable CSA within the infinitely compliant range of 21 – 380 mm2; assuming circular lumen cross-sections, this corresponded to a diameter of 5.2 – 22 mm. Pressure values above 380 mm2 (22-mm diameter) could be measured, but mechanical properties of the bag would be engaged above this distension range. Measurements from the impedance planimetry electrode pairs and the pressure transducer were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit.
Subjects underwent upper endoscopy in the left lateral decubitus position. Moderate sedation with 2–12 mg midazolam and 0–250 µg fentanyl was administered during the procedure. Propofol was used in addition to midazolam and fentanyl with anesthesia assistance at the discretion of their endoscopist in 4 patients. The FLIP probe was placed transorally and positioned with the distal 1–3 impedance sensors beyond the EGJ as confirmed by demonstration of a waist in the impedance planimetry segment at a bag distension volume of 20–30 ml. The endoscope was withdrawn before initiation of the FLIP study protocol. The FLIP assembly position was adjusted by the endoscopist during the study to maintain placement relative to the EGJ as visualized on real-time output. Simultaneous CSAs and intra-bag pressures were measured during 5 ml step-wise distensions beginning with 5 ml and increasing to target volume of 60 or 70 ml; Each step-wise distension volume was maintained for 5 – 20 seconds during a single distension protocol for each patient; examples of the distension protocol can be observed in the volume plots of figures illustrating FLIP topography. The distension protocol changed during the course of the evaluation period, initially with a limit of 60ml and later to a limit of 70 ml. The recording unit was set to stop infusing and display an alarm message if the intra-bag pressure exceeded 60 mmHg, which sometimes limited the extent of bag distension.
FLIP Data analysis
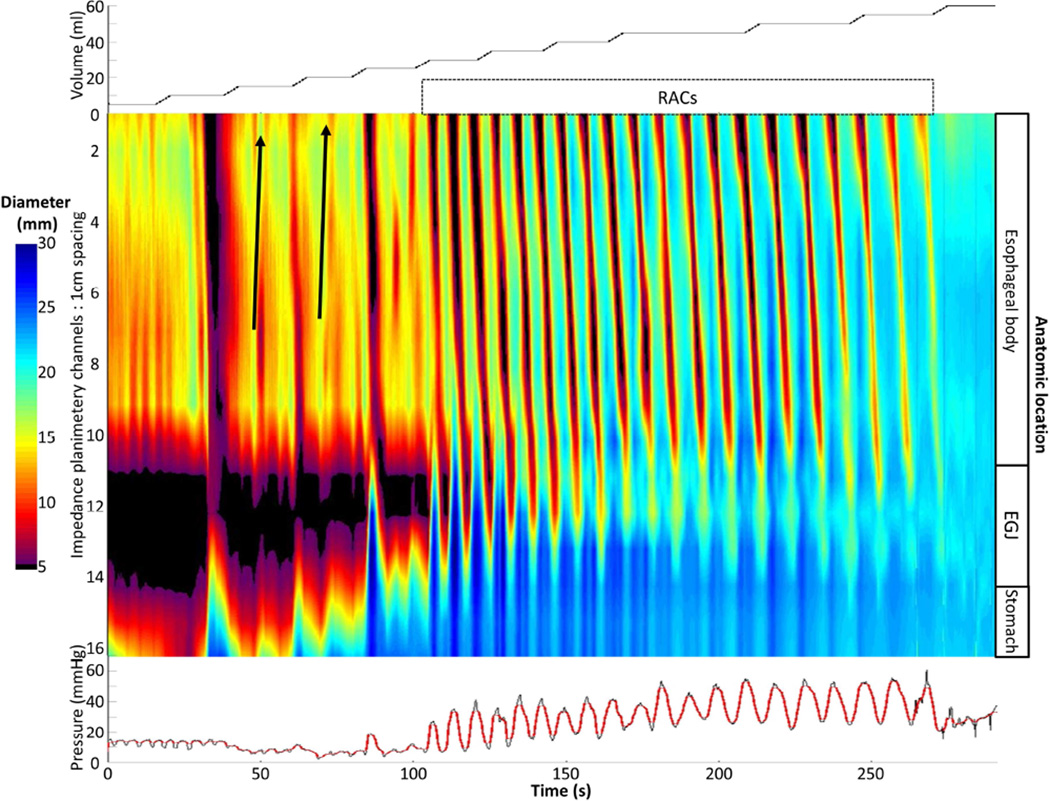
Data including distension volume, intra-bag pressure, and 16 channels of CSA measurements (via impedance planimetry) for the entire study for each subject were exported to MATLAB (The Math Works, Natick, MA, USA) for analysis using a customized MATLAB program. This program applied a filter to minimize vascular and respiratory artifact and then generated tracings of each channel’s measured luminal diameter. Interpolation of each channels’ diameter measurement was applied to generate color-coded topography plots with corresponding plots of volume distension and intra-bag pressure by time; (an example of a normal control is displayed in Figure 1). The program identified the EGJ-midline by searching for the minimal CSA of the distal impedance planimetry channels. The EGJ-distensibility index (EGJ-DI) was calculated by measuring the narrowest EGJ CSA and intra-bag pressure at each data sample (10 per second) obtained during the time course at distension volumes of 50 and 60-ml (corresponding to the 30 and 40-ml distension volumes previously reported using a shorter FLIP assembly, 10-cm in length).17, 21 The median values for narrowest EGJ CSA and intrabag pressure were then divided to calculate the EGJ-DI (CSA/pressure; mm2/mmHg) for both the 50 and 60-ml distension volumes.
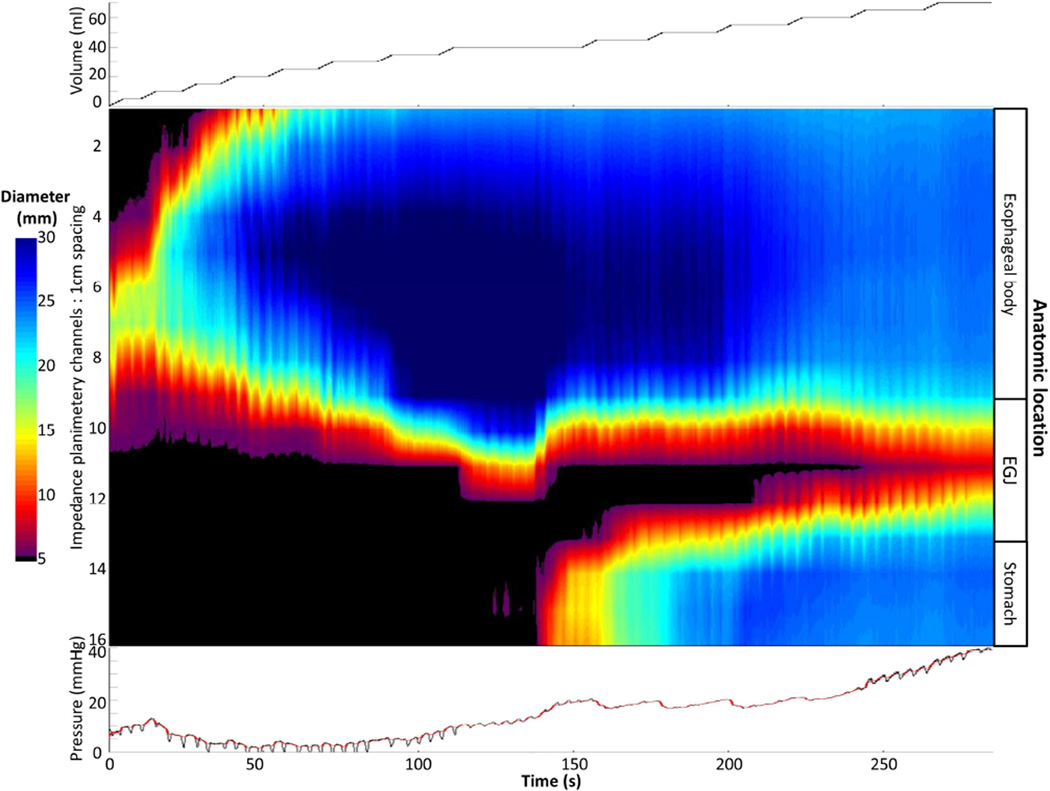
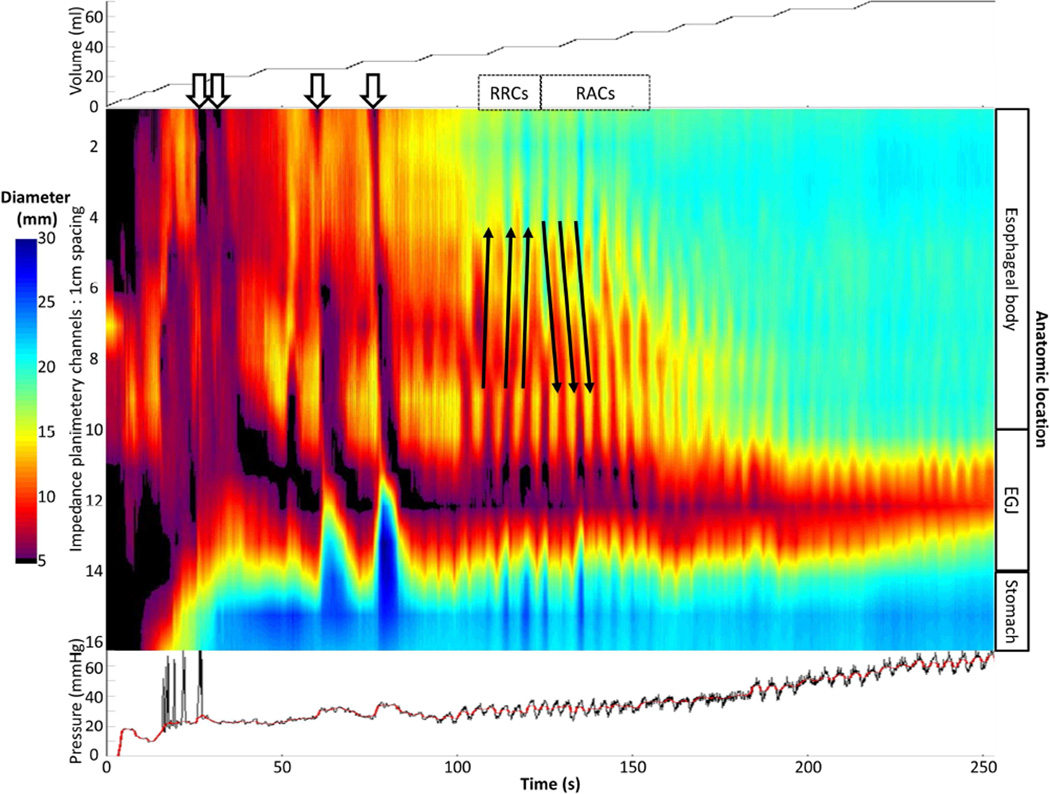
Figure 1. FLIP topography of a normal control.
Data output (Top: distension volume, thus representing the distension protocol; Middle: Topography; Bottom: Intra-bag pressure) generated by the customized MATLAB program over the course of the study protocol from a single control subject are displayed. Two retrograde contractions (noted with black arrows) are present in a period of contractility prior to the onset of repetitive, antegrade, contractions (RACs). Figure used with permission from the Esophageal Center at Northwestern.
Esophageal body contractions were identified by a transient decrease of ≥ 5 mm in the measured luminal diameter detected in ≥2 consecutive axial impedance planimetry channels. Though this 5-mm threshold was somewhat arbitrarily defined, this cutoff seemed reasonable to avoid measurement of residual vascular and respiratory fluctuations and/or changes associated with FLIP catheter position adjustment and also appeared to sufficiently identify the organized, contractile activity that was present in all ten normal controls. Esophageal contractions were identified and analyzed using the FLIP topography plots and 16 channel diameter tracing output. Propagation direction of contractions was determined by the slope of the tangent line placed on the onset of contraction, akin to the manometric contractile front velocity.18 Contractions with a positive slope were designated antegrade contractions; those with a negative slope, retrograde. Contractions were considered repetitive when ≥3 occurred consecutively. Repetitive, antegrade contractions (RACs) and repetitive, retrograde contractions (RRCs) were specifically identified as they represented distinct, frequently observed patterns potentially related to distension-mediated (secondary) peristalsis. Presence or absence of esophageal contractions (i.e. distension-related contractility), retrograde contractions, repetitive contractions, RACs, and RRCs were recorded as dichotomous variables. Categorization of contraction presence and contractile patterns was performed by two independent raters; if disagreement between the two raters occurred, a final classification was determined by consensus interpretation between the two raters. Percent agreement between the two raters was 100% (Fleiss kappa 1.0) for contractility and 92% (Fleiss kappa 0.89) for contraction patterns.
The narrowest luminal diameter achieved duration contractions were noted for each study; contractions achieving a luminal diameter ≤ 6 mm were defined as occlusive. Contractions were considered non-occlusive if the minimal luminal diameter reached was > 6mm. This threshold for occlusive contractions was determined by the expected diameter range of the occluded FLIP assembly that included the 3-mm catheter diameter plus the added diameter of the compressible, albeit fluid-filled, bag and additionally based on analysis of our normal subjects that identified sustained nadir diameters during contraction (thus interpreted as achieving an occlusive contraction) in 9/10 subjects with nadir diameters ranging from 4.5 – 5.8 mm. The presence of any occlusive contractions, only non-occlusive contractions, or no contractions was recorded as a categorical variable.
Esophagram
Barium esophagram was obtained in patients at the discretion of the patients’ treating physicians. When available, the maximum esophageal body width was measured between 3 and 10 cm proximal to the EGJ. We chose include this location to approximate the area evaluated by the FLIP. In patients that had a timed-barium esophagram (TBE), the barium column height above the EGJ was measured from images obtained 1 and 5 minutes after ingestion of 200-ml barium.
Statistical analysis
Results were expressed as median and interquartile range (IQR) unless otherwise stated. Statistical comparisons were made between normal controls and all achalasia patients, and among achalasia patients by manometric sub-type.3 Comparison of dichotomous and categorical variables between groups was assessed with Χ2 test or Exact tests. Non-parametric continuous variables were compared with Mann-Whitney U or Kruskal-Wallis tests. Statistical significance was considered at a two-tailed p-value < 0.05. Post-hoc comparison testing, as appropriate, was completed using a Bonferroni correction.
Results
Baseline patient characteristics
Baseline demographic, manometric, and radiologic data for controls and achalasia patients are displayed in Table 1. Esophagrams were obtained in 36 achalasia patients; 10 type I, 19 type II, and 7 type III; all were timed barium esophagrams, except for two type I and one type III patients. The controls as a group were younger than the achalasia patients. Esophageal width was greater in patients with type I and type II than in type III achalasia. Integrated relaxation pressure, basal EGJ pressure, and esophageal retention on TBE did not differ between achalasia subtypes (p-values 0.37 – 0.85).
Table 1. Subject characteristics.
Data are presented as median (interquartile range) unless otherwise stated. Integrate relaxation pressure (IRP) represents the median value of supine swallows from each patient. Basal esophagogastric junction (EGJ) was measured at end-expiration.
| Controls | Achalasia (all) | Type I Achalasia |
Type II Achalasia |
Type III Achalasia |
|
|---|---|---|---|---|---|
| N | 10 | 51 | 15 | 26 | 10 |
| 1Age, mean (SD); years | 27.1 (8.5) | 50 (17) | 53 (14) | 43 (17) | 64 (7) |
| Female/male | 6/4 | 21/30 | 6/9 | 12/14 | 3/7 |
| IRP; mmHg | 32 (24 – 43) | 33 (25 – 63) | 32 (25 – 39) | 30.8 (22 – 41) | |
| Basal EGJ pressure; mmHg | 27 (22 – 40) | 25 (17 – 45) | 26 (23 – 39) | 36.5 (27 – 45) | |
| 2,3Max esophageal width; cm | 4.1 (3 – 5) | 4.9 (4.1 – 7.5) | 4.3 (3.4 – 5.1) | 2.7 (1.9 – 3.0) | |
| Barium column height at 1 min; cm | 16 (12 – 23) | 15 (9 – 22) | 19 (12 – 23) | 18.3 (12 – 23) | |
| Barium column height at 5 min; cm | 14.5 (8 – 21) | 12.2 (7 – 23) | 14.6 (8 – 21) | 11.8 (0 – 18) |
p-value < 0.001 for 1normal controls vs all achalasia.
p-value <0.001 for type I vs type III.
p-value = 0.003 for type II vs type III.
FLIP topography
Detection of occluding and non-occluding contractions
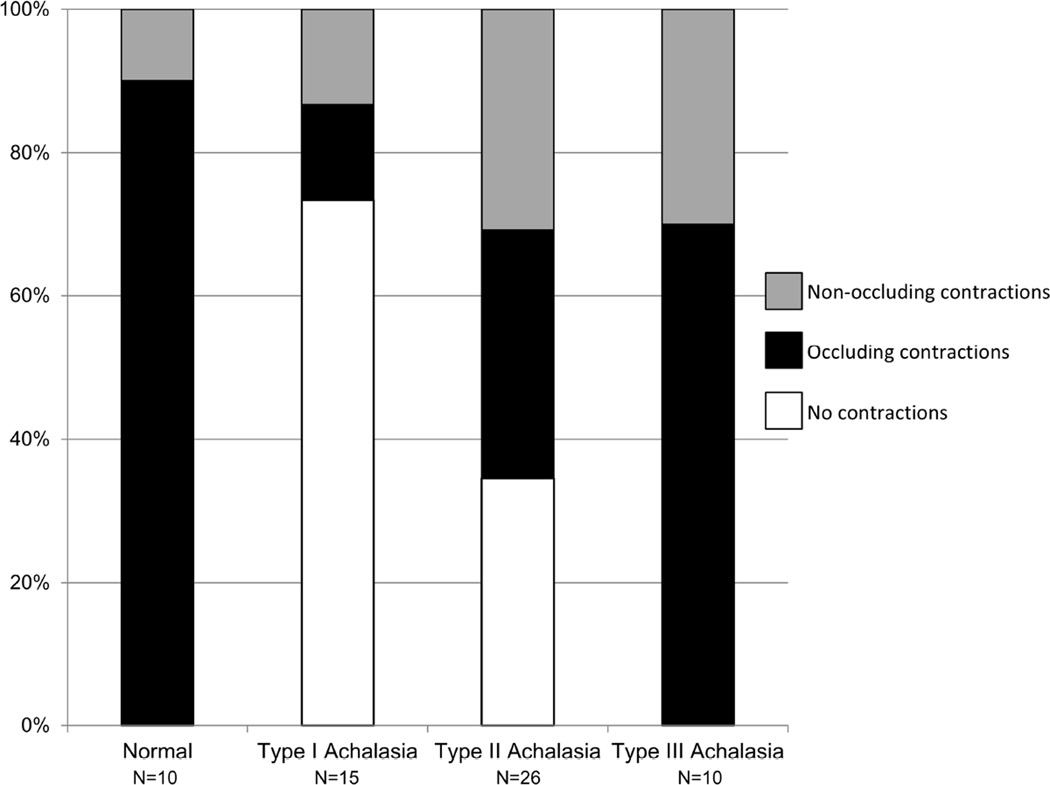
Nine of ten controls demonstrated occluding contractions, while the remaining control subject demonstrated only non-occluding contractions. Among the achalasia patients, 35% (18/51 patients: two type I, nine type II, and seven type III) had occluding contractions detected and 25% (13/51 patients: two type I, eight type II, and three type III) had only non-occluding contractions detected. The proportions of subjects with occluding and only non-occluding contractions detected by subtype are illustrated in Figure 2.
Figure 2. Non-occlusive contractions detected with FLIP.
Patients were considered to have occlusive contractions if any observed contraction on FLIP topography achieved a luminal diameter ≤ 6 mm and non-occlusive if the minimal diameter reached was > 6mm. Frequencies varied between normal controls and achalasia (p = 0.005) and among achalasia subtypes (p = 0.004: type I vs type II, p = 0.057; type I vs type III, p = 0.001; type II vs type III, p = 0.022).
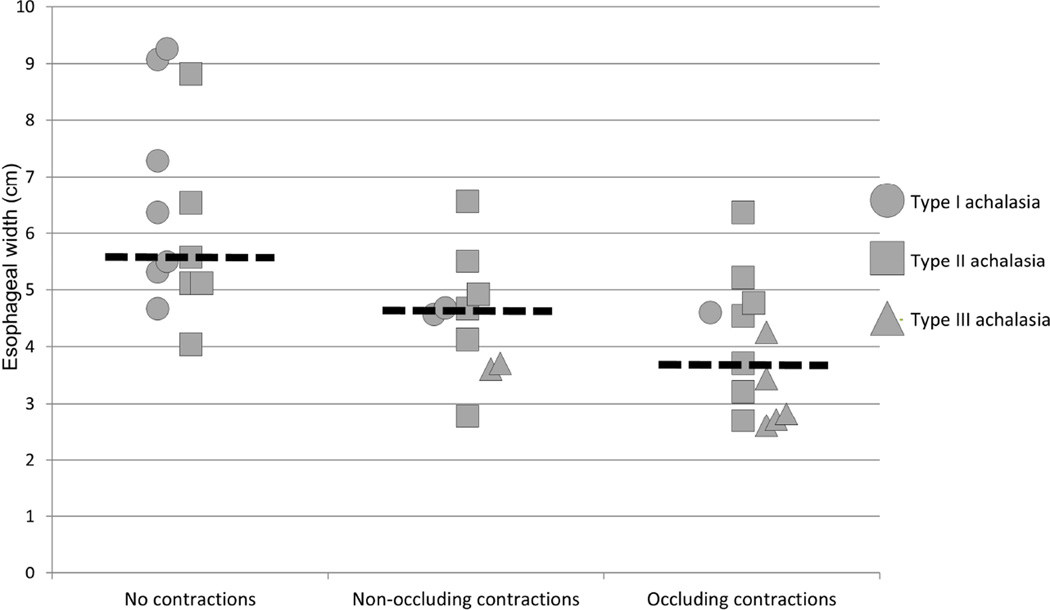
When evaluating the relationship of esophageal width on detection of occluding and/or non-occluding contraction in patients with available esophagrams, the median (IQR) esophageal width of patients without contractility detected was 5.1 cm (4.7 – 7.9), with only non-occlusive contractions detected was 4.1 cm (3 – 4.4), and with occlusive contractions detected was 3 cm (2 – 4.2), p = 0.001. Patients without contractility detected had a larger esophageal width than those with only non-occluding (p = 0.005) and occluding (p <0.001) contractions detected, but did not differ between patients with only non-occlusive and occlusive contractions (p = 0.21). The association of esophageal width and achalasia subtype as they relate to detection of non-occlusive and occlusive contractions is illustrated in Figure 3. There were seven patients (four type I, three type II) without contractions, three patients (two type II, one type III) with non-occluding contractions, and five patients (one type I, two type II, and two type III) with occluding contractions that did not have an esophagram available for review.
Figure 3. Association of esophageal width, achalasia subtype, and presence of occluding and non-occluding contractions.
Each patient that had an esophagram available for esophageal width measurement is represented according to their achalasia subtype. Median values within each contraction group are represented by the dashed horizontal lines.
Esophageal contractile patterns
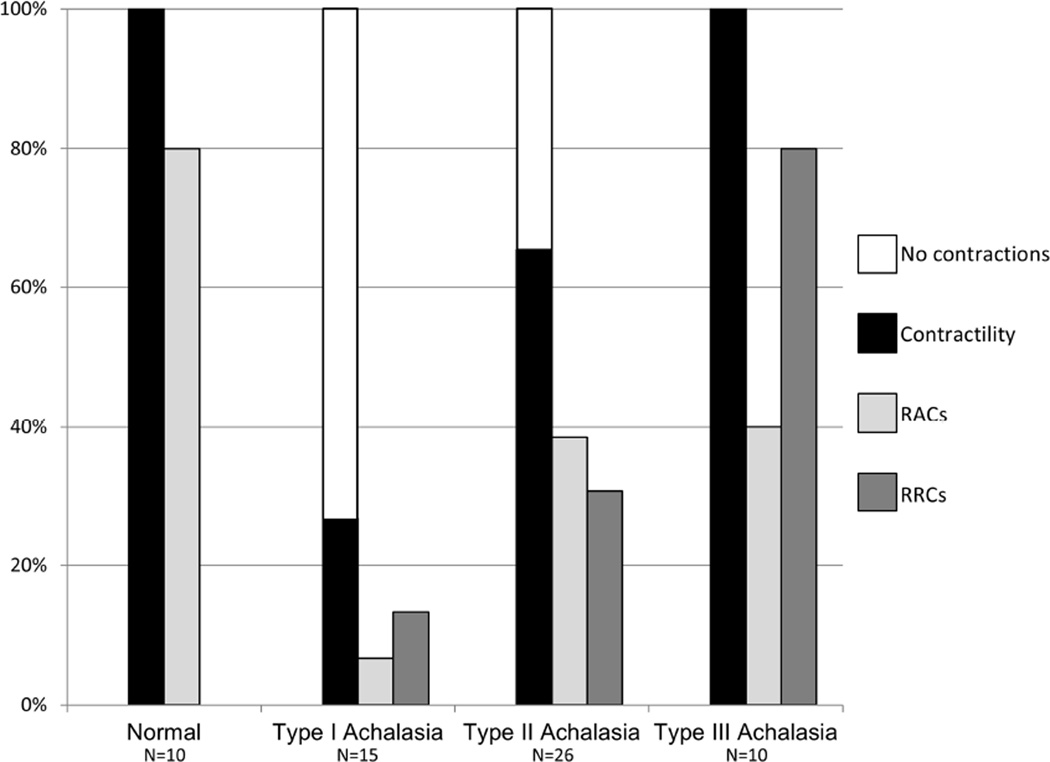
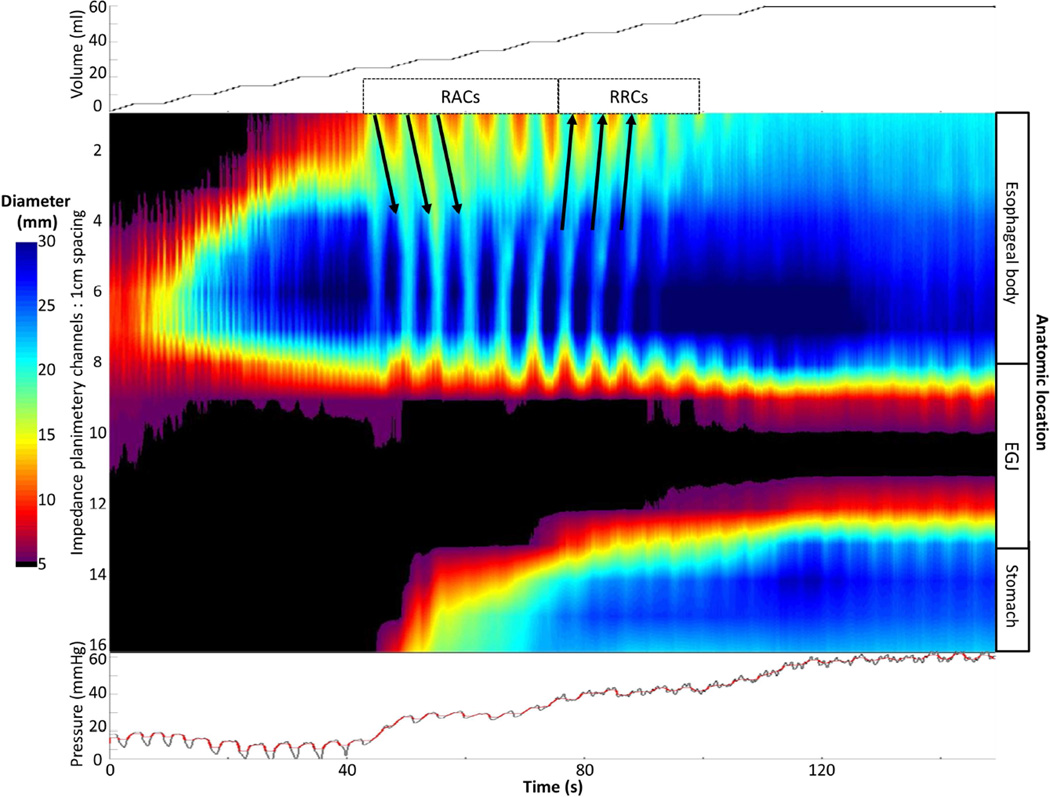
Esophageal contractility was observed with FLIP topography in all 10 of 10 controls. Eight of ten (80%) of controls demonstrated repetitive contractions; all of which propagated in the antegrade direction (i.e. 80% demonstrated RACs), an example is displayed in Figure 1. Retrograde contractions were observed in two of ten controls; no controls demonstrated RRCs.
Among all achalasia patients, 61% (31/51) demonstrated FLIP-detected contractility. Repetitive contractions were observed in 49% of patients with 29% demonstrating RACs and 35% demonstrating RRCs; nine patients (18%) had both RACs and RRCs. Retrograde contractions (non-repetitive and/or repetitive) were observed in 49% (25/51) of achalasia patients. Compared with controls, patients with achalasia (not differentiating by subtype) demonstrated contractility less frequently (p = 0.012), RACs less frequently (p = 0.004), and RRCs more frequently (p=0.021). Retrograde contractions (regardless of repetitive nature) appeared more frequently in achalasia patients than controls, but this was not statistically significant (p=0.088).
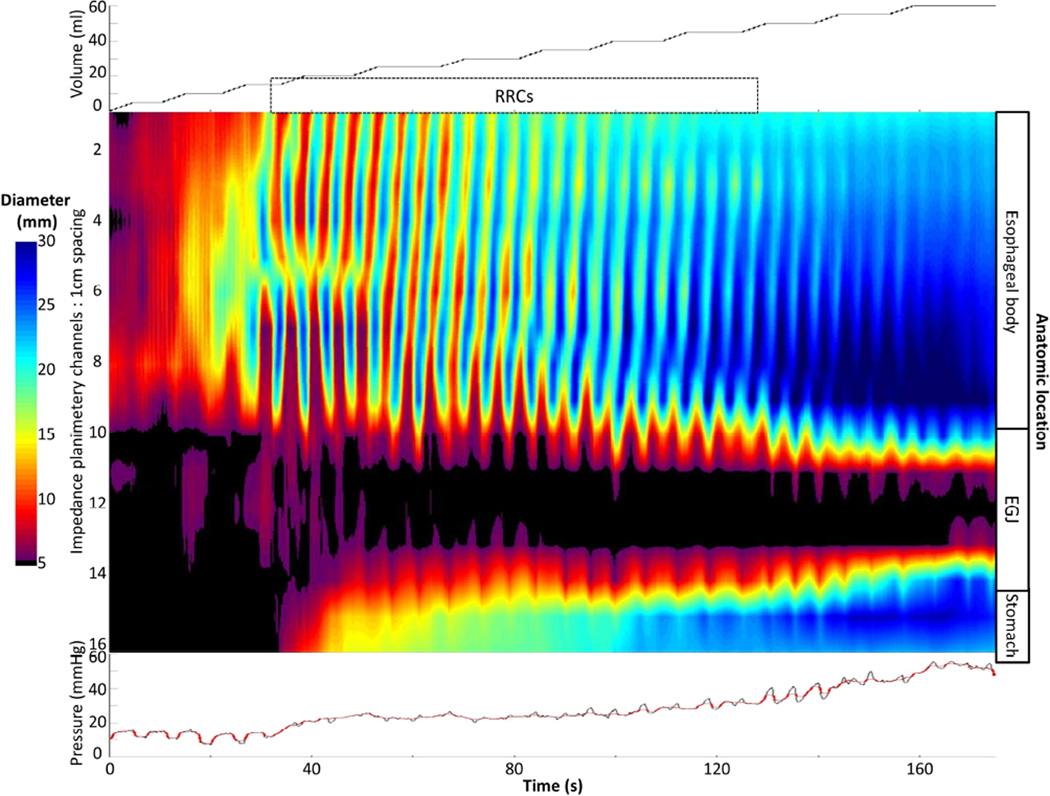
Differences in contractility patterns were also observed among achalasia subtypes (Figure 4). Examples of typical contractility patterns illustrated in both FLIP and pressure topography within each achalasia subtype are illustrated in Figure 5. Absent distension-related contractility was the predominant pattern observed in patients with type I achalasia, and some patients (35%) with type II achalasia, however 27% of patients with type I achalasia had some degree of contractility detected with FLIP; examples are displayed in Figure 6. There was heterogeneity among contractility patterns observed among patients with type II achalasia; 65% demonstrated some contractility. Contractility was detected less frequently in patients with type I achalasia than type II (p = 0.017) or type III (p <0.001) achalasia; there was a numeric trend toward decreased contractility observed between type II and type III (p = 0.032). A higher proportion of type III patients demonstrated RRCs than type I (p = 0.001) and type II (0.011), but the proportion of patients with RRCs did not differ between type I and type II (p = 0.210). Frequency of RACs did not statistically differ between achalasia subtypes (p = 0.071).
Figure 4. Frequencies of contractility patterns observed with FLIP topography among normal controls and achalasia subtypes.
Other than no contractions and contractility, which were mutually exclusive, a subject may have demonstrated more than one pattern. RACs - Repetitive, antegrade, contractions. RRCs - Repetitive, retrograde, contractions (RRCs).
Figure 5. Patterns of esophageal contractility detected with FLIP topography in achalasia.
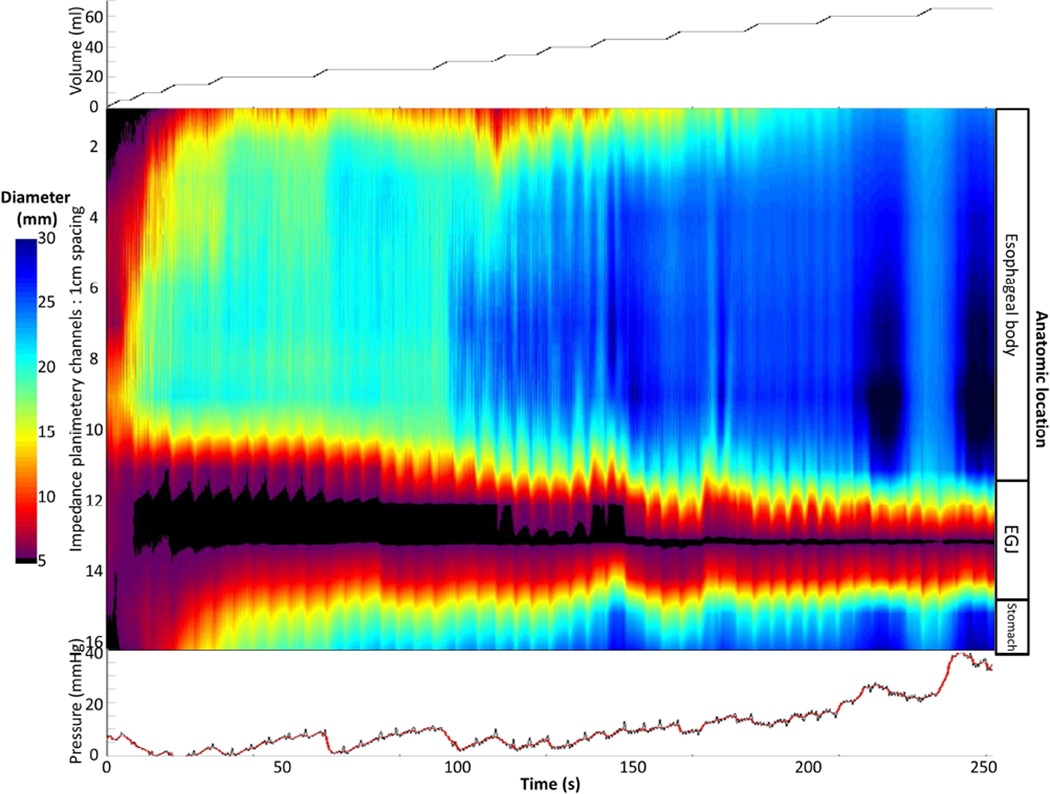
A). An example of absent contractility on FLIP topography is depicted in a patient with type I achalasia. B) In this example of FLIP topography in a type II patient, propagating contractions, both repetitive antegrade contractions (RACs; downward pointing black arrows)) and repetitive, retrograde contractions (RRCs; upward pointing black arrows) were observed. The narrowest luminal diameter achieved during contractions in this patient was 9.7 thus indicating non-occlusive contractions. C) On FLIP topography in this type III patient, RRCs, the most common pattern observed among type III achalasia patients, are observed. In each of the FLIP topographies (A – C) the narrow diameters at the esophagogastric junction (EGJ) throughout the distension protocol can be noted: The EGJ-distensibility index at in each of these patients was < 1.5 mm2/mmHg at 50 and 60-ml distension volumes. Figure used with permission from the Esophageal Center at Northwestern.
Figure 6. Potential for supplementary outcome prediction based on FLIP topography assessment.
Two swallows from two patients’ high-resolution manometries that met criteria for type I (A) and type II (C) achalasia are displayed. On FLIP topography, the type I patient (B) demonstrated contractility in the esophageal body, including both repetitive, antegrade contractions (RACs) and repetitive, retrograde contractions (RRCs); lumen occluding contractions were actually detected on FLIP topography (white arrows). Six months after 30-mm pneumatic dilation, the patient was asymptomatic. The FLIP topography for the type II patient (D) demonstrated absent contractility. After per-oral endoscopic myotomy and pneumatic dilation to 30 then 35- mm, the patient continued to have dysphagia on a daily basis. Figure used with permission from the Esophageal Center at Northwestern.
Esophagogastric junction distensibility-index
The EGJ-DI was calculated at 50 and 60-ml distension volumes in all 10 normal controls. There was a technical malfunction in the pressure sensor during studies of two achalasia patients (both with type II achalasia) that prevented EGJ-DI calculation. Thus, the EGJ-DI was calculated in 49 achalasia patients at 50-ml (15 type I, 24 type II, and 10 type III) and 47 patients at 60-ml (14 type I, 23 type II, and 10 type III).
The EGJ-DI was greater (i.e. EGJ was more distensible) in the controls than achalasia patients at both 50-ml [median (IQR) 7.1 mm2/mmHg (3.8 – 8.9) vs 1.0 (0.7 – 1.6); p < 0.001] and 60-ml [6.2 mm2/mmHg (4.6 – 8.1) vs 1.0 (0.5 – 1.2); p < 0.001] distension volumes. There was a difference in EGJ-DIs between achalasia subtypes at 50-ml (p= 0.036) with numeric trends toward a lower EGJ-DI in type III [0.7 mm2/mmHg (0.5 – 1.0)] than both type I [1.4 mm2/mmHg (1.0 – 1.7); p = 0.019] and type II [1.0 mm2/mmHg (0.8 – 1.7); p = 0.031]. Type I and type II had similar EGJ-DIs (p = 0.38). The difference in EGJ-DIs between achalasia subtypes was not significantly different at 60-ml (p = 0.13): Median (IQR) for Type I was 1.1 mm2/mmHg (0.8 – 1.7), type II was 0.9 (0.6 – 1.2), and type III was 0.5 (0.4 – 1.1).
Discussion
The major finding of this study was that by applying FLIP topography, a novel method to concurrently assess EGJ distensibility and distal esophageal contractility during volumetric distension, we frequently observed propagating contractions in achalasia patients with absent peristalsis on HRM/EPT. While we initially hypothesized that the FLIP would facilitate detection of non-luminal-occluding contractions in achalasia patients, we also detected luminal-occluding contractions in patients with all three achalasia subtypes. We also identified esophageal contractile patterns that varied in frequency between controls and achalasia subtypes, including a unique motor pattern not observed in our small cohort of control subjects: RRCs.
The conventional definition of achalasia includes a lack of peristalsis in the esophageal body in addition to impaired LES relaxation. With the advent of HRM/EPT and the description of the achalasia subtypes, achalasia with pan-esophageal pressurization was recognized as a subset of what had previously been grouped with ‘vigorous’ achalasia, a designation that would now be more appropriately limited to type III. Making this distinction improved prognostication of response to therapy.2 The mechanism of pan-esophageal pressurization has been attributed to contraction of esophageal longitudinal muscle.14, 22 Using a sophisticated methodology incorporating combined high-resolution impedance manometry and high-frequency intraluminal ultrasound, Hong et al examined esophageal longitudinal muscle contraction, identified by an increase in esophageal muscle CSA, in patients with achalasia: two with type I, seven with type II, and four with type III.14 They reported differing patterns of longitudinal muscle contraction among achalasia subtypes and identified longitudinal muscle contraction (with an absence of lumen occlusions) as the cause of luminal pressurization and esophageal emptying in type II and possibly type I achalasia. They also identified abnormal coordination of circular and longitudinal contraction in patients with type III achalasia. However, the authors acknowledged that their methodology could not detect non-occluding circular muscle contractions. Based on the current study, it appears that propagating contractions, which are likely due to circular muscle contraction, remain intact, in some patients with both type I and type II achalasia. Our detection of occluding contraction in several patients with type I achalasia was unexpected, though may be related to distension-induced esophageal stimulation from the FLIP balloon and/or air insufflation during endoscopy. Furthermore, the heterogeneity of FLIP topography patterns we observed among patients with type II achalasia may be indicative of there being more than one mechanism for generation of pan-esophageal pressurization: 1) longitudinal muscle contraction (associated with absent contractility with FLIP), 2) non-occluding circular muscle contractions causing luminal pressurization, and/or 3) occluding circular-muscle contractions concealed within pan-esophageal pressurization.
Retrograde contractions, including a unique pattern of RRCs, were frequently observed in achalasia patients with FLIP topography. Though retrograde contractions detected with manometry are occasionally observed on manometry in patients with and without achalasia, retrograde contractions and their potential clinical significance have not been previously been well described. One study using 24-hour ambulatory, water-perfused manometry reported retrograde pressure waves in four of eleven achalasia patients evaluated.23 Retrograde propagating contractions of circular muscle were also occasionally observed radiographically in a study of opossums with chronically obstructed esophagi resulting in dilatation.24 Though the mechanism of retrograde contractions remains unknown, an impairment of esophageal innervation has been proposed.23 Although we observed solitary retrograde contractions in two normal controls, RRCs were not seen in any of our ten controls; hence, RRCs may represent an aberrant response to persistent esophageal distension (i.e. secondary peristalsis) in the setting of impaired inhibitory innervation. Studies focused on secondary peristalsis in achalasia have not, to our knowledge, been previously reported. Another possible mechanism for retrograde contractions may be related to transient LES relaxation during which longitudinal muscle contraction has been reported to progress in a retrograde fashion in normal controls.25
Esophageal dilatation is a limiting factor to detection of esophageal contractions with both manometry and FLIP making luminal diameter a likely contributing determinant of achalasia subtype categorization. Previous reports of achalasia subtypes describe a greater degree of esophageal dilatation in patients with type I than with type II, which is sometimes greater than with type III.2, 4, 6, 7 While the initial description of achalasia subtypes reported severe esophageal dilatation determined by endoscopy was an independent predictor of poor treatment response (in addition to achalasia subtypes),2 another study using regression modeling found achalasia subtype, but not esophageal width, as an independent predictor of treatment response.4 Even though the FLIP bag distends to a diameter of 22 – 30 mm, it still may fail to detect esophageal contractility when the lumen is severely dilated. Given the association of esophageal width on the detection of contractility in our study, it is also unclear if distension of the FLIP catheter balloon induced esophageal contractility, akin to secondary peristalsis, or simply defined a threshold bag diameter necessary to detect non-occlusive esophageal contraction. While the clinical significance of non-occluding contractions in the dilated esophagus remains unclear, detection of contractions in patients with dilated esophagi may be a prognostic factor for an improved response to treatments that relieve EGJ outflow obstruction.
Based on our findings, the clinical criterion of achalasia regarding absent peristalsis may need to be revisited. Though the clinical significance of maintained contractility in achalasia requires additional study, its presence and pattern may be useful prognostic factors and could potentially be incorporated as a supplementary classification method to provided prognostication for response to treatment, particularly in patients with type I and type II achalasia. Just as pan-esophageal pressurization (which may occur from varying contributions of longitudinal and circular muscle) has been shown to facilitate esophageal emptying in type II achalasia,14 antegrade propagating contractility may also assist in esophageal emptying in some patients with achalasia. As return of peristalsis was associated with improved symptomatic and radiographic (timed-barium esophagram column height) outcomes,26 patients demonstrating RACs prior to intervention may be more likely to have a return of peristalsis and positive outcome following treatment (such as in Figure 6). Furthermore, achalasia sub-classification with FLIP topography may allow a method to further stage achalasia along a continuum of the disease course, similar to the proposed pathologic progression reflected by the manometric achalasia subtypes.27 However, use of FLIP topography for assessment of esophageal function is in its early phases of development and requires further study before definitive conclusions of clinical significance can be reached.
This study and the methodology employed have some inherent limitations. One lies within our definition of contractility (i.e. using a change in diameter of 5-mm as a threshold). While this may artificially dichotomize the presence or absence of contractility, the observation of repetitive contractions appears to be a unique esophageal motor pattern. Additionally, without concurrent manometry or intraluminal ultrasound, we are unable to determine the direct temporal association of FLIP topography findings with pan-esophageal pressurization and/or longitudinal muscle contractions; thus with the current methodology, these relationships are only inferred at this time. Additional limitations with the FLIP regard the concurrent use with endoscopy along with moderate sedation, the questionable reliability of intra-bag pressure measurement from a single pressure sensor at the distal end of the device (which lies in the stomach distal to the often closed EGJ), and the inability to distinguish between swallow-induced and distension-related contractility.18 Thus, continued study with FLIP topography, potentially incorporating concurrent radiographic, manometric, and/or ultrasound assessment, will be needed to refine analysis paradigms and improve its evaluation of esophageal function.
In conclusion, using a novel methodology (FLIP topography) to concurrently assess EGJ distensibility and distal esophageal contractility, we report the observation of propagating contractions in untreated patients with type I and type II achalasia. Additionally, while normal controls predominantly demonstrated RACs, which likely represent normal secondary peristalsis, we identified a unique contractile pattern in achalasia patients (particularly type III), RRCs, which may be a marker of impaired esophageal inhibitory neural function. Thus, FLIP topography offers a method to uncover propagating contractions that are not detected with manometry. Though additional longitudinal patient study is required to demonstrate the clinical significance of these observations, assessment of esophageal contractility in achalasia with FLIP topography may be a useful tool to supplement the manometric evaluation.
Acknowledgments
Grant support: This work was supported by T32 DK101363 (JEP) and R01 DK079992 (JEP) from the Public Health service.
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
John E. Pandolfino, Zhiyue Lin, and Peter J. Kahrilas: Patent filed for FLIP topography methodology with Crospon, Inc. through Northwestern University
Abbreviations
- CSA
cross-sectional area
- EGJ
esophagogastric junction
- DI
distensibility index
- EPT
esophageal pressure topography
- FLIP
functional lumen imaging probe
- HRM
high-resolution manometry
- IQR
interquartile range
- IRP
integrated relaxation pressure
- LES
lower esophageal sphincter
- RAC
repetitive, antegrade contraction
- RRC
repetitive, retrograde contraction
- TBE
timed barium esophagram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dustin A. Carlson, Joel Sternbach, Erica Eschelbach, Laurel Friesen, Zoe Listernick, Benjamin Mogni: None
Author contributions: DAC contributed to study concept and design, data analysis and interpretation of data, drafting of the manuscript, and approval of the final version. ZL contributed to data analysis, technical support, and approval of the final version. JS, EE, LF, ZL, BM, contributed to data acquisition and analysis and approval of the final version. PJK contributed to revising the manuscript critically and approval of the final version. JEP contributed to study concept and design, analysis and interpretation of the data, revising the manuscript critically, and approval of the final version.
References
- 1.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. The American journal of gastroenterology. 2013;108(8):1238–1249. doi: 10.1038/ajg.2013.196. quiz 50. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135(5):1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvador R, Costantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14(11):1635–1645. doi: 10.1007/s11605-010-1318-4. [DOI] [PubMed] [Google Scholar]
- 5.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. Journal of neurogastroenterology and motility. 2011;17(1):48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144(4):718–725. doi: 10.1053/j.gastro.2012.12.027. quiz e13-4. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Kim N, Kim SE, et al. Clinical characteristics and treatment outcomes of 3 subtypes of achalasia according to the chicago classification in a tertiary institute in Korea. Journal of neurogastroenterology and motility. 2013;19(4):485–494. doi: 10.5056/jnm.2013.19.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia: more the rule than the exception. JAMA surgery. 2013;148(2):157–164. doi: 10.1001/2013.jamasurg.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Factors involved in the return of peristalsis in patients with achalasia of the cardia after Heller's myotomy. The American journal of gastroenterology. 1995;90(5):713–717. [PubMed] [Google Scholar]
- 10.Zaninotto G, Costantini M, Anselmino M, et al. Onset of oesophageal peristalsis after surgery for idiopathic achalasia. The British journal of surgery. 1995;82(11):1532–1534. doi: 10.1002/bjs.1800821125. [DOI] [PubMed] [Google Scholar]
- 11.Mittal RK, Ren J, McCallum RW, et al. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. The American journal of physiology. 1990;258(2 Pt 1):G208–G215. doi: 10.1152/ajpgi.1990.258.2.G208. [DOI] [PubMed] [Google Scholar]
- 12.Schneider JH, Peters JH, Kirkman E, et al. Are the motility abnormalities of achalasia reversible? An experimental outflow obstruction in the feline model. Surgery. 1999;125(5):498–503. [PubMed] [Google Scholar]
- 13.Khan A, Ren-Fielding C, Traube M. Potentially reversible pseudoachalasia after laparoscopic adjustable gastric banding. Journal of clinical gastroenterology. 2011;45(9):775–779. doi: 10.1097/MCG.0b013e318226ae14. [DOI] [PubMed] [Google Scholar]
- 14.Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139(1):102–111. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon BP, Frokjaer JB, Drewes AM, et al. A new measurement of oesophago-gastric junction competence. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(5):543–546. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 16.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(6):496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27(7):981–989. doi: 10.1111/nmo.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Z, Kahrilas PJ, Xiao Y, et al. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therapeutic advances in gastroenterology. 2013;6(2):97–107. doi: 10.1177/1756283X12470017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(11):e765–e771. doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatek MA, Pandolfino JE, Hirano I, et al. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointestinal endoscopy. 2010;72(2):272–278. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tutuian R, Pohl D, Castell DO, et al. Clearance mechanisms of the aperistaltic oesophagus: the •pump gun• hypothesis. Gut. 2006;55(4):584–585. doi: 10.1136/gut.2005.085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Herwaarden MA, Samsom M, Smout AJ. Prolonged manometric recordings of oesophagus and lower oesophageal sphincter in achalasia patients. Gut. 2001;49(6):813–821. doi: 10.1136/gut.49.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Schulze-Delrieu K, Shirazi S, et al. Dynamic imaging of obstructed opossum esophagus. From altered load to altered contractility. Digestive diseases and sciences. 1994;39(7):1377–1388. doi: 10.1007/BF02088037. [DOI] [PubMed] [Google Scholar]
- 25.Babaei A, Bhargava V, Korsapati H, et al. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology. 2008;134(5):1322–1331. doi: 10.1053/j.gastro.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Nicodeme F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(2):131–137. doi: 10.1016/j.cgh.2012.10.015. quiz e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013;145(5):954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]