Abstract
Objectives
Determine if a brief intervention (BI) reduces the negative consequences of drug use/misuse among adult emergency department (ED) patients, and identify patients more likely to benefit from the BI.
Methods
This randomized, controlled trial enrolled 1,026 18–64 year-old ED patients whose drug misuse indicated a needed for a BI. Differences in total Inventory of Drug Use Consequences (InDUC) scores between the treatment (BI) and control arms (no BI) were assessed every 90 days over a one-year period. Regression models were constructed to identify demographic and clinical factors associated with greater reductions in total InDUC scores.
Results
Although total InDUC scores decreased for both the treatment and control arms, there were no differences in scores between the treatment and the control arms at baseline at each follow-up. In the regression analyses, participants who were not using drugs or received drug treatment in the past 90 days generally had lower InDUC scores at each follow-up.
Conclusions
Although negative consequences decreased in both study arms over time, receiving a BI did not lead to a greater reduction in the negative consequences of drug misuse than not receiving a BI. Of importance in the design of future ED drug misuse interventions, participants who were successful in stopping their drug misuse or receiving drug treatment did show fewer negative consequences of drug use/misuse.
Keywords: adult, emergency medical services/methods, emergency service, motivational interviewing, substance-related disorders/therapy, treatment outcome
1. INTRODUCTION
Validating anecdote and conventional wisdom, recent research has documented a high prevalence of drug use/misuse among adult emergency department (ED) patients in the United States (US; Blow et al., 2011; Wu et al., 2012; Johnson et al., 2013; Hankin et al., 2013; Sanjuan et al., 2014; Macias Konstantopoulos et al., 2014), which in some EDs is greater than the underlying general population that they serve (Bernardino, 2014). Despite this high prevalence, few ED patients with drug misuse problems have accessed drug treatment that might help them reduce or eliminate their drug use/misuse and its concomitant negative consequences (Rockett et al., 2003; Breton et al., 2007). In recognition of the high drug use/misuse prevalence and low access to treatment among ED patients, substance misuse researchers and clinicians have advocated for research to further understand how to capitalize on the captive audience of ED patients by identifying those who need treatment, providing initial interventions and encouraging follow up with treatment sources as they are available (Cunningham et al., 2009, Bernstein et al., 2009). Given the many devastating negative financial, health, psychological and social consequences of drug use/misuse, it is important that ED-based interventions not only are effective in reducing or eliminating drug use/misuse, but also have a meaningful impact on decreasing its negative consequences.
Insight into the creation of potential effective drug use/misuse interventions might be drawn from experience with ED-based alcohol use/misuse interventions. Although their use has been recommended (Cunningham et al., 2009), brief interventions (BIs) have had mixed results in reducing or eliminating alcohol consumption among adolescent and ED patients (Nilsen et al., 2007; Havard et al., 2008; Yuma-Guerrero et al., 2012; Cochran et al., 2014; Dent et al., 2008; Wojnar and Jakubczyk, 2014; , 2008; Sommers et al., 2013; Bernstein et al., 2010; Academic ED SBIRT Research Collaborative, 2010; Newton et al., 2013). Furthermore, researchers have identified either decreases (Longabaugh et al., 2001; Blow et al., 2006) or no impact (Mello et al., 2013; D’Onofrio et al., 2012) of BIs on ED adult patient reported negative consequences of alcohol consumption as compared to control conditions. In a review of applicable studies, Havard, et al. (2008) concluded that BIs as compared to standard care could reduce (Odds ratio [OR 0.59]) subsequent alcohol-related injuries among ED patients in the succeeding 6–12 months, but found that due to study heterogeneity conducting a meta-analysis on the impact of BIs on decreasing the negative consequences of alcohol use/misuse was not possible.
The impact of BIs on reducing or eliminating drug/misuse among ED patients has been less well studied. In a recent randomized, controlled trial, Bogenschutz et al. (2014) found that a BI with a telephone booster was not more efficacious in reducing self-reported days of drug use over a one-year period than minimal screening only or screening, assessment and referral to treatment. However, there are no published studies that have assessed if BIs can decrease the negative consequences of drug use/misuse among adult ED patients. Further research is needed to examine the efficacy of BIs in reducing drug use/misuse as well as their negative consequences before this approach can be recommended--or not recommended--for ED patients.
This investigation focused on evaluating the efficacy of a BI in reducing the negative consequences of drug use/misuse in a randomized, controlled trial: the Brief Intervention for Drug Misuse for the Emergency Department (BIDMED) study. The primary aim was to ascertain if a BI aimed at reducing drug use/misuse negative consequences among adult ED patients was more efficacious than no BI (study questionnaires only) over a one-year period. The secondary aim was to determine if there were demographic or clinical factors associated with decreases in the negative consequences of their drug misuse, such as severity of drug misuse at baseline enrollment and participation in a drug program, which might identify a sub-group of ED patients for whom a BI or other intervention is more beneficial.
2. METHODS
2.1 Study design and setting
BIDMED was a randomized clinical trial conducted over a 30-month period from July, 2010-December, 2012 in The Miriam Hospital and the Rhode Island Hospital EDs. The hospital institutional review board approved the study.
2.2 Study population
A random sample of ED patients was screened for study eligibility, recruited and enrolled from 8:00 am to midnight seven days a week when research assistants (RAs) were available to conduct the study. Prior to each shift of data collection, the RAs generated lists of the patient rooms in the EDs in random order with replacement using an internet-based random selection program (www.random.org). RAs reviewed the ED electronic medical records (EMRs) to determine the study eligibility of those patients whose rooms were selected. If potentially study eligible, these selected ED patients were interviewed to confirm their study eligibility. Preliminary study eligibility criteria were randomly selected ED patients who were 18–64 years-old; English- or Spanish-speaking; not critically ill or injured; not prison inmates, under arrest, nor undergoing home confinement; not presenting for an acute psychiatric illness; not requesting treatment for substance use/misuse; not intoxicated; and not having a physical or mental impairment that prevented providing consent or participating in the study.
Patients who met preliminary study eligibility were asked to take the Alcohol, Smoking and Substance Involvement Screening Test, Version 3 (ASSIST; Humeniuk et al., 2008) using an audio computer-assisted self-interviewer (ACASI). The adaptation, pilot testing, psychometric assessments and reading level of this and the other study instruments for the BIDMED study has been described previously (Merchant et al., 2014). ED patients with an ASSIST score of ≥ 4 points for any ASSIST drug category or those who ever injected drugs were invited to enroll in BIDMED. Per World Health Organization (WHO) recommendations, an ASSIST score ≥4 points for any drug category indicates a need for a BI, whereas a score ≥27 points indicates a need for a more intensive intervention (Humeniuk et al., 2008). However, in the BIDMED study, only a BI (or no BI, depending on randomization) was provided as part of the study.
2.3 Study protocol
After enrollment, participants were assigned into one of the two study arms (treatment arm [BI] or control arm [no BI]) using block randomization (1:1 assignment) with a block size of six. Afterwards, participants completed the Inventory of Drug Use Consequences (InDUC) questionnaire (Tonigan and Miller 2002), and the Treatment Services Review (TSR) questionnaire (McLellan, 1992). The 45-item InDUC provides a total score as well as subscores on five non-overlapping subscales: physical, interpersonal, intrapersonal, impulse control, and social responsibility. We used the InDUC to assess the negative consequences of drug use/misuse over the prior 90 days at baseline and each follow-up period. The TSR is a 5-minute questionnaire that participants used to indicate drug treatment programs that they attended or at least contacted within the prior 90 days. The study authors adapted the TSR so that participants could recognize the names of local substance misuse treatment programs and to permit its administration by ACASI.
After completing the study questionnaires, participants randomly assigned to the treatment arm received a BI by a trained interventionist who was not a part of the ED clinical staff. The interventionists met with the study investigators throughout the study to discuss clinical and procedural issues arising from the delivery of the BI. To ensure fidelity to the BI, each interventionist voice recorded his/her BI sessions and these were reviewed by the psychologist research team members and discussed with the interventionist. Deviations from the BI protocol were addressed and suggestions for improvement were provided at these review sessions.
At 3, 6, 9 and 12 months post-enrollment, participants repeated the baseline questionnaires via the Internet at a location of their choice. RAs sent reminders via email, letter or telephone before each follow-up time point. Participants received gift cards to a local pharmacy for completing the baseline and each successive follow-up.
2.4 BI description
The primary goal of the BI was to motivate participants to reduce or eliminate their drug use/misuse, seek appropriate treatment, and decrease the negative consequences of their drug misuse (See supplemental material for the BI outline). The BI sessions were approximately 20–30 minutes in duration and were based on two theoretically driven approaches to behavior change: motivational interviewing (Miller et al., 2002) and the health beliefs model (Rosenstock, 1974). During BI sessions, the interventionist asked each participant about his or her history and experiences with drug use/misuse and received feedback about their drug use/misuse. In addition, participants were queried about the negative consequences of their drug use/misuse and behaviors that support their drug use/misuse. Finally, the interventionist summarized statements about expressed change to drug use and help participants develop change plans. Participants were encouraged to seek further treatment commensurate with their need for a more intensive intervention.
2.5 Data analysis
The data analysis consisted first of an examination of enrollment and follow-up, followed by an analysis of the primary outcome of the impact of the BI on changes in negative consequences of drug misuse over time, as measured by InDUC scores. Next, secondary analyses were performed to examine the consistency of the primary outcome findings after accounting for missing data and propensity to remain or not remain in the study. Finally, factors associated with changes in InDUC scores in sub-groups were explored.
2.5.1 Participant enrollment, follow-up and study completion comparisons
We created a diagram depicting the results of enrollment and follow-up by study arm at baseline and completion of each follow-up. To assess differential loss to follow-up by study arm, demographic characteristics of participants were compared by study arm at baseline and for those who completed each follow-up using student’s t-test and Pearson Chi-square tests. An α=0.05 significance level was used to establish differences.
2.5.2 Primary outcome analysis
We first conducted a primary outcome analysis investigating the impact of the BI on InDUC scores and subscores over time using the unadjusted data (no adjustments for missing data) without inclusion of potential factors that might impact InDUC scores (e.g., demographic characteristics, type of drug misuse intervention needed at baseline enrollment, participation in a drug misuse treatment program, etc.). The primary analysis consisted of comparing total InDUC scores and subscores between the study arms at each follow-up. Differences in mean total InDUC scores and subscores were calculated along with corresponding 95% confidence intervals (CIs). Total InDUC scores and subscores by study arm and the differences in scores and subscores were plotted over time.
2.5.3 Imputation procedures for missing data
As expected for longitudinal studies, particularly among substance misusing populations, the data were incomplete due to participants not completing one or more of their required follow-up questionnaires. We first compared participant characteristics by study arm for those who completed vs. did not complete each follow-up to provide guidance on which covariates would need to be considered for missing data imputation. Covariates at an α=0.05 significance level were considered further to be included in models for imputation. Two approaches to account for missing data were used: regression-based multiple imputations, and propensity score-based multiple imputations. These complementary approaches permitted us to conduct secondary analyses that provided lesser (regression-based) or greater (propensity-based) weight to concerns about differential loss to follow-up by study arm at each follow-up period.
For the regression-based multiple imputations, we created two different regression models to impute InDUC scores and the missing covariates. First, linear models were constructed using subsets of participants who completed each follow-up (3, 6, 9 and 12 months). The outcomes for these models were the five InDUC subscores at each follow-up, and predictors were participant demographic characteristics (age, gender, race, insurance status, etc.) and all InDUC subscores before this follow-up. Separate models were constructed for each study arm (BI or no BI). Next, logistic regression was used to impute missing covariate values for the binary outcomes of any vs. no drug use, and any drug treatment vs. no drug treatment received during the preceding 90 days at each follow-up again using subsets of participants who completed that follow-up. Separate models were created for each study arm for each follow-up. We created 10 separate imputed datasets for each study arm for each follow-up.
For the propensity score-based multiple imputations, propensity scores were used to account for the propensity to remain vs. not remain in the study at each follow-up by study arm. Data from those who completed the study at each follow-up again were used for these calculations. We created five strata of propensity scores for remaining in the study at the quintile points of the calculated propensity score for each follow-up period. For each strata, we imputed the missing values (total InDUC scores, five InDUC subscores, use or no use of drugs within the past 90 days, and receipt of drug treatment within the past 90 days) using data from a randomly chosen participant in the same strata who completed data at that follow-up time point. In a manner similar to the regression-based multiple imputations, we created 10 separate imputed datasets for each study arm for each follow-up.
2.5.4 Secondary analyses using imputed datasets
We conducted a series of secondary analyses that repeated the aforementioned primary outcome analysis to examine consistency of the primary outcome results after accounting for missing data and propensity to remain or leave the study. For these secondary analyses, we used the regression-based and propensity-based multiple imputed datasets we created. In a similar manner as the primary outcome analysis, means, standard errors and differences in total InDUC score and subscores by study arm for each follow-up were calculated and the differences in scores and subscores along with 95% CIs of differences were plotted over time.
2.5.5 Identification of factors associated with changes in InDUC scores
We identified demographic and clinical factors associated with changes in InDUC scores from baseline through follow-up using two modeling approaches. In the first approach, changes in total InDUC scores between baseline and each follow-up (3, 6, 9, and 12 months) were modeled separately, adjusting for total InDUC scores at baseline. This analysis permitted a comparison of each follow-up against the baseline condition, which aimed to highlight when changes in InDUC scores and hence changes in negative consequences of drug misuse might be more apparent. These models endeavored to indicate times when the effect of the intervention might be more or less pronounced. In the second approach, changes in InDUC scores over time (a longitudinal/repeated measures analysis) taking into account the correlated nature of the data was assessed, again adjusting for total InDUC scores at baseline. This analysis provided a perspective of changes over time and possible patterns in those changes. Mirroring the analytic procedures undertaken for the primary and secondary analyses, models were constructed for these two approaches using the unadjusted dataset and then the two imputed datasets.
First, bivariable linear models were constructed to examine the relationship between the total InDUC scores at each follow-up and predictors of interest (participant demographic characteristics, study arm, type of WHO-recommended intervention based on ASSIST scores at study enrollment, drug misuse treatment utilization during the prior 90 days, and use of any drug in the prior 90 days), adjusting for total InDUC scores at baseline. Next, multivariable models were constructed that included baseline total InDUC scores, use of drugs within the past 90 days before each follow-up, receipt of drug treatment in the past 90 days before each follow-up, study arm, and WHO-recommended intervention (brief or more intensive) at baseline based on the ASSIST. In addition to these variables, demographic characteristic covariates significant at the α=0.05 level from the bivariable models were included in the multivariable models.
For the longitudinal/repeated measures analyses only, the cumulative effects of 90 day periods without drug use or ongoing/episodic utilization of drug treatment services (on a continuous scale of zero to four 90-day periods) were considered along with the short-term effects (immediate past 90-days). Given the possibility that time might exert a differential effect on InDUC scores at each follow-up period, follow-up periods were entered as a categorical rather than a continuous variable. Interactions between study arm, drug use cessation, and drug treatment at each follow to measure differential impact on total InDUC scores were assessed in these analyses. As further check for consistency of the outcomes measured in the longitudinal/repeated measures analyses, models for participants who completed all four follow-ups also were constructed and analyzed, in addition to the unadjusted and two imputed datasets. Because total InDUC scores approximated a normal distribution, no data transformations were performed.
Beta coefficients (βs) and corresponding 95% CIs were estimated for all linear regression models. Variable and model checking procedures (e.g., Hosmer-Lemeshow testing for logistic regression models; tests for homoscedasticity, normality and linearity for linear regression models; likelihood ratio testing to compare linear regression models with different forms or inclusion of variables, such as quadratic terms for time, etc.) were performed to verify model fitness and appropriateness.
3. RESULTS
3.1 Enrollment and follow-up retention
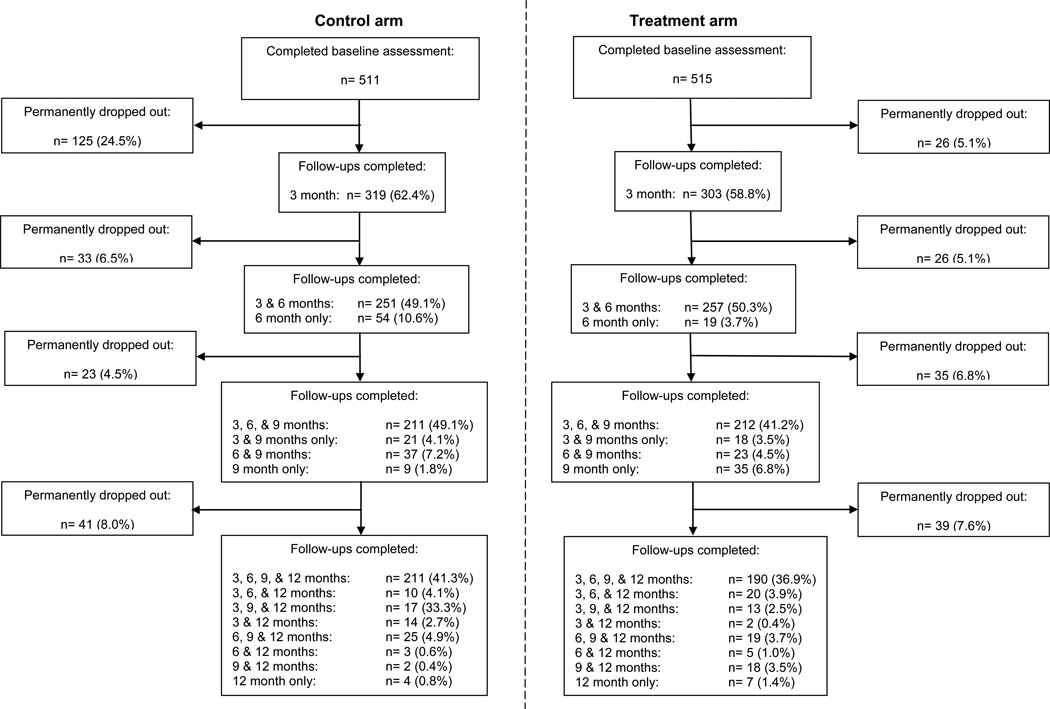
The enrollment and follow-up diagram is displayed in Figure 1. Of the 511 participants in the control arm, 62.3%, 55.9%, 58.0%, and 59.8% participants completed the 3, 6, 9, and 12-month follow-ups, respectively, and of the 515 participants in the treatment arm, these percentages were 59.4%, 57.9%, 54.9%, and 56.5%, respectively. The majority of the participants were male (54.2%), white/non-Hispanic (56.8%); most had twelve or more years of formal education (36.4%) and had governmental healthcare insurance (39.8%); were never married (52.1%), employed (36.2%), not homeless (79.3%), and received their medical care from an ED or urgent care center (42.3%). As shown in Table 1, the demographic characteristics of participants at baseline and those who completed each follow-up were similar between the two study arms. Supplemental Table 1 depicts the differences in participant characteristics by study arm at each follow-up for those who did vs. did not complete each follow-up.
Fig. 1.
Enrollment and follow-up during the Brief Intervention for Drug Misuse in the Emergency Department (BIDMED) study
Table 1.
Comparison of participant demographic characteristics and need for intervention at baseline by study arm, baseline through follow-ups
| Baseline |
3 month follow-up |
6 month follow-up |
9 month follow-up |
12 month follow-up |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Control arm |
Treatment arm |
Control arm |
Treatment arm |
Control arm |
Treatment arm |
Control arm |
Treatment arm |
Control arm |
Treatment arm |
|||||||
|
n=511 |
n=515 |
p-value |
n=321 |
n=304 |
p-value |
n=288 |
n=296 |
p-value |
n=299 |
n=283 |
p-value |
n=308 |
n=289 |
p-value | ||
| Demographic Characteristics | p< | p< | p< | p< | p< | |||||||||||
| Median age (year [IQR]) | 30 (24,43) | 30 (23,42) | 0.48 | 31 (25,44) | 31 (24,44) | 0.92 | 32 (25,44) | 32 (24,44) | 0.75 | 31 (25,44) | 32 (23, 44) | 0.35 | 31 (25, 44) | 32 (24,42) | 0.44 | |
| % | % | % | % | % | % | % | % | % | % | |||||||
| Gender (female) | 44.0 | 47.5 | 0.26 | 47.0 | 53.0 | 0.14 | 47.9 | 51.0 | 0.45 | 48.5 | 51.6 | 0.46 | 48.1 | 51.9 | 0.35 | |
| Race | 0.58 | 0.15 | 0.11 | 0.65 | 0.75 | |||||||||||
| White, non-Hispanic | 57.8 | 55.8 | 64.8 | 55.9 | 64.2 | 54.1 | 60.9 | 54.8 | 61.0 | 57.1 | ||||||
| Hispanic | 10.3 | 8.7 | 7.8 | 7.2 | 7.3 | 7.4 | 7.7 | 8.1 | 8.4 | 7.3 | ||||||
| Black/African-American, non-Hispanic | 23.0 | 26.7 | 21.5 | 27.6 | 21.5 | 29.4 | 23.4 | 27.6 | 23.1 | 26.3 | ||||||
| Other | 9.0 | 8.7 | 3.7 | 5.6 | 9.0 | 9.1 | 8.1 | 9.5 | 7.4 | 9.3 | ||||||
| Years of formal education | 0.17 | 0.18 | 0.12 | 0.14 | 0.28 | |||||||||||
| Less than 12 yrs | 34.6 | 40.1 | 32.4 | 37.8 | 31.3 | 37.2 | 30.1 | 38.2 | 32.8 | 38.1 | ||||||
| Grade 12 | 29.8 | 30.2 | 28.4 | 30.3 | 28.8 | 30.4 | 29.8 | 27.9 | 27.3 | 26.6 | ||||||
| More than 12 yrs | 35.6 | 29.7 | 39.2 | 32.0 | 40.0 | 32.4 | 30.1 | 33.9 | 40.0 | 35.3 | ||||||
| Insurance status | 0.95 | 0.74 | 0.89 | 0.71 | 0.79 | |||||||||||
| Private | 22.4 | 21.7 | 25.6 | 25.3 | 26.0 | 24.3 | 26.4 | 24.4 | 25.3 | 23.5 | ||||||
| Governmental | 38.9 | 39.7 | 38.0 | 40.8 | 39.9 | 40.9 | 38.5 | 41.7 | 38.6 | 41.2 | ||||||
| None | 38.7 | 38.6 | 36.5 | 33.9 | 34.0 | 34.8 | 35.1 | 33.9 | 36.0 | 35.3 | ||||||
| Marital status | 0.52 | 0.31 | 0.42 | 0.84 | 0.47 | |||||||||||
| Married | 12.1 | 11.8 | 15.9 | 13.2 | 16.0 | 12.2 | 13.0 | 14.1 | 13.0 | 13.8 | ||||||
| Divorced/Widowed/Separated | 14.6 | 17.8 | 12.2 | 16.5 | 13.5 | 17.2 | 13.7 | 15.9 | 13.3 | 17.7 | ||||||
| Never married | 52.5 | 51.6 | 52.0 | 48.4 | 49.3 | 50.0 | 52.8 | 50.2 | 52.9 | 48.4 | ||||||
| Unmarried couple | 20.8 | 18.8 | 20.0 | 22.0 | 21.2 | 20.6 | 20.4 | 19.8 | 20.8 | 20.0 | ||||||
| Homelessness | 0.72 | 0.19 | 0.31 | 0.93 | 0.69 | |||||||||||
| Currently/Homeless in past year | 19.7 | 21.7 | 15.6 | 21.1 | 16.3 | 21.3 | 17.8 | 18.4 | 18.1 | 20.8 | ||||||
| Never homeless | 80.4 | 78.3 | 84.4 | 79.0 | 83.7 | 78.7 | 82.3 | 81.6 | 81.8 | 79.2 | ||||||
| Employment status | 0.16 | 0.55 | 0.17 | 0.22 | 0.40 | |||||||||||
| Employed | 39.5 | 32.8 | 37.7 | 32.3 | 38.5 | 30.1 | 38.5 | 30.7 | 36.4 | 29.8 | ||||||
| Disability | 19.8 | 22.5 | 20.6 | 22.4 | 22.2 | 23.7 | 21.1 | 21.9 | 21.4 | 23.5 | ||||||
| Student | 7.8 | 8.0 | 9.4 | 9.5 | 7.6 | 9.8 | 8.7 | 11.7 | 9.4 | 10.4 | ||||||
| Unemployed | 32.9 | 36.8 | 32.4 | 35.9 | 31.6 | 36.5 | 31.8 | 35.7 | 32.8 | 36.3 | ||||||
| Usual source of medical care | 0.71 | 0.71 | 0.81 | 0.78 | 0.76 | |||||||||||
| Private clinic | 26.5 | 27.1 | 29.3 | 29.6 | 30.9 | 29.4 | 32.4 | 21.1 | 31.8 | 30.5 | ||||||
| Hospital or community clinic | 29.8 | 30.4 | 29.6 | 33.2 | 31.6 | 33.1 | 29.1 | 32.2 | 29.9 | 33.6 | ||||||
| Emergency department or urgent care center | 43.4 | 42.4 | 41.1 | 37.1 | 37.5 | 37.5 | 38.1 | 36.7 | 35.4 | 33.2 | ||||||
| Intervention needed at baseline | 0.81 | 0.48 | 0.82 | 0.83 | 0.73 | |||||||||||
| Brief intervention | 82.5 | 83.1 | 80.9 | 80.3 | 82.1 | 82.5 | 82.4 | 82.9 | 81.6 | 82.1 | ||||||
| Intensive intervention | 17.5 | 16.9 | 19.1 | 19.7 | 17.9 | 17.5 | 17.6 | 17.1 | 18.4 | 17.9 | ||||||
Key: IQR=Interquartile range; Control arm=No brief intervention arm; Treatment arm=Brief intervention arm
3.2. Primary outcome analysis examining impact of the BI on InDUC scores and subscores
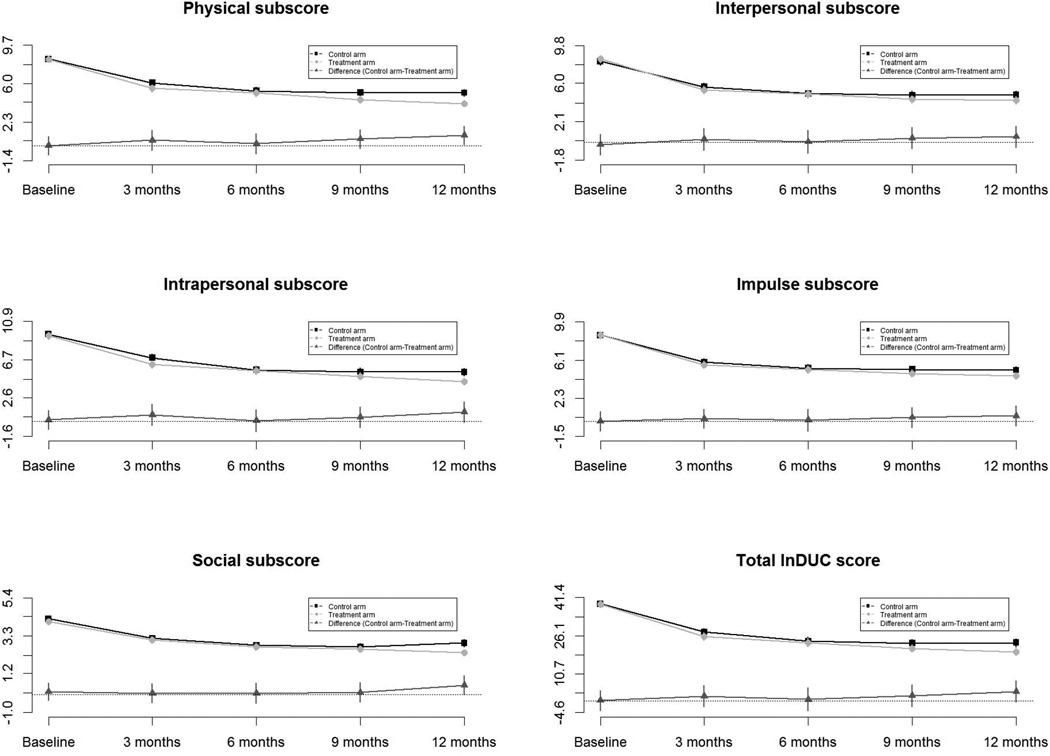
For the primary outcome analysis using the unadjusted dataset (no adjustment for missing data or propensity for remaining/not remaining in the study), the average scores in five subscale scores, total InDUC scores and difference in InDUC scores at the baseline and follow-up contacts by arm are summarized in Table 2. The patterns of differences in InDUC scores by study arm over time are displayed in Figure 2. As shown in Table 2 and Figure 2, total InDUC scores decreased 33.7% from baseline to 3-month follow-up and 57.6% from baseline to 12-month follow-up in the treatment arm, and by 28.9% and 44.6% in the control arm, respectively. Also as shown, there was a large decrease in total InDUC scores from baseline to the three-month follow-up, but total scores remained stable for the remainder of the 12-month follow-up period. However, there were no differences in total InDUC scores between study arms at each follow-up.
Table 2.
Differences in InDUC scores between control and treatment arms, baseline through follow-ups (unadjusted data)
| Control arm | |||||
|---|---|---|---|---|---|
| Baseline | 3 month follow-up | 6 month follow-up | 9 month follow-up | 12 month follow-up | |
| n=511 | n=319 | n=305 | n=278 | n=286 | |
| InDUC categories | x̄(SE) | x̄(SE) | x̄(SE) | x̄(SE) | x̄(SE) |
| Physical | 8.33 (0.31) | 6.06 (0.33) | 5.28 (0.34) | 5.11 (0.33) | 5.09 (0.33) |
| Interpersonal | 8.23 (0.38) | 5.63 (0.38) | 4.95 (0.40) | 4.79 (0.40) | 4.84 (0.39) |
| Intrapersonal | 9.48 (0.38) | 6.91 (0.42) | 5.60 (0.42) | 5.40 (0.41) | 5.39 (0.41) |
| Impulse control | 8.59 (0.34) | 5.91 (0.33) | 5.29 (0.37) | 5.17 (0.36) | 5.10 (0.36) |
| Social responsibility | 4.25 (0.18) | 3.15 (0.18) | 2.76 (0.19) | 2.68 (0.20) | 2.89 (0.20) |
| Total InDUC score | 38.90 (1.47) | 27.65 (1.49) | 23.88 (1.60) | 23.14 (1.58) | 23.31 (1.58) |
| Treatment arm | |||||
| Baseline | 3 month follow-up | 6 month follow-up | 9 month follow-up | 12 month follow-up | |
| n=515 | n=303 | n=276 | n=292 | n=254 | |
| InDUC categories | x̄(SE) | x̄(SE) | x̄(SE) | x̄(SE) | x̄(SE) |
| Physical | 8.32 (0.31) | 5.52 (0.36) | 5.07 (0.36) | 4.45 (0.33) | 4.07 (0.31) |
| Interpersonal | 8.46 (0.39) | 5.32 (0.41) | 4.89 (0.44) | 4.36 (0.38) | 4.27 (0.39) |
| Intrapersonal | 9.31 (0.38) | 6.18 (0.42) | 5.51 (0.44) | 4.92 (0.41) | 4.35 (0.38) |
| Impulse control | 8.58 (0.36) | 5.64 (0.36) | 5.16 (0.40) | 4.77 (0.37) | 4.54 (0.36) |
| Social responsibility | 4.08 (0.17) | 3.07 (0.20) | 2.68 (0.21) | 2.54 (0.19) | 2.35 (0.18) |
| Total InDUC score | 38.76 (1.47) | 25.73 (1.62) | 23.31 (1.73) | 21.04 (1.57) | 19.59 (1.51) |
| Difference in InDUC scores (Control arm - Treatment arm) | |||||
| Baseline | 3 month follow-up | 6 month follow-up | 9 month follow-up | 12 month follow-up | |
| InDUC categories | Δx̄(95% CI) | Δx̄(95% CI) | Δx̄(95% CI) | Δx̄(95% CI) | Δx̄(95% CI) |
| Physical | 0.01 (−0.86, 0.87) | 0.54 (−0.41, 1.48) | 0.21 (−0.77, 1.18) | 0.66 (−0.25, 1.57) | 1.02 (0.13, 1.92) |
| Interpersonal | −0.23 (−1.29, 0.83) | 0.31 (−0.79, 1.42) | 0.06 (−1.11, 1.22) | 0.43 (−0.67, 1.52) | 0.57 (−0.51, 1.66) |
| Intrapersonal | 0.17 (−0.87, 1.23) | 0.72 (−0.45, 1.89) | 0.09 (−1.09, 1.28) | 0.48 (−0.65, 1.61) | 1.03 (−0.08, 2.14) |
| Impulse control | 0.01 (−0.96, 0.98) | 0.27 (−0.69, 1.24) | 0.13 (−0.94, 1.20) | 0.40 (−0.61, 1.41) | 0.56 (−0.45, 1.57) |
| Social responsibility | 0.17 (−0.32, 0.65) | 0.08 (−0.45, 0.61) | 0.08 (−0.48, 0.64) | 0.13 (−0.41, 0.68) | 0.53 (0.00, 1.06) |
| Total InDUC score | 0.14 (−3.94, 4.23) | 1.92 (−2.40, 6.25) | 0.57 (−4.07, 5.20) | 2.10 (−2.28, 6.48) | 3.72 (−0.58, 8.02) |
Key: InDUC=Inventory of drug use consequences; SE=Standard error; x̄=Average of scores; Δx̄=Average of difference in scores; 95% CI=95% confidence interval
Fig. 2.
Changes and differences in the Inventory of Drug Use Consequences (InDUC) scores overtime (unadjusted data)
3.3 Secondary analyses examining impact of the BI on InDUC scores and subscores accounting for missing data and propensity to remain in the study
Using the regression-based multiple imputations dataset (Supplemental Table 2 and Supplemental Figure 1), the reductions in total InDUC scores were: treatment arm (27.1% from baseline to 3-month follow-up and 39.4% from baseline to 12-month follow-up) and control arm (26.4% reduction from baseline to 3-month follow-up and 39.0% from baseline to 12-month follow-up). Using the propensity score-based multiple imputations dataset, the reductions in total InDUC scores were: treatment arm (32.9% from baseline to 3-month follow-up and 49.7% from baseline to 12-month follow-up) and control arm (28.3% from baseline to 3-month follow-up and 40.0% from baseline to 12-month follow-up) (Supplemental Table 3 and Supplemental Figure 21). Consistent with the results from the primary outcome analysis, there also were no differences in total InDUC scores between study arms at each follow-up for both the regression-based and propensity score-based multiple imputations datasets in these secondary analyses.
3.4 Factors associated with changes in total InDUC scores
When considering the relationship between demographic characteristics and total InDUC scores at 3, 6, 9 and 12-month follow-up in the bivariable regression model analyses, only participant years of formal education was associated with a change in total InDUC scores (Supplemental Tables 4 – Supplemental Tables 72). In general, greater years of formal education was associated with lower total InDUC scores at each follow-up.
In the multivariable regression model analyses assessing change in total InDUC scores at each follow-up, lower scores were observed among those with 12 years of education, no drug use during the 90-day follow-up period, and utilization of drug treatment services at baseline and during the 90-day follow-up period for the unadjusted dataset. Scores were also lower at the 3-and 6-month follow-up for those who utilized drug treatment services at baseline and at the 12- month follow-up for those who used them during the 12-month follow-up period. Scores were higher for those with greater total InDUC scores at baseline, and at the 3- and 6-month follow-up for those whose ASSIST scores indicated a need for a more intensive intervention. In comparison, for the regression-based (Supplemental Table 83) and propensity score-based (Supplemental Table 94) multiple imputations datasets, lower total InDUC scores were noted for those with more years of formal education, no drug use at follow-up, and generally for those who utilized drug treatment at baseline and follow-up or at least at baseline. Higher scores again were observed among those with greater baseline total InDUC scores.
In the longitudinal/repeated measures analyses (Table 4), lower total InDUC scores were observed in the unadjusted and complete follow-up datasets for those with 12 years of formal education, no past 90-day drug use before any follow-up period (short-term effects), drug treatment services utilization before any follow-up period (short-term effects), and greater periods of no drug use over time (cumulative effects). Greater scores were noted among those with greater scores at baseline. There were no predictors of total InDUC scores in the two imputations datasets, and none of the interaction terms were predictive of scores for any of the four datasets.
Table 4.
Factors associated with greater changes in total InDUC scores by mixed effected model, multivariable analyses
| Unadjusted |
Completed only |
Regression based multiple imputations |
Propensity score based multiple imputations |
||
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
| Age (years) | −1.29 (−4.46, 1.89) | −3.41 (−7.19, 0.37) | 0.01 (−0.7, 0.72) | −0.17 (−1.21, 0.86) | |
| Years of formal education | |||||
| Less than 12 years | ref | ref | ref | ref | |
| Grade 12 | −5.54 (−8.71, −2.37) | −5.56 (−9.46, −1.67) | −0.36 (−1.36, 0.64) | −0.32 (−1.35, 0.70) | |
| More than 12 years | −3.83 (−6.83, −0.82) | −3.34 (−6.90, 0.23) | −0.37 (−1.37, 0.63) | −0.30 (−1.28, 0.68) | |
| Intervention needed at baseline | |||||
| Brief intervention | ref | ref | ref | ref | |
| Intensive intervention | 0.90 (−2.80, 4.61) | 4.80 (0.28, 9.33) | 0.16 (−0.66, 0.97) | −0.04 (−0.94, 0.85) | |
| Time effect | |||||
| 3 month follow-up | ref | ref | ref | ref | |
| 6 month follow-up | −3.68 (−8.61, 1.24) | −7.03 (−12.98, −1.07) | −0.24 (−1.6, 1.12) | −0.59 (−2.83, 1.65) | |
| 9 month follow-up | 3.49 (−2.07, 9.04) | 1.01 (−5.33, 7.35) | 0.21 (−1.26, 1.69) | 0.32 (−1.89, 2.53) | |
| 12 month follow-up | 1.58 (−4.13, 7.28) | −1.51 (−8.19, 5.17) | 0.10 (−1.40, 1.59) | −0.11 (−2.26, 2.03) | |
| Interaction Analysis* | |||||
| Study arm: 3 month follow-up | ref | ref | ref | ref | |
| Study arm: 6 month follow-up | 1.56 (−1.71, 4.83) | 0.13 (−3.57, 3.83) | −0.03 (−0.86, 0.79) | −0.09 (−1.32, 1.14) | |
| Study arm: 9 month follow-up | 2.13 (−1.18, 5.43) | 3.06 (−0.65, 6.77) | −0.09 (−0.93, 0.76) | −0.10 (−1.34, 1.14) | |
| Study arm: 12 month follow-up | 1.06 (−2.24, 4.36) | 2.14 (−1.57, 5.84) | −0.21 (−1.14, 0.73) | −0.29 (−1.65, 1.08) | |
| No drug use: 3 month follow-up | ref | ref | ref | ref | |
| No drug use: 6 month follow-up | −3.82 (−7.48, −0.16) | −2.36 (−6.65, 1.93) | −0.37 (−1.59, 0.86) | −0.02 (−1.35, 1.30) | |
| No drug use: 9 month follow-up | −3.30 (−7.39, 0.78) | −1.64 (−6.50, 3.22) | −0.31 (−1.48, 0.85) | 0.06 (−1.28, 1.41) | |
| No drug use: 12 month follow-up | −2.15 (−6.67, 2.36) | 1.80 (−3.61, 7.22) | −0.36 (−1.62, 0.89) | 0.11 (−1.28, 1.51) | |
| Drug treatment: 3 month follow-up | ref | ref | ref | ref | |
| Drug treatment: 6 month follow-up | 0.53 (−4.35, 5.40) | 0.80 (−1.97, 4.02) | 0.18 (−1.10, 1.46) | 0.38 (−1.56, 2.32) | |
| Drug treatment: 9 month follow-up | −7.04 (−12.78, −1.29) | 0.75 (−6.11, 7.61) | −0.48 (−2.18, 1.23) | −0.36 (−2.50, 1.79) | |
| Drug treatment: 12 month follow-up | −5.36 (−11.67, 0.94) | 4.44 (−3.58, 12.47) | −0.14 (−1.57, 1.29) | 0.32 (−1.78, 2.42) | |
| No drug use (past 90 days) | −5.25 (−8.01, −2.49) | −6.56 (−9.96, −3.15) | −0.11 (−0.8, 0.58) | −0.36 (−1.58, 0.86) | |
| Drug treatment (past 90 days) | −6.72 (−10.50, −2.93) | −10.24 (−14.89, −5.60) | −0.43 (−1.74, 0.88) | −1.05 (−3.69, 1.59) | |
| No drug use (accumulative) | −2.21 (−3.65, −0.77) | −2.79 (−4.36, −1.23) | −0.17 (−0.65, 0.30) | −0.21 (−0.80, 0.38) | |
| Drug treatment (accumulative) | 0.42 (−1.66, 2.51) | −2.00 (−4.42, 0.43) | −0.05 (−0.50, 0.40) | −0.14 (−0.78, 0.50) | |
| Baseline total InDUC score | 0.48 (0.44, 0.53) | 0.40 (0.34, 0.46) | 0.05 (−0.05, 0.15) | 0.03 (−0.03, 0.08) | |
n.b. Beta coefficients of each interaction terms were calculated separately
Key: InDUC=Inventory of drug use consequences; Ref=Reference; IQR=Interquartile range; β=Beta coefficients; 95% CI=95% confidence interval
4. DISCUSSION
Although it is disappointing to report and conclude, the BI employed in this study did not lead to greater reductions in the negative consequences of drug misuse than no BI (screening/answering questionnaires alone). This finding complements those from our investigation from this same population and study that observed no short-term (3-month follow-up) benefit from the BI in regards to reduced drug use or greater utilization of drug treatment services (Merchant et al.). Likewise, Woodruff et al. (2014) observed no differences in past 30-day drug use abstinence six months post-enrollment among approximately 700 San Diego ED patients randomly assigned to a BI on drug misuse vs. a BI on safer driving practices. Bogenschutz, et al. (2014) also recently reported no advantage to a BI with telephone boosters as compared with screening, assessment and referral to treatment or minimal screening only in terms of drug misuse at 3, 6, and 12 months post-enrollment among 1,285 adult ED patients across six recruitment sites. Along with similarly focused studies among outpatient clinic patients, these results consistently indicate that BIs, at least in their present form, are not useful for drug misuse (Saitz et al., 2014, Roy-Byrne et al., 2014).
When attempting to discern if there were particular participants or subgroups of ED patients (based on their demographic or clinical characteristics) who might in fact benefit from the BI or another intervention---despite the BI not being beneficial for the population as a whole---the results of the various analyses generally indicated that lower InDUC scores were associated with lack of drug use as well as utilization of drug treatment services. This impact was not dependent on receipt of a BI. Causality, however, cannot be proven for this type of study. The implication of this finding for future drug misuse interventions in the ED might be that an emphasis on drug use cessation and linkage to drug treatment services, rather than a BI, could lead to greater reductions in drug misuse negative consequences. Future research can investigate this hypothesis.
Similar to researcher’s observations from some ED-based alcohol misuse intervention studies (Blow et al., 2006; Longabaugh et al., 2001; D’Onofrio et al., 2012; Mello et al., 2013), we found that participation in this study perhaps alone (with or without receiving a BI) implies a decrease in the self-reported negative consequences of drug use/misuse over time. The reduction in total InDUC scores in both study arms from baseline could be interpreted as hopeful news, if they represent a true decrease in the negative consequences of drug use/misuse. This decrease appears to be greatest in the short-term (by the three-month follow-up point) and is sustained over time. This interpretation rests on the assumption that those who remained in the study were representative of those who did not, and therefore our imputation for missing data and adjustment for propensity to remain/not remain in the study was valid. If true, then perhaps by bringing attention to ED patient drug misuse through study participation alone (with or without receiving a BI), maybe from self-reflection through completing the study questionnaires, can lead to decreases in drug misuse negative consequences. By this interpretation, perhaps simply permitting ED patients to assess themselves might be a useful and seemingly low-cost approach to decreasing the negative consequences of drug use/misuse. An intervention that employs or enhances this self-reflection and change could be designed and evaluated in future studies. However, we do not have the benefit of knowing if there would be decreases in InDUC scores (and hence negative consequences of drug misuse) over time among those who were not study participants. That is, ED patients who were not asked about their drug use. In other words, we cannot know the trajectory of InDUC scores among a hypothetical comparison group of ED patients who did not complete questionnaires as part of the study, but were followed over time to measure their InDUC scores. As a consequence, we cannot infer causality of study participation alone (completing screening and questionnaires with or without a BI) in reducing the negative consequences of drug misuse.
On the other hand, there are other possible interpretations of the observed decrease in InDUC scores among study participants that call into question the value of screening only as a means of reducing the negative consequences of drug misuse. The large decreases in total InDUC scores, especially noticeable at the three-month follow-up point, instead could be due to differential loss to follow-up of those with high InDUC scores at baseline, and hence the score changes over time are not representative of changes in InDUC scores. Although the propensity score analyses were performed to help mitigate this possibility, propensity score and multiple imputation analyses could not take into account unmeasured factors that impact missing data or propensity to remain or not remain in the study. Accordingly, we cannot fully assess this alternative interpretation with these data. In addition, decreases in scores from baseline could be because of regression to the mean, which is an explanation might be supported by the lower scores over time. Because trends over time prior to study enrollment cannot be known, this alternative interpretation cannot be assessed. Furthermore, lower total InDUC scores at follow-up could be the result of social desirability biases, perhaps magnified by those who chose to remain in the study, and might not reflect any true change in behavior from being in the study.
This investigation had several limitations. The primary aim for the BIDMED study was to investigate if the BI led to a 25% greater decrease in drug use/misuse in the treatment vs. the control arm by three-month follow-up. For that primary aim, the estimated sample size was 550 per study arm for an 80% power and a two-sided Type I error rate of 0.05. The investigation on InDUC scores reported upon in this manuscript was a secondary aim of the BIDMED study, and hence we did not estimate an a priori sample size needed to compare total InDUC scores by study arm over time. As a consequence, this investigation might not have had adequate sample size for this comparison. One challenge was missing data due to loss-to-follow-up of participants and, consequently, how to impute missing InDUC scores at each time point. Approximately 30% of participants did not complete any one of the four follow-up questionnaires, although the missing data/study retention was comparable to studies in similar settings (D’Onofrio, 2012). Future studies evaluating substance misuse treatments need to consider innovative methods as well as those tried elsewhere to maximize study retention of ED participants. Although common approaches of imputing missing data based on other available data for participants remaining in the study were used, we cannot verify that these approaches were the optimal ways of overcoming this challenge. Even though the state where the study conducted has one of the highest illicit drug use/misusing prevalences in the US (Bernardino, 2014), the efficacy of BIs in decreasing drug use/misuse negative consequences maybe not the same in EDs of other states. Last but not least in importance, we cannot exclude the possibility that these self-reported data are not correct and could be overstated (social desirability bias), or if the InDUC is the best method to measure negative consequences of drug misuse.
Participants in the BIDMED study self-reported fewer negative consequences of drug misuse in both study arms over time, especially at the three-month follow-up, and these decreases were stable over time. However, the BI did not confer a greater decrease in negative consequences. The results do suggest that some ED patients can have fewer negative consequences of drug use/misuse, perhaps from simply completing questionnaires about their drug use/misuse and negative consequences. Furthermore, those who quit using drugs or utilize drug treatment services appear to have fewer negative consequences from drug use/misuse over time. Future interventions need to be designed and investigated that assess these potential pathways to reducing the negative consequences of drug use in this patient population.
Supplementary Material
Table 3.
Factors associated with total InDUC scores at each follow-up, multivariable analyses (unadjusted data)
| Δ 3 month follow-up |
Δ 6 month follow-up |
Δ 9 month follow-up |
Δ 12 month follow-up |
||
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
| Study arm (Control arm) | −2.99 (−6.51, 0.53) | −3.33 (−7.10, 0.44) | −0.69 (−4.75, 3.38) | −1.43 (−5.25, 2.38) | |
| Years of formal education | |||||
| Less than 12 years | ref | ref | ref | ref | |
| Grade 12 | −4.99 (−9.54, −0.45) | −6.29 (−11.12, −1.45) | −5.31 (−10.55, −0.08) | −5.26 (−10.18, −0.34) | |
| More than 12 years | −3.53 (−7.68, 0.62) | −2.74 (−7.17, 1.69) | −4.55 (−9.33, 0.22) | −2.82 (−7.31, 1.66) | |
| Intervention needed at baseline | |||||
| Brief intervention | ref | ref | ref | ref | |
| Intensive | 5.93 (0.64, 11.22) | 6.77 (1.07, 12.47) | 4.19 (−1.91, 10.29) | 1.74 (−4.00, 7.49) | |
| Drug use | |||||
| Using any drug at baseline and follow-up | ref | ref | ref | ref | |
| Using any drug at baseline, but not at follow-up | −9.12 (−12.89, −5.35) | −14.38 (−18.39, −10.37) | −12.99 (−17.21, −8.77) | −12.32 (−16.16, −8.48) | |
| Drug misuse treatment utilization | |||||
| No treatment at baseline and follow-up | ref | ref | ref | ref | |
| Treatment at baseline, but not at follow-up | −11.90 (−17.74, −6.06) | −10.48 (−20.42, −0.54) | 6.94 (−5.10, 18.98) | −6.44 (−18.35, 5.47) | |
| No treatment at baseline, but treatment at follow-up | 0.48 (0.40, 0.55) | −10.04 (−21.11, 1.03) | −8.76 (−20.89, 3.38) | −18.33 (−29.66, −6.99) | |
| Treatment at baseline and follow-up | −14.43 (−21.32, −7.43) | −15.43 (−23.90, −6.96) | −17.07 (−27.26, −6.88) | −22.16 (−31.38, −12.94) | |
| Baseline total InDUC score | 0.47 (0.39, 0.54) | 0.34 (0.27, 0.42) | 0.33 (0.25, 0.42) | 0.38 (0.30, 0.46) | |
Key: InDUC=Inventory of drug use consequences; Ref=Reference; IQR=Interquartile range; β=Beta coefficients; 95% CI=95% confidence interval
Highlights.
Self-reported drug use/misuse negative consequences, as measured by the Inventory of Drug Use Consequences (InDUC), decreased for ED patients enrolled in the study from baseline to three-month follow-up, regardless of whether or not an intervention was received.
Drug use/misuse negative consequences were not lower among those who received a BI as compared to those who did not receive a BI.
Drug use/misuse negative consequences were generally lower over time among those who received drug treatment during follow-up or quit using drugs.
Acknowledgements
The research team gratefully acknowledges the assistance of Ms. Vera Bernardino for preparing the data for analysis and publication, the research assistants who assessed patients for the study and helped coordinate the study (Naira Arellano, Vera Bernardino, Rosalie Berrios-Candelaria, Vianella Burgos, Ian Donaghy, Dora Estrela, Cindy Gonzalez, Alyssa Hozey, Michelle Leveillee, Stefanie Paolino, Ayanaris Reyes, and Becca Rose), and the support of the staff and patients at our two hospitals. The authors gratefully acknowledge the assistance of Michael J. Mello, MD, MPH, and Ted Nirenberg, PhD.
Role of Funding Source
This research was supported by grants from the National Institute on Drug Abuse (R01 DA026066) and the Lifespan/Tufts/Brown Centers for AIDS Research (P30 AI042853). ClinicalTrials.gov identifier: NCT01124591. The funders had no role in the design or execution of the study, nor in the preparation or review of the manuscript. This manuscript was prepared in partial fulfillment of Mr. Wentao Guan’s thesis requirements for his Master’s of Science in Biostatistics degree from Brown University.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:..
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Wentao Guan, MSc, Conduct of analyses, Preparation of manuscript
Tao Liu, PhD, Supervision of analyses, Review of manuscript
Janette R. Baird, PhD, Supervision of data collection, Supervision of intervention delivery, Review of manuscript
Roland C. Merchant, MD, MPH, ScD, Design of study, Supervision of study, Supervision of analyses, Supervision of manuscript preparation
Author Disclosures
Conflict of Interest
No conflict declared
REFERENCES
- Academic Ed Sbirt Research Collaborative. The impact of screening, brief intervention and referral for treatment in emergency department patients’ alcohol use: a 3-, 6- and 12-month follow-up. Alcohol Alcohol. 2010;45:514–519. doi: 10.1093/alcalc/agq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino VL, Baird JR, Liu T, Merchant RC. Comparison of substance-use prevalence among Rhode Island And The Miriam Hospital Emergency Department patients to state and national general population prevalence estimates. R. I. Med. J. 2014;98:30–34. [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Bernstein JA, Stein JB, Saitz R. Sbirt In emergency care settings: are we ready to take it to scale? Acad. Emerg. Med. 2009;16:1072–1077. doi: 10.1111/j.1553-2712.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- Bernstein J, Heeren T, Edward E, Dorfman D, Bliss C, Winter M, Bernstein E. A brief motivational interview in a pediatric emergency department, plus 10-day telephone follow-up, increases attempts to quit drinking among youth and young adults who screen positive for problematic drinking. Acad. Emerg. Med. 2010;17:890–902. doi: 10.1111/j.1553-2712.2010.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow FC, Barry KL, Walton MA, Maio RF, Chermack ST, Bingham CR, Ignacio RV, Strecher VJ. The efficacy of two brief intervention strategies among injured, at-risk drinkers in the emergency department: impact of tailored messaging and brief advice. J. Stud. Alcohol. 2006;67:568–578. doi: 10.15288/jsa.2006.67.568. [DOI] [PubMed] [Google Scholar]
- Blow FC, Walton MA, Barry KL, Murray RL, Cunningham RM, Massey LS, Chermack ST, Booth BM. Alcohol And drug use among patients presenting to an inner-city emergency department: a latent class analysis. Addict. Behav. 2011;36:793–800. doi: 10.1016/j.addbeh.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Donovan DM, Mandler RN, Perl HI, Forcehimes AA, Crandall C, Lindblad R, Oden NL, Sharma G, Metsch L, Lyons MS, Mccormack R, Macias-Konstantopoulos W, Douaihy A. Brief intervention for patients with problematic drug use presenting in emergency departments: a randomized clinical trial. JAMA Intern. Med. 2014;174:1736–1745. doi: 10.1001/jamainternmed.2014.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton AR, Taira DA, Burns E, O’leary J, Chung RS. Follow-up services after an emergency department visit for substance abuse. Am. J. Manag. Care. 2007;13:497–505. [PubMed] [Google Scholar]
- Cochran G, Field C, Caetano R. Injury-related consequences of alcohol misuse among injured patients who received screening and brief intervention for alcohol: a latent class analysis. Subst. Abuse. 2014;35:153–162. doi: 10.1080/08897077.2013.820679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham RM, Bernstein SL, Walton M, Broderick K, Vaca FE, Woolard R, Bernstein E, Blow F, D’onofrio G. Alcohol, tobacco, and other drugs: future directions for screening and intervention in the emergency department. Acad. Emerg. Med. 2009;16:1078–1088. doi: 10.1111/j.1553-2712.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, Degutis LC. Preventive care in the emergency department: screening and brief intervention for alcohol problems in the emergency department: a systematic review. Acad. Emerg. Med. 2002;9:627–638. doi: 10.1111/j.1553-2712.2002.tb02304.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, Busch SH, Bernstein SL, O’Connor PG. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann. Emerg. Med. 2012;60:181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, Pantalon MV, Degutis LC, Fiellin DA, Busch SH, Chawarski MC, Owens PH, O’Connor PG. Brief intervention for hazardous and harmful drinkers in the emergency department. Ann. Emerg. Med. 2008;51:742–750. doi: 10.1016/j.annemergmed.2007.11.028. E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AW, Weiland TJ, Phillips GA, Lee NK. Opportunistic screening and clinician-delivered brief intervention for high-risk alcohol use among emergency department attendees: a randomized controlled trial. Emerg. Med. Australas. 2008;20:121–128. doi: 10.1111/j.1742-6723.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- Hankin A, Daugherty M, Bethea A, Haley L. The emergency department as a prevention site: a demographic analysis of substance use among ed patients. Drug Alcohol Depend. 2013;130:230–233. doi: 10.1016/j.drugalcdep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Havard A, Shakeshaft A, Sanson-Fisher R. Systematic review and meta-analyses of strategies targeting alcohol problems in emergency departments: interventions reduce alcohol-related injuries. Addiction. 2008;103:368–376. doi: 10.1111/j.1360-0443.2007.02072.x. Discussion 377–378. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, De Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation Of The Alcohol, Smoking And Substance Involvement Screening Test (Assist) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Woychek A, Vaughan D, Seale JP. Screening for at-risk alcohol use and drug use in an emergency department: integration of screening questions into electronic triage forms achieves high screening rates. Ann. Emerg. Med. 2013;62:262–266. doi: 10.1016/j.annemergmed.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Longabaugh R, Woolard RE, Nirenberg TD, Minugh AP, Becker B, Clifford PR, Carty K, Licsw Sparadeo F, Gogineni A. evaluating the effects of a brief motivational intervention for injured drinkers in the emergency department. J. Stud. Alcohol. 2001;62:806–816. doi: 10.15288/jsa.2001.62.806. [DOI] [PubMed] [Google Scholar]
- Macias Konstantopoulos WL, Dreifuss JA, McDermott KA, Parry BA, Howell MI, Mandler RN, Fitzmaurice GM, Bogenschutz MP, Weiss RD. Identifying patients with problematic drug use in the emergency department: results of a multisite study. Ann. Emerg. Med. 2014;64:516–525. doi: 10.1016/j.annemergmed.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. initial studies of the treatment services review. J. Nerv. Ment. Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Mello MJ, Baird J, Nirenberg TD, Lee C, Woolard R, Longabaugh R. Dial: a randomised trial of a telephone brief intervention for alcohol. Inj. Prev. 2013;19:44–48. doi: 10.1136/injuryprev-2012-040334. [DOI] [PubMed] [Google Scholar]
- Merchant RC, Baird JR, Liu T. Short-term efficacy of a brief intervention to reduce drug misuse and increase drug treatment utilization among adult emergency department patients. Acad. Emerg. Med. In press doi: 10.1111/acem.12767. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant RC, Baird JR, Liu T, Taylor LE, Montague BT, Nirenberg TD. Brief intervention to increase emergency department uptake of combined rapid human immunodeficiency virus and hepatitis c screening among a drug misusing population. Acad. Emerg. Med. 2014;21:7527–67. doi: 10.1111/acem.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S, Conforti K. Motivational Interviewing: Preparing People For Change. 2nd Ed. New York: Guilford Press; 2002. [Google Scholar]
- Newton AS, Dong K, Mabood N, Ata N, Ali S, Gokiert R, Vandermeer B, Tjosvold L, Hartling L, Wild TC. brief emergency department interventions for youth who use alcohol and other drugs: a systematic review. Pediatr. Emerg. Care. 2013;29:673–684. doi: 10.1097/PEC.0b013e31828ed325. [DOI] [PubMed] [Google Scholar]
- Nilsen P, Baird J, Mello MJ, Nirenberg T, Woolard R, Bendtsen P, Longabaugh R. A systematic review of emergency care brief alcohol interventions for injury patients. J Subst Abuse Treat. 2007;35:184–201. doi: 10.1016/j.jsat.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Rockett IR, Putnam SL, Jia H, Smith GS. Assessing substance abuse treatment need: a statewide hospital emergency department study. Ann. Emerg. Med. 2003;41:802–813. doi: 10.1067/mem.2003.189. [DOI] [PubMed] [Google Scholar]
- Rosenstock IM. Historical origins of the health belief model. Health Educ. Monogr. 1974;2:328–335. [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, West Ii, Maynard C, Atkins DC, Graves MC, Joesch JM, Zarkin GA. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312:492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH. Screening and brief intervention for drug use in primary care: the Aspire Randomized Clinical Trial. JAMA. 2014;312:502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Rice SL, Witkiewitz K, Mandler RN, Crandall C, Bogenschutz MP. Alcohol, tobacco, and drug use among emergency department patients. Drug Alcohol Depend. 2014;138:32–38. doi: 10.1016/j.drugalcdep.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS, Lyons MS, Fargo JD, Sommers BD, Mcdonald CC, Shope JT, Fleming MF. Emergency department-based brief intervention to reduce risky driving and hazardous/harmful drinking in young adults: a randomized controlled trial. Alcohol. Clin. Exp. Res. 2013;37:1753–1762. doi: 10.1111/acer.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart IH, Ranney ML, Howland J, Mello MJ. A systematic review of emergency department interventions for college drinkers. J. Emerg. Med. 2013;45:962–968. doi: 10.1016/j.jemermed.2013.05.065. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR. The Inventory Of Drug Use Consequences (Induc): test-retest stability and sensitivity to detect change. Psychol. Addict. Behav. 2002;16:165–168. [PubMed] [Google Scholar]
- Wojnar M, Jakubczyk A. Brief interventions for hazardous and harmful alcohol consumption in accident and emergency departments. Front. Psychiatry. 2014;5:152. doi: 10.3389/fpsyt.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff SI, Clapp JD, Eisenberg K, Mccabe C, Hohman M, Shillington AM, Sise CB, Castillo EM, Chan TC, Sise MJ, Gareri J. Randomized clinical trial of the effects of screening and brief intervention for illicit drug use: the Life Shift/Shift Gears Study. Addict. Sci. Clin. Pract. 2014;9:8. doi: 10.1186/1940-0640-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Swartz MS, Wu Z, Mannelli P, Yang C, Blazer DG. Alcohol and drug use disorders among adults in emergency department settings in the United States. Ann. Emerg. Med. 2012;60:172–180. doi: 10.1016/j.annemergmed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuma-Guerrero PJ, Lawson KA, Velasquez MM, Von Sternberg K, Maxson T, Garcia N. Screening, brief intervention, and referral for alcohol use in adolescents: a systematic review. Pediatrics. 2012;130:115–122. doi: 10.1542/peds.2011-1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.