Abstract
The adult ventricular-subventricular zone (V-SVZ) of the lateral ventricle produces several subtypes of olfactory bulb (OB) interneurons throughout life. Neural stem cells (NSCs) within this zone are heterogeneous, with NSCs located in different regions of the lateral ventricle wall generating distinct OB interneuron subtypes. The regional expression of specific transcription factors appears to correspond to such geographical differences in the developmental potential of V-SVZ NSCs. However, the transcriptional definition and developmental origin of V-SVZ NSC regional identity are not well understood. In this study, we found that a population of NSCs in the ventral region of the V-SVZ expresses the transcription factor Nkx2.1 and is derived from Nkx2.1-expressing (Nkx2.1+) embryonic precursors. To follow the fate of Nkx2.1+ cells and their progeny in vivo, we used mice with an Nkx2.1-CreER “knock-in” allele. Nkx2.1+ V-SVZ NSCs labeled in adult mice generated interneurons for the deep granule cell layer of the OB. Embryonic brain Nkx2.1+ precursors labeled at embryonic day 12.5 gave rise to Nkx2.1+ NSCs of the ventral V-SVZ in postnatal and adult mice. Thus, embryonic Nkx2.1+ neural precursors give rise to a population of Nkx2.1+ NSCs in the ventral V-SVZ where they contribute to the regional heterogeneity of V-SVZ NSCs.
Keywords: Stem cell heterogeneity, neural stem cells, adult neurogenesis, V-SVZ, regional patterning, Nkx2.1
INTRODUCTION
The adult ventricular-subventricular zone (V-SVZ) harbors neural stem cells (NSCs) that are distributed throughout the walls of the lateral ventricles. While the V-SVZ as a whole produces numerous subtypes of olfactory bulb (OB) interneurons, V-SVZ NSCs are not equivalent (Lim and Alvarez-Buylla, 2014; Lledo et al., 2008). For instance, NSCs in the dorsal V-SVZ give rise to OB interneuron subtypes that are distinct from those produced by ventral V-SVZ NSCs (Ihrie et al., 2011; Merkle et al., 2007). Differences in V-SVZ NSC developmental potential appear to relate to regional transcription factor expression that divides the V-SVZ into numerous domains (Lim and Alvarez-Buylla, 2014; Lledo et al., 2008; Lopez-Juarez et al., 2013; Merkle et al., 2014). However, the gene expression that defines the different populations of V-SVZ NSCs is not well known, and it is also unclear how such regional differences are established.
The adult V-SVZ exhibits regional patterns of transcription factor expression similar to those observed in embryonic development, suggestive of a lineage relationship between region-specific embryonic neural precursors and NSCs of the adult V-SVZ (Alvarez-Buylla et al., 2008). For instance, the dorsal V-SVZ expresses Gsh2 (Lopez-Juarez et al., 2013) and other transcription factors of the lateral ganglionic eminence (LGE) (Kohwi et al., 2005; Waclaw et al., 2006). While the Gsh2-Cre transgene drives recombination in many adult V-SVZ cells and OB interneurons (Young et al., 2007), constitutive Cre-drivers do not generally establish a precise time at which cell labeling occurred.
The most compelling evidence for an embryonic origin of V-SVZ regional identity relates to the most dorsal V-SVZ NSC population. Using Emx1-CreER mice to label a cohort of E10.5 neural precursors in the developing pallium, Young et al. (2007) showed this population gives rise to adult NSCs in the dorsal V-SVZ. In contrast, the contribution of Nkx2.1-expressing (Nkx2.1+) neural precursors to adult OB neurogenesis has been unclear. Cells that express Nkx2.1 are found in the medial ganglionic eminence (MGE) – but not the LGE or pallium – and in utero fate-tracing analysis suggests that E13.5 MGE cells do not normally generate OB interneurons (Wichterle et al., 2001). However, in embryonic Nkx2.1-Cre mice, a small stream of labeled cells is observed migrating from the MGE to the OB (Xu et al., 2008), and E13.5 MGE cells transplanted into the OB can generate neurons (Wichterle et al., 1999). More recently, Merkle et al. (2014) demonstrate that Nkx2.1+ cells in the early postnatal brain generate a small number of OB neurons. However, whether Nkx2.1+ V-SVZ NSCs continue to produce OB neurons into adulthood has not been determined, and the embryonic origin of this population of NSCs has not been clearly demonstrated.
In this report, we show that postnatal and adult Nkx2.1+ V-SVZ cells in the ventral brain generate OB interneurons into adulthood and derive from embryonic Nkx2.1+ precursors. Our data indicate that embryonic Nkx2.1+ cells give rise to a regionally distinct population of V-SVZ NSCs in the adult brain, providing clear evidence that the ventral identity of neural precursors established in the early embryonic brain persists throughout development and into adulthood.
MATERIALS AND METHODS
Animal Husbandry and Procedures
All experiments were performed with mice of either sex and in accordance to protocols approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. C57BL/6 (wild type) mice were obtained from Jackson Laboratories. Mice homozygous for Ai14 (Madisen et al., 2010) were mated to mice with the Nkx2-1tm1.1(cre/ERT2)Zjh allele (Taniguchi et al., 2011). For the purpose of adult fate-tracing, >P60 mice received 5 mg tamoxifen (Sigma) dissolved in corn oil (Sigma) by oral gavage per 30 grams of body weight once a day for 5 consecutive days. For embryonic fate-tracing, timed pregnant dams with Nkx2.1-CreER; Ai14 embryos were given one dose of tamoxifen (5mg per 30g body weight) by oral gavage. Embryonic day 0.5 (E0.5) was estimated to be 12:00 pm on the day that vaginal plugs were observed. Bromodeoxyuridine (BrdU) (Sigma) was administered via drinking water (1mg/mL) for one week.
Immunohistochemistry
Embryonic brains were fixed in 4% Paraformaldehyde (PFA). Transcardiac perfusion was performed on postnatal and adult animals first with phosphate buffered saline (PBS) (pH 7.4) and subsequently with 4% PFA. To prepare specimens for cryosectioning, we equilibrated brains in PBS with 30% sucrose for cryoprotection and then embedded them in OCT (Tissue-Tek). 12 μm cryosections were obtained at −23°C in a Microm HM 525 cryostat (Thermo Scientific) and stored at −80°C. To generate vibratome sections, fixed brains were embedded in 1% agarose and sectioned at 50 μm with a Leica VT1000S vibratome. Sections were stored at 4°C in PBS with 0.1% sodium azide. Whole mount preparations were performed as previously described (Mirzadeh et al., 2010).
Immunohistochemistry (IHC) was performed using a blocking solution consisting of 1% BSA (Sigma), 0.3% Triton-X 100 (Sigma), 0.3M Glycine (Sigma), and either 10% Normal Goat Serum (Jackson Immunoresearch) or 10% Normal Donkey Serum (Jackson Immunoresearch). Cryosections were incubated in blocking solution at room temperature for 2 hours and then with primary antibodies at 4°C for at least 16 hours. NKX2.1 IHC was performed by incubating samples in primary antibody for 48 hours. Cryosections were incubated with Alexfluor conjugated secondary antibodies (Life Technologies) at room temperature for 30 minutes (1:500 dilution). Nuclear counterstain DAPI (Sigma) was applied at 0.1 μg/ml during the addition of secondary antibodies. For BrDU IHC, we performed antigen-retrieval by incubation in 2N HCL at 37°C for 45 minutes before incubation with anti-BrdU antibodies. Samples were then re-fixed with 4% PFA for 10 minutes at room temperature and then stained for additional antigens (Tang et al., 2007). Vibratome sections were incubated in blocking solution for at room temperature for 2 hours, and then with primary antibodies for 4°C for 16 hours. Secondary antibody incubation (1:500 dilution) was performed at room temperature for 90 minutes.
The primary antibodies used in this study include: BrdU (rat, 1:250, Abcam #Ab6326). Dcx (rabbit, 1:500, Abcam #Ab18723), Dcx (guinea pig, 1:500, Millipore #AB2253), Dlx2 (guinea pig, 1:2000, a kind gift from Dr. Kazuaki Yoshikawa), dsRed (rabbit, 1:1000, Clontech #632392), GFAP (chicken, 1:1000, Abcam #Ab4674), Ki67 (rabbit, 1:500, Vector #VP-K451), Nestin (chicken, 1:500, Aves #NES), Nkx2.1 (rabbit, 1:400, Santa Cruz #SC-13040), Nkx2.1 (mouse IgG1, 1:250, Leica #NCL-L-TTF-1), Phospho-vimentin, Ser55 (mouse IgG2b, 1:500, MBL #D076-3S), RFP (rat, 1:1000, Chromotek #5F8), Sox2 (goat, 1:200, Santa Cruz SC-17320), and S100β (mouse, 1:500, Sigma #S2532).
Imaging and quantification
Experiments using C57BL/6 mice were performed with 12 μm cryosections derived from at least 3 animals per time point (E12.5, E15.5, E15.5, P7, P60). Embryonic fate labeling experiments using Nkx2.1-CreER; Ai14 mice were performed with 3 animals at P7 and 6 animals at P60). To quantify the proportion of embryonically labeled cells that co-expressed NKX2.1 protein, we counted a total of 4 non-adjacent cryosections per animal (n=3) for each time point (P7 and P60). To quantify embryonically labeled cells in the OB at P60, we analyzed a total of 8 non-adjacent 50 μm vibratome sections per animal (n=3). Adult fate labeling using Nkx2.1-CreER; Ai14 mice were performed with 4 mice per time point (P60 and P120). To quantify P60-labeled OB cells, we analyzed 20 non-adjacent 50 μm vibratome sections per animal (n=4). To quantify P120-labeled OB cells, we analyzed 12 non-adjacent 50 μm vibratome sections per animal (n=4). To analyze of the initial and terminal dendritic branch point of Class 5 GCs born from adult Nkx2.1+ precursors, we quantified between 17–26 dendritic processes per animal (n=4).
Imaging was performed using either a DMI4000 B epifluorescent microscope with attached DFC345 FX camera or a TCS SP5 X confocal microscope (Leica). Image processing, including cropping and pseudo-coloring, was performed with Fiji (Schindelin et al., 2012) and Photoshop CC (Adobe). Quantification of tdTomato+ cells per unit area was performed using Fiji. Volume was then calculated by including slice thickness. Briefly, the border of the granular cell layer (GCL) was traced in Fiji using the “Freehand Selection” tool and the area was then calculated using the “Measure” tool. Relative positions of labeled cells within the GCL and EPL were calculated with the “Straight” tool. At the location of each labeled cell, the total radial thickness of the GCL was determined, and the position of the labeled cell was then calculated as a percentage (%) of this GCL thickness. Student t-tests were performed using Prism 6 (GraphPad).
RESULTS
NKX2.1 is expressed in the ventral germinal zone of the embryonic and early postnatal telencephalon
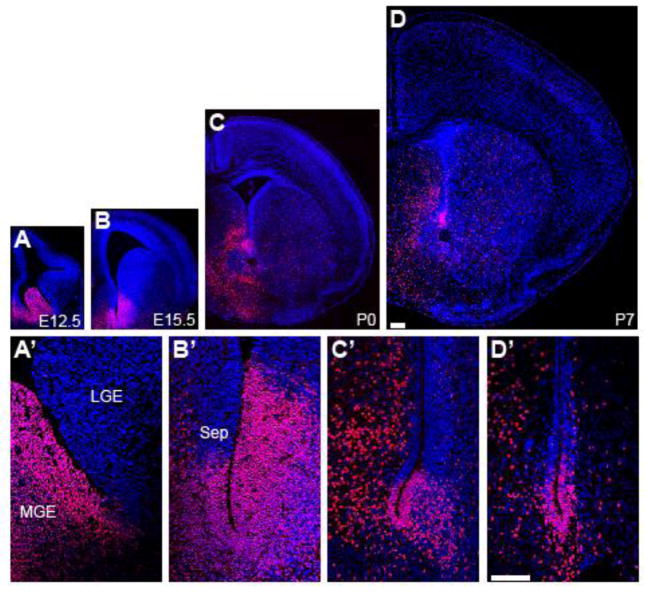
To determine the temporospatial expression of NKX2.1 in embryonic and postnatal telencephalic germinal zones, we performed immunohistochemical (IHC) analysis of coronal brain sections from E12.5, E15.5, P0, and P7 mice. Nkx2.1 is expressed in a restricted region of the early neural tube starting at around E9 (Price et al., 1992; Shimamura et al., 1995; Sussel et al., 1999). Consistent with previous results (Flames et al., 2007), we observed Nkx2.1 expression throughout the E12.5 MGE (Fig. 1A), with this domain of expression ending abruptly in the sulcus between the MGE and LGE (Fig. 1A′). Shortly after E14.5 the ganglionic eminences flatten, forming the lateral ventricle wall. At E15.5, NKX2.1+ cells were present in a ventral domain along both the medial and lateral walls of the lateral ventricle (Fig. 1B,B′). This ventral domain was also present postnatally at P0 and P7 (Fig. 1C,D). At all ages analyzed, the NKX2.1+ domain had distinct dorsal borders in both the medial and lateral walls (Fig. 1A′-D′).
Figure 1.
NKX2.1+ is expressed in ventral germinal regions throughout development. A–D, IHC analysis of coronal brain sections for NKX2.1 (red) and DAPI (blue) from mice at low power magnification at E12.5 (A, A′), E15.5 (B, B′) P0 (C, C′) P7 (D, D′). A′-D′, higher-magnification confocal images of A–D. Scale bars=100 μm.
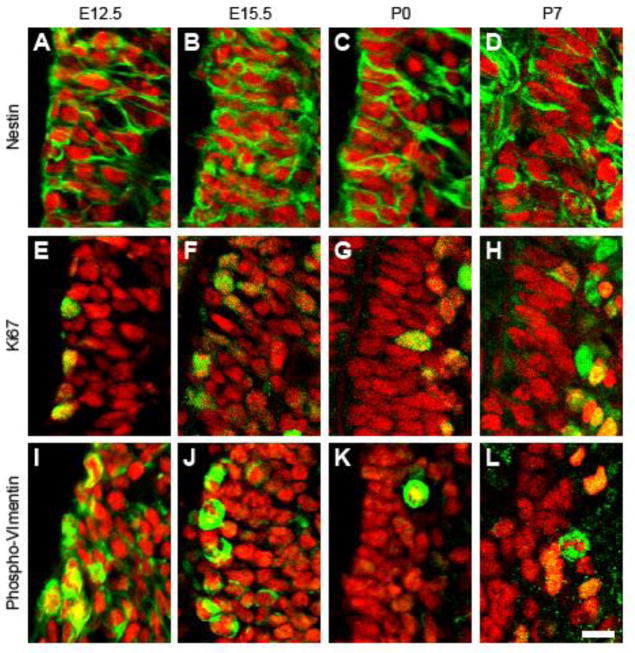
Radial glial cells (RGCs) are the primary neural precursor of the embryonic brain (Kriegstein and Alvarez-Buylla, 2009). At E12.5 and E15.5, many of the NKX2.1+ cells close to the ventricle wall exhibited typical RGC characteristics, including a long radial process and the expression of Nestin (Fig. 2A,B). Some NKX2.1+ cells expressed phospho-vimentin (Fig. 2I,J), which is detected in mitotic RGCs. At the early postnatal ages (P0 and P7), many NKX2.1+ cells were also Nestin+ (Fig. 2C,D) and proliferative, as evidenced by the expression of Ki67 (Fig. 2G,H) and Phospho-Vimentin (Fig. 2K,L). Thus, proliferative NKX2.1+ neural precursor cells exist in the ventral telencephalon through early postnatal development.
Figure 2.
NKX2.1+ cells in the embryonic and postnatal brain have neural precursor cell identity. A–L, IHC analysis of the ventral telencephalon for NKX2.1 (red) and neural precursor markers Nestin (A–D), Ki67 (E–H), and Phospho-Vimentin (I–L) (all in green) at E12.5 (A,E,I), E15.5 (B,F,J), P0 (C,G,K), and P7 (D,H,L). Scale bar=10 μm.
Adult Nkx2.1+ V-SVZ cells are neurogenic in vivo
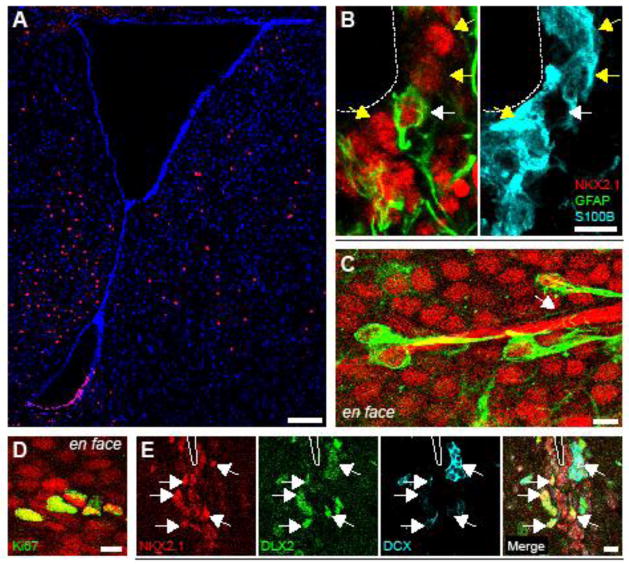
IHC of adult (>P60) brains revealed a domain of NKX2.1+ cells in the ventral V-SVZ (Fig. 3A). NKX2.1 immunoreactivity was not observed in the dorsal V-SVZ. The majority of NKX2.1+ cells corresponded to ependymal cells expressing S100β (Fig. 3B). Type B1 cells – the V-SVZ NSC population – do not express S100β but are immunopositive for glial fibrillary acidic protein (GFAP) (Didier et al., 1986; Doetsch et al., 1997; Mirzadeh et al., 2008). A small proportion of NKX2.1+ cells were negative for S100β but were immunopositive for GFAP (Fig. 3B). Type B1 cells characteristically also have endfeet upon blood vessels (Mirzadeh et al., 2008), and we observed GFAP+/NKX2.1+ cells with processes contacting blood vessels (Fig. 3C). Some NKX2.1+ cells were also Ki67+ (Fig. 3D), suggesting that these NKX2.1+ cells continue proliferating in adulthood. We also observed NKX2.1+ cells that co-expressed markers of the V-SVZ neurogenic lineage (Fig. 3E) including DLX2, a key transcription factor for the genesis and migration of interneurons (Ghanem et al., 2007), and DCX, a marker of migratory neuroblasts. Together, these data indicated that Nkx2.1 is expressed in V-SVZ cells of the adult neurogenic lineage.
Figure 3.
NKX2.1 is expressed in cells of the adult V-SVZ neurogenic lineage. A, IHC analysis of P60 coronal brain sections for NKX2.1 (red) and DAPI (blue). B, High-magnification confocal analysis of NKX2.1 (red), GFAP (green) and S100β (cyan) expression in the P60 ventral V-SVZ. Both panels are from the same focal plane. White dashed lined depicts lateral ventricle. White arrow points to NKX2.1+/GFAP+/S100β-cell. Yellow Arrows point to NKX2.1+/GFAP-/S100β+ cells. C, IHC analysis of whole mount preparations derived from P60 brain. en face view of NKX2.1 (red) and GFAP (green) expression in the ventral surface of lateral ventricle. White arrow points to basal process contacting a blood vessel (visible because goat-anti-mouse secondary antibody was used). D, NKX2.1 (red) and Ki67 (green) expression in the ventral surface of the P60 lateral ventricle, shown en face. E, IHC analysis of coronal P60 sections showing NKX2.1 (red), DLX2 (green), and DCX (cyan) in the ventral V-SVZ. White dashed lined depicts lateral ventricle. Scale bars: (A) 250 μm (B–E) 10 μm.
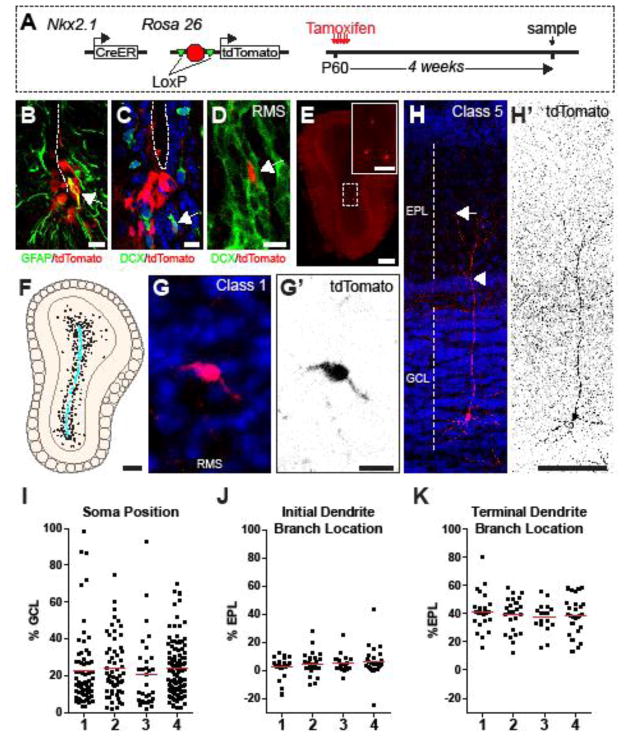
MGE-born cortical interneurons down-regulate Nkx2.1 expression prior to arriving in the cortex (Marin et al., 2000; Nobrega-Pereira et al., 2008). We did not detect any NKX2.1 immunopositive cells within the OB (data not shown), suggesting that the progeny of NKX2.1+ V-SVZ NSCs might down-regulate Nkx2.1 expression in a similar manner. To investigate whether Nkx2.1+ cells in the adult brain generate OB interneurons, we employed a genetic fate-tracing strategy with the Nkx2.1-CreER allele, in which the CreER expression cassette is inserted into the endogenous Nkx2.1 locus (Taniguchi et al., 2011). Administration of tamoxifen to Nkx2.1-CreER mice induces Cre-mediated recombination in Nkx2.1-expressing cells, thereby labeling these cells as well as their descendants (Taniguchi et al., 2011). To follow the fate of cells that had undergone Cre-mediated recombination, we used the Ai14 transgene, which expresses tdTomato after excision of a “floxed-stop” cassette (Madisen et al., 2010). We administered tamoxifen to Nkx2.1-CreER; Ai14 animals from P60–64 (Fig. 4A). After 5 d of tamoxifen administration, we observed tdTomato+ cells in the ventral V-SVZ, but not the OB (data not shown). After 4 weeks (wk), we observed tdTomato+ cells along the ventral tip of the lateral ventricles (Fig. 4B,C) consistent with the regional expression of NKX2.1 protein (Fig. 3A). To determine the identity of the tdTomato+ cells (hereafter referred to as Nkx2.1P60-labeled), we performed IHC for the type B1 cell marker GFAP, and for the ependymal cell marker S100β. We found that many Nkx2.1P60-labeled cells expressed S100β and had the morphology of ependymal cells. However, some Nkx2.1P60-labeled cells were located immediately below the ependymal cell layer and expressed GFAP (Fig. 4B), suggesting a type B1 cell identity. After being born in the V-SVZ, DCX+ neuroblasts migrate anteriorly along the lateral ventricle in chains that merge into the rostral migratory stream (RMS) before entering the core of the OB (Doetsch and Alvarez-Buylla, 1996; Doetsch et al., 1999; Lois and Alvarez-Buylla, 1994; Luskin, 1993). We also observed DCX+ / Nkx2.1P60-labeled cells within migratory chains along the walls of the V-SVZ (Fig. 4C) and within the RMS (Fig 4D). Together these data indicate that Nkx2.1+ precursors give rise to cells of the V-SVZ neurogenic lineage.
Figure 4.
Nkx2.1+ cells generate OB interneurons in the adult mouse brain. A, Adult in vivo Nkx2.1-labeling experimental design. High-magnification images of Nkx2.1P60-labeled (red) cells with GFAP (green) in ventral V-SVZ (B) and DCX (green) expression in ventral V-SVZ (C) and RMS (D). White dashed line depicts lateral ventricle. E, tdTomato expression in the OB after 28 days of Tamoxifen administration. F, Composite drawing of Nkx2.1P60-labeled (red) cells from in the OB 4 wk after labeling. Cyan dots depict Nkx2.1P60-labeled cells within the OB core. Black dots depict Nkx2.1P60-labeled cells outside of the OB core. Representative images of Class 1 (G) and class 5 (H) Nkx2.1P60-labeled (red) cells in the OB. G′-H′, Pseudo-colored image of tdTomato channel from G–H. EPL= external plexiform layer, GCL= granule cell layer. Arrowhead denoted initial branch point. Arrow denotes terminal branch location. I, Relative position of Nkx2.1P60-labeled soma within the GCL. J, Relative position of initial dendritic branch point of Nkx2.1P60-labeled cells within the EPL. K, Relative position of terminal dendrite branches within the EPL. Red lines in I–K represent mean value. Scale bars: (B–D,G′) 10 μm, (H,H′) 100 μm, (E–F) 250 μm.
In the OB, Nkx2.1P60-labeled cells were found within the granule cell layer (GCL) of all animals analyzed (Fig. 4E, n=4) at a density of 72.8 ± 35.7 cells/mm3 (317 cells total). After arriving in the OB, young neuroblasts exit the RMS and begin differentiating into interneurons. This progressive maturation of newly-born granular cell (GC) neurons can be categorized into five stages or (1–5) based on their location and morphology (Petreanu and Alvarez-Buylla, 2002). Stage 1 cells are the most immature, having the morphology typical of neuroblasts 5–7 d after their migration from the V-SVZ and located primarily in the OB core, which is the most rostral extent of the RMS (Petreanu and Alvarez-Buylla, 2002). We found that 18.4±10.1% (58/317, n=4) of Nkx2.1P60-labeled GC interneurons were class 1 (Fig. 4G,G′), suggesting that these cells had recently arrived from their origin in the adult V-SVZ. Class 4/5 cells are more mature, having elaborate branched apical dendrites, and represent 11–30 days of differentiation (Petreanu and Alvarez-Buylla, 2002). We observed class 4/5 tdTomato+ cells in 4 of 4 animals (Fig. 4H,H′), indicating that Nkx2.1P60-labeled V-SVZ cells give rise to mature OB granule cells interneurons.
To further characterize Nkx2.1P60-derived GC interneurons, we measured their relative position of their soma within the GCL. OB granule cells can be classified into three subtypes depending on the position of their cell body within the GCL: superficial, intermediate, or deep (Price and Powell, 1970). Deep GC interneurons are located in the first third (0–33%) of the GCL, close to the OB core, while intermediate (34–66%) and superficial (67–100%) interneurons are located more exteriorly. We found that the majority of Nkx2.1P60-labeled cells were located deep in the GCL and had an average relative soma position of 22.9±1.9% (Fig. 4I, n=4). GC interneurons can be further classified based on the position at which their dendrites synapse within the glomerular layer (Kelsch et al., 2007; Shepherd et al., 2007). We found that Nkx2.1P60-derived granule cells had initial dendrite branch points deep in the EPL and dendritic branches largely restricted to the deep EPL (92 cells, Fig. 4H,J,K). We did not observe tdTomato+ cells that co-expressed GCL markers CalR or NPY (data not shown). PGCs were rare in Nkx2.1P60-labeled brains (2 of 319) and were only observed in 2 of 4 brains. Thus, these data suggest that adult Nkx2.1+ NSCs predominantly generate deep GC interneurons.
To determine whether Nkx2.1+ precursors remain active in older mice, we administered tamoxifen to Nkx2.1-CreER; Ai14 mice from P120–P124 (Supplemental Fig. 1A). 4 wks later, we analyzed the OB and observed tdTomato+ cells in the GCL (n=4, 25.2±10.7 cells/mm3, Supplemental Fig. 1B). The soma of Nkx2.1P120-labeled granule cells were located in the deep GCL (30.1±12.1%, Supplemental Fig. 1C,D). Taken together, these data indicate that Nkx2.1+ adult V-SVZ precursors generate new neurons for the OB GCL throughout adulthood.
Postnatal and adult NKX2.1+ NSCs are derived from early embryonic Nkx2.1+ neural precursors
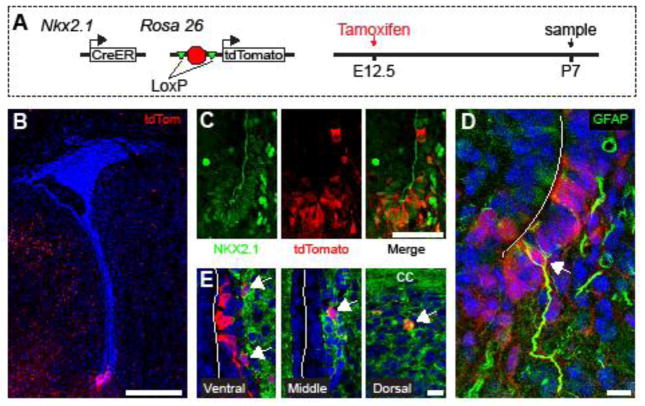
We next investigated whether embryonic Nkx2.1+ neural precursors give rise to the ventral NKX2.1+ NSCs of the postnatal V-SVZ. To follow the fate of embryonic Nkx2.1-expressing precursors into early postnatal brain development, we administered tamoxifen to Nkx2.1-CreER; Ai14 mice at E12.5 and analyzed brains (n=3) at P7 (Fig. 5A). Consistent with previous results (Taniguchi et al., 2011), we observed tdTomato+ (hereafter referred to as Nkx2.1E12.5-labeled) cells in the ventral germinal zone of the late embryonic brain. At P7, Nkx2.1E12.5-labeled cells were present in the ventral V-SVZ (Fig. 5B). Nkx2.1E12.5-labeled cells in the V-SVZ were restricted to the ventral tip in a pattern similar to that of NKX2.1 protein expression (Fig. 1D,D′). To determine whether Nkx2.1-labeled cells from E12.5 still expressed NKX2.1 protein at P7, we performed co-IHC for tdTomato and NKX2.1 (Fig. 5C), and found that 99.8±0.4%, of the Nkx2.1E12.5-labeled cells were also NKX2.1+ (n=3, 286 of 287 cells total).
Figure 5.
Postnatal V-SVZ NKX2.1+ precursors arise from embryonic Nkx2.1+ precursors. A, Embryonic in vivo Nkx2.1-labeling experimental design. B–E, IHC analysis of coronal sections from Nkx2.1E12.5-labeled brains at P7. B, Low-magnification image of Nkx2.1E12.5-labeled (red) cells with DAPI (blue) counterstain at P7. High-magnification confocal images of Nkx2.1E12.5-labeled (red) cells with (C) NKX2.1 (green) and (D) GFAP (green) expression in the ventral V-SVZ. Scale Bars: (B) 250 μm (C–E) 10 μm.
At around P7, type B1 cells emerge and begin to express GFAP (Merkle et al., 2004). In the P7 brain, we observed GFAP+ / Nkx2.1E12.5-labeled cells in the ventral V-SVZ (Fig. 5D). DCX+ / Nkx2.1E12.5-labeled cells were also observed among chains in the ventral, middle and dorsal V-SVZ regions (Fig. 5E). These data suggest that Nkx2.1E12.5-labeled precursors remain neurogenic in the postnatal brain.
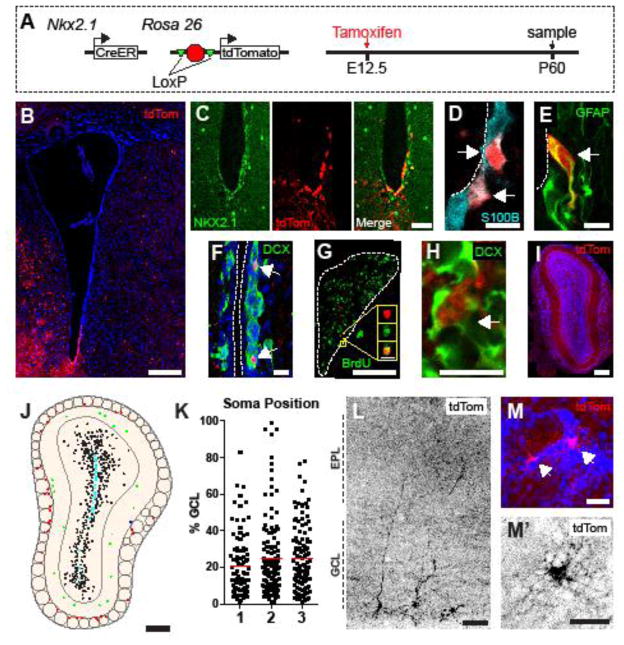
Next we sought to determine whether Nkx2.1-expressing embryonic precursors also contribute NSCs to the adult V-SVZ. For this, we analyzed P60 mouse brains (n=3) for Nkx2.1E12.5-labeled cells (Fig. 6A). As in the P7 animals, Nkx2.1E12.5-labeled cells in the V-SVZ of P60 brains were restricted to the ventral tip of the lateral ventricle (Fig. 6B). IHC revealed that 99.8±0.3%, (n=3, 219/220 cells total) of Nkx2.1E12.5-labeled cells in the V-SVZ were also NKX2.1+ (Fig. 6C). Ependymal cells arise from ventricle-contacting precursors during early postnatal development (Spassky et al., 2005) and constitute a significant proportion of the V-SVZ (Doetsch et al., 1997; Mirzadeh et al., 2008). As expected, the majority of Nkx2.1E12.5-labeled cells corresponded to S100β+ ependymal cells (Fig. 6D). However, a small number of Nkx2.1E12.5-labeled cells expressed GFAP (Fig. 6E), suggestive of a type B1 cell identity. As with the P7 brains, we also observed DCX+ / Nkx2.1E12.5-labeled cells among chains of neuroblasts in the lateral ventricle walls (Fig. 6F) as well as in the RMS (Fig. 6G,H). To confirm that Nkx2.1E12.5-labeled cells in the RMS were born in the adulthood, we continuously administered BrdU to the mice for 1 wk prior to analysis. IHC revealed that Nkx2.1E12.5-labeled cells in the RMS incorporated BrdU (Fig. 6G), suggesting that Nkx2.1E12.5-derived V-SVZ NSCs are neurogenic in adulthood.
Figure 6.
Postnatal Day 60 V-SVZ NKX2.1+ precursors arise from embryonic Nkx2.1+ precursors. A, Embryonic in vivo Nkx2.1-labeling experimental design. B, Low-magnification image of Nkx2.1E12.5-labeled (red) cells with DAPI (blue) counterstain at P60. High-magnification confocal images of Nkx2.1E12.5-labeled (red) cells and (C) NKX2.1 (green), (D) S100β (cyan), and (E) GFAP (green) expression in the ventral V-SVZ at P60. White dashed lines depict lateral ventricle. F, High-magnification image of Nkx2.1E12.5-labeled (red) cells and DCX (green) expression in the lateral V-SVZ. White dashed lines depict lateral ventricle Nkx2.1E12.5-labeled (red) cells in the RMS with (G) BrdU (green) and (H) DCX (green) IHC. White dashed line depicts border of RMS. I, tdTomato expression (red) in the OB at postnatal day 60 with DAPI counterstain (blue). J, Composite drawing of Nkx2.1E12.5-labeled cells in the P60 OB. Colored dots depict Nkx2.1E12.5-labeled cells within the OB core (cyan), GCL (black), EPL (green), and GL (red). K, Relative position of Nkx2.1E12.5-labeled soma within the GCL. Red lines represent mean value. Representative images of Nkx2.1E12.5-labeled (L) GC interneurons and (M,M′) PGCs. Scale Bars: (B,I–J) 250 μm, (C,G,L) 50μm, (M,M′) 25 μm, (D–F,H) 10 μm.
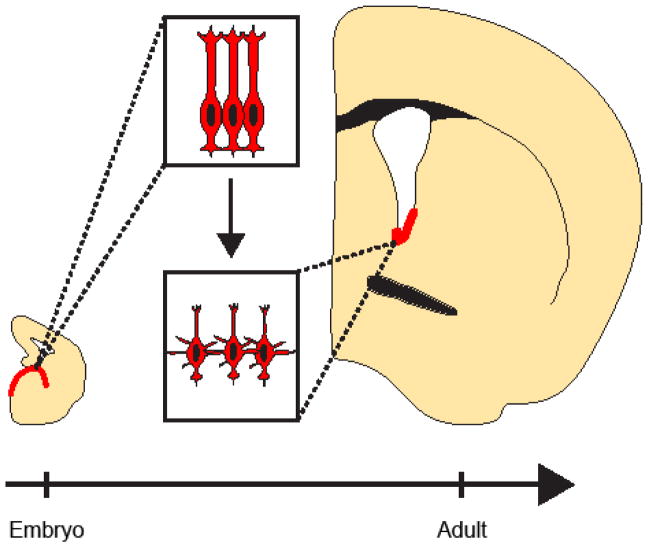
tdTomato+ cells were observed in the OB of Nkx2.1E12.5-labeled brains at P60. The majority of Nkx2.1E12.5-labeled cells were located in the GCL (Fig. 6I,J, 374/426 cells total, n=3). Of these, 7.0±4.5% (28/374 cells total, n=3) were located in the OB core and had a class 1 neuroblast morphology, suggesting that they were recently born in the adult brain. Nkx2.1E12.5-labeled GC interneurons were located in the deep GCL and had an average relative soma position of 23.6±2.4% (Fig. 6J,K). While the majority of Nkx2.1E12.5-labeled OB cells were GC interneurons (Fig. 6L), we also observed tdTomato+ PGCs (Figs. 6M,M′, 8.6±1.7%, 36/426 cells total, n=3) and external plexiform layer interneurons (4.0±3.5%, data not shown). Taken together, these data support a model in which early embryonic MGE neural precursors give rise to a population of adult V-SVZ NSCs that take residence in a restricted ventral domain and generate new OB neurons throughout adult life (Fig. 7).
Figure 7.
Regional identity of embryonic Nkx2.1+ precursors is maintained into adulthood. Model of Nkx2.1+ domain from embryogenesis into adulthood. Nkx2.1+ radial glia (depicted in upper inset) give rise to Nkx2.1+ type B1 cells (depicted in lower inset) of the adult V-SVZ.
DISCUSSION
Our results indicate that the ventral V-SVZ contains a population of Nkx2.1+ NSCs that continue to generate OB neurons throughout life. These adult NKX2.1+ NSCs derive from Nkx2.1-expressing cells in the early embryonic brain, indicating that ventral germinal zones contribute to the positional heterogeneity of the adult V-SVZ by giving rise to a regionally-specified population of NSCs.
Adult Nkx2.1+ V-SVZ NSCs primarily generated OB interneurons of the deep GCL. While NSCs in the dorsal V-SVZ give rise to superficial granule cells, NSCs in the ventral V-SVZ primarily generate deep granule cells (Merkle et al., 2007). The production of deep OB granule cells is therefore consistent with the ventral location of NKX2.1+ NSCs within the V-SVZ (Fig. 3). V-SVZ NSCs also have a rostral-caudal identity. While rostral V-SVZ NSCs produce many PGCs, the caudal V-SVZ produces very few (Merkle et al., 2007). Consistent with the caudal location of the Nkx2.1 domain within the V-SVZ (Fig. 3 and (Merkle et al., 2014), we observed very few PGCs born from adult Nkx2.1+ NSCs. Further characterization of GC interneurons born from Nkx2.1+ V-SVZ NSCs revealed that they extend dendritic arbors into the deep EPL (Fig. 4H,J–K). This deep lamina-specific dendritic targeting is consistent with adult born GC interneuron identity described by Kelsh et al. (2007). Therefore, the Nkx2.1 V-SVZ domain generated neurons consistent with the temporospatial identity of an adult, ventrocaudal NSC population.
In comparison to the dorsal-lateral regions of the V-SVZ, there are relatively few NSCs in the ventral regions of the lateral ventricle (Mirzadeh et al., 2008). Moreover, the adult Nkx2.1+ domain (Fig. 3) is a small proportion of the entire V-SVZ. Thus, the number of Nkx2.1-lineage OB interneurons born in the adult mouse brain (Fig. 4) appears to be commensurate with the relatively small size of the Nkx2.1 V-SVZ domain and the paucity of NSCs in this ventral region. For instance, Nkx2.1P60-derived neuroblasts contributed to 0.05% ± 0.037 (n=4) of the DCX+ cell population in the RMS (Fig. 4D). However, given that Nkx2.1+ NSCs continued to generate new OB neurons late into life (Supplemental Fig. 1), it is interesting to consider that the neurons generated by this spatially restricted population of NSCs might have unique and important functions for olfaction.
What is the developmental origin of the different V-SVZ NSC populations? While Emx1+ embryonic neural precursors of the developing cortex have been fate-traced to adult NSCs in the dorsal V-SVZ, the origin of NSCs in other regions of the adult lateral ventricle has been less clear. Multiple studies have implicated the LGE as an important origin of V-SVZ NSCs (Kohwi et al., 2005; Waclaw et al., 2006; Young et al., 2007). For example, E13.5 LGE cells produce many OB neurons during development (Wichterle et al., 2001), and when grafted into the adult brain, LGE cells can also generate neurons for the OB (Wichterle et al., 1999). Furthermore, the embryonic LGE and MGE express Gsh2, and adult Gsh2-Cre mice have labeled NSCs in the V-SVZ (Young et al., 2007). However, because Gsh2 is also expressed in the adult V-SVZ (Lopez-Juarez et al., 2013), the Gsh2-Cre transgene may label V-SVZ cells in both the embryonic and adult brain, making it difficult to establish an embryonic origin to the Gsh2+ cells of the adult V-SVZ. Similarly, while Nkx2.1-Cre labels cells in the ventral V-SVZ (Young et al., 2007), we found Nkx2.1 to be expressed throughout development and into adulthood (Figs. 1–3), again making it unclear when Nkx2.1-Cre induces recombination.
To more clearly establish a temporal relationship between a population of embryonic neural precursors and V-SVZ NSCs, we used the Nkx2.1-CreER allele to label a cohort of Nkx2.1+ precursors at E12.5, which gave rise to NSCs of the postnatal and adult V-SVZ. At E12.5, the expression of Nkx2.1 distinguishes the MGE from the LGE (Flames et al., 2007). While Nkx2.1 is expressed in other E12.5 ventral brain regions such as the septum and preoptic area (POA), the MGE does not express Dbx1, which is detected in these other ventral embryonic regions (Hirata et al., 2009). Mice with the Dbx1-Cre transgene do not appear to have labeled cells in the ventral V-SVZ (Young et al., 2007). Thus, in light of these other studies, our fate-tracing analysis of embryonic Nkx2.1+ cells suggests that the MGE is an important embryonic origin to the NSCs of the ventral V-SVZ.
Interestingly, we found that Nkx2.1+ precursors labeled during embryogenesis generated a more diverse population of OB interneurons than those labeled in adulthood. While the majority of Nkx2.1E12.5-labeled cells in the OB were GC interneurons (87.4±5.0%), we also observed a substantial population of labeled PGCs (8.6±1.7%, Figs. 6M,M′), which were exceptionally rare in Nkx2.1P60-labeled mice (n=2/4, 2 of 319 cells). Using a constitutively active, transgenic Nkx2.1-Cre driver, Xu et al. (2008) observed streams of labeled cells migrating from the MGE toward the OB, suggesting that Nkx2.1+ precursors generate OB interneurons during embryogenesis. Furthermore, analysis of Nkx2.1-Cre transgenic animals at P100 revealed labeled PGCs (Xu et al., 2008). These previous data, taken together with our results suggest that the majority of PGCs derived from Nkx2.1+ precursors arise during embryogenesis.
What accounts for the difference in the developmental potential of embryonic and adult Nkx2.1+ precursors? One possibility is that there is a single homogeneous population of Nkx2.1+ precursors whose fate becomes restricted over time. Another possibility is that there are multiple subpopulations of Nkx2.1+ precursors with different developmental potentials. Recent work by Fuentealba et al. (2015) suggests that V-SVZ NSCs are born from embryonic precursors between E13.5 and E15.5 but remain quiescent until postnatal life. After performing clonal analysis, they found that most individual V-SVZ NSCs generate either PGCs or GCs, but not both (Fuentealba et al., 2015). Therefore, it is possible that by administering tamoxifen to Nkx2.1CreER:Ai14 mice at E12.5 (Fig. 6), we labeled a population of precursors that are neurogenic during embryogenesis as well as a population of quiescent precursors that later become V-SVZ NSCs in postnatal life. The population of Nkx2.1+ precursors in the embryonic brain may contain clones that generate PGCs and GCs, while those in the adult brain may generate primarily GC cells.
Do transcription factors that determine regional identity in the embryonic brain continue to define developmental differences in V-SVZ NSCs of the adult? Gsh2 is expressed in neural precursors in the LGE and MGE, and the Gsh2-Cre driver labels cells that later become V-SVZ NSCs that are distributed along the entire dorsal-ventral extent of the lateral ventricle wall (Young et al., 2007). However, using both a Gsh2-GFP reporter mouse and GSH2 IHC analysis to detect active Gsh2 transcription, Lopez-Juarez et al. (2013) found that Gsh2 expression in the adult V-SVZ is restricted to the dorsolateral regions and is not detected in cells in the ventral V-SVZ (Lopez-Juarez et al., 2013). These results indicate that while a large proportion of the V-SVZ derives from cells that express Gsh2 at some point during development, Gsh2 expression is not maintained in most cells of the Gsh2-lineage. In contrast, embryonic Nkx2.1+ neural precursors give rise to a much more spatially restricted population of V-SVZ NSCs (Young et al., 2007), and virtually every Nkx2.1E12.5-labeled cell in the V-SVZ continued to express NKX2.1 protein in the postnatal and adult brain (Figs. 5C,6C). RGCs are considered to be the NSC population of the embryonic brain (Kriegstein and Alvarez-Buylla, 2009). In addition to generating many neurons and glia during embryogenesis, RGCs also give rise to V-SVZ NSCs during early postnatal development (Merkle et al., 2004). Taken together, our results suggest that the expression of Nkx2.1 in embryonic RGCs is “maintained” as they continue dividing throughout development and after they become NSCs of the adult brain (Fig. 7).
The regional identity of neural precursors is a critical aspect of normal brain development. How is Nkx2.1 expression maintained in a spatially precise pattern despite the rapid growth and the complex anatomical changes of the developing brain? While sonic hedgehog (SHH) signaling is required for the expression of Nkx2.1 and many other ventral transcription factors through mid-embryonic development (Fuccillo et al., 2004; Gulacsi and Anderson, 2006; Hebert and Fishell, 2008; Xu et al., 2005), it is unclear whether neural precursors require SHH-signaling to maintain the expression of these transcription factors later in development. Future studies may determine the extent to which external morphogens such as SHH and cell-intrinsic epigenetic mechanisms maintain the expression of important determinants of neural precursor regional identity.
Conclusions
We provide clear evidence that Nkx2.1-expressing precursors in the E12.5 embryo give rise to a specific regional population of V-SVZ NSCs that maintain Nkx2.1 expression throughout life. Consistent with their ventral position within the V-SVZ, Nkx2.1+ V-SVZ precursors generate a subpopulation of deep granule interneurons. Further studies regarding how Nkx2.1 expression is regulated over time will provide key insights into how NSC heterogeneity is maintained throughout development.
Supplementary Material
Nkx2.1+ cells generate OB interneurons throughout life. A, P120 in vivo Nkx2.1-labeling experimental design. B, Representative image of a Nkx2.1P120-derived GC interneuron and high mag pseudo-colored inset (B′). C, Composite drawing of Nkx2.1P120-labeled (red) cells in the OB 4 wk after labeling. Cyan dots depict Nkx2.1P60-labeled cells within the OB core. Black dots depict Nkx2.1P60-labeled cells outside of the OB core. D, Relative position of Nkx2.1P120-labeled soma within the GCL. Solid red bars denote mean of each animal, dashed rad bar denotes average across all 4 animals. Scale bars: (B,B′) 25 μm, (C) 250 μm.
Highlights.
Nkx2.1 expression defines a ventral domain of NSCs in the adult V-SVZ.
Adult Nkx2.1+ NSCs generate deep granule cells for the olfactory bulb.
Embryonic Nkx2.1+ cells give rise to adult Nkx2.1+ NSCs in the ventral V-SVZ.
A ventral domain of Nkx2.1+ NSCs persists throughout life in the telencephalon.
Acknowledgments
We thank L. Fuentealba and A. Alvarez-Buylla for helpful comments and suggestions, and Melanie Bedolli for assisting in the caesarean delivery of tamoxifen-treated embryos. This work was funded by: NIH New Innovator 1DP2OD006505-01, VA 2I01 BX000252-04, CIRM Predoctoral Fellowship (TG2-01153).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work Cited
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harbor symposia on quantitative biology. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Didier M, Harandi M, Aguera M, Bancel B, Tardy M, Fages C, Calas A, Stagaard M, Mollgard K, Belin MF. Differential immunocytochemical staining for glial fibrillary acidic (GFA) protein, S-100 protein and glutamine synthetase in the rat subcommissural organ, nonspecialized ventricular ependyma and adjacent neuropil. Cell and tissue research. 1986;245:343–351. doi: 10.1007/BF00213941. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A. Embryonic Origin of Postnatal Neural Stem Cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JL, Ekker M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulacsi A, Anderson SA. Shh maintains Nkx2.1 in the MGE by a Gli3-independent mechanism. Cerebral cortex. 2006;16(Suppl 1):i89–95. doi: 10.1093/cercor/bhk018. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nature reviews Neuroscience. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nature neuroscience. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, Alvarez-Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–262. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS biology. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends in neurosciences. 2014;37:563–571. doi: 10.1016/j.tins.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends in neurosciences. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, Campbell K, Nakafuku M. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes & development. 2013;27:1272–1287. doi: 10.1101/gad.217539.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nature neuroscience. 2014;17:207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell stem cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marin O. Postmitotic Nkx2–1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. Journal of cell science. 1970;7:631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Price M, Lazzaro D, Pohl T, Mattei MG, Ruther U, Olivo JC, Duboule D, Di Lauro R. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992;8:241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tang X, Falls DL, Li X, Lane T, Luskin MB. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5837–5844. doi: 10.1523/JNEUROSCI.5048-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature neuroscience. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. The Journal of comparative neurology. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nkx2.1+ cells generate OB interneurons throughout life. A, P120 in vivo Nkx2.1-labeling experimental design. B, Representative image of a Nkx2.1P120-derived GC interneuron and high mag pseudo-colored inset (B′). C, Composite drawing of Nkx2.1P120-labeled (red) cells in the OB 4 wk after labeling. Cyan dots depict Nkx2.1P60-labeled cells within the OB core. Black dots depict Nkx2.1P60-labeled cells outside of the OB core. D, Relative position of Nkx2.1P120-labeled soma within the GCL. Solid red bars denote mean of each animal, dashed rad bar denotes average across all 4 animals. Scale bars: (B,B′) 25 μm, (C) 250 μm.