Abstract
C. difficile CD37, a clinical isolate from the USA, does not produce toxin A, B or binary toxin. The aim of this study was to determine whether strain CD37 can protect mice against infection from a challenge with a toxigenic C. difficile strain. Three groups of mice (n=10) were pretreated with a antibiotics cocktail for 5 days, switched to sterile water for 2 days, and given one dose of clindamycin (10 mg/kg) one day (day-1) before challenge (day 0) with a toxigenic C. difficile strain. Group 1 (CD37+UK6) was given 107 C. difficile CD37 vegetative cells by gavage twice a day on days -1 and -2, followed by challenge with 106 spores of the toxigenic C. difficile UK6 (BI/NAPI/027) on day 0; Group 2 (UK6) was infected with 106 C. difficile UK6 spores on day 0; Group 3 (CD37) was challenged with 106 CD37 vegetative cells on day 0. Our data show that pre-inoculation with strain CD37 provided significant protection (survival, p < 0.001 between groups CD37+UK6 and UK6) against subsequent infection with the strain UK6, while mice infected with CD37 only did not develop any symptoms of C. difficile infection (CDI). Our results highlight the potential use of CD37 as a therapeutic strain for the prevention of primary and recurrent CDI in humans.
Introduction
Clostridium difficile is the leading cause of health care-associated infectious diarrhea [1]. Two toxins, TcdA and TcdB, are major virulence factors of C. difficile [2–4]. Both mortality and morbidity of C. difficile infection (CDI) have increased significantly in the last 15 years [5], partially due to the emergence of new virulent BI/NAP1/027 type C. difficile strains, some of which have increased toxin production and sporulation capacity, altered microbial resistance patterns (fluoroquilonone resistance) and produce an additional binary toxin [6, 7]. Interestingly, non-toxigenic C. difficile strains which lack the genes for TcdA, TcdB and the binary toxin exist in nature, and have been isolated from humans, although little is known about their biology [8].
Previous studies have shown that asymptomatic colonization by non-toxigenic C. difficile strains tend to decrease risk of CDI in humans [9]. Non-toxigenic C. difficile strains have been shown to prevent fatal CDI in hamsters and piglets [10] [11, 12]. C. difficile CD37 is a non-toxigenic clinical isolate from the USA [13]. In the strain CD37, the PaLoc (encoding toxin A and B) is absent, in its place is 115 bp of non-coding DNA [14].
CDI has been studied in a number of animal models, including hamsters, guinea pigs, rabbits, rats, germfree mice, conventional mice and germfree piglets [15–20]. The hamster model has been widely used. However, hamsters are extremely sensitive to C. difficile, rapidly develop clinical signs of CDI, and die within 2 to 3 days of infection dependent on strains used [21]. Therefore, this model does not represent the usual course and spectrum of CDI in human beings. The recently developed mouse and piglet CDI models are good alternatives for studying chronic CDI [19, 20], though large C. difficile challenging doses are required.
In the present study, we evaluated whether CD37 could be used as a therapeutic strain for the prevention of CDI in mice.
Materials and methods
Animals
C57BL/6 female mice (5 to 6 weeks old) were purchased from Charles River Laboratories, MA. All mice used in the experiments were housed in groups of 5 animals per cage under the same conditions. Food, water, bedding and cages were autoclaved. All studies followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Tufts University Institutional Animal Care and Use Committee (IACUC) under the Protocol #G2012-70.
Spore preparation
C. difficile strains UK6 and CD37 were kindly provided by Drs. Abraham L. Sonenshein and Dale Gerding [22]. C. difficile strain UK6 belongs to the 027/BI21/NAP1 type, and produces TcdA, TcdB and binary toxin. Spores of C. difficile UK6 were prepared as described [23]. C. difficile CD37 is a non-toxigenic clinical isolate from the USA [13]. Strain CD37 is a poor sporulator, and vegetative cells were used to infect mice.
Antibiotics administration and C. difficile challenge
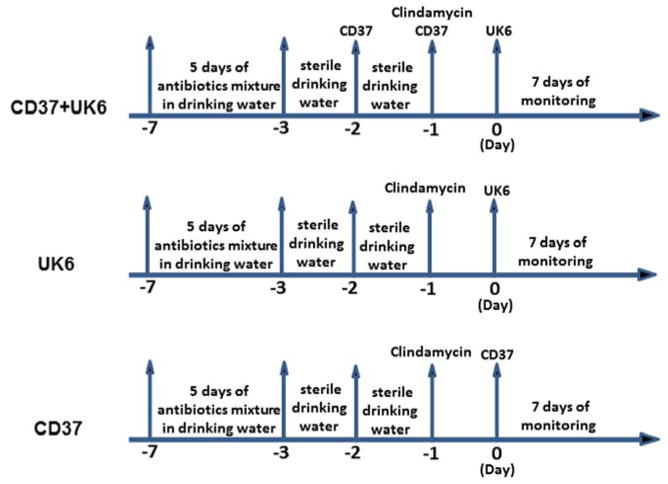
Antibiotic administration was based on a previously published protocol [20]. The experimental design is illustrated in Fig. 1. Three groups of mice (n=10) were given a mixture of six antibiotics including ampicillin (200 mg/kg), kanamycin (40 mg/kg), gentamycin (3.5 mg/kg), colistin (4.2 mg/kg), metronidazole (21.5 mg/kg) and vancomycin (4.5 mg/kg) in the drinking water for 5 days. After 5 days of antibiotic treatment, all mice were given autoclaved water for 2 days, followed by a single dose of clindamycin (10 mg/kg) intraperitoneally 1 day before (day-1) challenge with C. difficile UK6 spores by gavage (day 0). One group (CD37+UK6) was given 107 C. difficile CD37 vegetative cells by gavage twice a day on days -1 and -2, followed by challenge with 106 C. difficile UK6 spores on day 0; the second group (UK6) was infected with 106 C. difficile UK6 spores on day 0; and the third group (CD37) was challenged with 106 CD37 vegetative cells on day 0.
Fig. 1. Experimental scheme of pretreatment of mice with C. difficile CD37 in a mouse model of C. difficile infection.
After 5 days of antibiotic treatment, mice were given autoclaved water for 2 days, followed by a single dose of clindamycin (10 mg/kg) intraperitoneally 1 day before (day-1) challenge with C. difficile UK6 spores by gavage (day 0). The first group (CD37+UK6) was given 107 C. difficile CD37 vegetative cells by gavage twice a day on days -1 and -2, followed by challenge with 106 C. difficile UK6 spores on day 0; the second group (UK6) was infected with 106 C. difficile UK6 spores on day 0; the third group (CD37) was challenged with 106 CD37 vegetative cells on day 0. Mice were monitored for diseases for 7 days.
Fecal cytotoxicity
Fecal cytoxicity was determined as described previously [15]. Fecal samples were collected, and dissolved at 0.1g/ml in sterile PBS containing protease inhibitor cocktail. After centrifugation the supernatants were recovered and stored at −80°C. To detect toxin-mediated cytotoxicity in fecal samples, the supernatants were filtered and serially diluted before adding to monolayers of Vero grown in 96-well plates. Each sample and dilution was duplicated. Toxin titers were defined as the highest dilution to cause 100% cytopathic effect (cell rounding) after overnight incubation. The specific activity caused by C. difficile toxins from fecal samples was confirmed with goat anti-TcdA and -TcdB polysera.
Statistical analysis
Mouse survivals were analyzed by Kaplan-Meier survival analysis with a log rank test of significance using Prism. Mean relative weight was analyzed by multiple t tests using the Prism. Results are expressed as means ± standard errors of means.
Results
Pre-inoculation of C. difficile CD37 protected mice against infection with a virulent strain C. difficile UK6
The experimental scheme is illustrated in Fig 1. After challenge with C. difficile UK6 spores (groups: UK6 and CD37+UK6) or C. difficile CD37 cells (group: CD37) on day 0, three groups of mice (n=10) were monitored for mortality (Fig 2A), weight loss (Fig 2B) and diarrhea (Fig 2C). While all mice in group UK6 developed diarrhea, only 30 % mice from the group CD37+UK6 developed diarrhea (Fig 2C). Eighty percent of group CD37+UK6 mice survived, which is significantly higher than survival of group UK6 mice (20%) (p<0.01) (Fig 2A). Mice in group UK6 also lost significantly more weight than those in group CD37+UK6 (p<0.05, on post-infection days 2 and 3). As expected, mice from the control group CD37 did not develop any signs of disease, neither diarrhea (Fig 2C) nor weight loss (Fig 2B), and all mice survived (Fig. 2A). Mice from the group CD37+UK6 (Fig. 2E) excreted much less toxins in the feces as compared with the group UK6 (Fig 2D). No toxins were detected in the feces from group CD37 (Fig 2F).
Fig. 2. Pre-inoculation of C. difficile CD37 protected mice against infection with a virulent strain C. difficile UK6.
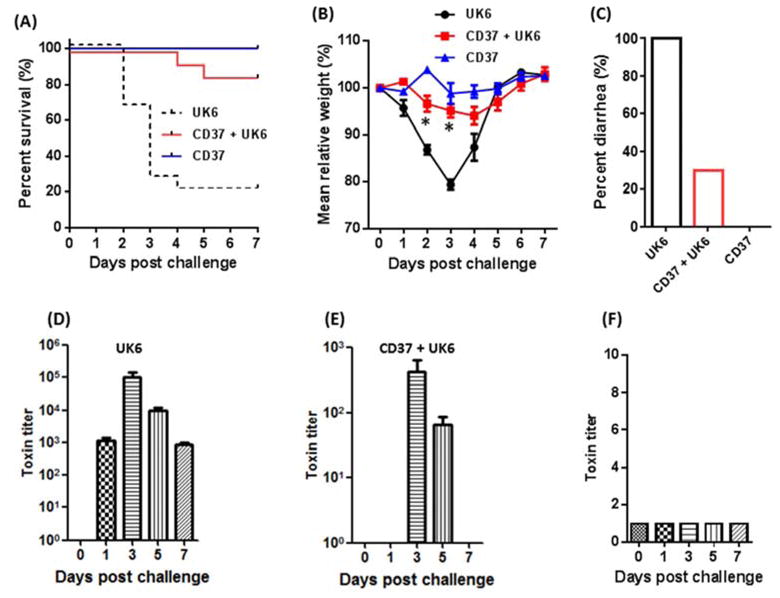
After challenge with UK6 or CD37, mice were monitored for survival (P <0.001 between groups UK6 and CD37+UK6) (A), weight loss (* p<0.05 on post-infection days 2 and 3 between groups UK6 and CD37+UK6) (B), and occurrence of diarrhea (C). C. difficile toxin levels in feces from group UK6 (D), CD37+UK6 (E) and CD37(F).
Discussion
The incidence of CDI is rising worldwide [24]. Standard therapy depends on treatment with vancomycin, fidaxomicin or metronidazole, none of which are fully effective [25]. Moreover, an estimated 15–35% of those infected with C. difficile relapse following treatment [26, 27]. In an effort to improve outcomes and reduce recurrences of CDI, interest has been renewed in the development of non-antibiotic and adjunct approaches to therapy. There has been considerable interest in the use of probiotics to prevent CDI.
It has been reported that non-toxigenic C. difficile can protect hamsters against challenge with historic and epidemic toxigenic BI/NAP1/027 C. difficile strains [12]. Non-toxigenic C. difficile has been used to colonize and prevent CDI in two patients who experienced multiple relapses of CDI, and has been proven safe [11, 28]. More recently, non-toxigenic C. difficile strain M3 was evaluated for prevention of recurrent CDI in 129 patients in a randomized clinical trial; and it was shown that the strain M3 was well tolerated, colonized the gastrointestinal tract and significantly reduced CDI recurrence [29]. In this regard, non-toxigenic C. difficile could be considered as probiotics. C. difficile CD37 is the first non-toxigenic strain sequenced. More importantly, CD37 is a poor sporulator, making it an ideal strain for potential use in humans for treatment/prevention of primary and recurrent CDI. Therefore, in this project we evaluated its efficacy in protecting CDI in mice, and found oral inoculation of mice with C. difficile CD37 vegetative cells provided significant protection to mice against challenge with a clinical toxigenic BI/NAP1/027 type of C. difficile strain.
C. difficile CD37-mediated protection is not due to immune response induced by CD37 against the toxigenic strain, since anti-C. difficile antibodies cannot be significantly induced in mice within one or two days. The most logic interpretation of our observations would be that CD37 pre-colonization prevented the subsequent colonization of the toxigenic C. difficile as proposed previously [30]. However, due to the difficulties in differentially enumerating CD37 and UK6 from mouse feces, the hypothesis of colonization competition is difficult to test. Historically, live attenuated bacteria and viruses have been used as vaccines for human and animals [31] [32]. In this regard, C. difficile CD37 could be used as a potential vaccine candidate to prevent primary and recurrent CDI. An ideal vaccine should target both toxins and bacterial colonization with a goal to cure the toxin-mediated symptoms and reduce/prevent C. difficile transmission. Since strain CD37 is a poor sporulator, it would be an ideal vaccine candidate if engineered to carry immunodominant toxin epitopes. However, we should also be cautious in the possibility of non-toxigenic strains conversion into toxigenic strains in vivo though not reported so far [33].
Acknowledgments
Financial support to X.SUN from NIDDK (grant K01DK092352), Tufts Collaborates! 2013 (grant V330421). We would like to thank Drs. Ellie J.C. Goldstein, Dale Gerding, Giovanni F. Widmer, Abraham L. Sonenshein, for critical comments of the manuscript.
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbut F, Corthier G, Charpak Y, Cerf M, Monteil H, Fosse T, Trevoux A, De Barbeyrac B, Boussougant Y, Tigaud S, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Archives of internal medicine. 1996;156(13):1449–1454. [PubMed] [Google Scholar]
- 2.Elliott B, Chang BJ, Golledge CL, Riley TV. Clostridium difficile-associated diarrhoea. Internal medicine journal. 2007;37(8):561–568. doi: 10.1111/j.1445-5994.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. The New England journal of medicine. 1994;330(4):257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 4.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18(2):247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173(9):1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. The New England journal of medicine. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. The New England journal of medicine. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 8.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 9.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351(9103):633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 10.Songer JG, Jones R, Anderson MA, Barbara AJ, Post KW, Trinh HT. Prevention of porcine Clostridium difficile-associated disease by competitive exclusion with nontoxigenic organisms. Vet Microbiol. 2007;124(3–4):358–361. doi: 10.1016/j.vetmic.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Villano SA, Seiberling M, Tatarowicz W, Monnot-Chase E, Gerding DN. Evaluation of an oral suspension of VP20621, spores of nontoxigenic Clostridium difficile strain M3, in healthy subjects. Antimicrob Agents Chemother. 2012;56(10):5224–5229. doi: 10.1128/AAC.00913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagaro KJ, Phillips ST, Cheknis AK, Sambol SP, Zukowski WE, Johnson S, Gerding DN. Nontoxigenic Clostridium difficile protects hamsters against challenge with historic and epidemic strains of toxigenic BI/NAP1/027 C. difficile. Antimicrob Agents Chemother. 2013;57(11):5266–5270. doi: 10.1128/AAC.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CJ, Markowitz SM, Macrina FL. Transferable tetracycline resistance in Clostridium difficile. Antimicrobial agents and chemotherapy. 1981;19(6):997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwer MS, Allan E, Mullany P, Roberts AP. Draft genome sequence of the nontoxigenic Clostridium difficile strain CD37. J Bacteriol. 2012;194(8):2125–2126. doi: 10.1128/JB.00122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Wang H, Zhang Y, Chen K, Davis B, Feng H. Mouse relapse model of Clostridium difficile infection. Infection and immunity. 2011;79(7):2856–2864. doi: 10.1128/IAI.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czuprynski CJ, Johnson WJ, Balish E, Wilkins T. Pseudomembranous colitis in Clostridium difficile-monoassociated rats. Infection and immunity. 1983;39(3):1368–1376. doi: 10.1128/iai.39.3.1368-1376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekety R, Silva J, Toshniwal R, Allo M, Armstrong J, Browne R, Ebright J, Rifkin G. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Reviews of infectious diseases. 1979;1(2):386–397. doi: 10.1093/clinids/1.2.386. [DOI] [PubMed] [Google Scholar]
- 18.Knoop FC. Clindamycin-associated enterocolitis in guinea pigs: evidence for a bacterial toxin. Infection and immunity. 1979;23(1):31–33. doi: 10.1128/iai.23.1.31-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele J, Feng H, Parry N, Tzipori S. Piglet models of acute or chronic Clostridium difficile illness. The Journal of infectious diseases. 2010;201(3):428–434. doi: 10.1086/649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Douce G, Goulding D. Refinement of the hamster model of Clostridium difficile disease. Methods Mol Biol. 2010;646:215–227. doi: 10.1007/978-1-60327-365-7_14. [DOI] [PubMed] [Google Scholar]
- 22.Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, Frost EH, Savelkoul P, Nicholson B, van den Berg RJ, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. Journal of clinical microbiology. 2008;46(2):431–437. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez J, Springthorpe VS, Sattar SA. Clospore: a liquid medium for producing high titers of semi-purified spores of Clostridium difficile. Journal of AOAC International. 94(2):618–626. [PubMed] [Google Scholar]
- 24.Evans CT, Safdar N. Current Trends in the Epidemiology and Outcomes of Clostridium difficile Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(Suppl 2):S66–71. doi: 10.1093/cid/civ140. [DOI] [PubMed] [Google Scholar]
- 25.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(3):302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 26.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38(6):2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonna I, Welsby PD. Pathogenesis and treatment of Clostridium difficile infection. Postgrad Med J. 2005;81(956):367–369. doi: 10.1136/pgmj.2004.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seal D, Borriello SP, Barclay F, Welch A, Piper M, Bonnycastle M. Treatment of relapsing Clostridium difficile diarrhoea by administration of a non-toxigenic strain. European journal of clinical microbiology. 1987;6(1):51–53. doi: 10.1007/BF02097191. [DOI] [PubMed] [Google Scholar]
- 29.Gerding DN, Meyer T, Lee C, Cohen SH, Murthy UK, Poirier A, Van Schooneveld TC, Pardi DS, Ramos A, Barron MA, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. Jama. 2015;313(17):1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 30.Nagaro KJ, Phillips ST, Cheknis AK, Sambol SP, Zukowski WE, Johnson S, Gerding DN. Nontoxigenic Clostridium difficile protects hamsters against challenge with historic and epidemic strains of toxigenic BI/NAP1/027 C. difficile. Antimicrobial agents and chemotherapy. 57(11):5266–5270. doi: 10.1128/AAC.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer WA, 2nd, Chason KD, Brighton M, Jaspers I. Live attenuated influenza vaccine strains elicit a greater innate immune response than antigenically-matched seasonal influenza viruses during infection of human nasal epithelial cell cultures. Vaccine. 2014;32(15):1761–1767. doi: 10.1016/j.vaccine.2013.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Wu Q. Research progress in live attenuated Brucella vaccine development. Curr Pharm Biotechnol. 2013;14(10):887–896. doi: 10.2174/1389201014666131226123016. [DOI] [PubMed] [Google Scholar]
- 33.Brouwer MS, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nature communications. 2013;4:2601. doi: 10.1038/ncomms3601. [DOI] [PMC free article] [PubMed] [Google Scholar]