Abstract
Background
Opioid dependence remains a significant public health problem worldwide with only three FDA-approved treatments, all targeting the mu-opioid receptor. Dronabinol, a cannabinoid (CB) 1 receptor agonist, is currently under investigation as a novel opioid withdrawal treatment. This study reports on safety outcomes of dronabinol among adults in opioid withdrawal.
Methods
Twelve adults physically dependent on short-acting opioids participated in this 5-week within-subject, randomized, double blind, placebo-controlled inpatient study. Volunteers were maintained on oral oxycodone 30mg qid. Double-blind placebo substitutions occurred for 21 hours before each of 7 experimental sessions in order to produce opioid withdrawal. A single oral test dose was administered each session (placebo, oxycodone 30 and 60mg, dronabinol 5, 10, 20, and 30mg [decreased from 40mg]). Heart rate, blood pressure, respiratory outcomes and pupil diameter were assessed repeatedly.
Results
Dronabinol 40mg produced sustained sinus tachycardia accompanied by anxiety and panic necessitating dose reduction to 30mg. Sinus tachycardia and anxiety also occurred in one volunteer after dronabinol 20mg. Compared to placebo, dronabinol 20 and 30mg produced significant increases in heart rate beginning 1 hour after drug administration that lasted approximately two hours (p<0.05). Dronabinol 5 and 10mg produced placebo-like effects. Oxycodone produced prototypic mu-opioid agonist effects (e.g., miosis).
Conclusion
Dronabinol 20mg and higher increased heart rate among healthy adults at rest who were in a state of opioid withdrawal, raising concern about its safety. These results have important implications for future dosing strategies and may limit the utility of dronabinol as a treatment for opioid withdrawal.
Keywords: Dronabinol, opioid withdrawal, safety, treatment, opioid dependence
1. INTRODUCTION
Opioid dependence is a significant public health problem worldwide that continues to grow, in large part, due to the prescription opioid epidemic. In 2013, within the United States (US), there were 1.9 million individuals with a prescription opioid use disorder and 712,000 with a heroin use disorder (SAMHSA, 2014). Currently, there are only three marketed FDA-approved medications (methadone, buprenorphine and naltrexone) for opioid dependence treatment, and all exert their efficacy through action at the mu-opioid receptor.
Dronabinol, a schedule III oral synthetic Δ9-tetrahydrocannabinol (THC) analog and cannabinoid-1 (CB1) receptor agonist, is currently approved for chemotherapy-induced nausea and vomiting and anorexia in patients with acquired immunodeficiency syndrome (AIDS). Interestingly, CB1 receptors are often co-localized with opioid receptors in brain regions involved in opioid withdrawal, drug reward and self-administration, and analgesia, including the locus coeruleus, nucleus accumbens, thalamus and spinal cord (Pickel et al., 2004; Scavone et al., 2010; Welch 2009). Thus, CB1 agonists may have a role in modulating opioid-mediated effects, including the expression of opioid withdrawal (Scavone et al., 2013).
Preclinical studies have reported that CB1 agonists (i.e., Δ9-THC) attenuate signs of opioid withdrawal (Lichtman et al 2001; Cichewicz and Welch 2003), while conversely; CB1 antagonists (SR 141716A) elicit opioid withdrawal signs (Navarro et al., 1998). One recent randomized placebo-controlled clinical study reported that dronabinol (30mg/day) briefly and modestly reduced subject-rated opioid withdrawal severity; however, open-label buprenorphine on day 2 and other ancillary medications on multiple days also were given and no physiologic data (e.g., heart rate) were reported (Bisaga et al., 2015).
Safety outcomes from a study evaluating the efficacy of acute doses of oral dronabinol (without adjuvant medications) for the treatment of opioid withdrawal are reported here. Because there were statistically and clinically significant effects on heart rate and there is interest in exploring this class of agents for opioid dependence, the study team believed these results warranted quick dissemination (primary efficacy analyses to be reported separately).
2. METHODS
2.1 Participants
Inclusion criteria were: age 18–50 years; self-report of non-medical use of short-acting opioids ≥21/30 days; opioid physical dependence; positive urine opioid test; and good general health as determined by physical exam, 12-lead ECG, and blood and urine chemistries. Exclusion criteria included: currently seeking treatment or pregnant/breastfeeding; physiologic dependence on alcohol or sedative/hypnotics requiring medical management; buprenorphine or methadone as primary drug of abuse; ongoing medical (e.g., chronic pain) or psychiatric (e.g., schizophrenia) illness; recent use of CYP3A4/2D6 medications; and use of marijuana > 15/30 days in order to exclude daily or near daily users who could be tolerant to the effects of Δ9-THC. The protocol was written and carried out in accordance with the Declaration of Helsinki, was approved by the University of Kentucky (UK) Institutional Review Board, and participants provided written informed consent.
2.2 Study setting
Participants resided as inpatients on the UK research unit for 5 weeks and were maintained on a caffeine-free diet. Urine was tested daily for the presence of illicit drugs and weekly for pregnancy (female). Cigarette smoking was allowed under staff supervision, except for 30 minutes before and throughout experimental sessions. Non-psychoactive medications (i.e., acetaminophen, colace) were available as needed, but restricted from midnight before session through session completion.
2.3 Study design, procedure and experimental sessions
This study employed a double-blind, randomized, within subject design. Participants were stabilized on oral oxycodone 30 mg qid (8:00, 12:00, 18:00, 22:00) for at least 5 days before completing a placebo training session followed by 7 experimental sessions at ≥72 hour intervals. Sessions were from 09:00 to 16:00. Oral oxycodone maintenance continued throughout the study except on session days, when double-blind placebo was substituted for the three oxycodone maintenance doses preceding each session to produce spontaneous opioid withdrawal (mean baseline visual analog scale score was 69.0 for “How severe is your OPIOID WITHDRAWAL?” scored from 0 “not at all” to 100 “extremely”). In addition, on session days, the 12:00 oxycodone maintenance dose was omitted because each session evaluated a single oral test dose at 10:00: dronabinol (5, 10, 20, or 40mg), oxycodone (30 or 60mg), or placebo. Only the first two subjects received dronabinol 40mg due to safety concerns (see results). This dose was then reduced to 30mg. Thereafter, the dose order was fully randomized except that dronabinol 20mg always preceded 30mg. With regard to dronabinol dose selection, the principal investigator (SLW) initially proposed 30mg as the highest test dose, but the sponsor required that the higher 40 mg dose be tested.
2.4 Drugs
Drugs were prepared by the UK Investigational Pharmacy under an Investigational New Drug Application (#69,214). Oxycodone HCl 30mg tablets (Mallinckrodt Inc., Hazelwood, MD), lactose monohydrate powder N.F. (Medisca Pharmaceuticals, Plattsburgh, NY), and dronabinol 5 and 10mg capsules (PAR Pharmaceutical, Spring Valley, NY) were used to prepare oxycodone, placebo, and dronabinol doses, respectively. All active doses were loose-filled with lactose before being over-encapsulated in order to maintain the blind.
2.5 Physiologic measures
During sessions, heart rate (HR), blood pressure, and oxygen saturation (Dinamap Non-Invasive Patient Monitor, GE Medical Systems, Tampa, FL) were measured every minute, beginning 30 minutes before drug administration. Respiration rate and end-tidal carbon dioxide (EtCO2) levels (N-85 Capnograph, Nellcor, Boulder, CO) along with pupil diameter (Pupillometer, PLR-200, NeurOptics, Irvine, CA) were assessed every 15 minutes, beginning 30 minutes before drug administration.
2.6 Data analysis
Physiologic measures collected every minute were averaged across 15-minute intervals and evaluated employing a two-factor repeated measures model (dose [placebo; oxycodone 15, 30mg; dronabinol 5, 10, 20, 30mg] x time). Peak maximum and minimum values were analyzed with a one-factor (dose) model. Significant results were evaluated with Dunnett post-hoc comparisons of placebo to active doses. Analyses utilized Proc Mixed in SAS 9.3 (SAS Institute, Inc., Cary, NC). Statistical significance was set at p<0.05. Means (standard errors) are reported.
3. RESULTS
3.1 Demographics
Twelve participants (6 females, all Caucasian) completed the study. They were 31.3 (±1.5) years old, completed 11.8 (±0.6) years of school, and used opioids (heroin and non-medical prescription opioids) 26.0 (±1.0) of the past 30 days. Nine were using heroin and prescription opioids, one was using heroin only, and two were using prescription opioids only. Six were injecting opioids. Eleven were tobacco smokers [Fagerstrom score: 4.4 (±0.7)]. Two used marijuana in the last 30 days (range: 1–2 days of use). Other substances used infrequently in the past 30 days included alcohol (n=4), benzodiazepines (n=2), and buprenorphine (n=4).
3.2 Physiologic outcomes
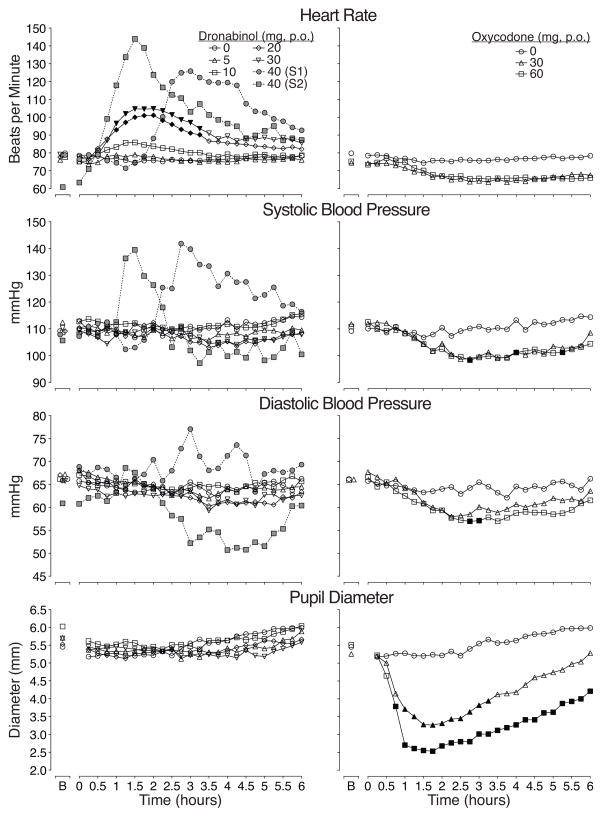
The first two subjects receiving dronabinol 40mg experienced sinus tachycardia (see Figure 1), and both described having an “anxiety attack” that was assessed as similar to a panic attack by the study psychiatrist. The tachycardia lasted approximately two hours. Systolic blood pressure (SBP) and heart rate increased for these two subjects, but diastolic blood pressure (DBP) response was more variable (see Figure 1). Neither experienced dizziness nor chest pain. Both reported feeling normal by the end of session with vital signs returning to near baseline levels.
Figure 1. Physiologic time course data for heart rate, blood pressure and pupil diameter.
Time action curves for heart rate, systolic and diastolic blood pressure, and pupil diameter are shown as mean data (n=12 for placebo, oxycodone 30 and 60mg, and dronabinol 5, 10, and 20, but n=9 for dronabinol 30 mg). B indicates baseline. Data from dronabinol dose conditions and placebo are shown in the left figures while the placebo and positive oxycodone control condition are shown in the right figures. Error bars are omitted for clarity; however, Table 1 shows the standard errors for each dose condition for these outcomes and illustrates the magnitude of variability in peak effects. Black symbols indicate a significant difference from placebo (p<0.05). Data from subjects 1 (S1) and 2 (S2), the only two volunteers (in gray) who received dronabinol 40 mg, are also displayed in the left figures (these data were not included in the analyses but are shown here for illustrative purposes).
There was a significant main effect of dose (F(6,63)=14.3, p<0.0001) and dose x time interaction for heart rate (F(150,1575)=2.0, p<0.0001) as well as for pupil diameter (main effect of dose: F(6,63)=15.2, p<0.0001; dose x time interaction: F(144, 1508)=2.2, p<0.0001). A significant effect of dose was found for systolic and diastolic blood pressure (SBP: F(6,63)=4.7, p=0.001; DBP: F(6,63)=5.3, p<0.0001).
The remaining ten subjects completed the amended protocol with 30mg as the highest dronabinol dose. One did not receive the 30mg dose because they were unable to tolerate the 20mg dose, complaining of anxiety and a fast heartbeat, with HR and SBP peaking at 154 bpm (baseline: 81 bpm) and 164 mmHg (baseline: 109 mmHg), respectively.
Time course data are shown in Figure 1 for HR, SBP, DBP, and pupil diameter. There were significant main effects of dose and time for all four outcomes and significant dose x time interactions for HR and pupils. Dronabinol 20 and 30mg significantly increased HR compared to placebo for approximately two hours. However, the effects of these doses and the lower dronabinol doses on SBP, DBP, and pupil diameter were not significantly different from placebo. Oxycodone 30 and 60mg decreased HR, blood pressure and pupil diameter (p<0.05) as expected, without causing adverse events.
There was a significant dose effect and dose x time interaction on oxygen saturation. Oxycodone and respiratory rate, 60mg significantly reduced oxygen saturation compared to placebo. For EtCO2 both oxycodone doses were different from placebo (p values <0.0001). Dronabinol produced no significant effects on any respiratory outcome with one exception; the 30mg dose produced significantly higher EtCO2 levels (37.9 [±0.2]) compared to placebo (35.9 [±0.2]).
Results from peak maximum and minimum analyses are displayed in Table 1. There were significant dose effects on all outcomes (except maximum DBP), which were primarily driven by differences between oxycodone and placebo. The exception was dronabinol 20 and 30mg, which produced peak maximum HRs that were approximately 20 and 30 bpm higher than placebo (p<0.0001).
Table 1.
Peak maximum and minumum values by dose condition
| F | Drug condition (mg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Oxycodone
|
Dronabinol
|
||||||
| 30 | 60 | 5 | 10 | 20 | 30 | |||
| Maximum values: | ||||||||
| End-tidal CO2 | 18.0 | 39.4 (0.8) | 42.8 (1.0) | 45.3 (1.0) | 39.6 (0.9) | 38.8 (0.9) | 40.2 (0.9) | 40.9 (1.1) |
| Respirations/min (#) | 2.7 | 18.9 (0.9) | 17.8 (0.6) | 17.4 (0.6) | 19.9 (1.0) | 19.2 (0.9) | 18.8 (0.7) | 18.9 (1.1) |
| Pupil diameter (mm) | 5.3 | 6.1 (0.2) | 5.6 (0.3) | 5.3 (0.4) | 6.0 (0.2) | 6.2 (0.2) | 5.9 (0.2) | 6.0 (0.3) |
| O2 saturation (%) | 3.6 | 99.3 (0.2) | 99.5 (0.1) | 99.0 (0.2) | 99.4 (0.2) | 99.5 (0.2) | 99.5 (0.2) | 99.8 (0.1) |
| Heart rate (bpm) | 23.2 | 84.4 (2.3) | 77.3 (1.8) | 79.9 (1.8) | 85.4 (2.4) | 91.7 (3.9) | 107.6 (6.2) | 112.6 (3.4) |
| SBP (mmHg) | 2.3 | 120.2 (2.2) | 118.6 (2.1) | 116.8 (1.8) | 117.5 (2.8) | 122.3 (2.3) | 123.8 (4.0) | 117.6 (2.7) |
| DBP (mmHg) | 1.9 | 71.1 (1.4) | 70.0 (1.3) | 68.0 (0.9) | 70.4 (1.7) | 70.7 (1.5) | 71.0 (1.6) | 67.7 (1.2) |
| Minimum values: | ||||||||
| End-tidal CO2 | 3.8 | 32.3 (0.5) | 34.7 (0.6) | 34.9 (0.7) | 33.4 (0.6) | 32.3 (0.6) | 33.8 (0.8) | 34.2 (0.9) |
| Respirations/min (#) | 3.2 | 13.3 (0.8) | 11.7 (0.7) | 11.9 (0.5) | 13.3 (0.7) | 13.3 (0.6) | 12.7 (0.6) | 13.1 (1.0) |
| Pupil diameter (mm) | 38.8 | 4.9 (0.4) | 2.9 (0.2) | 2.4 (0.1) | 4.8 (0.4) | 5.0 (0.3) | 4.9 (0.3) | 4.8 (0.4) |
| O2 saturation (%) | 7.6 | 97.2 (0.2) | 96.8 (0.3) | 95.8 (0.3) | 97.2 (0.2) | 97.3 (0.3) | 97.4 (0.3) | 96.9 (0.3) |
| Heart rate (bpm) | 14.4 | 70.5 (2.0) | 61.7 (1.7) | 62.1 (1.5) | 68.8 (1.4) | 70.9 (1.7) | 72.8 (2.4) | 75.0 (2.0) |
| SBP (mmHg) | 7.8 | 102.4 (2.2) | 94.0 (2.2) | 94.4 (1.5) | 100.7 (2.3) | 104.1 (1.7) | 98.9 (1.9) | 98.1 (3.2) |
| DBP (mmHg) | 6.1 | 59.8 (1.6) | 55.8 (1.5) | 53.9 (1.4) | 59.2 (1.6) | 60.3 (0.9) | 57.0 (1.4) | 55.9 (2.4) |
Bolded F and mean (SE) peak values indicate significant dose effect (p<0.05) and Dunnett post-hoc comparison to placebo, respectively. Sample size is n=12 for all drug conditions expect dronabinol 30 mg (n=9).
4. DISCUSSION
This randomized, double-blind, placebo-controlled study reports on the safety of a wide range of acute oral dronabinol doses among opioid dependent adults experiencing acute opioid withdrawal of moderate severity. Dronabinol 40mg was poorly tolerated producing sustained sinus tachycardia and anxiety/panic in the two volunteers who received it. Dronabinol 20 and 30mg were better tolerated; however, one subsequent volunteer also experienced tachycardia and anxiety after receiving 20mg. With both time course and peak maximum analyses, dronabinol 20 and 30mg doses produced statistically significant increases in HR. Thus, there are safety concerns with dronabinol 20, 30 and 40mg. Dronabinol otherwise produced physiological effects most similar to placebo, while oxycodone produced prototypic mu-opioid agonist effects (e.g., miosis, decreased respiratory function).
Dronabinol’s effect on HR is not entirely surprising because dronabinol, like other cannabinoids, has complex effects on the central nervous system and, in particular, sympathomimetic activity resulting in tachycardia, as acknowledged by the FDA-package insert list of its side effects. One early study infused IV THC into healthy men who smoked marijuana less than once per week (Kanakis et al., 1976), and they reported an increase in HR of 32 ± 7 BPM that was partially attenuated by pretreatment with propranolol. In another study conducted with daily marijuana smokers, volunteers were pretreated with atropine, propranolol, both atropine and propranolol, or placebo followed by IV THC (mean dose: 35.0 ug/kg; Benowitz et al., 1979). Pretreatment with atropine or propranolol each moderately attenuated THC’s increase in heart rate, while pretreatment with both atropine and propranolol completely blocked this heart rate change. Notably, drug administration in the current study occurred during acute opioid withdrawal, a state characterized by increased noradrenergic outflow. There may have been a synergistic effect between the already increased noradrenergic activity at baseline and the additional sympathomimetic effect of dronabinol, leading to an exaggerated HR response at higher doses of dronabinol. The dronabinol-induced tachycardia appears to be dose-related.
Other factors, in addition to the baseline activity of the noradrenergic system, may also affect whether CB1 agonists produce heart rate increases and the magnitude of effect. For instance, oral THC has highly variable absorption (McGilveray, 2005), which may explain why the time course of effects of dronabinol 40mg on heart rate and blood pressure varied between subject 1 and subject 2. Degree of individual tolerance to Δ9-THC also may be critical. Acute oral doses of dronabinol 10 and 20mg did not increase HR (Bedi et al., 2013), but 40 mg did (p<0.05) in marijuana users; however, the mean increase was quite small (6 bpm; Haney, 2007) compared to that observed in the current study. It is possible that the modest heart rate increase was due to tolerance to Δ9-THC as those subjects were regular marijuana users (and not opioid dependent), whereas our volunteers were specifically selected for low marijuana use to preclude confounding the primary outcomes with inter-individual differences in Δ9-THC tolerance. Review of the literature (e.g., Mendizabal and Adler-Graschinsky, 2007; Zubrzycki et al., 2014) reveals that the cardiovascular effects of CB1 agonists are somewhat conflicting, indicating a clear need for further research in this area.
Notably, this study administered single doses of dronabinol that were more than ten-fold higher than typical starting doses (2.5 mg) for its FDA-approved indications. Alternative dosing strategies could be employed to try to mitigate these heart rate effects if high doses of dronabinol were required. For instance, Bisaga and colleagues (2015) employed a dronabinol dose run-up (starting at 10 mg per day and increasing to 15 mg twice daily over two days) while simultaneously administering ancillary medications, such as clonidine and clonazepam, which effectively treat (and/or prevent) emergent tachycardia, high blood pressure and anxiety/panic (and potentially masked these effects in that study).
Overall, the current study demonstrates that acute oral doses of dronabinol 5 and 10mg were well tolerated, but higher doses of 20, 30 and 40 mg produced sustained elevations in HR, often above 100 bpm, among otherwise healthy adults who were at rest, but experiencing early signs and symptoms of opioid withdrawal. These findings suggest caution and close monitoring of HR with high dose dronabinol and consideration of alternative dosing strategies in future research and/or clinical use (as it is already FDA-approved and available for off-label use). Thus, while cannabinoid agonists may remain a rational target for treating opioid dependence, the cardiovascular effects of high dose oral dronabinol may limit its utility in treating acute opioid withdrawal.
Highlightss.
This study evaluated acute doses of oral dronabinol, a CB1 agonist, in adults during spontaneous opioid withdrawal.
Dronabinol 20 and 30 mg increased heart rate compared to placebo (p<0.05).
Dronabinol 40 mg produced sustained sinus tachycardia andanxiety/panic.
High dose dronabinol may have limited utility asa stand-alone agent to treat acute opioid withdrawal.
Acknowledgments
Role of Funding Source: Funding sources include NIDA DA033932 (Walsh) & NCATS UL TR000117. The NIDA project officer required that the highest proposed dose of dronabinol (30mg) be changed to 40mg. Otherwise, NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to acknowledge UK Investigational Drug Service, nursing and staff on the inpatient Center for Clinical and Translational Science research unit, and UK Center on Drug and Alcohol research staff, especially Victoria Vessels and Aurora Adams.
Footnotes
Contributors: Dr. Walsh designed the study, wrote the protocol, and provided primary study oversight. Dr. Babalonis assisted with recruitment and enrollment as well as study management. Dr. Lofwall helped with study design, provided medical oversight, and assisted in interpretation of data and writing of the manuscript. Ms. Jicha assisted with data analysis, literature review and wrote the first draft of the manuscript. Mr. Nuzzo arranged randomization, managed data, and completed all analyses. Dr. Elayi assisted in medical oversight. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Drs. Lofwall and Walsh have received honoraria from PCM Scientific for giving educational talks on opioid dependence andsalary support from Braeburn Pharmaceuticals for conducting clinical research at UK. Dr. Lofwall has consulted for CVS Caremark AND Orexo.
Mr. Nuzzo was a statistical consultant and project coordinator for the NIDA CTN Clinical Consulting Center and Johns Hopkins Behavioral Pharmacology Research Unit. Dr.Walsh has consulted for Sun Pharma, Camurus, World Meds, Durect, Novartis, Pfizer, Astra Zeneca, Cerecor, and Braeburn Pharmaceuticals. Dr. Elayi reports no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Crystal J. Jicha, Email: crystal.jicha@uky.edu.
Paul A. Nuzzo, Email: pnuzz2@uky.edu.
Shanna Babalonis, Email: babalonis@uky.edu.
Samy Claude Elayi, Email: samy-claude.elayi@uky.edu.
Sharon L. Walsh, Email: sharon.walsh@uky.edu.
References
- Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. 2013;18:872–881. doi: 10.1111/j.1369-1600.2011.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther. 1979;25:440–446. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, Raby WN, Levin FR, Carpenter KM, Mariani JJ, Nunes EV. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015;154:38–45. doi: 10.1016/j.drugalcdep.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Kanakis C, Jr, Pouget JM, Rosen KM. The effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac performance with and without beta blockade. Circulation. 1976;53:703–707. doi: 10.1161/01.cir.53.4.703. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta (9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- Mendizabal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Chowen J, Rocio ACM, del Arco I, Villanua MA, Martin Y, Roberts AJ, Koob GF, de Fonseca FR. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- Zubrzycki M, Liebold A, Janecka A, Zubrzycka M. A new face of endocannabinoids in pharmacotherapy. Part I: protective role of endocannabinoids in hypertension and myocardial infarction. J Physiol Pharmacol. 2014;65:171–181. [PubMed] [Google Scholar]