Abstract
Objective
To investigate if sexual activity moderated menstrual cycle-related shifts in cytokines associated with helper-T type 1 (Th1) cells (e.g., interferon-γ, IFN-γ) and helper T type 2 (Th2) cells (e.g., interleukin-4, IL-4). Immune activity shifts across the menstrual cycle, with higher follicular-phase Th1 cell activity, but higher luteal-phase Th2 cell activity There is little known about how social behaviors alter Th1/Th2 ratios, despite evidence that psychosocial factors can influence immunity. Of particular interest is how sexual activity influences immune responses that may support conception, such as the Th1/Th2 balance.
Design
Participants provided saliva samples at four timepoints (menstrual, follicular, ovulatory, and luteal), which were assayed using enzyme-linked immunosorbent assays (ELISA).
Setting
Academic laboratory.
Participants
Thirty healthy premenopausal women (16 sexually abstinent, 14 sexually active), not taking hormonal or immunoactive medications.
Interventions
None.
Main outcome measures
Salivary estradiol (E2), progesterone (P4), IFN-γ, IL-4.
Results
Sexually active, but not abstinent, women were significantly more likely to express Th2-like cytokine ratios (IFN-γ < IL-4) in the luteal phase than other phases. Similarly, sexually active women had significantly higher P4, and higher P4 to E2 (P/E) ratios, in the luteal phase than did abstinent women. The P/E ratio mediated menstrual variations in cytokine ratios in sexually active women.
Conclusion
These results support the hypothesis that shifts in immune response across the menstrual cycle may reflect tradeoffs between reproduction and immunity. These findings point to the need for further research on the interaction between sexual behavior, the menstrual cycle, and immune response.
Keywords: immunity, sex differences, menstrual cycle, Th1/Th2, cytokine
Introduction
There is increasing interest in the mechanisms underlying sex differences in immune health. In women, a coordinated shift in immune parameters occurs during reproductive events such as pregnancy and parturition to accommodate the changing needs of mother and offspring (1). Intriguingly, changes in immunity have also been documented across the menstrual cycle, which are similarly thought to represent tradeoffs between reproduction and immunity. For example, several studies have documented lower inflammation and other innate immune parameters at midcycle, corresponding to ovulation, relative to other phases of the menstrual cycle (2-4); this is thought to prevent immune interference with conception during fertile windows.
Similarly, researchers have suggested there may be cycle-related changes in adaptive immunity, particularly in T-helper cells, which coordinate activity of other immune actors such as B-cells and macrophages. Several studies have tracked shifts in the relative proportions of cytokines, immune signaling molecules, associated with different subtypes of T-helper cells. These studies have shown cytokines expressed during the follicular phase are predominantly those associated with T-helper 1 cells (Th1), but luteal phase cytokines are more predominantly those associated with T-helper 2 cells (Th2) (5-7) but see also (8). Typical development of Th cells requires instruction from dendritic cells that locks in what type of cytokines (and thus, what subtype of Th cell) they will secrete. The Th1-associated cytokines (e.g., Interferon-γ; IFN-γ) drive intracellular defense, whereas Th2-associated cytokines (e.g., Interleukin-4; IL-4) are characteristic of extracellular defense as well as self-cell tolerance (9). Higher levels of Th2-type cytokines are expressed during pregnancy, as these cytokines facilitate embryo implantation and placentation (10). In contrast, higher levels of Th1-type cytokines stimulate macrophages and cytotoxic T cells, key components of immune defense in the tissues. Thus, the ratio of Th1/Th2-associated cytokines can be characterized as reflecting immune priorities: defending against viruses and bacteria (Th1-dominant) vs. preparing for and/or tolerating the semiallogenic fetus (Th2-dominant)(11).
Progesterone (P4) and estradiol (E2), steroid hormones that regulate much of the endocrine-immune coordination during pregnancy, fluctuate across the menstrual cycle, with the luteal phase characterized by a high P/E ratio (that is, the ratio concentrations of unbound P4 and E2). Similarly, pregnancy is associated with a marked increase in the P/E ratio. Due to the parallels between luteal phase and pregnancy in endocrine and immune factors, the female body has been described as being in a “pregnancy-like” state (7). These reproductive-immune interactions may also explain sex differences in the Th1/Th2 ratio, with females typically expressing more Th2-like cytokine ratios than males (12). Of note, sexually active women typically express higher luteal-phase P4, and E2 across the menstrual cycle, than women who are sexually abstinent (13). Thus, there may also be significant differences in how Th1/Th2 ratios change across the menstrual cycle in sexually active and abstinent women.
Understanding the potential role for partnered sexual behaviors in Th1/Th2 ratios is also important given increasing research suggesting that social behaviors moderate immune activity (14). In addition to indirect effects on stress or mood (15), social interactions can provide information about one's environment or life events (e.g., reproduction) that may require immune modulation (16). In particular, partnered sexual behavior may signal conception is possible, and as such, downshift immune responses that may interfere with reproduction and/or upregulate immune responses that may promote conception. Among women who are sexually abstinent, however, there would be no such signal, and thus, potentially, no significant shifts in the Th1/Th2 ratio. In support of this hypothesis, several studies have shown that sexual activity may modulate immune activity. Increased frequency of partnered sexual activity is associated with lower levels of secretory immunoglobulin A, an antibody important for first-line immune defense (17, 18). Moreover, sexual activity appears to moderate shifts in inflammation, with increased frequency of sexual activity associated with greater mid-cycle decrease in C-reactive protein, a marker of inflammation (2). It is unknown whether sexual activity within a relationship impacts cytokine ratios, or moderates changes in cytokine ratios across the menstrual cycle. If shifts in cytokine ratios are related to coordination of tradeoffs between reproduction and immunity, they may be more critical for reproductively active (i.e., sexually active) than reproductively inactive (i.e., sexually abstinent) women. As such, partnered sexual activity – even that which occurs outside the fertile window and thus cannot directly lead to a conception – may promote fertility via priming the immune system to engage in shifts that promote reproduction.
In the present study, we examined changes in IL-4 (a Th2-associated cytokine) and IFN-γ (a Th1-associated cytokine), and their ratio, across the menstrual cycle. We compared these changes in women who were sexually active with a partner and women who were sexually abstinent. We hypothesized that sexually active women would demonstrate significant shifts in cytokine ratios across the menstrual cycle; however, such shifts in sexually abstinent women would be weaker and potentially non-detectable. Extending the findings of Faas et al. (7), we hypothesized that sexually active women, and to a lesser extent abstinent women, would show a significantly more Th2-like cytokine ratios in the luteal phase. Also, following the findings of Prasad et al. (13) we predicted luteal phase P4 would be higher in sexually active vs. abstinent women. Novel to this study, we predicted that the P/E ratio would be associated with cytokine ratios, such that higher P/E ratios in the luteal phase would mediate the effect of group (sexually active vs. abstinent) on cytokine ratios.
Materials and Methods
Participants
All study procedures were approved by the Indiana University Institutional Review Board, and all participants provided informed consent. Thirty-five healthy premenopausal women were recruited from the community. Inclusion criteria were: premenopausal, with regular menstrual cycles every 26 – 34 days. Exclusion criteria were: any self-reported use of hormonal medications or medications with a known immune effect (e.g., antibiotics), regular use of any other medication type (prescription or non-prescription), pregnancy or lactation within the past 12 months, or any medical condition known to impact immune response (e.g., cancer). Women reporting vitamin or herbal supplement use, or occasional (< 1×/week) use of over-the-counter antihistamines or analgesics (acetaminophen, aspirin or non-steroidal anti-inflammatories) were included. Sexually abstinent participants included women who reported no partnered genital sexual activity within the last four months; however, women with a lifetime history of sexual activity were included. Sexually active participants included women who reported penile-vaginal intercourse at least once a week, with only one current sexual partner. As women taking oral contraceptives (OC) were excluded, sexually active participants used either condoms or non-hormonal intrauterine devices as contraception. Study size was determined by prospective power analyses, which indicated 12-16 participants per group would adequately (∼85% power) capture small effect sizes (d = .20). Of the 35 women recruited, 3 dropped out mid-study and 2 could not produce adequate saliva volumes to be assayed, leaving data from 30 participants.
Procedures: Sample collection
Participants completed two laboratory visits: one at menses (within two days of onset of menstrual bleeding) and one at ovulation (within two days of ovulation). Date of ovulation was estimated via backwards counting according to the onset of menstrual bleeding and their typical cycle length (19). To further confirm date of ovulation, participants were given a packet of 5 dipstick urine tests for luteinizing hormone (OneStep Urine Ovulation Test, BlueCross Biomedical, Beijing, China) at their first laboratory visit (i.e., at menses). They were instructed to complete the tests daily, between 1-5pm, in the days prior to their estimated ovulation date; if participants noticed a positive test strip before the estimated date of ovulation, they were scheduled for a lab visit within 48 hours. If participants had not noticed a positive test strip by the estimated date of ovulation, they were instructed to continue daily testing, and come into the lab as soon as they did notice a positive test. All participants had a positive test strip (i.e., evidence of ovulation) within 48 hours of the second lab visit (the “ovulation” sample).
During laboratory visits, participants completed surveys and were measured for height, weight, and body fat with a floor-unit body composition scale (FitScale 585F, Tanita Corporation, Illinois USA). All participants (sexually active and abstinent) also completed urine tests for human chorionic gonadotropin at both lab visits; thus, we confirmed no participant was pregnant during the study. All participants provided information on any illness and significant stressful events during the study period via survey measures completed alongside sample collection (see below). Sexually active participants additionally reported on each sexual event during the study via online diary measures; from these, sexual intercourse events were tallied.
As salivary measures of cytokines have been shown to correlate well with serum measures (20), but are significantly less invasive to collect, we used saliva as our main medium for endocrine and cytokine measures. During lab visits, participants provided saliva samples in polypropylene tubes via a passive drool with no stimulation; samples were frozen immediately after collection. Additionally, participants completed two at-home saliva samples, which were frozen in their home freezer and transported frozen to the lab on deep-freeze ice packs in Styrofoam boxes (19). At-home samples were collected during the follicular phase (7-10 days following menses onset), and luteal phase (7-10 days following ovulation). All saliva samples were stored at -80C until analysis, and no sample was subjected to more than 2 freeze-thaw cycles.
Procedures: Cytokine and hormone assay
Saliva samples were assayed for P4, E2, IFN-γ and IL-4 with commercially available enzyme-linked immunosorbent assay (ELISA) kits, using procedures recommended by kit manufacturers (P4 and E2, kits from Salimetrics LLC, Pennsylvania, USA; IFN-γ and IL-4, Cytoset kits from Invitrogen Corporation, Maryland, USA). Intra-assay and inter-assay coefficients of variance were low (4.71 – 6.35%, and 2.24 – 10.48% respectively). Sensitivity limits for the assays were as follows: IFN-γ: 3.9 pg/mL, IL-4: 2.0 pg/mL, P4 = 5.0 pg/mL, E2: 0.1 pg/mL. P4 and E2 were measured in pmol/L to standardize across different molecular weights.
Analytic plan
Undetectably low cytokine values were replaced with the lowest detectable value for the assay. A total of 11% of IL-4 and 38% of IFN-γ values were below the limit of detection, which is typical for a sample of young, healthy participants (20). In addition, 8 participants did not provide luteal-phase samples, leading to 27% of missing data in the luteal phase (7% of total values) missing. As the missingness of these samples was due to pragmatic reasons (e.g., samples collected at home were returned to the lab unfrozen) not related to the underlying relationships to be tested, for the purposes of our statistical tests, these values were considered missing at random (21). We further confirmed luteal phase samples were missing at random via Dixon's test, in which the mean values of key variables are compared across missing and non-missing observations (22). In no case were key variables (IL-4, IFN-γ, P4, or E2) at menses, follicular or ovulation timepoints significantly different in women with or without missing luteal phase samples. Missing values were addressed using bootstrapping and other statistical techniques robust to missing data (see below). We used a natural log transformation to correct for right-skew of cytokine distributions.
To examine changes in IL-4, IFN-γ, P4, E2, and the ratio of P/E across the menstrual cycle, we used repeated measures mixed generalized linear models, specifying subject-level intercepts (which accounted for individual differences in baseline cytokine levels) and controlling for important covariates. We included age, race (dummy coded as white/nonwhite), and BMI as covariates as these have been shown to be important sources of variation in of cytokine values in healthy individuals in both cross-sectional studies (23-25) and short-term longitudinal studies (e.g., predicting changes in Th1/Th2 profiles across pregnancy (26)). In each model we included the following fixed effects: group (sexually active vs. sexually abstinent), time (menstrual, follicular, ovulatory, luteal) as a repeated measure, and the interaction of group and time.
To examine changes in the cytokine ratio across the menstrual cycle, we used the methods recommended by Lin et al. (27) to characterize within-subject shifts in cytokine ratios. We first calculated the standardized (i.e., z-scored) value for each cytokine within each participant. That is, the value for each time point was compared to the other values collected from the same participant. These scores represented the relative expression of cytokine within the cycle: high values would indicate high expression of that cytokine and vice versa. We were thus able to characterize the relative expression of IFN-γ/IL-4 into two categories: Th1-like (z-scored IFN-γ > z-scored IL-4), or Th2-like (z-scored IFN-γ < z-scored IL-4). The average absolute difference between IFN γ and IL-4 standardized cytokine values within a timepoint was z = 0.61 (that is, 0.61 standard deviations from each other), and in only 21 cases (18% of total data) were the standardized cytokine values within 0.10 standard deviations from each other. In other words, cytokine ratios were generally either strongly Th1-like or Th2-like, with few values close to the classification cutoff.
We conducted a repeated measures generalized estimating equation (GEE) with cytokine ratio category (Th1-like vs Th2-like) as the outcome variable, again entering group, time and their interaction as fixed effects, and controlling for age, race, and BMI. Finally, to test for mediation of differences between groups in change in Th1/Th2 ratio via hormonal factors, we conducted bootstrapped path analyses testing indirect effects. All analyses were performed with IBM SPSS Statistics version 22.0 (Windows); path analyses were conducted via the PROCESS and MEMORE macros for SPSS (28, 29). Contrasts were considered significant if p< 0.05, and marginally significant if p < 0.10.
Results
Participant demographics
A total of 16 sexually abstinent and 14 sexually active women were enrolled; full demographics are presented in Table 1. Participants reported their race as predominantly White (70%), with 16% East Asian and 13% other; one White woman reported Hispanic/Latina ethnicity. The average age was 23.44 (SD = 5.31). There was a significant difference in relationship status between sexually active and abstinent participants (χ2= 27.47, p < 0.001), as all sexually active participants reported being in a sexually active relationship, while only three participants in the sexually abstinent group reported being in a non-sexual dating relationship. As all sexually active participants were in a relationship, these variables were collinear and thus we were unable to control for relationship status in the analyses below. Average length of current relationship was 4.14 years (SD = 7.38 years). On average, sexually active women reported 6.67 intercourse events during the study period (range = 1 - 18); there was no significant difference across menstrual cycle phases in the number of intercourse events (F(3, 7.55) = 1.34, p =0.34, Cohen's f = 0.04).
Table 1. Demographics and baseline characteristics by sexual activity status (unadjusted).
| Sexually Active | Sexually Abstinent | Total | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | F | p | |
|
|
||||||||
| Age | 24.87 | 7.02 | 22.16 | 2.92 | 23.44 | 5.31 | 2.214 | 0.155 |
| Years of education | 15.96 | 4.12 | 15.53 | 2.42 | 15.73 | 3.24 | 0.003 | 0.960 |
| Body mass index | 23.54 | 3.19 | 23.5 | 4.67 | 23.31 | 3.6 | 0.001 | 0.978 |
|
|
||||||||
| N | % | N | % | N | % | χ2 | p | |
|
|
||||||||
| Race | 4.974 | 0.083 | ||||||
| White | 12 | 86% | 8 | 50% | 20 | 67% | ||
| Asian | 2 | 14% | 5 | 31% | 7 | 23% | ||
| Mixed Race/Other | 0 | 0% | 3 | 19% | 3 | 10% | ||
| Ethnicity | 1.182 | 0.277 | ||||||
| Hispanic/Latina | 1 | 7% | 0 | 0% | 1 | 3% | ||
| Not Hispanic/Latina | 13 | 93% | 16 | 100% | 29 | 97% | ||
| Relationship status | 21.233 | 0.000 | ||||||
| Married/cohabiting | 6 | 43% | 0 | 0% | 6 | 20% | ||
| Dating/ In a relationship | 8 | 57% | 3 | 19% | 11 | 37% | ||
| Single/casually dating | 0 | 0% | 13 | 81% | 13 | 43% | ||
Participants had an average body mass index (BMI) of 23.31 (SD = 3.60), and average body fat percentage of 27.09% (SD = 7.43%). There was no significant change in body fat (t(31) = .62, p = 0.49) nor BMI (t(31) = 0.82, p = .23) from menses to ovulation, and thus in analyses below we used the average across timepoints for each participant as a covariate. No participant reported significant traumatic events or colds/infections during the study period. There were no significant differences between sexually active and abstinent participants on any demographic variable other than relationship status (Table 1 and Supplementary Table 1).
Endocrine measures
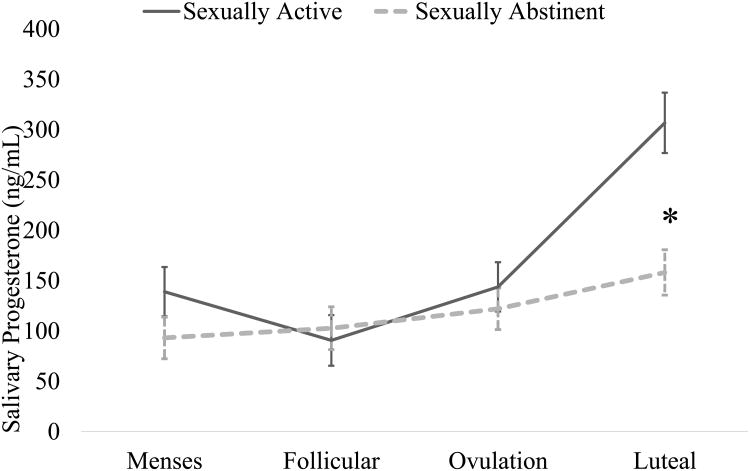
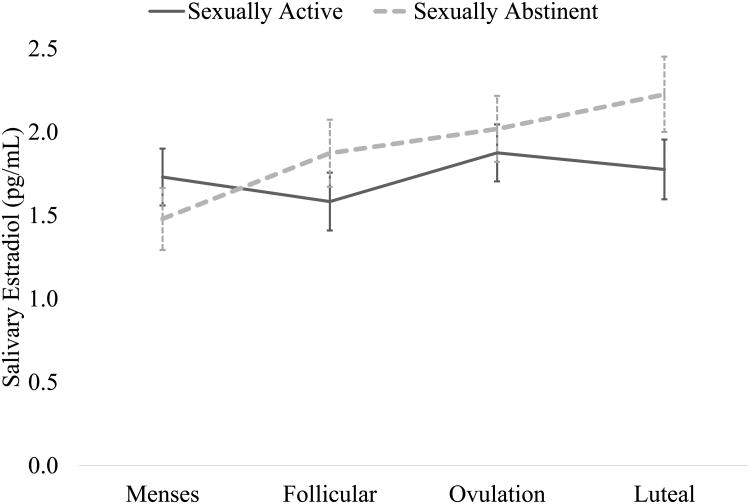
The interaction between group and time significantly predicted P4 (F(3, 85.90) = 5.26, p < 0.01, Cohen's f = 0.32). Across groups, P4 was highest during the luteal phase (main effect of time, F(=3, 85.95) = 15.76, p < 0.001, Cohen's f = 0.48); however, sexually active women had significantly greater P4 during the luteal phase than did sexually abstinent women (Mean difference = 148.70 pg/mL, SE = 37.41, p(contrast) < 0.001; see Figure 1). Similarly, the interaction between group and time significantly predicted E2 (F(3, 85.54) = 3.25, p < 0.05, Cohen's f = 0.18). In this case, the effect was driven by differences in the sexually abstinent group, as there was no significant change over time in E2 among sexually active women (all specific contrast ps = ns). Among sexually abstinent women, E2 at menses was significantly lower than at the follicular timepoint (Mean difference = -.39 pg/mL, SE = .15, p(contrast) < 0.05), which in turn was significantly lower than at the luteal timepoint (Mean difference = -.353, SE = .16, p(contrast) < 0.05, see Figure 2). Finally, the P/E ratio was significantly predicted by the interaction of group and time (F(3, 85.55) = 7.39, p < 0.001, Cohen's f = 0.41; Supplemental Figure 1). As with P4, sexually active women had significantly higher P/E ratios than sexually abstinent women in the luteal phase (Mean difference = 104.48, SE = 20.74, p(contrast) < 0.001).
Figure 1.
Progesterone concentration across the menstrual cycle, separated by sexual activity status (adjusted for age, body mass index, and race). Luteal-phase progesterone was significantly higher in sexually active vs. sexually abstinent women.
Figure 2.
Estradiol concentration across the menstrual cycle, separated by sexual activity status (adjusted for age, body mass index, and race). There were no significant differences between sexually active and abstinent women in estradiol at any individual timepoint. Among sexually abstinent women, there was a significant increase in estradiol from menses to follicular phase, and from follicular phase to luteal phase. There was no significant change in estradiol across the cycle in sexually active women.
Cytokine measures
For IFN-γ and IL-4, neither group nor time, nor their interaction, were significant predictors (Supplemental Table 1). However, the interaction of group and time on IFN-γ was marginally significant (F(3, 26.95) = 2.82, p = 0.064, Cohen's f = 0.12). Sexually active women had a marginally significant decrease in IFN-γ between ovulation and luteal phases (Mean difference = -.56 pg/mL, SE = .34, p(contrast) = 0.108), while sexually abstinent women did not (Mean difference = .08 pg/mL, SE = .26, p(contrast) = 0.777).
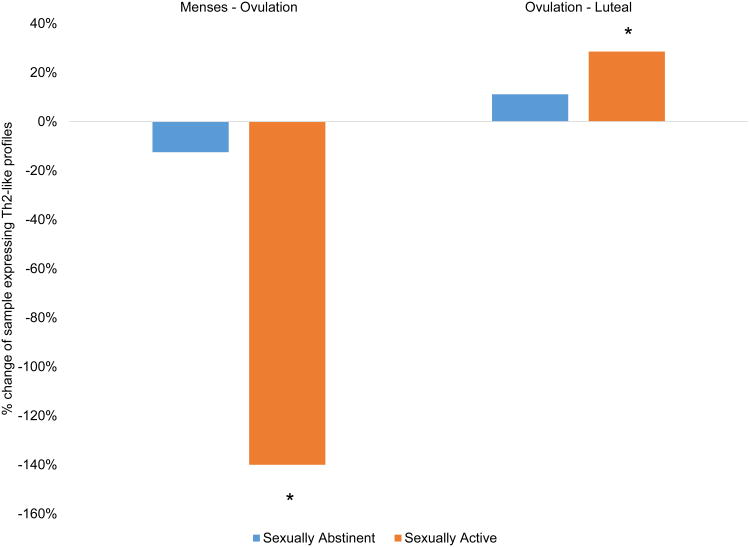
For the cytokine ratio, there was a significant interaction between group and time (Wald χ2= 7.53, p < 0.05, Cramer's V = 0.29). In sexually abstinent women, there was no significant change in cytokine ratio across the menstrual cycle (χ2= .62, p = 0.89, Cramer's V = 0.10). However, among sexually active women, the proportion of women showing Th2-like cytokine ratios significantly shifted from 35% during the follicular phase and ovulation, shifting to 78% at luteal phase (χ2= 10.75, p < 0.05, Cramer's V = 0.59; Figure 3).
Figure 3.
Percent change in number of participants expressing a Th2-like cytokine ratio (standardized IFN-γ < IL-4) across phases of the menstrual cycle, separated by sexual activity status. There was no significant change in cytokine ratios across the cycle in sexually abstinent women. Among sexually active women, there was a significant decrease between menses and ovulation, and significant increase between ovulation and the luteal phase, in the proportion of the sample expressing Th2-like cytokine ratios.
Interactions between endocrine and cytokine measures
We tested the association of P/E ratio and cytokine ratio, controlling for the effects of cycle phase, age, BMI, and race. There was a significant association between the P/E ratio and cytokine ratio (F(1, 86.65) = 3.70, p = 0.05, Cohen's f = 0.19), such that samples with Th2-like cytokine ratios had higher P/E ratios (M=77.53) than did samples with Th1-like cytokine ratios (M=58.96; Mean difference = 18.56, SE = 9.65, p(contrast) = 0.05).
We then examined if endocrine measures could explain the observed group-level differences in cytokine ratio change across the menstrual cycle. We specified a moderated mediation model, with cycle phase as the exposure, Th1/Th2 ratios as the outcome, P/E ratios as the mediator, group (sexually active vs. abstinent) as the moderator of the mediator, and age, BMI, and race as covariates (effect estimates are presented in Supplemental Table 2, and a conceptual diagram in Supplemental Figure 2). The confidence intervals of the estimated index of moderated mediation did not include zero (index estimate = 0.17, SE = 0.17, 90% CI = [0.01, 0.54]), thus supporting a moderated mediation. Specifically, among sexually active women, the confidence intervals of the conditional indirect effect of cycle phase on Th1/Th2 ratio (mediated by P/E ratio) did not include zero (index estimate = 0.17, SE = 0.16, 90% CI = [0.01, 0.49]). Among sexually abstinent women, however, the confidence intervals of the conditional indirect effect did include zero (index estimate = 0.00, SE = 0.06, 90% CI = [-0.08, 0.10]. In other words, there was evidence that P/E ratios mediated cycle-related change in Th1/Th2 ratios in sexually active, but not abstinent women.
As the predominant group-level differences in the Th1/Th2 ratio were observed in the luteal phase, we examined if the P/E ratio mediated the effect of group (sexually active vs. abstinent) on the Th1/Th2 ratio in the luteal phase only. Here, we specified a mediation model, with group (sexually active vs. abstinent) as the exposure, Th1/Th2 ratios as the outcome, P/E as the mediator, and age, BMI and race as covariates. The confidence intervals of the estimated indirect effect included zero (effect estimate = 1.62, SE = 11.67, 90% CI =[-24.55, 25.00]); thus, this mediation effect was not supported.
Discussion
The present study was the first to examine how partnered sexual behaviors influence cytokine ratios in healthy women, and how these ratios change across the menstrual cycle. There was a marginally significant decrease in IFN-γ during the luteal phase among sexually active women, but no significant change in IL-4 across the menstrual cycle for either sexually active or abstinent women. When considering both cytokines in relation to each other via the Th1/Th2 cytokine ratio, sexually active, but not abstinent, women expressed more Th2-like cytokine ratios (IFN-γ < IL-4) in the luteal phase than other phases. Finally, there were significant differences in endocrine ratios between groups, with sexually active women showing greater increase of P/E ratios in the luteal phase than did sexually abstinent women.
Many studies have shown immune effects of intimate relationships, such as increased inflammatory cytokine expression in individuals in hostile or unsatisfying marriages (30, 31); similarly, there has been considerable work suggesting poor relationship and sexual satisfaction is associated with infertility (32, 33). However, beyond the indirect role of stress (34), the mechanisms linking intimate relationships with fertility is unclear. The present study is among the first to examine these effects with respect to a specific, and evolutionarily unique, social behavior, namely partnered sexual behavior. Our findings support the broader hypothesis that sexual activity within a relationship – even that which does not result in conception – serves a variety of functions that impact reproductive health, from improving pair bonds (35) and improving relationship satisfaction across the lifespan (36) to engaging endocrine and immune responses (this study) that may promote fertility.
Specifically, as predicted, there were significant differences between sexually active and abstinent women in Th1/Th2 cytokine ratios across the menstrual cycle. Patterns of Th1 and Th2 cells are associated with a variety of clinically relevant reproductive outcomes, such as pregnancy and infertility (37). A Th2-dominant ratio is predictive of normal pregnancy while Th1-type cytokines are predictive of pre-eclampsia (38). This may be because Th1 cytokines, such as IFN-γ, can promote production of proteases such as thrombin, which disrupt the placental vasculature (39). Thus, a Th2-like cytokine ratio during the luteal phase may help prepare the female body prepare for possible pregnancy.
In the few days before and immediately after ovulation, however, a Th1-dominant cytokine ratio may be more beneficial. Th1-associated cytokines suppress vaginal immune responses that impair sperm motility (40), and promote production of IL-1, which assists in embryo implantation (41, 42). Thus, shifting from a Th1-like cytokine ratio around ovulation, to a Th2-like cytokine ratio during the luteal phase, may promote reproduction. Indeed, several studies have documented a mid-cycle surge in IL-1, a Th1-associated cytokine (43, 44). In the present study, shifts in Th1/Th2 cytokine ratios occurred in sexually active but not abstinent women, suggesting that partnered sexual behavior itself may act as a trigger for these immune events necessary for reproduction. If so, this may help explain why women attempting to conceive have greater success with increased regularity of sexual activity, even that which occurs well outside the fertile window (45).
Of note, although the greatest group-wise differences in cytokines were seen in the luteal phase, there was no evidence that absolute hormone levels in the luteal phase mediated the difference between sexually active and abstinent women. This implies that effects were driven by patterns of change in hormones and cytokines, rather than absolute levels per se. One study similarly found that although absolute levels of ovarian hormones did not determine women's inflammation levels at any one timepoint within the cycle, differential patterns of change in ovarian hormones between sexually active and abstinent women predicted differential patterns of change in inflammation (46). As each cycle phase represents different reproductive priorities, the immune system likely uses relative endocrine signals – such as increases or decreases – to determine the response most appropriate to that phase.
Subtle shifts in the Th1/Th2 ratio may be relevant in caring for patients with autoimmune conditions in which cytotoxicity is chronically high (associated with very high Th1/Th2 ratio, e.g., Type 1 diabetes) or low (associated with very low Th1/Th2 ratio, e.g., systemic erythematous lupus). Our findings, along with other studies examining menstrual cycle-related shifts in immunity (47), indicate the need to consider cycle phase, and likely partnered sexual behavior, in the clinical interpretation of immune biomarkers such as Th1/Th2 ratios. These findings call for further study of the behavioral health implications of sexual activity within a relationship above and beyond the traditional sexual risk-taking paradigm. Moreover, cytokines such as IFN-γ and IL-4 are neuroactive, and their central action has been implicated in mood disturbances (48) including in menstrual cycle-related mood disorders (49). Separately, it has been noted that women reporting regular sexual intercourse (several times per month) are significantly more likely to meet criteria for premenstrual dysphoric disorder than women reporting infrequent sexual intercourse (a few times per year) (50). Our data suggest a link between these separate findings: specifically, it is possible that, for vulnerable individuals, (normal) cyclical variations in Th1-associated cytokines are exacerbated by sexual activity which then lead to greater premenstrual mood disturbance.
Shifts in the cytokine ratio were associated with corresponding shifts in the P/E ratio, suggesting coordination between endocrine and immune systems. Many prior studies have similarly documented an effect of menstrual hormones on Th1/Th2 ratios (51, 52). These findings imply hormonal manipulations may be used clinically to replicate immune effects of sexual activity. However, previous studies attempted to manipulate Th1/Th2 ratios ex vivo in blood samples from women at follicular and luteal phases, but failed to find any significant differences across increasing concentrations of P and/or E (12). It is likely that endocrine factors work in concert with other systems (e.g., the autonomic nervous system, which is immunoregulatory (53) and which facilitates female sexual arousal (54)) to regulate the impact of partnered sexual activity on women's immunity.
These findings also support the need to consider the broader context of interactions across behaviors, and endocrine and immune systems. That is, there are likely ongoing inputs from behavioral patterns that modulate endocrine activity that, in turn, modulates immune activity. For example, it is possible that sexual activity and relationship quality interact to amplify effects on immune response. We were unable to control for relationship status in the present study, as all participants in the sexually active group were in relationships; future work examining women in long-distance or otherwise pragmatically sexually abstinent but committed relationships may help tease apart these effects.
Limitations to the present study include a small sample of predominantly White participants. We were also limited in the number of menstrual cycles we could sample. There is much variation in hormonal measures between cycles within a woman, let alone differences between ethnic groups (55). This limited sampling may be why we did not find that higher E2 among sexually active women, an effect which has been previously noted to be significant but small (13). It is also possible that the effect of sexual activity on E2 across the cycle is moderated by parity; as our sample was predominantly nulliparous young women, this hypothesis could not be tested. However, at least one study has shown that parity does not differentially predict the effect of intimate relationship status on E2 or P4 across the menstrual cycle (56), suggesting that the effect of sexual activity on ovarian hormones may not depend on parity. Similarly, our sample was relatively young women early in their reproductive careers. Life history theory suggests that among older women who have fewer future opportunities for reproduction, tradeoffs such as the ones observed in this study should be shifted in favor of reproduction (57). That is, among older (but still reproductive age) women, we should expect the immunomodulatory effect of sexual activity to be larger than that seen in this young sample. In women past reproductive age (that is, post-menopausal), however, the expected effects of sexual activity are unclear. Certainly there are significant changes to both endocrine and immune systems during menopause, and there are also aging-related changes in responsivity to environmental or psychosocial cues (58, 59). However, many aspects of response to sexual stimuli (e.g., vaginal arousal) are similar in pre- and post-menopausal women, including hormonal response (60). Further study is needed to examine the immunomodulatory effects of sexual activity across the lifespan.
We examined women not currently taking oral contraceptives (OCs), and found evidence for mediation of partnered sexual activity's impact on immune function via hormonal changes across the cycle. Thus it is likely that, to the extent that OCs limit the degree of hormonal fluctuation across the menstrual cycle, the effect of sexual activity on cytokine ratios would be similarly limited. Although likely not the case in our sample (as all women showed evidence of ovulation), it is also possible that more broadly, immune differences in sexually active vs. abstinent women are at least partially driven by differences in rates of ovulation (13). As women taking OCs are significantly less likely to ovulate (this being one of the main mechanisms by which OCs prevent pregnancy), we would expect fewer immune effects of sexual activity than in women not taking OCs.
As most of the sexually active women in this study were in committed, long-term monogamous sexual relationships with men, is also unknown if these results would extend into other types of sexual relationships, e.g., with multiple partners, with women, or with casual sexual partners outside of a steady relationship. It is likely that the immune response to a novel partner would differ from a regular sexual partner, both in terms of exposure to the partner's microbiome (61) and in the psychosocial context of the sexual behavior. In short, this work would benefit from replication in a broader population, with longer follow-up, canvassing a broader array of sexual behaviors. Such follow-up studies may be able to tease apart the specific effects of vaginal penetration/stimulation as compared to (or in concert with) the effects of sexual activity on relationship satisfaction and other psychosocial factors. Of note, there may be interactions between these effects and exposure to chronic stress, pathogens, and other immune challenges that were not observed in this sample of young, healthy women living in a post-industrial environment.
The ratio of Th1/Th2 cells was originally proposed as a marker of immune dysregulation, particularly in autoimmune and allergic conditions (62). However, the “Th1/Th2 paradigm” does not universally explain reproductive events (37) nor directly account for the more recently discovered Th17 cells (63). It is also possible that measures of stimulated lymphocyte cytokine production, or other measures of immune activity, would have differing results from our measures of circulating cytokine levels. Finally, this preliminary study investigated immune responses in healthy women who were actively contracepting, and as such do not directly speak to the impact of the observed effects on fertility or infertility. As such, much more work is needed to contextualize our findings in the broader scope of the immune system, and to examine the proposed mechanisms on clinically relevant outcomes such as pregnancy.
Conclusion
The findings from this study suggest that partnered sexual behavior is related to cycle-related shifts in endocrine and cytokine parameters in healthy women. Recent studies have shown interactions between menstrual cycling and sexual activity on other aspects of immunity such as mucosal antibodies (64), C-reactive protein (2), and antiviral activity (65, 66). This work underscores the importance of considering the environmental – including social – context of endocrine-immune interactions (67), and points to a novel behavioral paradigm for research and clinical care in reproductive-aged women.
Supplementary Material
Supplemental Figure 1. Progesterone: Estradiol ratio across the menstrual cycle, separated by sexual activity status (adjusted for age, body mass index, and race). Luteal-phase progesterone: estradiol ratios were significantly higher in sexually active vs. sexually abstinent women.
Supplemental Figure 2. Conceptual diagram for mediation analyses.
Supplemental Table 1. Peripheral unstimulated serum cytokine concentrations, by cycle phase and sexual activity status (unadjusted). There were no significant differences in the mean cytokine values between groups in any timepoint.
Supplemental Table 2. Effect estimates for mediation analyses.
Acknowledgments
This work was partially funded by the Office of the Vice Provost of Research at Indiana University-Bloomington through the Collaborative Research and Creative Activity Funding Award, and partially by the American Psychological Foundation's Henry P. David Award for Research in Human Reproductive Behavior and Population Studies. Dr. Lorenz is supported by grant T32HD049336-09 from the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams ET, Miller EM. The roles of the immune system in women's reproduction: Evolutionary constraints and life history tradeoffs. Am J Phys Anthropol. 2011;146:134–54. doi: 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz T, Demas GE, Heiman JR. Partnered sexual activity moderates midcycle decreases in inflammation in healthy women under review. doi: 10.1016/j.fertnstert.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wander K, Brindle E, O'Connor KA. C - reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136:138–46. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 4.Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev. 2014;36:71–82. doi: 10.1093/epirev/mxt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Northern ALD, Rutter SM, Peterson CM. Cyclic Changes in the Concentrations of Peripheral Blood Immune Cells During the Normal Menstrual Cycle. Exp Biol Med. 1994;207:81–8. doi: 10.3181/00379727-207-43795. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kim J, Jang B, Hur S, Jung U, Kil K, et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. The Journal of Immunology. 2010;185:756–62. doi: 10.4049/jimmunol.0904192. [DOI] [PubMed] [Google Scholar]
- 7.Faas M, Bouman A, Moesa H, Heineman MJ, de Leij L, Schuiling G. The immune response during the luteal phase of the ovarian cycle: a Th2-type response? Fertil Steril. 2000;74:1008–13. doi: 10.1016/s0015-0282(00)01553-3. [DOI] [PubMed] [Google Scholar]
- 8.López-Karpovitchs X, Larrea F, Cardenas R, Valencia X, Piedras J, Diaz-Sanchez V, et al. Peripheral blood lymphocyte subsets and serum immunoglobulins in Sheehan's syndrome and in normal women during the menstrual cycle. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 1992;45:247–53. [PubMed] [Google Scholar]
- 9.Saito S, Nakashima A, Shima T, Ito M. REVIEW ARTICLE: Th1/Th2/Th17 and Regulatory T - Cell Paradigm in Pregnancy. Am J Reprod Immunol. 2010;63:601–10. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 10.Kwak-Kim J, Bao S, Lee SK, Kim JW, Gilman-Sachs A. Immunological Modes of Pregnancy Loss: Inflammation, Immune Effectors, and Stress. Am J Reprod Immunol. 2014;72:129–40. doi: 10.1111/aji.12234. [DOI] [PubMed] [Google Scholar]
- 11.Swain SL, Bradley LM, Croft M, Tonkonogy S, Atkins G, Weinberg AD, et al. Helper T - cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991;123:115–44. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 12.Giron-Gonzalez J, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, et al. Consistent production of a higher TH1: TH2 cytokine ratio by stimulated T cells in men compared with women. European journal of endocrinology. 2000;143:31–6. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 13.Prasad A, Mumford SL, Louis GMB, Ahrens KA, Sjaarda LA, Schliep KC, et al. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm Behav. 2014;66:330–8. doi: 10.1016/j.yhbeh.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein SL, Nelson RJ. Influence of social factors on immune function and reproduction. Rev Reprod. 1999;4:168–78. doi: 10.1530/ror.0.0040168. [DOI] [PubMed] [Google Scholar]
- 15.Xiang L, Del Ben KS, Rehm KE, Marshall GD., Jr Effects of acute stress-induced immunomodulation on TH1/TH2 cytokine and catecholamine receptor expression in human peripheral blood cells. Neuropsychobiology. 2011;65:12–9. doi: 10.1159/000328160. [DOI] [PubMed] [Google Scholar]
- 16.Bartolomucci A, Palanza P, Gaspani L, Limiroli E, Panerai AE, Ceresini G, et al. Social status in mice: behavioral, endocrine and immune changes are context dependent. Physiol Behav. 2001;73:401–10. doi: 10.1016/s0031-9384(01)00453-x. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz T, van Anders SM. Interactions of sexual activity, gender, and depression with immunity. J Sex Med. 2014;11:966–79. doi: 10.1111/jsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SG, Morrison L, Calibuso MJ, Christiansen BA. The menstrual cycle and sexual behavior: Relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–44. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Anders SM, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: Methodological considerations. Arch Sex Behav. 2014;43:231–50. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 20.Byrne ML, O'Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM, et al. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav Immun. 2013;34:164–75. doi: 10.1016/j.bbi.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Heitjan DF, Basu S. Distinguishing “missing at random” and “missing completely at random”. The American Statistician. 1996;50:207–13. [Google Scholar]
- 22.Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–202. [Google Scholar]
- 23.Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–90. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DP, Genc M, Vardhana S, Babula O, Onderdonk A, Witkin SS. Ethnic differences of polymorphisms in cytokine and innate immune system genes in pregnant women. Obstet Gynecol. 2004;104:293–300. doi: 10.1097/01.AOG.0000133486.85400.5e. [DOI] [PubMed] [Google Scholar]
- 25.Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, et al. Increased T-helper interferon-γ-secreting cells in obese children. European Journal of Endocrinology. 2006;154:691–7. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 26.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. The Journal of Clinical Endocrinology & Metabolism. 2007;92:969–75. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 27.Lin D, Smith MA, Elter J, Champagne C, Downey CL, Beck J, et al. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 2003;71:5163–8. doi: 10.1128/IAI.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montoya AK, Hayes AF. Annual Convention of the Association for Psychological Science. New York, NY: May, 2015. Estimating and Testing Indirect Effects in Within-Subject Mediation Analysis: A Path-Analytic Framework. [Google Scholar]
- 29.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. 1st. New York: Guilford Press; 2013. [Google Scholar]
- 30.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 31.Donoho CJ, Crimmins EM, Seeman TE. Marital quality, gender, and markers of inflammation in the MIDUS cohort. Journal of Marriage and Family. 2013;75:127–41. doi: 10.1111/j.1741-3737.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benazon N, Wright J, Sabourin S. Stress, sexual satisfaction, and marital adjustment in infertile couples. J Sex Marital Ther. 1992;18:273–84. doi: 10.1080/00926239208412852. [DOI] [PubMed] [Google Scholar]
- 33.Monga M, Alexandrescu B, Katz SE, Stein M, Ganiats T. Impact of infertility on quality of life, marital adjustment, and sexual function. Urology. 2004;63:126–30. doi: 10.1016/j.urology.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Morreale M, Balon R, Tancer M, Diamond M. The impact of stress and psychosocial interventions on assisted reproductive technology outcome. J Sex Marital Ther. 2010;37:56–69. doi: 10.1080/0092623X.2011.533584. [DOI] [PubMed] [Google Scholar]
- 35.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 36.Heiman JR, Long JS, Smith SN, Fisher WA, Sand MS, Rosen RC. Sexual satisfaction and relationship happiness in midlife and older couples in five countries. Arch Sex Behav. 2011;40:741–53. doi: 10.1007/s10508-010-9703-3. [DOI] [PubMed] [Google Scholar]
- 37.Chaouat G, Ledée-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 38.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1: Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark DA, Manuel J, Lee L, Chaouat G, Gorczynski RM, Levy GA. Ecology of Danger-dependent Cytokine-boosted Spontaneous Abortion in the CBA × DBA/2 Mouse Model. I. Synergistic Effect of LPS and (TNF-α + IFN-γ) on Pregnancy Loss. Am J Reprod Immunol. 2004;52:370–8. doi: 10.1111/j.1600-0897.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- 40.Suarez S, Pacey A. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 41.Sheth K, Roca G, Al-Sedairy S, Parhar R, Hamilton C, al-Abdul JF. Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor (s) in preimplantation embryo culture fluid. Fertil Steril. 1991;55:952–7. doi: 10.1016/s0015-0282(16)54305-2. [DOI] [PubMed] [Google Scholar]
- 42.Finkelman F, Katona I, Urban J, Holmes J, Ohara J, Tung A, et al. IL-4 is required to generate and sustain in vivo IgE responses. The Journal of Immunology. 1988;141:2335–41. [PubMed] [Google Scholar]
- 43.Polan ML, Loukides JA, Honig J. Interleukin-1 in human ovarian cells and in peripheral blood monocytes increases during the luteal phase: Evidence for a midcycle surge in the human. Am J Obstet Gynecol. 1994;170:1000–7. doi: 10.1016/s0002-9378(94)70093-1. [DOI] [PubMed] [Google Scholar]
- 44.Cannon J, Dinarello C. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247–9. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox AJ, Weinberg CR, Baird DD. Timing of Sexual Intercourse in Relation to Ovulation — Effects on the Probability of Conception, Survival of the Pregnancy, and Sex of the Baby. N Engl J Med. 1995;333:1517–21. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz T, Worthman C, Vitzthum VJ. Links between inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications under revision. doi: 10.1093/emph/eov029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev. 2014;36:71–82. doi: 10.1093/epirev/mxt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 49.Bertone-Johnson E, Ronnenberg A, Houghton S, Nobles C, Zagarins S, Takashima-Uebelhoer B, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. 2014;29:1987–94. doi: 10.1093/humrep/deu170. [DOI] [PubMed] [Google Scholar]
- 50.Nowosielski K, Drosdzol A, Skrzypulec V, Plinta R. Sexual Satisfaction in Females with Premenstrual Symptoms. The Journal of Sexual Medicine. 2010;7:3589–97. doi: 10.1111/j.1743-6109.2010.01927.x. [DOI] [PubMed] [Google Scholar]
- 51.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–71. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1-and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109:30–3. doi: 10.1016/s0165-5728(00)00299-x. [DOI] [PubMed] [Google Scholar]
- 53.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 54.Lorenz TA, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women's physiological sexual arousal. Psychophysiology. 2012;49:111–7. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Windham GC, Elkin E, Fenster L, Waller K, Anderson M, Mitchell PR, et al. Ovarian Hormones in Premenopausal Women: Variation by Demographic, Reproductive and Menstrual Cycle Characteristics. Epidemiology. 2002;13:675–84. doi: 10.1097/00001648-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Barrett ES, Tran V, Thurston SW, Frydenberg H, Lipson SF, Thune I, et al. Women who are married or living as married have higher salivary estradiol and progesterone than unmarried women. American Journal of Human Biology. 2015 doi: 10.1002/ajhb.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vitzthum VJ. The ecology and evolutionary endocrinology of reproduction in the human female. Am J Phys Anthropol. 2009;140:95–136. doi: 10.1002/ajpa.21195. [DOI] [PubMed] [Google Scholar]
- 58.Fang CY, Egleston BL, Manzur AM, Townsend RR, Stanczyk FZ, Spiegel D, et al. Psychological reactivity to laboratory stress is associated with hormonal responses in postmenopausal women. J Int Med Res. 2014:0300060513504696. doi: 10.1177/0300060513504696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15:251–9. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laan E, Lunsen Rv. Hormones and sexuality in postmenopausal women: a psychophysiological study. Journal of Psychosomatic Obstetrics & Gynecology. 1997;18:126–33. doi: 10.3109/01674829709085579. [DOI] [PubMed] [Google Scholar]
- 61.Kort R, Caspers M, van de Graaf A, van Egmond W, Keijser B, Roeselers G. Shaping the oral microbiota through intimate kissing. Microbiome. 2014;2:41. doi: 10.1186/2049-2618-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 63.Awasthi A, Kuchroo VK. TH17 Cells in Health and Disease. Springer; 2011. From TH1/TH2 Paradigm to TH17 Cells: Le Roi Est Mort, Vive Le Roi; pp. 3–25. [Google Scholar]
- 64.Brown SG, Morrison LA, Calibuso MJ, Christiansen TM. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–44. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortenberry JD, Rogers M, Fordyce K, King S, Aronoff DM, Van Anders SM. HIV Inhibition and Variation in Anti-Microbial Peptides Associated With Intercourse. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. [Google Scholar]
- 66.Aronoff DM, Fordyce K, Salzman E, Fortenberry JD, Rogers M, King S, et al. Society for Gynecologic Investigation. Florence, Italy: 2014. Antiviral Activity of Vaginal Secretions Is Menstrual Cycle Phase-Dependent. [Google Scholar]
- 67.Demas GE, Carlton ED. Ecoimmunology for psychoneuroimmunologists: Considering context in neuroendocrine–immune–behavior interactions. Brain Behav Immun. 2015;44:9–16. doi: 10.1016/j.bbi.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Progesterone: Estradiol ratio across the menstrual cycle, separated by sexual activity status (adjusted for age, body mass index, and race). Luteal-phase progesterone: estradiol ratios were significantly higher in sexually active vs. sexually abstinent women.
Supplemental Figure 2. Conceptual diagram for mediation analyses.
Supplemental Table 1. Peripheral unstimulated serum cytokine concentrations, by cycle phase and sexual activity status (unadjusted). There were no significant differences in the mean cytokine values between groups in any timepoint.
Supplemental Table 2. Effect estimates for mediation analyses.