Abstract
Background
Recent meta-analyses support the utility of urinary biomarkers for the diagnosis and prognosis of acute kidney injury. It is critical to establish optimal sample handling conditions for short-term processing and long-term urinary storage, prior to widespread clinical deployment and meaningful use in prospective clinical trials.
Study Design
Prospective study.
Setting & Participants
Eighty children (median age, 1.1 [IQR, 0.5–4.2] years) undergoing cardiac surgery with cardiopulmonary bypass at our center. Fifty percent of the patients had acute kidney injury (defined as a 50% or greater increase in serum creatinine from baseline).
Predictors
We tested the effect on biomarker concentrations of short-term urine storage in ambient, refrigerator, and freezer conditions. We also tested the effects of multiple freeze-thaw cycles, as well as prolonged storage for five years.
Outcomes
Urine concentrations of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), and interleukin 18 (IL-18).
Measurements
All biomarkers were measured using commercially available kits.
Results
All three biomarkers were stable in urine stored at 4°C for 24 hours, but showed significant degradation (5.6%-10.1% from baseline) when stored at 25°C. All three biomarkers showed only a small although significant decrease in concentration (0.77%-2.9% from baseline) after three freeze-thaw cycles. Similarly, all three biomarkers displayed only a small but significant decrease in concentration (0.84%-3.2%) after storage for 5 years.
Limitations
Only the three most widely studied biomarkers were tested. Protease inhibitors were not evaluated.
Conclusions
Short-term storage of urine samples for measurement of NGAL, KIM-1 and IL-18 may be performed at 4°C for up to 24 hours, but not at room temperature. These urinary biomarkers are stable at −80°C for up to five years of storage. Our results are reassuring for the deployment of these assays as biomarkers in clinical practice, as well as in prospective clinical studies requiring long term urine storage.
Keywords: acute kidney injury (AKI), urinary biomarker, biomarker stability, urine storage, freeze-thaw cycle, sample handling conditions, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), interleukin 18 (IL-18), enzyme-linked immunosorbent assay (ELISA), acute renal failure, children, pediatric patients
The use of urinary biomarkers for early detection and prognostication of acute kidney injury (AKI) is a rapidly developing field within nephrology research. In particular, the most promising biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL),1 kidney injury molecule 1 (KIM-1),2 and interleukin 18 (IL-18)3 have now been investigated to the point that systematic reviews and meta-analyses of their diagnostic utility have recently appeared.4-7 A number of pivotal prospective clinical studies are now collecting serial urine samples at various points in time, for batch analysis of biomarkers at a much later date. In addition, clinical platforms for the measurement of select urinary biomarkers of AKI (e.g. NGAL) have been launched.8 It is therefore critical to establish optimal sample handling conditions for both short-term as well as long-term urinary storage.9
In the present study, we investigated the stability of urine NGAL, KIM-1 and IL-18 in a cohort of children undergoing cardiac surgery with cardiopulmonary bypass at our center. We first evaluated the effect on biomarker concentrations of short term storage over 24 hours in ambient, refrigerator, and freezer conditions. These findings are directly pertinent to obtaining reliable and reproducible results in the clinical setting. We then analyzed the effect of multiple freeze-thaw cycles, as well as the stability of these urinary biomarkers after prolonged storage (for 5 years). These results are highly relevant to clinical and translational research studies aimed at biomarker measurements in urine samples following long-term storage.
METHODS
Patient Population
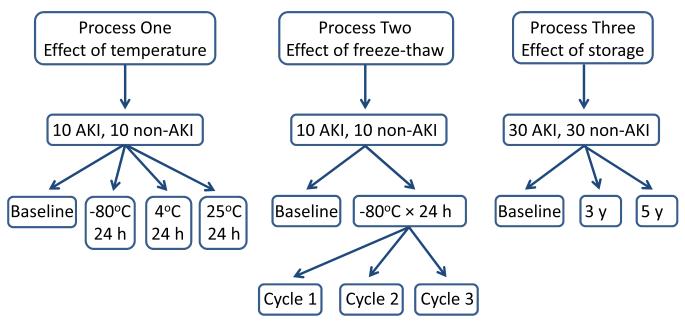
This study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center. All patients younger than 18 years undergoing cardiac surgery with cardiopulmonary bypass at our center from January 2004 through July 2007 were approached for study inclusion. The demographics of the original cohort of 220 patients have been previously described.10 The percentage increase in serum creatinine was calculated during the index hospitalization, with AKI defined as a 50% increase in serum creatinine from baseline. Urine samples for biomarker analysis were obtained at baseline and at various time points after initiation of cardiopulmonary bypass. For this study, we chose urine samples from 80 patients obtained at 2-6 hours after bypass, since this time point yielded the highest biomarker concentration in previous studies.10 The samples were chosen based on availability, from patients selected for even gender distribution and other characteristics representative of the original cohort. All urine samples were centrifuged upon collection, the supernatant divided into 1 ml aliquots, and processed in one of the following three methods, as illustrated in Figure 1. No protease inhibitors were employed.
FIGURE 1.
Schematic representation of the study design
Processes
Process 1: Effect of Temperature on Short-term Storage
Twenty urine samples were aliquoted and subjected to immediate biomarker testing (baseline, with testing initiated within 15 minutes of aliquoting), or tested after storage for 24 hours at −80°C, 4°C, or at 25°C (each on a separate aliquot).
Process 2: Effect of Freeze–Thaw Cycles
The effect of multiple freeze cycles on biomarker stability was evaluated in the same twenty subjects as for process 1. After immediate biomarker testing (baseline), samples were tested after a series of three sequential freeze-thaw cycles following storage for 24 hours at −80°C.
Process 3: Effect of Long-term Storage
Sixty urine samples were aliquoted and subjected to immediate biomarker testing (baseline), or tested after storage at −80°C for 3 or for 5 years (separate aliquots).
Biomarker Measurements
Single measurements of each biomarker were accomplished in a blinded fashion as previously described.10 The urine NGAL ELISA was performed using a commercially available assay (NGAL ELISA Kit 036, Bioporto, Grusbakken, Denmark) that specifically detects human NGAL and utilizes monoclonal capture and detection antibodies. The starting dilution factor for this assay is 1:500. The lower limit of detection for the NGAL ELISA is 4 pg/ml. The intra-assay and inter-assay coefficients of variation were 2.1% and 9.1%, respectively. The urine KIM-1 ELISA was constructed using commercially available reagents (DuoSet DY1750, R&D Systems, Minneapolis, MN) as described previously11. The KIM-1 ELISA uses affinity purified polyclonal capture and detection antibodies. The starting dilution for the KIM-1 ELISA is 1:1. The lower limit of detection is 59 pg/ml. Intra- and inter-assay coefficients of variation were 2.0% and 7.8%, respectively. The urine IL-18 ELISA kit was from Medical and Biological Laboratories, Nagoya, Japan. The IL18 ELISA uses monoclonal capture and detection antibodies. The starting dilution for the IL18 ELISA is 1:1. The lower limit of detection is 12.5 pg/ml. Intra- and inter-assay coefficients of variation were 7.5% and 7.3%, respectively.
Statistical Analysis
Biomarker concentrations are reported as median (interquartile range [IQR]). Analysis of variance (ANOVA) with repeated measures was used to evaluate change in biomarker concentrations during each process. We applied a log transformation (natural log) to biomarker concentrations in our statistical analyses, since the data were not normally distributed. Significance was set at p < 0.05 after Bonferroni correction for multiple comparisons (six comparisons for processes 1 and 2 and three comparisons for process 3). All statistical analyses were performed using SAS 9.3 statistical software (SAS Institute Inc).
RESULTS
Study Participants
The median age of the entire cohort (N = 80) was 1.1 (IQR, 0.5-4.2) years. Twenty unique patients (10 with AKI and 10 without AKI) participated in processes 1 and 2, and 60 additional patients (30 with AKI and 30 without AKI) were analyzed in process 3. All samples had biomarker levels within the range for each assay, and none of the samples were below the lower limits of detection for all three biomarkers. The patient characteristics for each process are shown in Table 1. There were no significant differences between the two groups.
TABLE 1.
Patient Characteristics
| Characteristic | Processes 1 and 2 (n=20) |
Process 3 (n=60) |
|---|---|---|
| Age, y | 2.90 [0.46-4.32] | 0.77 [0.50-4.15) |
| Female sex | 10 (50) | 31 (52) |
| AKI | 10 (50) | 30 (50) |
| Increase in serum creatinine | ||
| Non-AKI | 6.25% [0%-20.0%] | 20.0% [0%-25.0%] |
| AKI | 70.8% [66.7%-100.0%] | 77.5% [66.7%-116.7%] |
Note: Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range]. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: AKI, acute kidney injury
Processes
Process 1: Effect of Temperature on Short-term Storage
Baseline measurements of NGAL, KIM-1 and IL-18 were 56 (IQR, 20-88.5) ng/ml, 720.6 (IQR, 333.8-1469) pg/ml, and 68.7 (IQR, 28.9-200) pg/ml, respectively, as shown in Table 2. There was no significant difference between concentration measurements at baseline, 24 hours at 4°C, or 24 hours at −80°C. However, there was a significant decrease in the concentration measurements of all three biomarkers after 24 hours at 25°C (P < 0.05), with an NGAL concentration of 53.5 (IQR, 18.5-81) ng/ml, KIM-1 of 668.6 (IQR, 320.8-1348.9) pg/ml and IL-18 of 62 (IQR, 27.25-173.95) pg/ml. This corresponded to a median percentage decrease from baseline of 5.6% for NGAL, 6.1% for KIM-1, and 10.1% for IL-18 when measured after 24 hours of storage at 25°C.
TABLE 2.
Process 1: Effect of temperature on urinary concentrations of biomarkers after short-term storage
| NGAL | KIM-1 | IL-18 | |
|---|---|---|---|
| Baseline | 56 (20-88.5) | 720.6 (333.8-1469.0) | 68.7 (28.9-200.0) |
| short-term storage at −80°C | 55.5 (19.5-87.5) | 718.26 (331.77-1465.42) | 68.0 (28.75-198.0) |
| Decrease from baseline | 0.2% (0%-2.1%) | 0.3% (0.1%-0.6%) | 1.1%(0.6%-1.6%) |
| short-term storage at 4°C | 55.5 (19.5-87.5) | 719.17 (331.48-1464.75) | 68.0 (28.75-198.0) |
| Decrease from baseline | 0.9% (0%-2.3%) | 0.5%(0.2%-1.0%) | 1.0%(0.4%-2.1%) |
| short-term storage at 25°C | 53.5 (18.5-81.0)* | 668.6 (320.8-1348.88)* | 62.0 (27.25-173.95)* |
| Decrease from baseline | 5.6% (4%.5-7.8%) | 6.1% (4.0%-8.4%) | 10.1% (8.1%-14.2%) |
Note: Values are given as median [interquartile range]. Biomarkers were measured either immediately (baseline) or 24 hours after storage at the temperatures indicated. Unless otherwise indicated, NGAL expressed in ng/mL; KIM-1, in pg/mL; and IL-18, in pg/mL.
significant difference in measurement from baseline, short-term storage at −80°C, and short-term storage at 4°C at p<0.05 with Bonferroni correction from analysis of variance with biomarker modeled with natural log transformation.
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; IL-18, interleukin 18.
Process 2: Effect of Freeze–Thaw Cycles
The NGAL, KIM-1, and IL-18 values were measured at baseline and after 3 consecutive freeze-thaw cycles following 24 hours of storage at −80°C. Overall, all three biomarkers demonstrated a small but consistent trend to decrease with subsequent freeze-thaw cycles, as shown in Table 3. There was a significant decrease from baseline in all three biomarker levels by cycle 2, and a significant decrease from both baseline and cycle 1 after cycle 3. Although a statistically significant decrease, the total percentage decrease remained relatively small, ranging from 0.77% for KIM-1 to 2.9% for NGAL when comparing cycle 3 to baseline values.
TABLE 3.
Process 2: Effect of multiple freeze-thaw cycles on urinary concentrations of biomarkers
| NGAL | KIM-1 | IL-18 | |
|---|---|---|---|
| Baseline | 56 (20-88.5) | 720.6 (333.8-1469.0) | 68.7 (28.9-200.0) |
| Cycle 1 | 55.5 (19.5-87.5) | 718.3 (331.8-1465.4)* | 68.0 (28.8-198.0)* |
| Decrease from baseline | 0.25% (0%-2.0%) | 0.28% (0.089%-0.62%) | 1.1% (0.56%-1.6%) |
| Cycle 2 | 55.0 (19.0-86.5)* | 718.3 (331.9-1464.2)* | 67.5 (28.3-197.0)** |
| Decrease from baseline | 2.0% (0.12%-3.5%) | 0.51% (0.18%-0.70%) | 1.7% (1.3%-3.1%) |
| Cycle 3 | 54.5 (19.0-85.5)** | 716.1 (331.0-1448.4)† | 67.5 (28.2-194.7)** |
| Decrease from baseline | 2.9% (0.1%-5.0%) | 0.77% (0.63%-1.0%) | 2.5% (1.5%-3.9%) |
Note: Values are given as median [interquartile range]. Biomarkers were measured either immediately (baseline) or after 24 hours of storage at −80°C followed by freeze-thaw cycles as indicated. Values are given as median (interquartile range). Percent changes are for each cycle in comparison to baseline. Significance set at p<0.05 with Bonferroni correction from ANOVA with biomarker modeled with natural log transformation. Unless otherwise indicated, NGAL expressed in ng/mL; KIM-1, in pg/mL; and IL-18, in pg/mL.
significantly different from baseline;
significantly different from baseline and from cycle 1;
significantly different from baseline, cycle 1, and cycle 2
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; IL-18, interleukin 18.
Process 3: Effect of Long-term Storage
Baseline measurements of NGAL, KIM-1, and IL-18 were 59.3 (IQR, 18.8-221.24) ng/ml, 298.2 (IQR, 175-699.8) pg/ml, and 36.5 (IQR, 21.6-85) pg/ml, respectively. Overall, there was a small but significant trend for each biomarker to decrease over time, as shown in Table 4. After three years of storage, urinary NGAL levels decreased by 0.42%, KIM-1 decreased by 0.38%, and IL-18 decreased by 1.31%. Small but significant decreases were again observed after five years, with a decrease of 0.84% for NGAL, 1.98% for KIM-1, and 3.20% for IL-18. The results were similar when dividing the groups into those with versus without AKI, with significant yet relatively small decreases over five years (median decrease ranges of 0.68%-3.0% and 1.1%-4.2%, respectively).
TABLE 4.
Process 3: Effect of long term storage on urinary concentrations of biomarkers
| Biomarker | NGAL | KIM-1 | IL-18 |
|---|---|---|---|
| Total Process 3 Cohort (n=60) | |||
| Baseline | 59.3 (18.8-221.24) | 298.2 (175.0-699.8) | 36.5 (21.6-85.0) |
| 3 y | 60.2 (18.4-222.0) | 295.0 (173.6-697.8)* | 36.3 (21.1-84.3)* |
| Decrease from baseline | 0.42% (0.04%-1.24%) | 0.38% (−0.02%-1.02%) | 1.31% (0.43%-2.99%) |
| 5 y | 58.7 (18.5-221.3)* | 290.3 (171.2-691.8)** | 35.8 (19.9-82.0)** |
| Decrease from baseline | 0.84% (0.094%-1.87%) | 1.98% (0.73%-2.92%) | 3.20% (2.21%-4.69%) |
| Non-AKI (n=30) | |||
| Baseline | 18.84 (12.7-30.96) | 182.83 (103.78-340.45) | 21.6 (14.04-30.82) |
| 3 y | 18.4 (12.69-30.33) | 183.45 (104.96-336.19) | 21.14 (12.88-28.85)* |
| Decrease from baseline | 0.97% (0.27%-2.1%) | 0.36% (−0.37%-1.0%) | 2.9% (1.3%-4.2%) |
| 5 y | 18.53 (12.2-29.52)* | 177.61 (101.2-330.24)* | 20.84 (12.36-28.61)** |
| Decrease from baseline | 1.1% (0.28%-3.5%) | 2.9% (2.0%-3.0%) | 4.2% (2.4%-6.5%) |
| AKI (n=30) | |||
| Baseline | 221.24 (154.43-490.89) | 605.92 (290.36-990.66) | 84.98 (43.97-98.54) |
| 3 y | 222.0 (155.7-490.0) | 603.94 (288.44-984.88)* | 84.28 (43.09-97.22) |
| Decrease from baseline | 0.15% (−0.12%-0.49%) | 0.38% (0.13%-1.3%) | 0.93% (0.13%-1.3%) |
| 5 y | 221.26 (154.36-490.44)* | 601.14 (282.97-980.8)** | 82.05 (42.66-95.58)* |
| Decrease from baseline | 0.68% (0.05%-1.0%) | 1.1% (0.23%-2.0%) | 3.0% (2.0%-3.9%) |
Note: Values are given as median [interquartile range]. Biomarkers were measured either immediately (baseline) or after storage at −80°C for 3 or 5 years as indicated. Significance set at p<0.05 with Bonferroni correction from analysis of variance with biomarker modeled with natural log transformation. Unless otherwise indicated, NGAL expressed in ng/mL; KIM-1, in pg/mL; and IL-18, in pg/mL.
significantly different from baseline;
significantly different from baseline and from year 3
Abbreviations: NGAL, neutrophil gelatinase-associated lipocalin; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; AKI, acute kidney injury.
DISCUSSION
This study comprehensively investigated the effects of both short-term processing and long-term storage of the three most promising and extensively studied urinary biomarkers for AKI, namely NGAL, KIM-1 and IL-18. We demonstrated that firstly, storage at 4°C for up to 24 hours does not significantly affect biomarker levels, while storage at 25°C should be avoided. Secondly, up to three sequential freeze-thaw cycles caused only a small decrease in biomarker concentration. Thirdly, long-term storage of urinary biomarkers resulted in only a relatively small decrease in concentrations at 5-year follow up, representing only a 1%-3% decrease from baseline values. Therefore, long-term storage of urine for up to 5 years can be reliably accomplished with only minimal degradation of the tested biomarkers. In AKI patients, for all three processes, the small changes in biomarker concentration did not result in a decrease of the tested biomarkers to below the thresholds proposed for the clinical diagnosis of AKI. 4-7
Identification of novel biomarkers for early detection and risk stratification of AKI continues to be a rapidly developing field, yet little is still known about their stability both over time and by various storage methods. Previous studies have provided some insights into the effects of various short term storage conditions on biomarker stability, including centrifugation, protease inhibitors, and temperature. In regards to short-term storage, Van de Vrie et al. found that NGAL and KIM-1 remained stable at 4°C for up to 48 hours.12 However, they found a significant decrease in urinary KIM-1 concentration when samples were stored at 4°C beyond 48 hours. Sample stability was not affected by the addition of protease inhibitors. Parikh et al. tested varying processing conditions for short-term handling of multiple biomarkers.13 Conditions included storing urine samples at 4°C for 48 hours prior to freezing at −80°C, centrifugation with storage at 25°C for 48 hours before freezing at −80°C, and immediate freezing at −80°C without centrifugation. Their investigation showed that storage at 4°C for up to 48 hours and lack of centrifuging did not affect biomarker levels of NGAL, KIM-1, or IL-18. However, IL-18 demonstrated suboptimal stability when stored at 25°C. Similarly, Han et al. showed decreased biomarker concentrations after storage at room temperature.14 Our results indicate that urine samples should not be stored at 25°C for even short (24-hour) periods of time as this leads to significant decreases in concentrations of NGAL, KIM-1, and IL-18. Although ideally all samples would be processed immediately, our data support the notion that biomarker testing can be delayed for up to 24 hours at 4°C without compromising results. In this way, a urine sample collected overnight could be processed later that day without concerns for biomarker degradation. Our standard operating procedure calls for all obtained urine samples to be immediately stored at 4°C. Samples are processed at least twice daily by laboratory technicians, ensuring that sample processing is completed within approximately 12 hours after collection. Following centrifugation to remove particulate matter, the urine samples are aliquoted and promptly frozen at −80°C for future analysis. Our results indicate that urinary NGAL, KIM-1 and IL-18 are sufficiently stable to allow for routine clinical testing of samples stored at 4°C for up to 24 hours.
We demonstrated that repeated freeze-thaw cycles do cause a decrease in biomarker values. However, the percentage decrease, while statistically significant, remained small and is likely not clinically relevant unless more than three freeze-thaw cycles are employed. The analysis of multiple freeze-thaw cycles is relevant to current laboratory practice as well as to prospective clinical research studies. Clinically, a 1-ml aliquot of urine is often more than enough for any one test, and therefore the same sample may be frozen and reused at a later time for another test. In research studies, samples are routinely frozen and batch analyzed at a later date. In both circumstances, the relative stability of the measured biomarkers after up to three freeze-thaw cycles as shown in this study is encouraging.
The few studies that have previously analyzed the effect of long-term freezing on biomarker stability have yielded conflicting results. Nauta et al. investigated the effect of −80°C storage on multiple urinary biomarkers, including NGAL and KIM-1, and found a gradual decrease in the median concentration of these biomarkers over the course of one year.15 However, others have reported better stability of urinary biomarkers after long-term storage. Van de Vrie et al. found that samples stored for up to 6 months at −80°C did not result in a decrease in concentration of NGAL or KIM-1.12 Similarly Pennemans et al. also demonstrated that urinary samples can be stored for 1.5 years without significant decrease in KIM-1 values 16. The reasons for these discrepant findings are not clear. Methodological differences, in particular storage for even short periods of time at room temperature prior to freezing, may have differentially affected biomarker levels. However, our data confirm that with optimal initial handling and processing of samples, urinary concentrations of NGAL, KIM-1, and IL-18 remained remarkably stable after long-term storage.
Our study is the first to confirm the stability of urinary NGAL, KIM-1, and IL-18 for up to five years of storage at −80°C. Other strengths include examining patients both with and without AKI, which allows for a wide dynamic range of biomarker concentrations for testing. One limitation of our study is the evaluation of only the three most promising biomarkers of AKI that have been most extensively studied to date. The encouraging results for NGAL, KIM-1, and IL-18 reported herein may not be applicable for other biomarkers, including the very recently described markers of cell cycle arrest that hold promise for the prediction of severe AKI.17 Another limitation pertains to the lack of protease inhibitor use. It is possible that even the small degree of degradation measured after five years of storage in our study may have been prevented by the addition of protease inhibitors at baseline. An additional limitation pertains to the lack of urine creatinine measurements in the present study. Urinary biomarkers are often expressed as a ratio with urine creatinine, and we did not measure the effect of the three processes on urine creatinine. Finally, this study was a single center cohort study of a select population of children undergoing cardiac surgery with cardiopulmonary bypass. The results may not be generalizable to other patient populations, such as adults or centers with different laboratory practices.
In conclusion, our study demonstrates that short-term storage of urine samples for measurement of NGAL, KIM-1, and IL-18 should be performed at 4°C and within 24 hours of sample acquisition. Up to three freeze-thaw cycles, and long term storage at −80°C for up to five years, result in only a minimal decrease in biomarker levels. Our results therefore indicate that long-term storage and retesting can be accomplished with excellent stability of urinary biomarkers of AKI examined in this study. Our results are reassuring for the deployment of these assays as biomarkers of AKI in clinical practice, as well as for prospective clinical studies requiring long term storage prior to batch measurement.
ACKNOWLEDGEMENTS
Support: This study was supported by National Institutes of Health grant P50 DK 096418 to Dr Devarajan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Devarajan is a co-inventor on submitted patents for the use of NGAL as a biomarker of kidney injury. The other authors declare that they have no relevant financial interests.
Contributions: Research idea and study design: MP, EN, MB, CDK, PD; data acquisition: QM, CH, MB, CDK, PD; data analysis and interpretation: MP, EN, MB, CDK, PD; statistical analysis: MP, EN, MB, PD; supervision or mentorship: PD. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. PD takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
REFERENCES
- 1.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 2.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 4.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51(Pt 3):335–51. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9(1):e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;62(6):1058–1067. doi: 10.1053/j.ajkd.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter JL, Lamb EJ. Evaluating new biomarkers for acute kidney injury: putting the horse before the cart. Am J Kidney Dis. 2014;63(4):543–546. doi: 10.1053/j.ajkd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58(22):2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5(2):128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van de Vrie M, Deegens JK, van der Vlag J, hilbrands LB. Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) Am J Kidney Dis. 2014;63(4):573–576. doi: 10.1053/j.ajkd.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Parikh CR, Butrymowicz I, Yu A, et al. Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis. 2014;63(4):567–572. doi: 10.1053/j.ajkd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4(5):873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauta FL, Bakker SJ, Lambers Heerspink H, et al. Effect of frozen storage on urinary concentration of kidney damage markers. Am J Kidney Dis. 2012;59(4):586–589. doi: 10.1053/j.ajkd.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Pennemans V, Rigo JM, Penders J, Swennen Q. Collection and storage requirements for urinary kidney injury molecule-1 (KIM-1) measurements in humans. Clin Chem Lab Med. 2011;50(3):539–543. doi: 10.1515/CCLM.2011.796. [DOI] [PubMed] [Google Scholar]
- 17.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]