Abstract
Background
Alcohol is often mixed with various nonalcoholic beverages. While consumption of food with alcohol will decrease peak breath alcohol concentrations (BrAC), recent evidence has suggested that mixing alcohol with diet beverages can result in higher BrAC when compared with mixing the same amount of alcohol with sweetened beverages. The purpose of this study was to examine this phenomenon using two different moderate alcohol doses.
Methods
Twenty participants (10 males) attended five sessions where they received 1 of 5 doses (0.91 ml/kg vodka + 3.64 ml/kg of diet soda, 0.91 ml/kg vodka + 3.64 of regular soda, 1.82 ml/kg vodka + 7.28 ml/kg diet soda, 1.82 ml/kg vodka + 7.28 ml/kg regular soda, and a placebo beverage). BrAC was recorded repeatedly up to 180 minutes after dose administration.
Results
Participants had significantly higher BrAC when the mixer was diet as compared to regular for both alcohol dose conditions. No gender differences were observed.
Conclusions
Mixing alcohol with diet beverages can result in higher BrAC when compared to the same amount of alcohol administered with a similar sweetened beverage. Individuals who consume diet mixers with alcohol may reduce caloric intake but increase the harms associated with higher BrACs.
Keywords: alcohol, mixers, breath alcohol concentrations, artificial sweeteners
INTRODUCTION
Alcohol contains calories and consumers often limit added calories to alcohol via caloric mixers. However, limited evidence suggests that mixing alcohol with diet beverages results in higher breath and blood alcohol concentrations (BrAC) when compared with the same dose of alcohol mixed with a sweetened mixer (e.g., Marczinski and Stamates, 2013; Rossheim and Thombs, 2011; Wu et al., 2006). The difference can be noticeable with one study reporting an 18% higher BrAC when the carbonated soda mixer was diet versus caloric (Marczinski and Stamates, 2013). However, this previous study had limited generalizability in that drinks were prepared to best study pharmacokinetics around intoxicating levels (i.e., peak BrAC of .08 g%). Previous work has incorporated limited amounts of mixer since it is thought that sugar-sweetened mixers delays gastric emptying time when compared to non-caloric mixers (Wu et al., 2006).
Despite concerns about the generalizability of current laboratory-based studies, this observation has also been found in a field study in bar patrons who self-selected their mixers (Rossheim and Thombs, 2011). Further, preliminary survey research has determined that almost half of college student female drinkers report the use of diet mixers with alcohol and consumption of alcohol with diet mixers was associated with greater alcohol-related harms even when the amount of alcohol consumed is controlled (Stamates et al., 2015). Given the limited findings that consumption of alcohol with diet mixers may pose as a risk factor for greater BrACs which can result in heightened risks to brain and liver health, additional research is needed to test the reliability of this effect for low to moderate alcohol doses using drinks prepared for optimal taste (i.e., with a greater mixer to alcohol ratio).
For this study, participants attended five sessions where they received 1 of 5 doses (0.91 ml/kg vodka + 3.64 ml/kg of diet soda, 0.91 ml/kg vodka + 3.64 of regular soda, 1.82 ml/kg vodka + 7.28 ml/kg diet soda, 1.82 ml/kg vodka + 7.28 ml/kg regular soda, and a placebo beverage). BrAC was recorded repeatedly up to 180 minutes after dose administration. We predicted that the alcohol + diet beverage would result in higher BrAC compared to the alcohol + regular beverage for both alcohol doses.
METHOD
Participants
Twenty adults (10 men) between the ages of 21 and 30 (M = 22.65, SD = 2.56) participated in this study. The sample included 19 Caucasians and 1 African American, who were recruited using established procedures reported elsewhere (Marczinski and Stamates, 2013). The Northern Kentucky University Institutional Review Board approved this study. All participants provided informed consent prior to participation. Participants received $160 upon completion of the study.
Measures
The Personal Drinking Habits Questionnaire (PDHQ; Vogel-Sprott, 1992) was used to assess the weekly frequency of drinking, the number of standard drinks over the hourly duration of a typical drinking occasion, and the customary dose of alcohol. The Intoxilyzer Model 400 (CMI Inc., Owensboro, KY) with a disposable mouthpiece was used to collect each breath sample from a participant to assess current Breath Alcohol Concentration (BrAC).
Dose administration
Participants received 1 of 5 doses (0.91 ml/kg vodka + 3.64 ml/kg diet soda, 0.91 ml/kg vodka + 3.64 regular soda, 1.82 ml/kg vodka + 7.28 ml/kg diet soda, 1.82 ml/kg vodka + 7.28 ml/kg regular soda, and a 3.64 ml/kg regular soda as the placebo beverage) based on body weight. For the alcohol conditions, 40% alcohol/volume Smirnoff Red Label vodka, No. 21 (Smirnoff Co., Norwalk, CT) was used and the alcohol doses were reduced to 87% for female participants.
The mixers were carbonated soft drinks which included Squirt and Diet Squirt (Dr. Pepper Snapple Group, Plano, TX) resulting in a 1:4 (alcohol/soft drink) ratio. A 12 oz. can of Squirt contains 140 calories and 37g of sugar. A 12 oz. can of Diet Squirt contains zero calories and is sweetened by aspartame. In the placebo condition, 3.64 ml/kg of Squirt was administered with 10 ml of vodka poured on the surface to give an alcohol scent.
Procedure
Subject screening, dose administration, and testing procedures followed the protocol in a psychopharmacology lab described in Marczinski and Stamates (2013). Participants were scheduled for 5 separate individual sessions with a minimum of 24 hours between sessions. Session start times ranged from 10 a.m. to 4 p.m., with a maximum deviation in start times within a subject of 2 hours. Participants were required to fast for 2 hours, abstain from any form of caffeine for 8 hours, and abstain from alcohol for 24 hours, before every test session (which was assessed via self-report). Participants were asked to provide a urine sample on each test session to test for 10 common drug metabolites that would be contraindicated for alcohol administration (uVera Diagnostics, Norfolk, VA). A baseline breathalyzer reading using an Intoxilyzer Model 400 (CMI Inc., Owensboro, KY) ensured a zero BrAC at the start of each session.
Dose administration was double-blind and the order of doses was counterbalanced between subjects. Participants were asked to consume the drink within 10 minutes. BrACs were measured at 30, 40, 70, 80, 90, 120, 150, and 180 min. after drinking was initiated and including placebo sessions. Upon completion of testing, participants were given a meal and relaxed in the lab until BrAC was below .02 g/210 l.
Data Analytic Strategy
Mean BrAC were analyzed separately for the 0.91 ml/kg and 1.82 ml/kg alcohol dose conditions. For the 0.91 ml/kg alcohol doses, mean BrAC were analyzed using a 2 (Mixer: regular vs. diet) × 3 (Time: 30, 40, 70 min.) × 2 (Gender) mixed design ANOVA. For the 1.82 ml/kg alcohol doses, mean BrAC were analyzed using a 2 (Mixer) × 8 (Time) × 2 (Gender) mixed design ANOVA. Mixer and Time were treated as within-subjects factors and Gender was treated as a between-subjects factor. For significant interactions, paired sample t-tests were used, applying the Bonferroni correction for multiple comparisons. The alpha level was set at .05 for all statistical tests and SPSS 17.0 (IBM Corporation, Armonk, NY) was used to conduct all analyses.
3. RESULTS
3.1. Drinking Characteristics
From the PDHQ, the sample reported a mean (SD) weekly alcohol drinking frequency of 1.10 (0.73), with a mean (SD) number of standard drinks per occasion of 3.48 (1.65) over a mean (SD) duration of 3.33 (1.15) hours. The sample self-reported a mean (SD) typical alcohol dose of 0.84 g/kg (0.45) per occasion. No gender differences were observed, ps > 0.26.
3.2. Breath Alcohol Concentrations
0.91 ml/kg alcohol dose BrAC
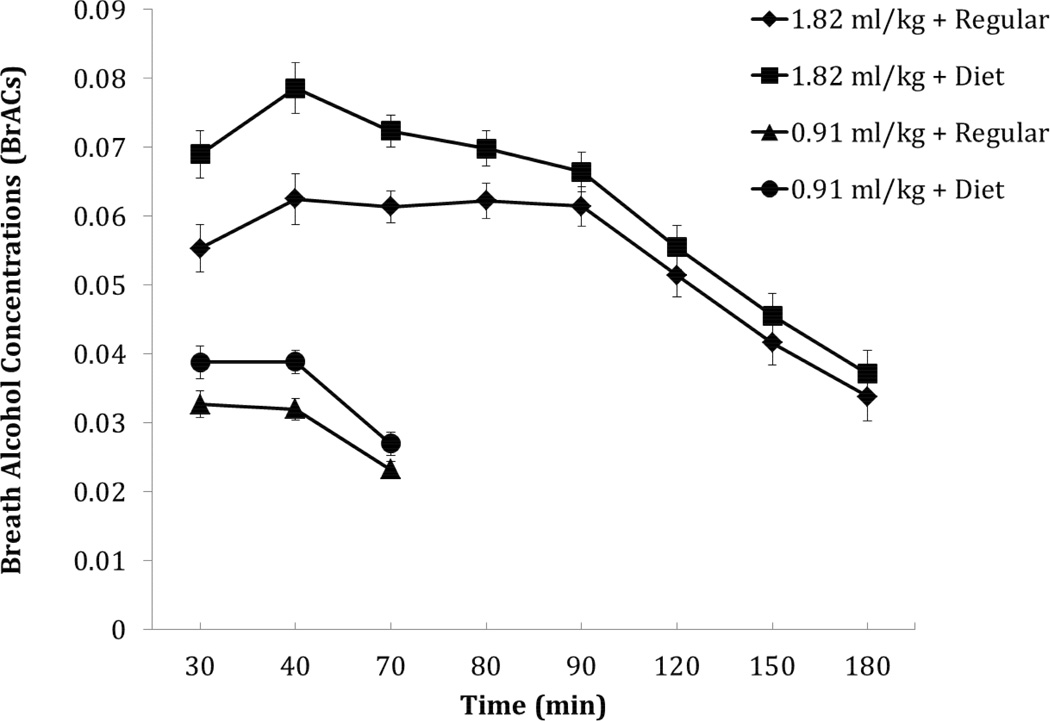
Results of the 2×3×2 ANOVA revealed a main effect of Mixer, F(1,18) = 14.66, p = .001, η2 = 0.449, and a main effect of Time, F(2,17) = 51.47, p < .001, η2 = 0.858. Paired samples t-tests adjusted for multiple comparisons were used to compare the conditions and confirmed a significantly higher value at each time point for the diet condition compared to the regular condition, ps < .014 (see Figure 1). No other effects or interactions were observed.
FIGURE 1.
Mean breath alcohol concentrations (BrAC) for the 0.91 ml/kg and 1.82 ml/kg alcohol dose conditions in combination with the regular or diet mixers. Standard errors are represented in the figure by the error bars attached to each symbol.
1.82 ml/kg alcohol dose BrAC
Results of a 2×8×2 ANOVA revealed a main effect of Mixer, F(1,18) = 18.49, p < .001, η2 = 0.507, and a main effect of Time, F(7,12) = 74.50, p < .001, η2 = 0.978. Figure 1 shows that the mean BrAC for the diet mixer was higher at each time point compared with the regular mixer. Paired samples t-tests adjusted for multiple comparisons were used to compare the conditions and confirmed greater values at the 30, 40, and 70 minute times for the diet condition compared to the regular condition, ps < .001. No other effects or interactions were observed.
4. DISCUSSION
The purpose of this study was to determine if there are BrAC differences when alcohol is mixed with a diet versus sweetened mixer. The results from both alcohol doses indicated that BrAC was elevated at every time point when a diet mixer was used as compared to a sweetened mixer. The difference in BrAC was not only statistically significant but also practically significant. For the 0.91 ml/kg dose of alcohol, the mixer type resulted in a 22% difference in BrAC (when measured at 40 minutes after dose administration). For the 1.82 ml/kg dose of alcohol, the mixer type resulted in a 25% difference in BrAC when measured at this same time point. These differences that were observed in this current study are similar to our previously reported finding of an 18% higher BrAC when the carbonated soda mixer was diet versus caloric (Marczinski and Stamates, 2013). This consistency occurred despite methodological differences between the two studies.
In both the current and previous study (Marczinski and Stamates, 2013), no gender differences in BrAC were observed. While all alcohol consumers should be made aware of this phenomenon, it appears more likely that women would select alcoholic beverages with a diet mixer given that they are more likely to conscious of calories in their drinks. Young women may be particularly vulnerable as they frequently use diet mixers with alcohol and they also restrict food intake when drinking to control calorie consumption and ultimately body weight (Stamates et al., 2015).
It is important to highlight the public health implications of these results although these implications are somewhat speculative and will need further investigation. First, women are more likely to experience harms associated with alcohol consumption when compared to men. While it is known that repeated chronic use of alcohol and high blood alcohol levels cause damage to a variety of body systems and lead to mortality, these risks are heightened for women (Zheng et al., 2015). Even after only one drinking occasion, the harmful impact of alcohol in female participants can be observed by assessing the status of reproductive hormones (Schliep et al., 2015). Despite such findings, young women now engage in binge drinking at high rates that are similar to young men. In cases of unplanned and unintended pregnancies, drinking to high blood alcohol concentrations can lead to Fetal Alcohol Syndrome (FAS) which is the primary and preventable cause of mental retardation. This is particularly a concern given that first trimester drinking and especially binge drinking to high BrACs can result in FAS (May et al., 2013). In many cases, early first trimester drinking occurs even before women are aware of their pregnancy status. FAS occurs on a spectrum and even some limited exposure to high BrACs can cause harm.
Finally, the findings of this study will need to be applied to standard drink calculators used by consumers to estimate BrAC. In the context of driving, the differences of up to 25% higher BrAC levels may impact those consumers who think they are safe to drive when they are not. Similarly, alcohol prevention materials should include this information to inform consumers that the harms to health associated with higher BrACs may outweigh the benefits of saving some calories with diet mixers.
Highlights.
Lab study examined breath alcohol concentrations for alcohol with mixers
For both alcohol doses, diet (vs. sweetened) mixers resulted in higher breath alcohol contentration (BrAC) levels
Risks of higher BrAC levels may outweigh the benefits of reduced caloric intake
Acknowledgments
Role of Funding Source
This research was funded by NIH grants AA019795 and GM103436, awarded to CA Marczinski. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH. The NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors (Marczinski, Stamates & Maloney) contributed fully to the study design, data analysis, writing the manuscript, and the decision to submit the paper for publication. Data collection was completed by AL Stamates and SF Maloney.
Conflict of Interest
No conflict declared.
REFERENCES
- Marczinski CA, Stamates AL. Artificial sweeteners versus regular mixers increase breath alcohol concentrations in male and female social drinkers. Alcohol. Clin. Exp. Res. 2013;37:696–702. doi: 10.1111/acer.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, DeVries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CD, Hoyme HE, Tabachnick B, Seedat S. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013;133:502–512. doi: 10.1016/j.drugalcdep.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossheim ME, Thombs DL. Artificial sweeteners, caffeine, and alcohol intoxication in bar patrons. Alcohol. Clin. Exp. Res. 2011;35:1891–1896. doi: 10.1111/j.1530-0277.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- Schliep KC, Zarek SM, Schisterman EF, Wactawski-Wende J, Trevisan M, Sjaarda LA, Perkins NJ, Mumford SL. Alcohol intake, reproductive hormones, and menstrual cycle function: a prospective cohort study. Am. J. Clin. Nutr. 2015 doi: 10.3945/ajcn.114.102160. Article ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Barry KL, Fleming MF. Detection of problem drinkers: the alcohol use disorders identification test (AUDIT) South. Med. J. 1995;88:52–59. [PubMed] [Google Scholar]
- Stamates AL, Linden-Carmichael AN, Lau-Barraco C. Mixing alcohol with diet beverages: what are the risks? Alcohol. Clin. Exp. Res. 2015;39S1:212A. [Google Scholar]
- Vogel-Sprott M. Alcohol Tolerance and Social Drinking: Learning the Consequences. New York: Guilford; 1992. [Google Scholar]
- Wu KL, Chaikomin R, Doran D, Jones KL, Horowitz M, Rayner CK. Artificially sweetened versus regular mixers increase gastric emptying and alcohol absorption. Am. J. Med. 2006;119:802–804. doi: 10.1016/j.amjmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Zheng YL. Alcohol intake and associated risk of major cardiovascular outcomes in women compared with men: a systematic review and meta-analysis of prospective observational studies. BMC Public Health. 2015;15:773. doi: 10.1186/s12889-015-2081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]