Abstract
Objectives
The goal of this paper is to examine the correlation of catheter (cath) based and echocardiographic assessment of aortic stenosis in a community-based academic hospital setting, particularly in the degree that decision to refer for surgery is altered.
Background
Current guidelines discourage aortic stenosis (AS) evaluation by invasive pressure measurement if echocardiography (echo) is adequate, but several studies show sizable differences between echo and cardiac catheterization lab (CCL) measurements. We examine this correlation using high quality CCL techniques.
Methods
Sequential patients with suspected AS by echo (n=40) aged 61-94 underwent catheterization with pressure gradients via left ventricular pressure wire and ascending aorta catheter. The echos leading to the catheterization were independently reviewed by an expert panel to assess the quality of community-based readings.
Results
CCL changed assessment of severity of aortic valve area (AVA) by more than 0.3cm2 in 25% and 0.5cm2 in 8%. Values changed to over or under the surgical threshold of AVA <1cm2 in 30% of the patients. Pearson correlation of 0.35 between measurements of AVA by echo and CCL is lower than earlier studies, which often reported correlation values of 0.90 or greater. Echo expert reviews provided minimal improvement in discrepancies (Pearson correlation of 0.46), suggesting quality of initial interpretation was not the issue.
Conclusions
Cath-echo correlation of AS severity is lower in contemporaneous practice than previously assumed. This can alter the decision for aortic valve replacement. Sole reliance on echo-derived assessment of AS may at times be problematic.
Keywords: Aortic stenosis, Catheterization, Echocardiography, Aortic valve replacement
Introduction
Assessment of aortic stenosis severity, historically by hemodynamic methods in the cardiac catheterization lab (CCL), is increasingly determined by echocardiography (echo). In fact, current Valvular Heart Disease Guidelines discourage routine hemodynamic valvular measurements in the CCL before aortic valve replacement, making it a Class III indication since 2006 (1), unless there are discrepancies between the non-invasive and clinical assessment. There is even a published editorial suggesting that crossing an aortic valve for hemodynamic assessment is “unacceptable”(2). Many of these sentiments are based on comparative data decades old that show superb correlation between non-invasive and invasive assessments of aortic stenosis (3),(4),(5),(6),(7). However in more recent evaluations, such good correlation does not always occur (8),(9),(10),(11),(12). Using ACC Guideline recommendations for valve replacement with an aortic valve area (AVA) < 1.0cm2, one recent comparison of catheterization to echo measurements found 20% more patients had an AVA <1.0 cm2 in the echo group than in the CCL group, possibly avoiding aortic valve replacement after invasive hemodynamic assessment in some patients (9). Thus even small differences in valvular parameters can affect critical patient management decisions. Comparative studies have been criticized for sub-optimal catheterization measurement techniques such as using a catheter pullback rather than simultaneous LV-aorta pressure measurements or having the transvalvular catheter contribute to the loss of valvular cross-sectional area (8), (13). Some CCLuse an estimated Fick rather than a directly measured cardiac output, or report aortic valvular results only as peak-to-peak gradients, rather than the more comprehensive valve area, or use an estimated Fick rather than a directly measured cardiac output, which would impact any comparison to echo findings(14,15). In fact aortic valvular pressure measurements have evolved over the last few decades, from single catheter pullback to the multiple transducer recordings currently felt optimal (15). Echo measurements also have potential technical issues, such as the inability to find the peak Doppler gradient (16), errors due to pressure recovery and accurately measuring the left ventricular outflow tract (LVOT) (16), and the inability to measure the cardiac output independently, relying on calculations based on velocity signals. Of course any comparative study between these two methods might show differences due to temporal alterations in pre-and after-load and contractility effecting pressure gradients, although a calculated valve area would be theoretically more consistent.
We sought to evaluate this issue using contemporary high quality simultaneous measurement techniques; simultaneously measuring pressures on either side of the aortic valve using a high fidelity pressure wire in the left ventricle (17), (18) and a catheter in the ascending aorta beyond the area of ‘pressurerecovery’ (19). Aortic valve severity was then assessed using simultaneously derived cardiac outputs and compared to the non-invasively derived study that had led to the catheterization. We performed an independent, expert review of all the echocardiograms to minimize the influence of variability in community echo interpretation. The goal of this paper is to re-examine whether the catheter-echocardiogram correlation in aortic stenosis is replicable in a community-based academic hospital setting, particularly in the degree that the decision to refer patients to aortic valve replacement surgery is altered. We believe our results lead to concern in relying solely on non-invasively derived assessments of aortic stenosis.
Methods
Consecutive patients between 2009 and 2012 with both right and left heart catheterizations by two experienced interventional cardiologists who have used the pressure wire technique were identified by a search query of the CCL database. Cases were further reviewed to determine whether or not aortic stenosis was being specifically evaluated with this catheterization. For patients in whom a native aortic valve area (AVA) was reported, a search for pre-catheterization echocardiograms was initiated and clinical records reviewed. Patients with existing prosthetic valves, multiple valvular abnormalities or more than mild aortic insufficiency, or low ejection fractions (<30%) were excluded. Only patients with pre-catheterization echocardiograms within 90 days were included in the final analysis.
Catheterization was done by standard femoral access. The aortic valve was crossed retrograde, usually using a hydrophilic straight wire (Terumo) and then with a 6F catheter or at times directly with the pressure wire alone (Radi, Burlington, MA). When a catheter was advanced across the valve, it was a relatively straight tip such as an Amplatz or right Judkins. Pigtail catheters were not used. Ventriculography was not performed. When the aortic valve was crossed with a catheter, the pressure wire was then advanced into the distal catheter tip, and after equalization, advanced into the left ventricle. The catheter was then withdrawn to the distal part of the ascending aortic, in order to avoid any artifact due to pressure recovery (19), (20), (21). Care was taken during this maneuver to keep the pressure wire in the body of the left ventricle, avoiding apical entrapment or LVOT locations (19), (20). This pressure wire technique should also eliminate a trans-valvular catheter affecting measured pressures by its cross-sectional area (22),(13). Once a stable position of the pressure wire was achieved, simultaneous pressures were recorded while the cardiac output was assessed by thermo dilution. The aortic valve area was determined by computerized integration (Xper, Phillips, Miami FL). The Gorlin formula was used,
Since these were simultaneous pressures close to each other anatomically, no “phase shifting” of the pressure waveforms was required before hemodynamic analysis. The software program also generated a mean transvalvular gradient. Cases in which neither a catheter nor a wire could cross the valve were excluded from further analysis.
Our primary comparison is the reported ECHO-derived AVA to the catheterization-derived AVA as this is often a primary factor used in determination of subsequent therapy (9), especially aortic valve replacement (AVR), in symptomatic patients (1). Comparisons of mean aortic gradients were also made.
Echocardiographic assessment of aortic stenosis was performed at individual labs per their own protocols. All of the labs obtained the highest peak velocity across the aortic valve usually in multiple views. Peak and mean gradients were calculated from the flow envelope. In all cases the aortic valve area was calculated using the continuity equation, AVA = (AOT × VOT) ÷ VAV, where AOT= area of the LVOT, VOT= peak velocity in the outflow tract, AVA = area of the stenotic aortic valve, and VAV= maximum velocity across the aortic valve. Left ventricular ejection fraction was determined by visual estimation.
To minimize echocardiogram interpretation variability by cardiologists in the community, an expert review was carried out. In this review, two experienced cardiologists were asked to independently review all echocardiograms used in this study. Digital copies of the original images were used, and both readers were blinded to all patient data. Each assessed the imaging quality (“poor,” “adequate,” “good”), mean gradient, peak velocity and calculated an AVA. Issues that were felt to degrade quality were inadequate views, sub-optimal quality and too much angulation between the Doppler beam and long axis of LVOT/Aortic valve among others. Discrepant expert reads of AVA > 0.2 cm2 were resolved by consensus review of the echocardiogram by both readers.
Review of catheterization-derived values included manually examining each case as documented in the computerized CCL database to insure that the AVA was a thermo dilution-derived value in order to prevent the known inaccuracies of Fick cardiac outputs using estimated oxygen consumption(14). Thermo dilution-derived cardiac output and valve area values were used in all CCL assessments and calculations.
Statistical analysis
Correlation of echo and cath measurements was assessed by linear regression analysis and by variance component analysis. The Pearson correlation (r) derived from linear regression, assesses the rank-ordering of variable values whereas the intra-class correlation (ICC), derived from variance component analysis assesses the degree of agreement between variable values. The ICC is the appropriate statistic for assessing agreement between tests purporting to measure the same thing on the same scale of measurement. The ICC ranges between 0, indicating no agreement, and 1, indicating perfect agreement. Scatter plots of the paired echo-cath variables included the regression line as well as a 45 degree line of equality along which the paired variables would lie if there was perfect agreement between echo-cath measurements (as would the regression line). Bland-Altman plots were also constructed as complementary assessments of echo-cath measurements.
For all analyses, we included echocardiogram site as a random effect to account for variability between sites.
Results
The initial database query yielded 75 patients who had undergone right and left heart catheterization in the preceding three years (Table 1). After applying exclusion criteria as noted above, forty patients had complete invasive and non-invasive data for analysis. The patients were predominantly male (24, 65%) and ranged in age from 61 to 94 (Table 2). Pre-catheterization echocardiograms were obtained at five different hospital and practice laboratories in our community and read by 18 certified cardiologists. Two patients had trans-esophageal echos; all others had trans-thoracic echos. Echos were completed anywhere from 0 to 88 days prior to catheterization (median 24 days). No patient had an abrupt change in clinical course to mandate catheterization; all were being electively studied, usually to assess the suitability of valve replacement.
Table 1. Study criteria.
| Right and left heart catheterizations | 75 | |
| Aortic stenosis not study of interest | 7 | |
| EF <30% | 16 | |
| Priorvalvular replacement | 5 | |
| Echocardiography imaging unavailable | 4 | |
| Catheter unable to cross aortic valve | 1 | |
| Multiple valves/aortic insufficiency | 2 | |
| Final patients included in study | 40 |
Table 2. Patient Demographics.
| Age Range | 61-94 |
| Gender | |
| Male | 25 (63%) |
| Female | 15 (38%) |
| Hypertension | 16 (40%) |
| Diabetes | 19 (48%) |
| Coronary disease (>70% stenosis) | 21 (53%) |
| Hyperlipidemia | 14 (35%) |
| Tobacco abuse | 9 (23%) |
| # echos completed per site | |
| Site 1 | 18 |
| Site 2 | 16 |
| Site 3 | 3 |
| Site 4 | 2 |
| Site 5 | 1 |
Patients were monitored for post-procedure complications and none were noted to have any complications. No special evaluation was done to assess for sub-clinical neurologic events.
Valvular measurements
Pre-catheterization echocardiography mean values were: AVA of 0.85cm2, mean gradient of 39, and maximum velocity of 378. Invasive data values were: mean AVA of 0.89cm2 and a mean gradient of 44.5. Complete measurements are listed in Table 3. Aortic valve areas correlated poorly between the two techniques, with an R value of 0.35 and a weak intra-class correlation of 0.32 (Figure 1). “The average AVA difference between the two techniques was 0.25 cm2 (range 0–1.59).
Table 3. Echo Cath Measurement Details.
| CATH MEASUREMENTS (Thermodilution based) | ECHO MEASUREMENTS | Echo-Cath Delay (Days) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | Ao Press | LV Press | P mean | HR | SEP (MS) | CO | AVA | AVA | EF | LVOT | BP | HR | P mean | P max | V max | CO | |
| 1 | 157/72 | 183/12 | 34.7 | 78 | 321 | 5.20 | 0.8 | 1.10 | 60 | 2.1 | 168/61 | 65 | 25 | 41 | 322 | - | 79 |
| 2 | 113/73 | 168/6 | 44.3 | 90 | 298 | 4.57 | 0.58 | 0.70 | 65 | 2.2 | 118/85 | 80 | 24 | 38 | 310 | - | 67 |
| 3 | 96/54 | 218/17 | 82.6 | 99 | 269 | 6.39 | 0.52 | 0.59 | 60 | 2.2 | 143/63 | 102 | 75 | 107 | 517 | 5.9 | 2 |
| 4 | 164/78 | 222/7 | 52.7 | 80 | 319 | 6.08 | 0.74 | 0.99 | 65 | 2 | 128/25 | 71 | 41 | 71 | 421 | - | 11 |
| 5 | 144/79 | 204/17 | 59.5 | 81 | 326 | 6.40 | 0.8 | 0.49 | 45 | 2 | 132/32 | 76 | 51 | 73 | 426 | 3.6 | 0 |
| 6 | 99/58 | 147/22 | 40.8 | 60 | 326 | 6.43 | 0.85 | 0.64 | 65 | 1.8 | 123/62 | 67 | 54 | 86 | 463 | 5.6 | 0 |
| 7 | 148/56 | 158/7 | 28.2 | 84 | 208 | 6.72 | 1.63 | 1.50 | 30 | 1.7 | 99/36 | 68 | 10 | 22 | 233 | 5.5 | 0 |
| 8 | 109/33 | 147/1 | 39.7 | 52 | 382 | 5.09 | 0.92 | 0.81 | 55 | 2.2 | 96/52 | 57 | 43 | 74 | 424 | - | 46 |
| 9 | 115/72 | 146/22 | 31.3 | 83 | 282 | 4.17 | 0.72 | 1.00 | 35 | 2.4 | 148/65 | 65 | 39 | nc | 340 | - | 3 |
| 10 | 98/60 | 157/5 | 50.5 | 62 | 355 | 6.11 | 0.88 | 0.90 | 55 | 2 | 120/64 | 75 | 58 | 106 | 477 | 4.8 | 70 |
| 11 | 115/54 | 204/5 | 81.9 | 63 | 340 | 6.29 | 0.73 | 0.81 | 60 | 1.8 | 129/59 | 76 | 72 | 109 | 521 | - | 15 |
| 12 | 141/61 | 179/0 | 47.5 | 73 | 327 | 5.15 | 0.71 | 0.92 | 60 | 2.1 | 120/49 | 94 | 38 | 61 | 389 | - | 67 |
| 13 | 130/67 | 136/4 | 10.3 | 59 | 335 | 4.51 | 1.6 | 1.25 | 55 | 1.8 | 135/59 | 88 | n/a | n/a | n/a | - | 45 |
| 14 | 162/77 | 228/6 | 67.8 | 82 | 262 | 3.76 | 0.48 | 0.61 | 55 | 2.3 | 108/42 | 77 | 59 | 98 | 493 | 5.2 | 1 |
| 15 | 144/74 | 157/6 | 20.3 | 57 | 312 | 4.12 | 1.1 | 1.10 | 50 | n/a | 137/82 | 86 | 12 | 24 | 244 | 3.7 | 2 |
| 16 | 182/66 | 208/3 | 28.4 | 81 | 306 | 3.91 | 0.67 | 1.00 | 70 | 2.4 | 173/76 | 82 | 23 | 41 | 320 | - | 3 |
| 17 | 116/46 | 177/9 | 47.4 | 61 | 389 | 4.17 | 0.58 | 0.73 | 65 | 2.1 | 124/65 | n/a | 56 | 96 | 490 | - | 88 |
| 18 | 168/61 | 171/13 | 79.7 | 61 | 468 | 5.67 | 0.5 | 0.71 | 50 | na | 156/70 | 58 | 44 | 67 | 410 | - | 81 |
| 19 | 118/85 | 172/10 | 45.5 | 85 | 313 | 9.04 | 1.14 | 1.00 | 65 | 2.2 | 120/70 | n/a | 45 | 67 | 409 | - | 58 |
| 20 | 143/63 | 212/2 | 67 | 83 | 302 | 7.21 | 0.79 | 0.68 | 60 | 2.1 | 150/80 | 57 | 59 | 101 | 523 | 5.7 | 1 |
| 21 | 128/25 | 140/15 | 24.1 | 54 | 370 | 4.85 | 1.4 | 0.90 | 60 | 2.1 | 125/60 | 70 | 22 | 39 | 314 | 4.2 | 5 |
| 22 | 132/32 | 147/9 | 21.3 | 58 | 367 | 14.0 | 1.6 | 1.40 | 65 | 2.1 | 130/49 | 58 | 39 | 65 | 403 | 9.9 | 1 |
| 23 | 123/62 | 169/9 | 36.5 | 61 | 354 | 3.66 | 0.6 | 0.62 | 60 | 2 | 114/65 | 80 | 24 | 37 | 305 | - | 74 |
| 24 | 99/36 | 113/10 | 20.8 | 82 | 304 | 2.20 | 0.45 | 0.87 | 30 | 2.1 | 110/56 | 78 | 18 | 30 | 272 | 4.2 | 9 |
| 25 | 96/52 | 122/78 | 26 | 52 | 170 | 4.96 | 2.36 | 0.77 | 55 | 2.1 | 105/65 | 127 | 20 | 33 | 288 | 3.4 | 4 |
| 26 | 148/65 | 209/1 | 68.9 | 74 | 330 | 8.07 | 0.9 | 1.10 | 70 | 2 | 160/70 | 84 | 55 | 84 | 458 | - | 84 |
| 27 | 120/64 | 154/8 | 37.4 | 67 | 318 | 4.80 | 0.87 | 0.70 | 45 | 2 | 130/65 | 75 | 41 | 72 | 424 | - | 15 |
| 28 | 129/59 | 149/1 | 15.3 | 71 | 260 | 4.67 | 1.46 | 0.71 | 35 | 2 | 135/65 | 78 | 16 | 28 | 263 | 3.1 | 7 |
| 29 | 120/49 | 151/1 | 28.9 | 61 | 318 | 4.92 | 1.07 | 1.10 | n/a | 2 | 130/70 | 71 | n/a | n/a | n/a | - | 1 |
| 30 | 135/59 | 187/0 | 51 | 70 | 321 | 3.36 | 0.47 | 0.30 | 40 | 1.9 | 140/70 | 58 | 41 | 61 | 390 | 2.2 | 4 |
| 31 | 108/42 | 121/4 | 18.1 | 79 | 262 | 4.18 | 1.07 | 0.88 | 40 | 2 | 115/55 | 67 | 12 | 29 | 270 | 4.4 | 2 |
| 32 | 145/64 | 183/2 | 48.5 | 69 | 318 | 3.51 | 0.52 | 1.00 | 65 | 2 | 150/75 | 69 | 60 | 81 | 449 | 6.9 | 73 |
| 33 | 132/81 | 151/2 | 25.1 | 80 | 238 | 4.69 | 1.11 | 0.64 | 60 | 2 | 135/70 | 71 | 41 | 67 | 409 | 5.0 | 0 |
| 34 | 127/52 | 166/6 | 42 | 63 | 336 | 4.93 | 0.81 | 0.60 | 45 | 1.9 | 130/65 | 82 | 37 | 61 | n/a | - | 0 |
| 35 | 124/71 | 144/7 | 24.6 | 79 | 274 | 4.33 | 0.91 | 1.10 | 55 | 2 | 125/70 | 73 | 28 | 48 | 345 | - | 58 |
| 36 | 99/41 | 141/0 | 36.1 | 72 | 312 | 3.65 | 0.61 | 0.87 | 35 | 1.7 | 104/60 | 88 | 42 | 66 | 407 | - | 11 |
| 37 | 115/70 | 148/1 | 35.9 | 83 | 291 | 5.93 | 0.92 | 1.10 | 66 | 1.9 | 110/56 | 57 | 30 | 49 | 350 | - | 1 |
| 38 | 121/76 | 200/3 | 61 | 115 | 263 | 5.23 | 0.5 | 0.61 | 60 | 2 | 132/66 | 65 | 70 | 107 | 516 | - | 77 |
| 39 | 95/44 | 224/4 | 107 | 70 | 353 | 4.54 | 0.4 | 0.50 | 40 | 1.9 | 103/56 | 73 | n/a | n/a | n/a | - | 13 |
| 40 | 142/71 | 155/8 | 20.2 | 55 | 308 | 4.20 | 1.1 | 1.12 | 50 | 2 | n/a | 70 | 11 | 25 | 243 | - | 35 |
Figure 1. Cath Derived AVA (cm2) versus Echo Derived AVA (cm2).
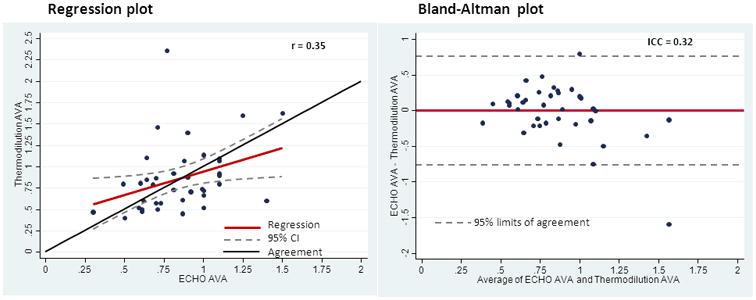
R = 0.35
ICC = 0.32
These measurements differed by 0.3cm2 in 25% of the patients and 0.5cm2 in 8% of the patients. Of note, catheterization changed the echo assessment of whether or not the valve area was over or under the surgical threshold of an AVA <1cm2 in 12 patients (30%), in five going from an echo valve area of <1.0cm2 to a catherization valve area >1.0cm2, and in seven going from an echo valve area of >_1.0cm2 to an catherization valve area of <1.0cm2. There was no obvious difference in clinical characteristics between the groups in which AVA differed by 0.3 cm2, (6/10 (60%) male, 5/10(50%) hypertension, 4/10 (40%) diabetes, 5/10 (50%) coronary disease, 4/10 (40%) hyperlipidemia, 2/10 (20%) tobacco abuse) compared to those whose AVA differed by less (19/30(63%) male, 11/30 (37%) hypertension, 14/30 (47%) diabetes, 16/30 (53%) coronary disease, 10/30 (33%) hyperlipidemia, 7/30(23%) tobacco abuse. (p=NS for all) Only two patients had both pressure wire and dual-lumen pigtail measurements made. In these the AVA agreed within 0.1 cm2 and the pressure measurements were virtually identical.
The mean transvalvular pressure comparison between echo and catheterization values demonstrated the best correlation with an R value of 0.83 (Figure 2) and a strong ICC value of 0.83. Cardiac outputs were reported in 17 echo patients and correlated with those done by catheterization with an r value of 0.74. The variance of catheterization thermo dilution measurements was 0.00601.
Figure 2. Cath Derived Mean Transvalvular Pressure (mmHg) versus Echo Derived Mean Transvalvular Pressure (mmHg).
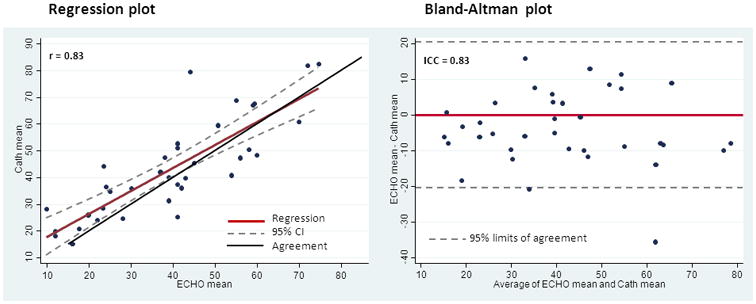
R = 0.83
ICC = 0.83
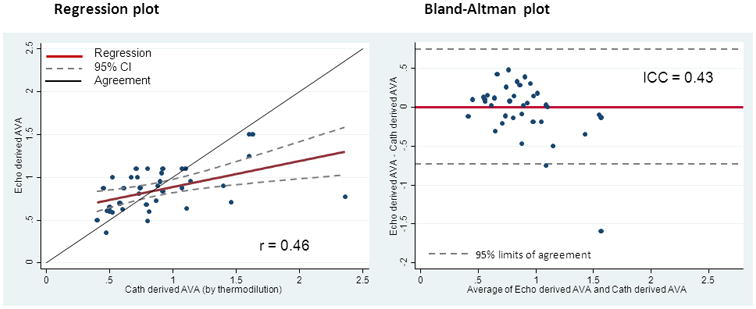
When the echocardiograms were independently reviewed by expert readers, there were disagreements with the community readings in 11 of the 40 echoes, although there were no disagreements between the blinded expert readers themselves. Table 4 shows the reasons for disagreement with community readings, which can be broadly categorized as those based on the quality of visualization and those based on reader interpretation. Not all the original echocardiograms were rated for quality in the original report but several were felt to be of poor quality by the expert readers. There were also several instances in which expert readers disagreed with the calculated AVA and suspected that there were differences between their outflow tract assessments compared with the original read. In three patients, the expert readers simply disagreed with the final AVA calculated by the original reader's report. In the remaining 29 cases, both expert readers and the original reader reports varied by less than 0.1cm2 and in no case did it change the categorization of “mild/ moderate/ severe” aortic stenosis using the ACC Guidelines criteria. The correlation of the congruent expert reader AVA determination with the CCL AVA was also poor, with a Pearson's of 0.46 and an ICC of 0.43. (Figure 3)
Table 4. Echo Reader Congruence.
| All Readers agree (community reader and both core lab readers) | 29/40 (72%) | |||
| Core lab readers disagree with community readers | 11/40(28%) | Reasons for disagreement | Inadequate views to assess valve | 4 |
| Outflow tract measurement faulty | 4 | |||
| Miscellaneous | 3 | |||
Figure 3. Comparison of Congruent Echocardiograms: Cath Derived AVA (cm2) versus Echo Derived AVA (cm2).
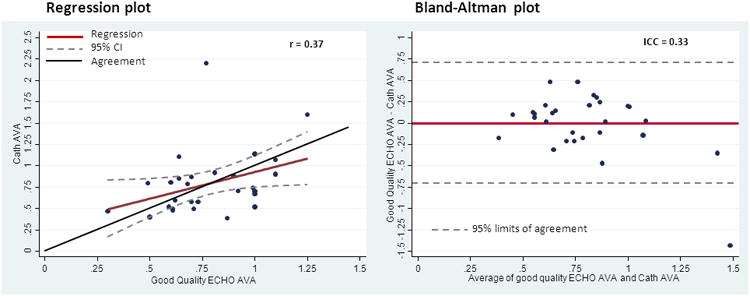
Subsequent analysis was undertaken to compare only the echocardiograms that were deemed to be of “good quality” (e.g. all readers were able to adequately visualize and calculate AVA) to the catheterization results (Figure 4). Correlational measures did not improve with a Pearson's calculation of 0.37 and an ICC of 0.33. Changes in valve replacement recommendations were however less frequent, 6/40 (15%) and many of the measured differences were minimal, with the calculated AVA differing by 0.1-0.2 cm2. An intensive review of the study interpretations, both echo and cath, were conducted for those “good quality” studies with AVA discrepancies >0.3 cm2. No errors in interpretation were uncovered, and it was felt that the reported values were the appropriate ones.
Figure 4. Comparison of Congruent and Good Quality Echocardiograms: Cath Derived AVA (cm2) versus Echo Derived AVA (cm2).
AVA calculations in the CCL are dependent on accurate measurement of cardiac output. Table 5 illustrates the changes in AVA if the cardiac output changed by 0.5L/min in either direction.
Table 5. AVA changes with CO +/- 0.5 L/min.
| Pt | Therm AVA | AVA CO +0.5 | AVA CO - 0.5 | Therm CO |
|---|---|---|---|---|
|
| ||||
| 1 | 0.80 | 0.87 | 0.72 | 5.20 |
| 2 | 0.58 | 0.64 | 0.51 | 4.57 |
| 3 | 0.52 | 0.56 | 0.48 | 6.39 |
| 4 | 0.74 | 0.80 | 0.68 | 6.08 |
| 5 | 0.80 | 0.84 | 0.76 | 6.40 |
| 6 | 0.85 | 0.91 | 0.79 | 6.43 |
| 7 | 1.63 | 1.76 | 1.51 | 6.72 |
| 8 | 0.92 | 1.01 | 0.83 | 5.09 |
| 9 | 0.72 | 0.81 | 0.63 | 4.17 |
| 10 | 0.88 | 0.95 | 0.81 | 6.11 |
| 11 | 0.73 | 0.79 | 0.67 | 6.29 |
| 12 | 0.71 | 0.78 | 0.64 | 5.15 |
| 13 | 1.60 | 1.78 | 1.43 | 4.51 |
| 14 | 0.48 | 0.54 | 0.42 | 3.76 |
| 15 | 1.10 | 1.19 | 1.02 | 4.12 |
| 16 | 0.67 | 0.75 | 0.58 | 3.91 |
| 17 | 0.58 | 0.65 | 0.51 | 4.17 |
| 18 | 0.50 | 0.55 | 0.46 | 5.67 |
| 19 | 1.14 | 1.20 | 1.07 | 9.04 |
| 20 | 0.79 | 0.85 | 0.74 | 7.21 |
| 21 | 1.40 | 1.55 | 1.26 | 4.85 |
| 22 | 1.60 | 1.73 | 1.48 | 14.00 |
| 23 | 0.60 | 0.69 | 0.52 | 3.66 |
| 24 | 0.45 | 0.56 | 0.35 | 2.20 |
| 25 | 2.36 | 2.51 | 2.21 | 4.96 |
| 26 | 0.90 | 0.95 | 0.84 | 8.07 |
| 27 | 0.87 | 0.96 | 0.78 | 4.80 |
| 28 | 1.46 | 1.62 | 1.30 | 4.67 |
| 29 | 1.07 | 1.17 | 0.96 | 4.92 |
| 30 | 0.47 | 0.54 | 0.40 | 3.36 |
| 31 | 1.07 | 1.20 | 0.94 | 4.18 |
| 32 | 0.52 | 0.59 | 0.44 | 3.51 |
| 33 | 1.11 | 1.23 | 0.99 | 4.69 |
| 34 | 0.81 | 0.89 | 0.73 | 4.93 |
| 35 | 0.91 | 1.02 | 0.81 | 4.33 |
| 36 | 0.61 | 0.69 | 0.53 | 3.65 |
| 37 | 0.92 | 1.00 | 0.85 | 5.93 |
| 38 | 0.50 | 0.55 | 0.45 | 5.23 |
| 39 | 0.40 | 0.45 | 0.36 | 4.54 |
| 40 | 0.89 | 0.98 | 0.81 | 5.32 |
No patient had another catheterization to measure AVA within 6 months of the index procedure. We identified two patients who underwent a second echo procedure in this time frame, and in both cases the measured AVA was within 0.2 cm2 of the index measurement.
Including echocardiogram site as a random effect to account for variability between sites, variance components for site in all analyses were virtually zero, the largest was 0.006, indicating little, if any, effect of site on the results. Thus, all results listed above are from analyses ignoring site.
Discussion
This study shows that the correlation between non-invasive and invasive measurements of aortic stenosis is lower in contemporary clinical practice than previously reported. Early reports of this correlation were excellent, with correlation values close to 0.90(3),(4),(6),(7). Zoghbi et al reported a correlation value of 0.94 in 39 patients(3), and Oh et al reported a correlation value of 0.83 in 100 patients who underwent both studies(6). However our correlations were poorer, in fact the intra-class correlation was very weak, surprisingly low for techniques that purport to measure the same valvular parameters. Potential errors in CCL measurement and echo measurement, as outlined in the introduction, could contribute to this discrepancy. CCL sometimes report aortic stenosis results as peak-to-peak gradients rather than valve area, may use the relatively less accurate estimated Fick cardiac output (14) or use pullback or femoral pressure measurements that are felt to be suboptimal (13). Poorly calibrated or inaccurate transducer equipment may exist. Sources of variability in echo assessment of aortic valves include cardiac output calculation issues, confusion of mid-cavity versus aortic valvular gradients, the inability to find peak Doppler gradients, technical issues such as image quality and adequacy, and measurement of the LVOT(16). In our incongruent cases, these latter 2 problems were the most frequent. Of course any comparative study between these two methods might show differences due to temporal alterations in pre-and after-load and contractility effecting pressure gradients, although a calculated valve area would be theoretically more consistent. In the case with the largest echo/cath discrepancy, there appeared to be an over-estimate of the echo mean pressure gradient compared to direct measurement in the cath lab, as well as a possible error in LVOT measurement. An intensive review of the data failed to provide an explanation of other differences, as both the echo and cath data interpretations appeared appropriate.
Nonetheless we feel that it is possible that the invasively measured values are more accurate, as these use simultaneously recorded pressure and flow measurements, the essentials for what is ultimately a hydraulic calculation. This may be an advantage over echo measurements, where much is inferred from velocity measurements, without direct pressure or flow assessment.
Patients included in this evaluation underwent simultaneous pressure measurements using a pressure wire passed across the stenotic valve and a fluid-filled catheter in the ascending aorta. We believe our technique avoids most of the potential serious limitations of invasive measurements (13) and may represent the best ‘state of the art’ for such assessment in routine clinical practice. A dual-lumen pigtail avoids most of the pitfalls of trans-aortic pressure measurements as well and may be equivalent to our technique, although the small lumens are prone to waveform damping which can art factually elevate the transvalvular gradient (23). Another advantage of the pressure wire approach is that no catheter crosses the valve during pressure measurements which may increase the gradient (22) and the location of the aortic catheter may be adjusted to decrease the pressure recovery phenomenon (19), (20), (21). Thus our trans-aortic gradient measurement with a pressure wire in the left ventricle and an ascending aorta catheter is felt to be inferior only to “multitransducer micromanometer catheters”, (15) equipment rarely found in clinical laboratories.
No comparison was attempted between the peak velocity measured by echo as there is no direct correlate of this measurement routinely made in the CCL(4). The “peak-to-peak” gradient occasionally reported in CCL data is not a simultaneous measurement of pressures.
We reviewed the echocardiograms that led to the catheterization using independent, expert and blinded readers. This improved the correlation of AVA and mean pressures minimally, and still left considerable discrepancies. This suggests that the quality of aortic valve assessment in our echocardiographic community was not the main source of the differences observed. We do not have a definite explanation for the cause of the persistent variability in the congruent cases. We do believe that simultaneous measurement of pressures and cardiac output is an intrinsic advantage of the CCL approach and may explain some of the discrepancies.
While it may appear that the absolute differences between AVA, for instance, are not very large, it must be stressed that small differences in this measurement can lead to very different treatment strategies. The aortic valve area is one of the most-used criteria for aortic valve assessment (1)(9), and is one of the three criteria for grading AS according to the ACC guidelines. An AVA of 1.2 cm2 for instance will often result in ‘watchful waiting,’ while an AVA 0.3 cm2 less, 0.9 cm2 will often lead to a recommendation of surgical valve replacement. Thus for 30% of our patients the recommendation for or against aortic valve replacement would potentially be changed compared to the recommendation from echo only. We found differences of 0.3cm2 in 25% and 0.5cm2 in 3 of 40 (8%) patients. In each of these 3 latter cases, the echo AVA calculation was relatively low despite only moderately elevated pressure measurements, (Table 3) emphasizing the importance of LVOT measurement.
Concern has been expressed about the possibility of embolic phenomena after retrograde aortic valve crossing, based in part on a MRI study that showed a surprising high frequency (22%) of MRI defects after such crossing (24). However, this study was criticized for the techniques used (25) and was unusual in that the frequency of overt clinical stroke (3%) was considerably greater than that experienced in typical clinical practice. A subsequent study failed to confirm this high rate of MRI defects after retrograde crossing, with no clinical neurological events and only 1 patient (2%) having an asymptomatic MRI defect (26).
But it must be remembered that the rate of clinically evident neurologic, as well as systemic, complications, could be larger after unnecessary aortic valve replacement (or not) if AVA was miscalculated (27). Cardiac surgery itself has a finite risk of causing procedural MRI defects(28) and almost one-half of patients develop MRI defects after valve or bypass surgery (29),(30). One could argue that the improved diagnostic ability that valve crossing infers more than compensates for the risk of avoiding aortic valve surgery if not needed, or not recommending surgery if in fact the valve is critically stenosed.
Although non-invasive aortic valve measurements may allow avoidance of some invasive procedures, they have no intrinsic quantitative advantage over direct pressure measurement and it is important to note that non-invasive studies became accepted when it was shown that they correlated very well with invasive measurements. The current study suggests that this correlation may well be less today in community practice. Given the sizable discrepancies we found, and the number of patients whose surgery/medical management decision was altered, we consider it worthwhile to continue to make invasive measurements to assess aortic stenosis, the ACC guidelines notwithstanding.
The dangers of echo underestimation of AVA due to lack of pressure recovery adjustment has been described, as the true hemodynamic load on the left ventricle is best represented by the recovered pressure, as measured hemodynamically in the CCL (21). However we also found that echo measurements may overestimate AVA in a substantial number of cases. Whether this represents an inability to capture the peak velocity gradient by echo (16) is unknown. Finally, the accurate measurement of aortic stenosis may become more important as high-risk patients are being considered for transcutaneous aortic valve replacement (TAVR)(14).
Limitations
Our study was significantly limited by being a single center study and by using the echocardiograms done in the community that led to the catheterization for comparison. We did not have comparison pressure measurements for most patients using dual-lumen pigtails, for instance, a commonly used alternative technique. The catheterization measurements were not repeated on any patient, and repeat echo in only two of the patients, so any inherent lack of reproducibility of these techniques were limitations. In these two patients the reproducibility appeared to be acceptable. There was often a delay between the echo and CCL studies, and temporal changes in hemodynamic status, as evidenced in Table 3, are possible. It would have been ideal to have the echocardiograms done in closer temporal fashion, although our goal was to compare invasive measurements to current community practice non-invasive measurements. We tried to minimize this issue through the use of the independent, expert readings. The difficulties in measuring gradients and valve area with echocardiography were not addressed other than the review by an expert panel, and remain a limitation. There are subgroups of aortic stenosis such as “low gradient, low output”. We did not attempt to stratify our small group by these criteria, since our main concern was comparing the measured pressure and valve areas by the two techniques. Finally the sample size is relatively small.
Conclusion
The assessment of aortic stenosis with invasive measurements often differs from that obtained by non-invasive techniques. Given the critical importance of these measurements for treatment decisions, especially surgical valve replacement, which changed in 30% of our patients, we think the concerns regarding the invasive assessment of aortic stenosis should be reconsidered.
Evolution of CCL technique, including usage of a thin pressure wire for simultaneous pressure measurements, may provide greater benefits with lower risk. Although only conjecture with such a small study, this approach points out the potential differences in assessing the severity of aortic stenosis and may offer a lower risk procedure to reassess the grading of aortic stenosis. This may be especially important in the case of potential discrepancy of clinical and echocardiography assessment or when other potential causes can not be ruled out as the potential source of patients' symptoms.
Abbreviations Used
- AS
aortic stenosis
- AVA
aortic valve area
- AVR
aortic valve replacement
- Cath
catheterization
- CCL
cardiac catheterization laboratory
- Echo
echocardiography
- ICC
intra-class correlation
- LVOT
left ventricular outflow tract
- TAVR
transcutaneous aortic valve replacement
Bibliography
- 1.Bonow R, Carabello B, Chatterjee K, et al. ACC/AHA 2006 practice guidelines for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease) J Am Coll Cardiol. 2006;48:598–675. [Google Scholar]
- 2.Chambers J, Bach D, Dumesnil J, et al. Editorial: Crossing the Aortic Valve in Severe Aortic Stenosis: No Longer Acceptable? The Journal of Heart Valve Disease. 2004;12:344–346. [PubMed] [Google Scholar]
- 3.Zoghbi WA, Farmer KL, Soto JG, Nelson JG, Quinones MA. Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation. 1986;73:452–9. doi: 10.1161/01.cir.73.3.452. [DOI] [PubMed] [Google Scholar]
- 4.Currie PJ, Seward JB, Reeder GS, et al. Continuous-wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation. 1985;71:1162–9. doi: 10.1161/01.cir.71.6.1162. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe WM, Roche AH, Coverdale HA, McAlister HF, Ormiston JA, Greene ER. Clinical evaluation versus Doppler echocardiography in the quantitative assessment of valvular heart disease. Circulation. 1988;78:267–75. doi: 10.1161/01.cir.78.2.267. [DOI] [PubMed] [Google Scholar]
- 6.Oh JK, Taliercio CP, Holmes DR, Jr, et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol. 1988;11:1227–34. doi: 10.1016/0735-1097(88)90286-0. [DOI] [PubMed] [Google Scholar]
- 7.Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation. 1985;72:810–8. doi: 10.1161/01.cir.72.4.810. [DOI] [PubMed] [Google Scholar]
- 8.Sakthi C, Yee H, Kotlewski A. Overestimation of aortic valve gradient measured by Doppler echocardiography in patients with aortic stenosis. Catheter Cardiovasc Interv. 2005;65:176–9. doi: 10.1002/ccd.20324. [DOI] [PubMed] [Google Scholar]
- 9.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–8. doi: 10.1136/hrt.2009.181982. [DOI] [PubMed] [Google Scholar]
- 10.Fischer JL, Haberer T, Dickson D, Henselmann L. Comparison of Doppler echocardiographic methods with heart catheterisation in assessing aortic valve area in 100 patients with aortic stenosis. Br Heart J. 1995;73:293–8. doi: 10.1136/hrt.73.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burwash J, Dickson A, Teskey R, Tam J, KL C. Aortic valve area discrepancy by Gorlin equation and Doppler echocardiography continuity equation: relationship to flow in patients with valvular aortic stenosis. Can J Cardiol. 2000;8:985–92. [PubMed] [Google Scholar]
- 12.Baumgartner H, Stefenelli T, Niederberger J, Schima H, Maurer G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–61. doi: 10.1016/s0735-1097(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 13.Turi ZG. Whom Do You Trust? Misguided Faith in the Catheter- or Doppler-Derived Aortic Valve Gradient. Catheterization and Cardiovascular Interventions. 2005;65:180–182. doi: 10.1002/ccd.20386. [DOI] [PubMed] [Google Scholar]
- 14.Gertz ZM, Raina A, O'Donnell W, et al. Comparison of invasive and noninvasive assessment of aortic stenosis severity in the elderly. Circ Cardiovasc Interv. 2012;5:406–14. doi: 10.1161/CIRCINTERVENTIONS.111.967836. [DOI] [PubMed] [Google Scholar]
- 15.Kern MJ. The Cardiac Catheterization Handbook. 5th. Philadelphia, PA: Saunders; 2011. p. 79. [Google Scholar]
- 16.Smith MD, Kwan OL, DeMaria AN. Value and limitations of continuous-wave Doppler echocardiography in estimating severity of valvular stenosis. JAMA. 1986;255:3145–51. [PubMed] [Google Scholar]
- 17.Parham W, Shafei AE, Rajjoub H, Ziaee A, Kern MJ. Retrograde Left Ventricular Hemodynamic Assessment Across Bileaflet Prosthetic Aortic Valves: The Use of a High-Fidelity Pressure Sensor Angioplasty Guidewire. Catheterization and Cardiovascular Interventions. 2003;59:509–513. doi: 10.1002/ccd.10596. [DOI] [PubMed] [Google Scholar]
- 18.Doorey AJ, Gakhal M, Pasquale MJ. Utilization of a pressure sensor guidewire to measure bileaflet mechanical valve gradients: hemodynamic and echocardiographic sequelae. Catheter Cardiovasc Interv. 2006;67:535–40. doi: 10.1002/ccd.20675. [DOI] [PubMed] [Google Scholar]
- 19.Schobel WA, Voelker W, Haase KK, Karsch KR. Extent, determinants and clinical importance of pressure recovery in patients with aortic valve stenosis. Eur Heart J. 1999;20:1355–63. doi: 10.1053/euhj.1998.1479. [DOI] [PubMed] [Google Scholar]
- 20.Assey ME, Zile MR, Usher BW, Karavan MP, Carabello BA. Effect of catheter positioning on the variability of measured gradient in aortic stenosis. Cathet Cardiovasc Diagn. 1993;30:287–92. doi: 10.1002/ccd.1810300405. [DOI] [PubMed] [Google Scholar]
- 21.Bahlmann E, Cramariuc D, Gerdts E, et al. Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: a SEAS substudy. JACC Cardiovasc Imaging. 2010;3:555–62. doi: 10.1016/j.jcmg.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Adele C, Vaitkus PT, Tischler MD. Evaluation of the significance of a transvalvular catheter on aortic valve gradient in aortic stenosis: a direct hemodynamic and Doppler echocardiographic study. Am J Cardiol. 1997;79:513–6. doi: 10.1016/s0002-9149(96)00799-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura RA, Carabello B. Hemodynamics in the Cardiac Catheterization Laboratory of the 21st Century. Circulation. 2012;125:2138–2150. doi: 10.1161/CIRCULATIONAHA.111.060319. [DOI] [PubMed] [Google Scholar]
- 24.Omran H, Schmidt H, Hackenbroch M, et al. Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet. 2003;361:1241–6. doi: 10.1016/S0140-6736(03)12978-9. [DOI] [PubMed] [Google Scholar]
- 25.Meine TJ, Harrison JK. Should we cross the valve: The risk of retrograde catheterization of the left ventricle in patients with aortic stenosis. American Heart Journal. 2004;148:41–42. doi: 10.1016/j.ahj.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Hamon M, Gomes S, Oppenheim C, et al. Cerebral microembolism during cardiac catheterization and risk of acute brain injury: a prospective diffusion-weighted magnetic resonance imaging study. Stroke. 2006;37:2035–8. doi: 10.1161/01.STR.0000231641.55843.49. [DOI] [PubMed] [Google Scholar]
- 27.Jilaihawi H, Chakravarty T, Weiss RE, Fontana GP, Forrester J, Makkar RR. Meta-analysis of complications in aortic valve replacement: comparison of Medtronic-Corevalve, Edwards-Sapien and surgical aortic valve replacement in 8,536 patients. Catheter Cardiovasc Interv. 2012;80:128–38. doi: 10.1002/ccd.23368. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz N, Schoenburg M, Mollmann H, et al. Cognitive decline and ischemic microlesions after coronary catheterization. A comparison to coronary artery bypass grafting. Am Heart J. 2011;162:756–63. doi: 10.1016/j.ahj.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129:2253–61. doi: 10.1161/CIRCULATIONAHA.113.005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knipp S, Matatko N, Wilhelm H, et al. Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008:872–879. doi: 10.1016/j.athoracsur.2007.10.083. [DOI] [PubMed] [Google Scholar]


