Abstract
Nonalcoholic fatty liver disease (NAFLD) represents a rapidly growing cause of chronic liver disease in the United States and is associated with significant morbidity and mortality, including progression to liver cirrhosis and hepatocellular carcinoma. NAFLD comprises a spectrum of liver conditions, ranging from simple steatosis to steatosis with inflammation (steatohepatitis) and progressive fibrosis. Weight loss represents a first line therapeutic modality for the management of NAFLD. Herein, we review the evidence base for medical, surgical, and endoscopic approaches to weight loss and their potential impact on the natural history of NAFLD.
Keywords: Fatty liver, Nonalcoholic steatohepatitis, Weight loss, Exercise, Pharmacotherapy, Endoscopic therapy, Bariatric surgery
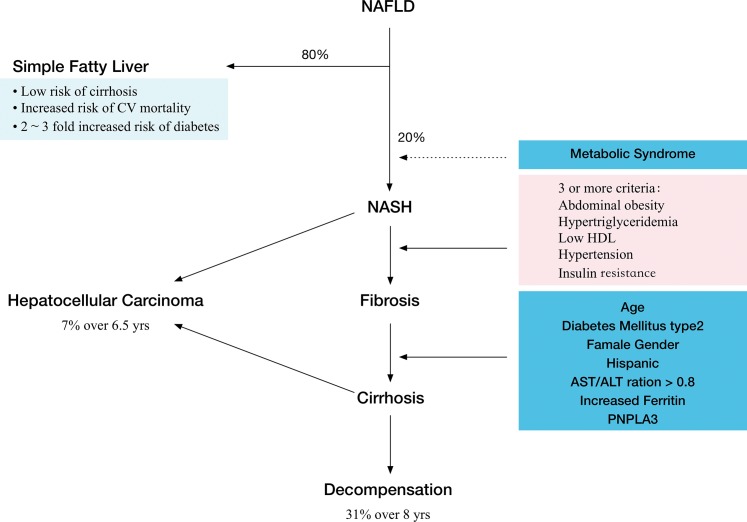
Spectrum of nonalcoholic fatty liver disease (NAFLD)
Over the past few decades we have seen a steep increase in the rates of obesity and its associated conditions, such as diabetes and metabolic syndrome, as more countries adopt the sedentary Western lifestyle and high caloric diet.
Epidemiological and research data have clearly established a link among obesity, diabetes, and NAFLD.1 The overall prevalence of NAFLD varies between 20% and 50% in Western countries, and it now represents the most common type of chronic liver disease.2,3 Fatty liver and NAFLD occur in all age groups, and its prevalence increases with increases in body weight. Fatty liver is found in 10–15% of normal weight individuals and 70% of obese subjects.4 The location of excess fat is paramount, as increased visceral fat increases the risk of hepatic steatosis both in lean and overweight individuals.5
NAFLD comprises a spectrum of liver conditions, ranging from simple steatosis to steatosis with inflammation and fibrosis (nonalcoholic steatohepatitis (NASH), liver cirrhosis, and liver cancer).6,7 These may also represent consecutive stages in disease progression. The diagnosis of NAFLD requires the presence of fat in hepatocytes, usually stored as triglyceride, and exclusion of other causes of hepatic steatosis, such as alcohol use, hepatitis C, and medication use, among others. A liver biopsy is usually needed to determine if the patient has NASH or fatty liver without inflammation.8
Retrospective and limited prospective data suggest that fatty liver without fibrosis and inflammation is unlikely to progress to liver cirrhosis. Presently, there is insufficient information available regarding the natural history of NASH, although it is generally accepted that these patients are at higher risk for developing advanced liver disease compared to patients with simple hepatic steatosis.4,6,9 This is based mostly on observational and retrospective studies. However, even “simple” fatty liver is not so benign: experimental and epidemiological studies have shown that hepatic steatosis significantly increases risk for developing type 2 diabetes6 and is associated with increased mortality from cardiovascular disease, cancer, and liver disease compared with age and gender-matched populations within the same county.
The levels of serum alanine aminotransferase (ALT) are used clinically to detect NAFLD. Accepting an ALT value of >40 U/L as a cut-off diagnosed steatosis with a sensitivity and specificity of 45% and 100%, respectively.10,11 A significant proportion of patients with biopsy-proven NASH have normal ALT levels.12 Ultrasound (US) is inexpensive, noninvasive, and widely available but has lower specificity for detecting mild steatosis. Newer modalities, such as magnetic resonance spectroscopy (MRS), are becoming more available and have proven to be more sensitive in quantifying liver fat,13 but their use is limited to academic centers at present. Several formulas that use clinical parameters have been developed to predict the presence of hepatic steatosis, such as the Fatty Liver Index,14 as the presence of liver fat can increase the risk of cardiovascular disease.
Liver biopsy remains the gold standard to distinguish NAFLD from NASH.15 However, liver biopsy can lead to serious complications and is limited by sampling variability.16 It should be reserved for patients with US-proven fatty liver who have risk factors for NASH or elevated liver enzymes without clear diagnosis.17 Features of fatty liver disease are scored using the system devised by the NASH Clinical Research Network (NASH CRN). The NAFLD Activity Score (NAS) is used as a composite measure of injury. It includes assessment of steatosis, ballooning degeneration, and lobular inflammation.18 Newer imaging modalities, such as transient elastography, can be used to predict the presence of liver fibrosis. A recent meta-analysis showed a pooled area under the curve of 0.94 for advanced fibrosis.19 Regarding fibrosis prediction, the only validated model based on clinical data is the NAFLD Fibrosis Score; it can adequately exclude advanced fibrosis in most patients and avoid the need for liver biopsy.20
Pathophysiology and natural history
The hallmark histologic feature of NAFLD is the accumulation of fat in the form of triglycerides in hepatocytes. Most of the triglycerides are re-esterified fatty acids that come from adipose tissue lipolysis into the liver. Adipose tissue insulin resistance is present in the majority of patients with NAFLD, whether they are obese or not,21,22 and adipose tissue lipolysis provides approximately 60% of the fatty acids used for hepatic triglyceride synthesis. The rest of the hepatic fatty acids come from de novo lipogenesis within the liver (25%) and dietary intake (15%).23
Hepatic lipid content is regulated by balancing hepatic lipid uptake, synthesis, oxidation, and export. Triglycerides are exported from the liver as very low-density lipoprotein (VLDL). Besides beta-oxidation in the mitochondria, lipid export is the only way to reduce hepatic lipid content. Excess hepatic fat leads to increased VLDL secretion and some of the serum lipid abnormalities noted in metabolic syndrome and NAFLD, including hypertriglyceridemia, decreased HDL, and higher low-density lipoprotein (LDL).24 However, the increased export of triglycerides as VLDL is unable to compensate for the increase in intrahepatic triglycerides. The high serum glucose and insulin associated with insulin resistance further perturb liver lipid metabolism by increasing the activity of carbohydrate response element-binding protein (ChREBP) and sterol regulatory-element binding protein 1c (SREBP-1c), the master regulator of hepatic de novo lipogenesis.25 Net lipid accumulation, specifically buildup of the triglyceride precursors diacylglycerol, results in activation of a serine kinase cascade. This, in turn, inhibits insulin signaling, leading to insulin resistance in the liver.26
Insulin resistance has a strong association with both hepatic steatosis and NASH.22 The pathogenesis of NASH is frequently described by the “two-hit hypothesis”: the first hit is the accumulation of fat in the liver and dysregulation of insulin metabolism, and the second hit, the progression from steatosis to steatohepatitis, is due to various inflammatory insults.27 At present, the precise factors driving inflammation remain unclear, with various metabolites, cytokines, inflammatory cells, and dysregulated processes, such as oxidative stress and autophagy, being implicated.28 Among clinical associations, diabetes and metabolic syndrome, advanced age, Hispanic ethnicity, female sex, and obesity are all related to more aggressive liver histology but without a clear distinction of cause and effect.29 Based on observational studies, it is assumed that simple steatosis will rarely progress to steatohepatitis and more aggressive liver disease.30 Patients with simple fatty liver on initial biopsy have a low chance of dying from liver disease. However, compared to the general population, patients with NAFLD have a significantly higher all-cause mortality,19,31,32 increased risk of developing diabetes,33,34 and higher incidence of cardiovascular disease and cancer.35,36
Patients with steatohepatitis may progress to liver cirrhosis and develop hepatocellular carcinoma. Roughly 25% of NASH patients will develop fibrosis and liver cirrhosis, and about 10% will develop end-stage liver disease (Fig. 1). Cirrhosis secondary to NASH is projected to become the most common indication for liver transplantation in next 20–30 years.9
Fig. 1. Natural history of NAFLD.
The effect of weight loss and treatment options
The complex pathophysiology of NAFLD and NASH has led to a number of pharmacologic treatment options targeting various mechanisms, but none of them are universally accepted. As NAFLD and the other components of metabolic syndrome are tied to obesity, weight loss constitutes the primary therapeutic goal (Table 1).
Table 1. Evidence summary for weight loss interventions for nonalcoholic fatty liver disease (NAFLD).
| Intervention | Author, year, country of origin |
Study design/controls | N cases/controls length of follow-up |
Liver–related outcomes | Results |
|---|---|---|---|---|---|
| Diet/exercise | Palmer38 1990, U.S. Diet/Wt loss |
Retrospective case control | 39 NAFLD/11 with other liver disease | ALT; AST | For every 1% of body weight lost, ALT improved by 8.1%; in pts who lost >10% of body weight, ALT normalized in 12/13 pts. |
| Ueno39 1997, Japan Diet/exercise |
Prospective case control | 15 NAFLD intervention/10 NAFLD no intervention; 3 mos |
ALT; AST; BMI | BMI: 31±5 (0); 28±4 (3 mos); p=0.05; ALT: 83±46 (0); 27±4 (3 mos); p=0.001; AST: 66±30 (0); 27±5 (3 mos); p=0.001. |
|
| Lewis40 2006, Australia Optifast VLCD |
Prospective cohort | 18 cases; 6 wks |
1H-MRS liver size | 43% reduction in mean liver fat after 6 wks (p=0.02); median EWL was 15%. |
|
| Shah41 2009, U.S. Diet (30% F; 20% P; 50% CH) or diet/exercise | Prospective trial | 9 diet/9 diet + exercise; 6 mos |
1H-MRS liver | 50% improved liver fat after 6 months; no diff if diet only or diet/exercise. | |
| Lazo42 2010, U.S. 1,200–1,500 kcal/d exercise |
Prospective cohort (Look Ahead Trial) | 96 cases; 12 mos |
1H-MRS liver | Liver fat: from −50.8% (0) vs. −22.8% (12 mos); p=0.04. | |
| Perez-Guisado49 2011, Spain Medi diet |
Prospective cohort | 14 cases: 4 mos |
ALT, AST, BMI | ALT: 72±4 (0); 37±6 (4 months); AST: 48±3 (0); 30±1 (4 months); BMI: 37±1 (0); 32±1 (4 months). |
|
| Shai50 2008, Israel, Germany, U.S. Medi diet; low-fat; low-CH |
Prospective RCT | 109 (medi diet); 104 (low-fat); 109 (low-CH); 24 mos |
ALT; weight (kg) | ALT: lower by 3.4±11 in medi group and 2.6±8.6 in low-fat group at 24 months (p<0.05). Weight: lower by −2.9±4.2 kg in low-fat diet; −4.4±6.0 kg in Medi group; and −4.7±6.5 kg in low-CH group. |
|
| Promrat51 2010, U.S. multicenter Diet/exercise |
RCT | 20 cases/10 controls (education); 12 months | NAS (liver biopsy); BMI |
BMI: 34±5 to 31±6; no change in controls (p=0.04); NAS: 4±1 to 2±1.5; controls 5±1 to 4±2, p=0.05. |
|
| Thoma45 2012, Diet/exercise interventions |
Meta-analysis | 23 studies: 11 diet only; 2 exercise only; 19 diet/exercise. |
Liver fat; AST/ALT; liver histology |
Weight loss of 4–14% led to decrease in liver fat of 35–81%. | |
| Medication | Harrison58 2009, U.S. Orlistat + Vit E/diet vs Vit E/diet (1,400 kcal/d) |
RCT | 23 cases Orlistat/Vit E/diet/18 controls diet/Vit E; 36 mos |
Liver histology | %Wt loss: 8.3 (cases) vs. 6 (controls); NS Steatosis and NAS decreased with wt loss independent of cases vs. controls. |
| Bariatric Surgery | Taitano68 2015, U.S. 92% RYGB; 8% LAPB |
Case series/none | 160 cases; 31±26 mos |
Liver biopsy (2nd biopsy done during unrelated 2nd surgery). | Steatohepatitis decreased from 26% to 3%; 27% new portal inflammation (p<0.001). Fibrosis decreased from 65% to 37% (p<0.001). De novo fibrosis in 21% after surgery. Worse fibrosis in 14% after surgery. |
| Rabl66 2012, Spain RYGB |
Prospective cohort | 26 cases; 16±3 mos |
Liver histology | NASH decreased from 96% to 15.3% (p<0.001). Fibrosis decreased from 31% to 19% (NS). |
|
| Cazzo69 2014, Brazil RYGB |
Prospective cohort | 63 cases; 12 mos |
Liver histology | NAS decreased from 1.1±1.3 to 0.1±1 (p=0.04). | |
| Dixon70 2004, Australia LAGB |
Retrospective case series | 36 cases: 25.6±10 mos |
Liver histology (2nd biopsy done during unrelated 2nd surgery or if NAS>2). | 23 pts had NASH before LAGB, only 4 had NASH after; fibrosis stage 3 improved in 9/10 pts; Wt loss of 34±17 kg. |
|
| Karcz71 2011, France SG |
Prospective cohort | 236 cases; 1, 3 yrs |
AST/ALT | ALT, (NASH pts): 42±25 (0) to 12±2 after 3 yrs, p<0.01. AST, (NASH pts): 28±14 to 17±2 after 3 yrs, p<0.01. BMI: 46±8 (0) to 31±5 (3 yrs), p<0.001. |
|
| Kral72 2004, Canada, U.S. BPD |
Retrospective cohort | 104 cases; 41±25 mos |
Liver biopsy (2nd biopsy done during unrelated 2nd surgery). | Steatosis: 1.6±1 (0) to 0.5±0.7, p<0.01. Fibrosis: 1.4±1.1 (0) to 1.56±1.2, p=0.05. Mean wt loss: 38±18 kg. |
|
| Keshishian73 2005, U.S. Duodenal switch |
Retrospective cohort | 78 cases; 6 to 36 mos |
Liver histology (2nd biopsy done during 2nd unrelated surgery) ALT; AST |
Mean AST (U/L) increased from 26 to 28 (p=0.02) at 6 mos; after 3 yrs down to 25. Mean ALT (U/L) increased from 32 to 38 (p<0.0001) at 6 mos; 3 yrs down to 31. Increased inflammation noted at 6 mos. |
|
| Mathurin74 2009, France | Prospective cohort | 381 cases; 1, 5 yrs |
Liver histology done per protocol at 0, 1, 5 yrs. | Increased fibrosis at 5 yrs: 0.3±0.6 to 0.4±0.6, p=0.001. NAS improved at 5 yrs: 2±1.3 to 1.1±1.3, p<0.001. |
|
| Burza77 2013, Sweden Bariatric surgery vs. usual care LABG (376 cases) VBG (1,369 cases) RYGB (265 cases) |
Prospective nonrandomized controlled trial | 1,775 cases; 1,795 controls. 2, 10 yrs. Controls: diet/exercise |
AST/ALT | AST and ALT decreased in cases at 2-yr proportional to wt loss, but not in controls. After 10 yrs, AST increased over baseline, ALT was lower than baseline in cases (p<0.001, surgery vs. control group). Wt gain was associated with increases in both AST and ALT after 2 and 10 yr follow-up. |
|
| Chavez-Tapia67 2010 | Meta-analysis (no RCTs found) |
21 studies: 15 prospective cohort; 5 retro cohort; 1 combined. |
Liver histology, weight loss |
Reduction in body weight most important positive effect of BS on NAFLD. Worsening in fibrosis noted in four studies. |
|
| Endoscopic devices | Lee80 2012, singapore intragastric balloon |
RCT (bioenterics balloon used, 6 mos of treatment) |
8 cases (IGB + diet)/10 controls (sham endo + diet) | Liver histology | NAS outcome, cases vs. controls: 2±0.8 vs. 4±2.3, p=0.03 |
| De Jonge82 2013, Netherlands Duodenal-jejunal bypass liner (DJBL) |
Prospective cohort 24 wks (DJBL out) 48 wks |
17 cases | AST ALT Wt (kg) |
AST: 35±4 (0) to 23±2 (24) to 34±3 (48), ns. ALT: 54±5 (0) to 28±2 (24) to 37±3 (48), p=0.01. Wt: 116±6 (0) to 103±6 (24) to 107±6 (48), p=0.01. |
|
| Popov79 2014, intragastric balloon |
Meta-analysis (bioenterics balloon, 6 mos of treatment, all trials) |
10 studies: 1 RCT, 2 pros case control; 7 cohorts |
ALT GGT BMI |
ALT, mean change: −9.9 U/L (95% CI: −13, −6.7). GGT, mean change: −10.4 U/L (95% CI: −13, −7.6). BMI, mean change: −5 kg/m2 (95% CI: −4, −5.8). |
Units used: AST/ALT (U/L); BMI (kg/m2); weight (kg).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; RYGB, Roux–en-Y gastric bypass; LAGB, laparoscopic adjustable gastric band; VBG, vertical band gastrectomy; BPD, biliopancreatic diversion; SG, sleeve gastrectomy; VLCD, very low calorie diet (800 kcal/day); F, fat; CH, carbohydrates; P, protein; mos, months; wks, weeks; yrs, years; wt, weight; Medi diet, Mediterranean diet; pts, patients.
NAFLD patients are inclined to consume more calories and simple carbohydrates than healthy controls.37 Studies have demonstrated the benefits of weight loss on hepatic fat and liver transaminases and prevention of incident NAFLD.38–42 At present, the American Association for the Study of Liver Diseases guidelines only recommend weight loss accomplished through lifestyle changes43 for both NAFLD and NASH.
Lifestyle/dietary therapy
Counseling in the office on lifestyle changes and outcomes of NAFLD is a good starting point, but referral to a structured intensive nutritional program may be a more effective approach. A recent study randomized patients in the community to one of two groups: an intensive lifestyle intervention with weekly sessions with dieticians over the course of 12 months and one counseling session by a clinician. Although neither group (77 patients in each arm) changed their exercise habits by the end of the study, the intervention group had significantly lower intrahepatic triglycerides, as measured by MRS (mean difference 4.5%, p<0.001), improved liver transaminases, and lower serum triglycerides and LDL cholesterol. The resolution of NAFLD was correlated with weight loss.44
A review by Thoma and colleagues summarized the effectiveness of physical exercise alone and physical exercise combined with dietary approaches to reduce intrahepatic fat.45 Energy restriction and weight reduction of 4–14% resulted in significant decreases in hepatic steatosis of 35–81%. The decrease in liver fat had the strongest correlation with degree of weight loss. Regular exercise may modestly reduce steatosis even without a change in weight.
The role of various macronutrients in the pathophysiology of NAFLD was recently reviewed in detail.46,47 Increased intake of ω-3 fatty acids and monounsaturated fatty acids and decreased intake of fructose were associated with reduced hepatic steatosis.
Various diets have been shown to improve components of metabolic syndrome and weight loss.48 The Mediterranean diet has probably the best evidence to support its use for reducing body weight and intrahepatic fat.49 The Spanish Mediterranean diet consists of high doses of virgin olive oil and ω-3 fatty acids from fish as the main source of fat, fish as the main source of protein, green vegetables/salads as the main source of carbohydrate, and moderate red wine intake. In a prospective study of 14 subjects with US-confirmed NAFLD associated with elevated liver enzymes (ALT >40 IU) and a mean initial body mass index (BMI) of 36 kg/m2, 12 weeks of the Mediterranean diet led to significant weight loss and improved hepatic steatosis and liver enzymes (average weight decreased from 109.79 kg to 95.86 kg, average ALT from 71.92 U/L to 37.07 U/L, and average aspartate aminotransferase (AST) from 47.71 U/L to 29.57 U/L). Complete fatty liver regression was observed in 21.4% of the patients, and an overall reduction of steatosis was found in 92.9% of the patients.
The largest study to date, by Shai et al., compared the Mediterranean diet to low-fat and low-carbohydrate diets and followed 322 subjects for 2 years. Weight loss was more significant with the Mediterranean and low-carbohydrate diets, and the glycemic index was improved with the Mediterranean diet. ALT levels were significantly reduced from baseline to 24 months in the Mediterranean diet and the low-carbohydrate groups (reductions of 3.4±11.0 and 2.6±8.6 units per liter, respectively; p<0.05 in comparison with baseline in both groups).50
Weight loss induced by dietary intervention can lead to improved histology for NASH. Thirty-one patients with biopsy-proven NASH were randomized to standard counseling vs. intensive intervention (1,000–1,500 kcal/d diet and 200 minutes of moderate aerobic activity weekly).51 The treatment group had an improved histological response after 48 weeks, with NAS reduction from 4.4 to 2.0 in the treatment group and NAS reduction of 4.9 to 3.5 in the standard counseling group (p=0.05). Intensive intervention was significantly correlated with weight loss, and patients with greater than 7% weight loss achieved the greatest decrease in inflammatory parameters.
Recently, a prospective study of 293 patients with histologically proven NASH who underwent a 12 month intensive lifestyle intervention showed that weight loss correlated with levels of improvement in histology. Patients who were able to achieve greater than 10% weight loss had the highest rates of NAS reduction and fibrosis regression.52
The biggest caveat of interventions that use dietary measures for weight loss is their lack of durability.53,54,55 It is unclear how weight regain will affect the natural history of NAFLD/NASH.
Studies have suggested that the severity of insulin resistance was correlated with the likelihood of progression from benign steatosis to NASH and the development of fibrosis. Therefore, therapies that improve insulin sensitivity or target other elements of the metabolic syndrome, such as dyslipidemia and hypertension, have been shown to reverse inflammation, steatosis, and ballooning degeneration and have been reviewed elsewhere.56,57
Pharmacotherapy for weight loss
There is limited data on the effect of weight loss medication on NAFLD. Orlistat was evaluated in several trials, but the results were inconclusive. Fifty patients with biopsy-proven NASH and BMI >27 kg/m2 were randomized to receive either Orlistat 120 mg tid + 1400 kcal/day diet + Vitamin E or 1400 kcal/d diet + Vitamin E.58 Both groups showed similar improvements in inflammation, ballooning, and steatosis. The Orlistat group lost a mean of 8.3% body weight compared to 6.0% in the diet plus vitamin E group (not significant). Orlistat did not provide any additional benefit.
Pentoxifylline, although not specifically used to target weight loss, was associated with a small amount of weight reduction in a trial evaluating its effect on NASH.59 At the end of the study, BMI change from baseline was −0.28 kg/m2 (±1.0) in the pentoxifylline group compared to 0.52 kg/m2 (±1.8) in the placebo group (p=0.052). Pentoxifylline treatment had a favorable effect on liver fibrosis and improved NAS score (p<0.001). The change in BMI was significantly associated with NAS improvement.
More recently, a daily injection of 1.8 mg of liraglutide, a glucagon like peptide 1 (GLP-1) analogue, was shown to significantly improve liver histology in patients with biopsy-proven NASH after 48 weeks of therapy. This was associated with reduced weight, BMI, and fasting glucose. More data from phase 3 trials are needed to validate the use of GLP-1 agonists as a therapeutic option for NASH, but initial data appear promising.60,61
Overall, data on the effect of weight loss for NAFLD are insufficient. A Cochrane review from 2011 could not find sufficient number of trials to perform meta-analysis for the effect of weight reduction on NAFLD, both for lifestyle or orlistat, and concluded that further randomized controlled trials with low risk for bias are needed.62 A recent prospective cohort study by Glass et al. demonstrated that weight loss greater than 10% total body weight in NASH patients was associated with a significantly higher rate of fibrosis regression (63.2 vs. 9.1%, p<0.001).
Bariatric surgery
To date, the most convincing evidence that sustained weight loss can cure or improve NAFLD and NASH comes from studies of patients who have undergone bariatric surgery. As the prevalence of obesity has increased over the past few decades, the number of bariatric procedures has grown exponentially, to over 220,000 procedures performed annually in the United States. As many as 90% of these patients with extreme obesity have NAFLD prior to surgery, but the incidence of serious liver disease is lower.63 Kleiner et al. evaluated 635 perioperative biopsies (median BMI 46) and found bridging fibrosis and cirrhosis in only 4.2% of liver biopsies,64 and 13.7% of liver biopsies had an NAS score greater than 5.
Presently, the most common bariatric procedures performed include Roux-en-Y gastric bypass, laparoscopic adjustable gastric band (LAGB), sleeve gastrectomy, vertical band gastrectomy, and biliopancreatic diversion (BPD), the last two performed only rarely today. Since 1995, more than 15 prospective studies have evaluated changes in liver histology after Roux-en-Y gastric bypass surgery.65,66 Time to follow-up liver biopsy varied between 10 to 21 months after surgery. Most studies reported improved hepatic steatosis, inflammation, and fibrosis. However, in a Cochrane review from 2010, four studies had noted worsening in fibrosis score over time, and two trials reported NASH global score deterioration.67
A recent paper by Taitano et al. followed 160 patients after bypass surgery.68 The time to repeat liver biopsy was 31±26 months (median 20 months), and the repeat biopsy was performed only if another abdominal surgery was needed. BMI decreased from 52±10 kg/m2 to 33±8 kg/m2. The authors reported improvements in all major NAS points: steatosis and lobular inflammation had resolved in 75%, portal inflammation in 49%, and fibrosis of any grade in 58%, without any deterioration in fibrosis scores. Compared to other studies, this study had a shorter follow-up to second biopsies, and they were coinciding with weight loss nadir. In another cohort of 63 patients with paired liver biopsies during and 12 months after surgery, resolution of fibrosis was achieved 1 year after Roux-en-Y bypass surgery.69
Weight loss induced by LAGB can improve liver histology in NAFLD. Thirty-six obese patients underwent paired liver biopsies. The first biopsy was performed at the time of LAGB placement, and the second biopsy was performed at a mean interval of 25.6±10 months after the procedure. Weight loss after LAGB surgery resulted in significant improvement in liver histology.70
In the literature review, we found no studies of follow-up biopsies for NAFLD in LSG. Karcz et al. reported on the effect of LSG on NASH, diagnosed at the time of surgery, and NASH-related comorbidities using clinical and biological data at 1 and 3 year follow-ups.71 A significant improvement of AST, ALT, triglyceride, and HDL levels was shown, but there was no histological data.
Two prospective cohort studies have evaluated the effect of biliopancreatic diversion procedures on NAFLD.72,73 In both studies, repeat biopsies were only performed if the patients needed reoperations for any reason. In the study by Keshishian et al., NAS scores were not reported, but the authors mentioned that improvements in inflammation were seen beyond 12 months after surgery.73 By 3 years, the degree of steatosis had decreased by 60%. Kral et al. compared repeat liver biopsies in 104 patients who had cirrhosis at the time of BPD and found most of them had improvements in inflammation and fibrosis in follow-up liver biopsies.72 However, three other patients developed de novo liver cirrhosis during follow-up. One patient with cirrhosis at the time of initial surgery regained all of the lost weight after a reversal of BPD and died of liver failure 40 months later.
The largest prospective cohort study followed 381 patients after BPD, LAGB, or gastric bypass and planned a full clinical assessment and repeat biopsies at 1 and 5 years after surgery.74 Biopsies were obtained from 362, 267, and 211 participants at baseline, 1 year, and 5 years respectively. This study reported major reductions in steatosis and ballooning degeneration; no change in inflammation; and a small increase in fibrosis score at both 1 and 5 years compared with the baseline biopsies. Presence of fibrosis on the index biopsy predicted fibrosis progression. There were 99 patients (27%) diagnosed with definite or probable NASH. In those patients, steatosis, ballooning, and NAS improved significantly after 5 years, but fibrosis and inflammation did not. Patients with progressing fibrosis had higher BMIs (around 45 kg/m2 at the time of the repeat biopsy), and they were more insulin resistant. Deterioration of fibrosis may have been related to a more severe natural history of NASH and lower benefits of bariatric surgery rather than to exacerbation related to the surgery. Studies of NASH patients have estimated that fibrosis will worsen by at least one stage in 37–53% of patients at 3–10 years.75,76
Long-term data form the Swedish Obese Subjects study showed that bariatric surgery was associated with lower ALT at 2 and 10 year follow-ups.77 AST levels were lower in the surgery group at the 2 year of follow-up but increased to higher than baseline levels at the 10 year follow-up. This prospective nonrandomized controlled trial included 3,570 obese subjects. The reason for the increase in AST after 10 years is unclear.
Most of the presented data came from observational studies. A Cochrane review examining the effect of bariatric surgery on NASH did not find any randomized controlled trial that fulfilled the inclusion criteria to make an assessment of benefit. Furthermore, they described eight studies with deterioration in fibrosis or overall histological score.67
Endoscopic weight loss methods
New minimally invasive endoscopic weight loss modalities are becoming more available.78 Intragastric balloons (IGB) have been shown to decrease liver enzymes and improve liver histology.79,80 Histological response to IGB therapy was evaluated in a single-blinded randomized controlled trial.80 The control arm received dietary intervention and sham endoscopy. Liver biopsies were performed in both cases and controls before balloon placement and within one month of balloon removal. At the end of treatment, the decrease in BMI was significantly greater in the IGB group than in the controls: 1.5 kg/m2 (0.36–3.37) vs. 0.8 kg/m2 (−0.74 to 1.33), p=0.0008. The median NAS was lower in IGB group than the sham group: 2±0.75 vs. 4±2.25, p=0.03. There was no difference in NAS prior to balloon insertion between the cases and the sham group: 5±1.00 vs. 5±2.25, p=0.52. IGBs have been used outside of the United States for more than 20 years and are expected to be approved soon in the US. IGBs can induce weight loss of about 10–12% and have low rates of serious adverse events. The most common adverse event associated with their use is persistent nausea and vomiting, leading to early removal of the balloon in 5–10% of cases.81
The duodenal-jejunal bypass liner improves biochemical parameters of NAFLD.82 As endoscopic devices become more available, their utility and safety in patients with NAFLD and NASH need to be further defined in randomized controlled trials.
Conclusions
In summary, NAFLD and its more severe form, NASH, are chronic diseases whose natural history in many cases may be stalled by appropriate interventions, including weight loss. Regression of fibrosis, a key disease end point, gives strong support for the AASLD weight loss guidelines. Weight loss of 10% is effective for the control of NASH and regression of fibrosis when follow-up is extended up to 5 years, regardless of whether weight was lost by medical management, bariatric surgery, or the new endoscopic minimally invasive weight loss devices, such as IGBs.
New medication targeting fibrosis development will have a strong impact on disease progression. However, these therapies should not be viewed as an isolated histological improvement but need to be assessed in their effect on metabolic syndrome in general. The close association between obesity, insulin resistance, and liver disease progression is a hallmark of NAFLD and NASH. Treatment of NAFLD requires a multidisciplinary approach that is directed to all aspects of metabolic syndrome. Weight loss represents a substantial part of this comprehensive approach.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BPD
biliopancreatic diversion
- BMI
body mass index
- ChREBP
carbohydrate response element-binding protein
- CRN
Clinical Research Network
- GLP-1
glucagon like peptide 1
- IGB
intragastric balloon
- LAGB
laparoscopic adjustable gastric band
- LDL
low-density lipoprotein
- MRS
magnetic resonance spectroscopy
- NAFLD
non-alcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- US
ultrasound
- VLDL
very low-density lipoprotein
References
- 1.Angulo P. Nonalcoholic fatty liver disease. Rev Gastroenterol Mex. 2005;70:52–56. [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Bedogni G, Miglioli L, Masutti F, Castiglione A, Crocè LS, Tiribelli C, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387–1391. doi: 10.1002/hep.21827. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 4.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 9.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 12.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 13.Kahl S, Straßburger K, Nowotny B, Livingstone R, Klüppelholz B, Keßel K, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. 2014;9:e94059. doi: 10.1371/journal.pone.0094059. doi: 10.1371/journal.pone.0094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859–871. doi: 10.1016/j.jhep.2013.05.044. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 16.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 17.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 20.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007–1019. doi: 10.1016/j.jhep.2012.11.021. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. doi: 10.1172/JCI200523621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725, e6. doi: 10.1053/j.gastro.2012.02.003. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 29.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 31.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 32.Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403–1411. doi: 10.1002/hep.23135. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T, Fukatsu M, Suzuki S, Wada T, Yoshida T, Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352–356. doi: 10.1111/j.1440-1746.2009.05998.x. doi: 10.1111/j.1440-1746.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- 34.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Targher G, Zoppini G, Day CP. Risk of all-cause and cardiovascular mortality in patients with chronic liver disease. Gut. 2011;60:1602–1603. doi: 10.1136/gut.2010.230656. author reply 1603–1604. doi: 10.1136/gut.2010.230656. [DOI] [PubMed] [Google Scholar]
- 36.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Capristo E, Miele L, Forgione A, Vero V, Farnetti S, Mingrone G, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH) Eur Rev Med Pharmacol Sci. 2005;9:265–268. [PubMed] [Google Scholar]
- 38.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–1413. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 39.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. doi: 10.1016/S0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 40.Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson CH, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701. doi: 10.1381/096089206777346682. doi: 10.1381/096089206777346682. [DOI] [PubMed] [Google Scholar]
- 41.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell LN, Wang J, Muralidharan S, Chalasani S, Fullenkamp AM, Wilson LA, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56:1311–1318. doi: 10.1002/hep.25805. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr. 2014;33:186–190. doi: 10.1016/j.clnu.2013.11.003. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18:91–112. doi: 10.1016/j.cld.2013.09.009. doi: 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Guisado J, Muñoz-Serrano A. The effect of the Spanish Ketogenic Mediterranean Diet on nonalcoholic fatty liver disease: a pilot study. J Med Food. 2011;14:677–680. doi: 10.1089/jmf.2011.0075. doi: 10.1089/jmf.2011.0075. [DOI] [PubMed] [Google Scholar]
- 50.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 51.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015(Apr 10) doi: 10.1053/j.gastro.2015.04.005. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Diabets prevention program research group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:677–686. doi: 10.1016/S0140-6736(09)61457-4. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson DA, Anton SD, Han H, Champagne CM, Allen R, LeBlanc E, et al. Adherence is a multi-dimensional construct in the POUNDS LOST trial. J Behav Med. 2010;33:35–46. doi: 10.1007/s10865-009-9230-7. doi: 10.1007/s10865-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burra P, Germani G. Orthotopic liver transplantation in non-alcoholic fatty liver disease patients. Rev Recent Clin Trials. 2014;9:210–216. doi: 10.2174/1574887109666141216105224. doi: 10.2174/1574887109666141216105224. [DOI] [PubMed] [Google Scholar]
- 56.Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;1:CD005166. doi: 10.1002/14651858.CD005166.pub2. doi: 10.1002/14651858.CD005166.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism. 2011;60:1278–1284. doi: 10.1016/j.metabol.2011.01.011. doi: 10.1016/j.metabol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 59.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armstrong MJ. In: International Liver Congress. Vienna, Austria: 2015. Liraglutide is effective in the histological clearance of non-alcoholic steatohepatitis in a multicentre, double-blinded, randomised, placebo-controlled phase II trial. [Google Scholar]
- 61.Armstrong MJ, Barton D, Gaunt P, Hull D, Guo K, Stocken D, et al. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open. 2013;3:e003995. doi: 10.1136/bmjopen-2013-003995. doi: 10.1136/bmjopen-2013-003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng L, Wang J, Li F. Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev. 2011:CD003619. doi: 10.1002/14651858.CD003619.pub3. doi: 10.1002/14651858.CD003619.pub3. [DOI] [PubMed] [Google Scholar]
- 63.Shalhub S, Parsee A, Gallagher SF, Haines KL, Willkomm C, Brantley SG, et al. The importance of routine liver biopsy in diagnosing nonalcoholic steatohepatitis in bariatric patients. Obes Surg. 2004;14:54–59. doi: 10.1381/096089204772787293. doi: 10.1381/096089204772787293. [DOI] [PubMed] [Google Scholar]
- 64.Kleiner DE, Berk PD, Hsu JY, Courcoulas AP, Flum D, Khandelwal S, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Semin Liver Dis. 2014;34:98–107. doi: 10.1055/s-0034-1371083. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes. 2013;2013:839275. doi: 10.1155/2013/839275. doi: 10.1155/2013/839275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin Liver Dis. 2012;32:80–91. doi: 10.1055/s-0032-1306428. doi: 10.1055/s-0032-1306428. [DOI] [PubMed] [Google Scholar]
- 67.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM. Bariatric surgery improves histological features of nonalcoholic Fatty liver disease and liver fibrosis. J Gastrointest Surg. 2015;19:429–437. doi: 10.1007/s11605-014-2678-y. doi: 10.1007/s11605-014-2678-y. [DOI] [PubMed] [Google Scholar]
- 69.Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-y Gastric Bypass on Nonalcoholic Fatty Liver Disease Evaluated Through NAFLD Fibrosis Score: a Prospective Study. Obes Surg. 2015;25:982–985. doi: 10.1007/s11695-014-1489-2. doi: 10.1007/s11695-014-1489-2. [DOI] [PubMed] [Google Scholar]
- 70.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 71.Karcz WK, Krawczykowski D, Kuesters S, Marjanovic G, Kulemann B, Grobe H, et al. Influence of Sleeve Gastrectomy on NASH and Type 2 Diabetes Mellitus. J Obes. 2011;2011:765473. doi: 10.1155/2011/765473. doi: 10.1155/2011/765473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kral JG, Thung SN, Biron S, Hould FS, Lebel S, Marceau S, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48–58. doi: 10.1016/j.surg.2003.10.003. doi: 10.1016/j.surg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Keshishian A, Zahriya K, Willes EB. Duodenal switch has no detrimental effects on hepatic function and improves hepatic steatohepatitis after 6 months. Obes Surg. 2005;15:1418–1423. doi: 10.1381/096089205774859290. doi: 10.1381/096089205774859290. [DOI] [PubMed] [Google Scholar]
- 74.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–540. doi: 10.1053/j.gastro.2009.04.052. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 75.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 76.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Burza MA, Romeo S, Kotronen A, Svensson PA, Sjöholm K, Torgerson JS, et al. Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PLoS One. 2013;8:e60495. doi: 10.1371/journal.pone.0060495. doi: 10.1371/journal.pone.0060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryou M, Ryan MB, Thompson CC. Current status of endoluminal bariatric procedures for primary and revision indications. Gastrointest Endosc Clin N Am. 2011;21:315–333. doi: 10.1016/j.giec.2011.02.004. doi: 10.1016/j.giec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Popov V, Kumar N, Thompson CC. In: Obesity Week. Boston, Massachusetts, USA: November 2014. Intragastric balloon as a treatment for nonalcoholic fatty liver disease in adults: a systematic review and meta-analysis. [Google Scholar]
- 80.Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756–760. doi: 10.1016/j.gie.2012.05.023. doi: 10.1016/j.gie.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 81.Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18:841–846. doi: 10.1007/s11695-007-9331-8. doi: 10.1007/s11695-007-9331-8. [DOI] [PubMed] [Google Scholar]
- 82.de Jonge C, Rensen SS, Koek GH, Joosten MF, Buurman WA, Bouvy ND, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves plasma parameters of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:1517–1520. doi: 10.1016/j.cgh.2013.07.029. doi: 10.1016/j.cgh.2013.07.029. [DOI] [PubMed] [Google Scholar]