Abstract
To determine whether a progestational agent can modify inflammation-induced preterm cervical ripening, mice on day 15 of gestation were given an intrauterine injection of (1) saline, (2) lipopolysaccharide, (3) an intramuscular injection of medroxyprogesterone acetate alone prior to lipopolysaccharide, or (4) medroxyprogesterone acetate alone. Cervices were obtained 6 hours later, then fixed, sectioned, and processed to stain collagen structure or to identify immune cells or nerve fibers. Cervical remodeling was induced by lipopolysaccharide treatment compared with that in saline controls, an effect blocked by medroxyprogesterone acetate pretreatment. Moreover, lipopolysaccharide reduced macrophages and enhanced neutrophils in the cervix, effects also forestalled by medroxyprogesterone acetate pretreatment. Although the density of nerve fibers was not altered by lipopolysaccharide, medroxyprogesterone acetate reduced innervation in the cervix. Thus, progestational treatment forestalls the inflammation-induced reduction in collagen structure and immune cell traffic through a mechanism that is independent of nerve fiber density. These findings raise the possibility that progestational treatment may regulate ripening of the cervix early in the process leading to preterm birth.
Keywords: Parturition, macrophages, neutrophils, collagen, preterm birth
INTRODUCTION
Preterm birth is a significant contributor to perinatal morbidity and mortality, yet the mechanisms by which preterm labor occur remain unclear. Clinical interventions have focused on approaches that reduce myometrial contractile activity to forestall the progression of an early onset of labor.1,2 However, more recent approaches have demonstrated that the use of progestational agents can reduce the risk of preterm birth in some populations.3–8 The mode of delivery, vaginal versus systemic treatment, choice of progestational agent, timing of treatment as well as patient history and presence of twins are important considerations for sustaining pregnancy in women at risk for premature birth.9 There has been insufficient data to support the hypothesis that these progestational agents promote myometrial quiescence in these patients. However, recent evidence by Facchinetti et al indicated that treatment with 17-alpha hydroxyprogesterone caproate reduced the rate of preterm delivery and attenuated shortening of the cervix in patients after standard tocolysis treatment had arrested preterm labor.10 Furthermore, vaginal administration of progesterone reduces the risk of preterm birth in patients with short cervix,7 suggesting that progestational agents may specifically target premature cervical ripening. The effects of progesterone on the process of cervical ripening in preterm birth have received little attention.
Studies in rodents have proven useful for understanding the role of progestational agents in preterm parturition. Intrauterine treatment with the endotoxin lipopolysaccharide (LPS) elevates proinflammatory cytokines and induces preterm parturition.11–13 These actions of LPS are forestalled by prior treatment with medroxyprogesterone acetate (MPA).14,15 At term, a similar rise in proinflammatory cytokines is proposed to be a component of processes associated with cervical ripening.13,16,17 Moreover, in rodents, increased numbers of macrophages and nerve fibers are associated with reduced collagen structure in the cervix by the day before birth,18,19 but effects of LPS or progestational agents on the process of cervical ripening are yet to be studied. The availability of cervix tissue from our previous study in which MPA blocked LPS-induced preterm birth13 led to the objective of the current study, which was to determine whether the inflammation-induced preterm cervical ripening involves trafficking of immune cells or hypertrophy of innervation and to test the hypothesis that the progestational agent MPA forestalls activation of processes associated with cervical ripening.
METHODS
Cervices were obtained from mice that were used in a previous study of the inflammation-induced preterm birth.11 Specifically, 4 groups of time-dated pregnant CD-1 mice received the following treatment after anesthesia on day 15 of pregnancy (n = 3/group): (1) saline controls received intrauterine injection of sterile saline vehicle (Sal; 0.1 mL); (2) to induce inflammation, another group of mice were given an intrauterine injection between the lower 2 gestational sacs of LPS (250 μg L2880; Sigma Chemical, St Louis, Mo) as previously detailed11,20; (3) dams were injected with MPA (1 mg/ dam, intramuscularly) 1 hour prior to intrauterine LPS (MPA + LPS); and (4) to control progestin treatment, an additional group of mice were injected with MPA alone. Medroxyprogesterone acetate was chosen for progestational treatment because of improved efficacy over progesterone to block LPS-induced preterm birth.14 The cervix of each mouse was obtained 6 hours after treatment. Cervices were immediately immersion fixed in 4% paraformaldehyde, then paraffin-embedded and cross-sectioned at 10 μm. Sections were stained for collagen structure with picrosirius red or processed by immunohistochemistry to identify resident immune cells, that is, macrophages with BM8 antisera, neutrophils with 7/4 antisera, or nerve fibers with peripherin antisera to neurofilaments.18,19,21 Although the 7/4 antisera cross-reacts with naïve monocytes, this epitope is lost when monocytes differentiate into macrophages in tissue, and systemic depletion of neutrophils reduces cell staining in the peripartum mouse cervix.21 The small number of stained cells that remained in the murine cervix after systemic neutrophil depletion suggests, at best, a very limited presence of monocytes and that staining is predominantly for neutrophils.
Analysis of Collagen Structure
Collagen content and complexity of structure was evaluated as previously described.19 In each of the 3 sections, birefringence of polarized light was evaluated in 9 nonoverlapping sequential placements of a 10 × 10 grid in an eyepiece reticle using a 20× objective. A black and white photograph was taken of each grid and mean optical density (OD) assessed using NIH ImageJ software (gray scale threshold calibrated with Rodbard standard curve22). Optical density of transmitted light was inversely proportional to density of collagen content and structure. Areas of bright red birefringence, indicative of high collagen content and complex dense cross-linking structure, appeared as white regions. The high transmittance of light in these white regions produced low OD numbers, an indication of high collagen density and complexity. By contrast, areas with low birefringence, indicative of low collagen content and diffuse structure, were dark and had proportionally high OD values. Data were normalized to cell nuclei density in the cervix of each mouse to account for variability in tissue hypertrophy or with respect to treatment. The mean number of cell nuclei (±SE)/μm3 ×104 for Sal, LPS, MPA + LPS, and MPA groups were 7.09 ± 0.42, 6.29 ± 0.57, 9.01 ± 0.84, and 7.52 ± 0.36, respectively (P > .05, analysis of variance [ANOVA]).
Census of Immune Cells and Nerve Fiber Density
Stereological procedures were used to enumerate immune cells and area with nerve fibers in the subepithelium and stromal regions of each cervix. Epithelium, blood vessels, and lumen were excluded from consideration. Individual stained immune cells were counted in 10 to 19 nonoverlapping placements of a 10 × 10 grid using a 40× objective as previously described.18 The density of nerve fibers in cervix was assessed as previously detailed.19 Briefly, nerve fibers were stained dark brown with an antibody to the type III neurofilament peripherin. Boxes that contained nerve fibers in 11 to 22 nonoverlapping 10 × 10 grid placements were counted. The area of these boxes provided an estimate of the distribution of nerve fibers in the cervix. To compensate for the hypertrophy of the cervix that occurs among mice and with respect to treatment, a correction for variations in extracellular matrix area, the number of immune cells, and estimated area of nerve fiber density were normalized to average cell nuclei counts per area in tissue sections from each mouse. Data were based on analyses of 2 sections per mouse (n = 3 mice/group) and were evaluated by ANOVA (SPSS, Chicago, Ill). When Levine’s test for homogeneity of variance was not statistically significant, the least squares difference test was used for individual comparisons. The Kruskal-Wallis test was used when data were not normally distributed; P < .05 was considered significant.
RESULTS
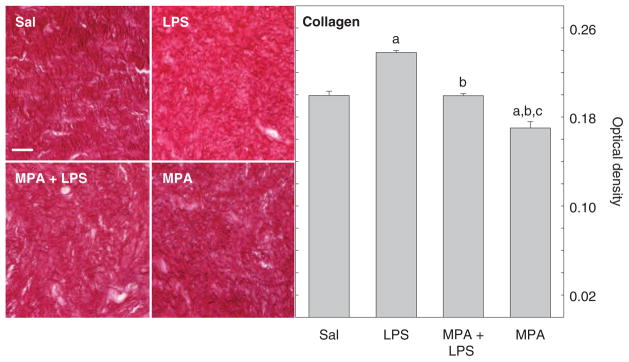
Morphology and density of collagen in cervices from pregnant mice varied with respect to treatment. Picrosirius red–stained collagen fibers were densely packed and regularly arranged in the region between luminal epithelium and stroma in the saline-treated controls (Figure 1, left). By 6 hours after intrauterine injection of LPS, birefringence of polarized light had increased because the intensity of staining was reduced. These effects of LPS were blocked by prior treatment with MPA. Moreover, MPA treatment alone enhanced stain intensity compared with groups administered LPS as well as the saline-treated controls.
Figure 1.
Left: Photomicrographs of picrosirius red–stained collagen structure in the murine cervix. Right: The OD of polarized light of sections of cervix in mice following treatment with Sal or LPS to induce preterm birth (LPS) or MPA prior to LPS to block the inflammation-induced preterm birth or MPA alone as a control. Data are the mean OD (±SE) of 9 nonoverlapping grid placements normalized to cell nuclei density (3 sections/mouse, 3 mice/group). OD was inversely related to collagen content and structure; low OD indicates denser collagen content and structure as described in “Methods.” MPA blocked collagen degradation induced by LPS. Letter symbol over each bar indicates P < .05 compared to a specific group: “a” versus Sal, “b” versus LPS, and “c” versus MPA + LPS. Scale bar in top left panel applies to all photomicrographs and indicates 50 μm. Sal = saline; LPS = lipopolysaccharide; MPA = medroxyprogesterone acetate.
As an indication of collagen content and structure, OD of polarized light from cervical tissue varied with respect to treatment. In the cervix from saline-treated mice, low OD, due to high birefringence, indicated dense collagen content and complex structure (Figure 1, right). Intrauterine injection of LPS significantly increased mean OD compared with that in the saline-treated controls, an indication that collagen structure declined by 6 hours after treatment (P < .0001 ANOVA; df = 3, F = 55.70). Pretreatment of pregnant mice with MPA blocked this effect of LPS. Mean OD of picrosirius red–stained sections were reduced in MPA + LPS-treated mice compared with those administered LPS but not significantly different from controls given saline. However, MPA treatment alone reduced mean OD compared with all groups. Thus, density and structure of collagen were enhanced by treatment with this progestational agent.
Brown-stained BM8-labeled macrophages populated the subepithelial stroma, between smooth muscle in the perimetrium and around blood vessels in the cervix. The presence of stained cells varied with treatment (Figure 2, left). Fewer macrophages were seen in cervices from pregnant mice after LPS or MPA treatments compared with that in the saline-treated controls (P < .041, Kruskal-Wallis). Stereological estimates of the cell numbers indicated that LPS induced a significant decline in resident macrophage (Figure 2, right). Prior treatment with MPA blunted the reduction in resident macrophage in the cervix of the LPS-treated mice. The census of resident macrophages in the cervix of MPA + LPS-treated mice was equivalent to that in mice given MPA alone.
Figure 2.
Left: Photomicrographs of cervix with macrophages stained dark brown with BM8 antibody. Right: Number of macrophage in the cervix of mice 6 hours after treatment. Data are the mean macrophage numbers normalized to cell nuclei density (±SE; evaluation of 10–19 nonoverlapping vertical and horizontal grid placements in 2 sections/mouse in 3 mice/group) to adjust for variability in hypertrophy of cervix. Scale bar in top left panel equals 50 μm and applies to all photomicrographs. Sal = saline; LPS = lipopolysaccharide; MPA = medroxyprogesterone acetate.
As with macrophages, cells stained brown with the 7/ 4 monoclonal antibody (mAb) were found in similar locations within the cervix. These 7/4 mAb-stained cells are likely to reflect neutrophils though some staining of monocytes cannot be completely excluded with certainty. In contrast to the reduced numbers of macrophages after LPS treatment, more neutrophils were present in the cervix of the LPS-treated mice than in any other group (Figure 3, left). The increased census of resident neutrophils, especially in the subepithelial stroma, was significant compared with all other groups (Figure 3, right; P < .025 ANOVA; df = 3, F = 5.5). Medroxyprogesterone acetate treatment blocked the LPS-induced rise in resident neutrophils, whereas MPA alone did not affect the neutrophil numbers in the cervix compared with saline controls.
Figure 3.
Left: Photomicrographs of representative sections of cervix from mice in various treatment groups stained with 7/4 antisera, which predominantly identifies resident neutrophils though staining of monocytes cannot with certainty be excluded. Note presence of stained neutrophils or naïve monocytes within blood vessels in Sal and LPS groups. Right: Number of neutrophil in cervix normalized to cell nuclei density (±SE; evaluation of 7–12 nonoverlapping vertical and horizontal grid placements in 2 sections/mouse, 3 mice/group). “b” indicates P < .05 versus LPS group. Scale bar in top left panel indicates 50 μm for all photomicrographs. Sal = saline; LPS = lipopolysaccharide; MPA = medroxyprogesterone acetate.
Nerve fibers were prominent in the cervix of all mice especially in stroma, around blood vessels, and, with less frequency, in regions between smooth muscle cells (Figure 4, left). The area of cervix that contained nerve fibers was the same in mice injected with LPS compared with that in the saline controls (Figure 4, right). However, compared with Sal controls, treatment with MPA reduced nerve fibers in the cervix whether or not mice were given LPS (P < .037 ANOVA; df = 3, F = 4.64). Thus, treatment with a progestational agent significantly reduced the area with nerve fibers in the cervix of pregnant mice.
Figure 4.
Left: Photomicrographs of cervix with nerve fibers in sections from the cervix of mice 6 hours after treatment as described in Figure 1 legend. Right: Area of the cervix with nerve fibers was normalized to cell nuclei density (mean area with nerve fiber ±SE of 11–22 nonoverlapping vertical and horizontal grid placements in 2 sections/mouse; n = 3 mice/group) to account for variations in hypertrophy among mice and with respect to treatment as described in Figure 1 legend. “a” indicates P < .05 versus Sal group. Scale bars in each panel equal 50 μm. Sal = saline; LPS = lipopolysaccharide; MPA = medroxyprogesterone acetate.
DISCUSSION
Based on the evidence in this mouse model for preterm birth, the findings support the hypothesis that inflammation-induced ripening of the cervix is forestalled by a progestational agent. Within 6 hours of an intrauterine injection of endotoxin, collagen content and structure in the cervix were reduced. The LPS-mediated restructuring of the cervix occurred well before preterm birth in this model.11 With significant remodeling of the cervix by 6 hours after endotoxin administration, approximately 18 hours prior to observed preterm birth, the present findings support the conclusion that cervical ripening is an early event in preterm birth. Associated with restructuring of the cervix is a decline in serum progesterone concentrations,11 though levels remain many fold higher than that in circulation in the prepartum mouse at 1 day before normal term.23 The finding that pretreatment with the progestational agent MPA blocked the LPS-induced preterm birth11 was extended by findings in the current study to include its ability to forestall cervical remodeling. Thus, in this mouse model for inflammation-induced premature birth, progestational agent treatment arrests remodeling of the cervix early in the process of preterm parturition.
Treatment with MPA also countered the effects of endotoxin to regulate the number of immune cells that reside in the cervix. By 6 hours after intrauterine LPS injection, the census of macrophages was reduced as the number of resident neutrophils increased in the cervix. This finding raises the possibility that immune cell traffic or turnover may be dynamic characteristic of the initial process associated with the inflammation-induced preterm cervical remodeling. In another respect, macrophage activation is part of an early proinflammatory response system.24,25 Whether activation or egress of macrophages from the cervix contribute to the effects of intrauterine LPS to increase systemic concentrations of interleukin (IL)-6 and IL-10 in these mice is not known.11 The latter cytokine, IL-10, is an important anti-inflammatory mediator that limits the LPS-induced innate immune responses26,27 and may mitigate systemic effects of LPS treatment on both the mother and fetus.28,29 The present findings do not exclude the possibility that macrophage numbers may initially go up then decline or change phenotype in the cervix within 6 hours of LPS treatment. The time course of immune cell trafficking and cascade of proinflammatory activities may be critical for promoting uterine contractile activity and, by 24 hours post-LPS treatment, premature birth. Another alternative hypothesis that apoptosis reduces the census of resident macrophages—this possibility is not supported by findings in the cervix30 or by evidence that apoptosis of immune cells is associated with the resolution, not initiation of inflammation.31–33 These consideration add to other reports that suggest cervical ripening may differ between pathophysiological processes and normal term birth.13,34,35 More information is needed about the time course of proinflammatory mediators in association with remodeling of the cervix in preterm and term birth. Clearly, intervention with the progestational agent MPA forestalled aspects of this cascade by blunting the decrease in resident macrophages, blocking the rise in neutrophils, and, as previously reported, increasing serum IL-10.11 Increased systemic IL-10 is associated with a transient leukocytosis and reduced proinflammatory cytokine production.36 Collectively, the present findings suggest that actions by a progestational agent to arrest advancement of inflammation-associated processes prevents restructuring of the cervix and, based on the previous findings in these mice, forestalls preterm birth.11
The findings fail to support the hypothesis that inflammation-induced preterm ripening and birth affects innervation in the cervix. At normal term, increased innervation is found in cervix of prepartum C3H/HeN and C57Bl6 mice.19,37 However, in this study, the area of cervix with nerve fibers was unchanged following LPS treatment. The difference in innervation of the cervix between endotoxin-induced preterm birth and normal term parturition suggests that neural pathways may be more important for regulating remodeling of the cervix and the process birth at normal term. Vasodilatation, collagenolysis, and infiltration of immune cells are characteristics of neurogenic inflammation38,39 that may mediate similar processes in the mechanism for cervical ripening at term.19 However, present findings do not exclude the alternative consideration that neural activity, rather than density of innervation, may contribute to inflammatory processes in the cervix associated with preterm birth.
The clinical use of 17-hydroxyprogesterone caproate (17-OHPC) is supported by the American College of Obstetrics and Gynecology to high-risk patients. Although 17-OHPC is administered systemically, the efficacy of vaginal administration of progesterone for preterm birth varied in other trials.3,9,40 Although the current study suggests that MPA can modify inflammation-induced cervical ripening, it remains unclear whether this is an essential mechanism by which other progestational agents prevent preterm birth in humans. If prevention and/or cessation of cervical ripening is an essential mechanism by which progestational agents prevent preterm birth, then it becomes critical to understand whether all progestational agents are equal in their ability to target cervical ripening and whether the route of administration affects efficacy. Further efforts are needed to forestall activation of pathways that lead to preterm delivery in patients at risk for premature cervical ripening.
In summary, findings in the current study support the hypothesis that progestational agent treatment forestalls the endotoxin-induced immigration of neutrophils and a decline in macrophages that are associated with ripening of the cervix. Although MPA reduces the area in the cervix with nerve fibers, hypertrophy of innervation does not appear to participate in the process of endotoxin-induced preterm birth. Therefore, the capability of progestational agent treatment to block ripening of the cervix and, as previously reported, arrest preterm birth adds to accumulating evidence that progesterone may be effective early in the process to prevent preterm birth.
Acknowledgments
We thank Thomas Lechuga for technical assistance. This study was supported in part by the March of Dimes (MAE), the NIH (K12 award, WRHR, University of Pennsylvania to MAE, and HD054931 to SMY), and the Dean of LLUSM.
References
- 1.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 2.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19:763–772. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 3.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–680. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 5.Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol. 1980;56:692–695. [PubMed] [Google Scholar]
- 6.Hauth JC, Gilstrap LC, III, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983;146:187–190. doi: 10.1016/0002-9378(83)91051-7. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 8.Rouse DJ, Caritis SN, Peaceman AM, et al. for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 10.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453–454. doi: 10.1016/j.ajog.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Elovitz MA, Mrinalini C. The use of progestational agents for preterm birth: lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–1010. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Erez O, Espinoza J. Intrauterine infection, pre-term labor, and cytokines. J Soc Gynecol Investig. 2005;12:463–465. doi: 10.1016/j.jsgi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 15.Elovitz MA, Mrinalini C. Can medroxyprogesterone acetate alter Toll-like receptor expression in a mouse model of intrauterine inflammation? Am J Obstet Gynecol. 2005;193:1149–1155. doi: 10.1016/j.ajog.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 17.Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10:323–338. doi: 10.1016/s1071-5576(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 18.Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod. 1999;61:879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- 19.Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005;12:578–585. doi: 10.1016/j.jsgi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J Matern Fetal Neonatal Med. 2008;21:223–230. doi: 10.1080/14767050801923680. [DOI] [PubMed] [Google Scholar]
- 21.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–245. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 22.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, MD: 1997–2007. Available at: http://rsb.info.nih.gov/ij/ [Google Scholar]
- 23.Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974;95:1486–1490. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 24.Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. Philadelphia, PA: WB Saunders Co; 2000. pp. 299–302. Section IV. [Google Scholar]
- 25.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 26.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Schmidtke P, Zepp F, Meyer CU. Boosting interleukin-10 production: therapeutic effects and mechanisms. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:465–475. doi: 10.2174/156800805774912926. [DOI] [PubMed] [Google Scholar]
- 28.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Muller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol. 2006;36:3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 29.Salminen A, Paananen R, Vuolteenaho R, et al. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res. 2008;63:280–286. doi: 10.1203/PDR.0b013e318163a8b2. [DOI] [PubMed] [Google Scholar]
- 30.Kemp B, Rath W, Winkler M, Reineke T, Beier HM, von Rango U. Is cervical dilatation during parturition at term associated with apoptosis? J Perinat Med. 2005;33:137–143. doi: 10.1515/JPM.2005.026. [DOI] [PubMed] [Google Scholar]
- 31.Kettritz R, Wilke S, von Vietinghoff S, Luft F, Schneider W. Apoptosis, proliferation and inflammatory infiltration in ANCA-positive glomerulonephritis. Clin Nephrol. 2006;65:309–316. doi: 10.5414/cnp65309. [DOI] [PubMed] [Google Scholar]
- 32.Mangan DF, Welch GR, Wahl SM. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 33.Hou FF, Boyce J, Zhang Y, Owen WF., Jr Phenotypic and functional characteristics of macrophage-like cells differentiated in pro-inflammatory cytokine-containing cultures. Immunol Cell Biol. 2000;78:205–213. doi: 10.1046/j.1440-1711.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194:1334–1340. doi: 10.1016/j.ajog.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez JM, Xu H, Elovitz MA. Preterm and term cervical ripening: similar or divergent molecular mechanisms? [abstract] Reprod Sci. 2008;15:262A. doi: 10.1095/biolreprod.108.075309. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs AC, Granowitz EV, Shapiro L, et al. Clinical, hematologic, and immunologic effects of interleukin-10 in humans. J Clin Immunol. 1996;16:291–303. doi: 10.1007/BF01541395. [DOI] [PubMed] [Google Scholar]
- 37.Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008;78:438–444. doi: 10.1095/biolreprod.107.063404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 39.Niissalo S, Hukkanen M, Imai S, Tornwall J, Konttinen YT. Neuropeptides in experimental and degenerative arthritis. Ann N Y Acad Sci. 2002;966:384–399. doi: 10.1111/j.1749-6632.2002.tb04239.x. [DOI] [PubMed] [Google Scholar]
- 40.Defranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]