Abstract
Objective
We compared the performance of the 50-g glucose challenge test (GCT) in singleton versus twin pregnancies and investigated the need for adjusting GCT cutoff values for gestational diabetes mellitus (GDM) in twin pregnancies among Korean women.
Methods
A retrospective chart review was performed in women who underwent GCT at 24 to 28 weeks' gestation and delivered in our department between January 2000 and April 2008. GCT performance was compared between singleton and twin pregnancies for an ideal cutoff value of the GCT for GDM screening.
Results
GCT results were available in 3,578 pregnancies (3,435 singleton and 143 twin pregnancies). The mean GCT value was higher in the twin group than in the singleton group. Women in the twin group had a higher mean GCT value (P=0.043) and a higher incidence of GCT ≥130, ≥135, and ≥140 mg/dL (P=0.014, 0.005, and 0.015, respectively). The false positive rate for GCT ≥140 mg/dL was significantly higher in the twin than in the singleton group (P=0.042). The optimal GCT screening cutoff value appears to be ≥145 mg/dL in twin pregnancies.
Conclusion
Our study demonstrates that the GCT is associated with a higher false positive rate in twin rather than singleton pregnancies. This study suggests we should consider adjusting the GCT cutoff value for GDM in Korean twin pregnancies.
Keywords: Diabetes, gestational; Pregnancy, twin; Screening test
Introduction
Gestational diabetes mellitus (GDM) frequently affects women during pregnancy [1,2]. According to recommendations by the American College of Obstetrics and Gynecology (ACOG), a universal screening for GDM using a 50-g glucose challenge test (GCT) is advocated. Women with abnormal GCT (serum glucose levels above a threshold of 130 to 140 mg/dL) should undergo the definitive diagnostic 3-hour 100-g oral glucose-tolerance test (OGTT) [1].
Overall, physiologic changes are amplified in multiple gestation pregnancies compared with singleton pregnancies [3,4]. Multiple gestations, such as twin pregnancies, have larger placentas, which result in higher hormone levels. Higher levels of estrogen, placental lactogen, and progesterone affect insulin sensitivity [4,5,6]. Another important contributor to insulin resistance during pregnancy is weight gain, which has also been shown to be higher in twin pregnancies [7,8]. Of these physiologic changes, those that affect glucose control are significantly influenced by differences in gestation number.
Considering these differences between twin and singleton pregnancies, it has been hypothesized that the accuracy and characteristics of the GCT may differ between these two groups [9,10]. Although the incidence of twin pregnancies has increased in recent years, studies related to twin pregnancy and GDM are clearly lacking.
Thus, this study aimed to compare the performance of the GCT in twin versus singleton pregnancies and to evaluate the ideal GCT cutoff value in twin pregnancies among Korean women.
Materials and methods
A retrospective chart review was performed in pregnant women who delivered between January 2000 and April 2008 at the obstetrics and gynecology department in Severance Hospital, Yonsei University College of Medicine. The study group included all women who underwent a GCT at 24 to 28 weeks' gestation. Maternal age, parity, body mass index, weight gain during pregnancy, gestational age at delivery, family history of diabetes mellitus, and neonatal outcomes were reviewed using medical records. Maternal age was categorized into <35 and ≥35 years. Pregnancies complicated by any of the following conditions were excluded from the study: pregestational diabetes mellitus, delivery at <24 weeks gestational age, and birth weight <500 g. Pregestational diabetes mellitus was defined as glucose intolerance that occurred before pregnancy.
Diagnosis of GDM was based on a two-step strategy. Patients underwent the GCT at 24 to 28 weeks' gestation. During the study period, all patients with a GCT result ≥140 mg/dL underwent OGTT. GDM was diagnosed if two of four values on the OGTT were abnormal, based on Carpenter-Coustan cutoffs [11].
Data analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). The Student t-test was used to compare continuous variables between the groups, and the chi-square test was used for categorical variables. A P-value <0.05 was considered statistically significant.
Results
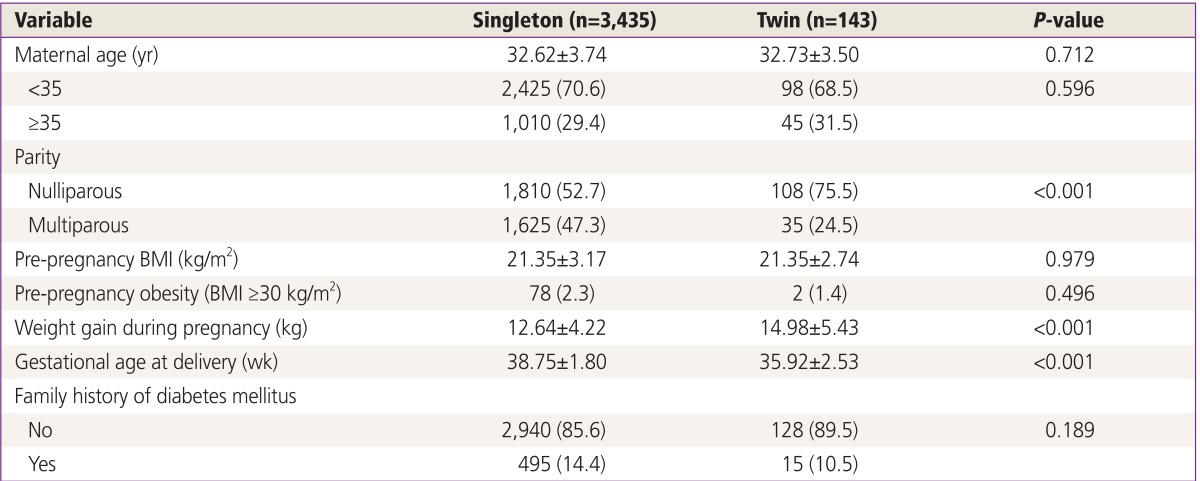
The results of the GCT were available in 3,578 pregnancies, of which 3,435 were singleton and 143 were twin. The characteristics of the singleton and twin groups are presented in Table 1. The twin group gained more weight during pregnancy, had a higher rate of nulliparity, and delivered earlier in comparison with the singleton group. Although the rate of advanced maternal age in the twin group was close to 30%, it was not significantly different from that of the singleton group.
Table 1. Baseline characteristics in twin and singleton pregnancies.
Values are presented as mean±standard deviation or number (%).
BMI, body mass index.
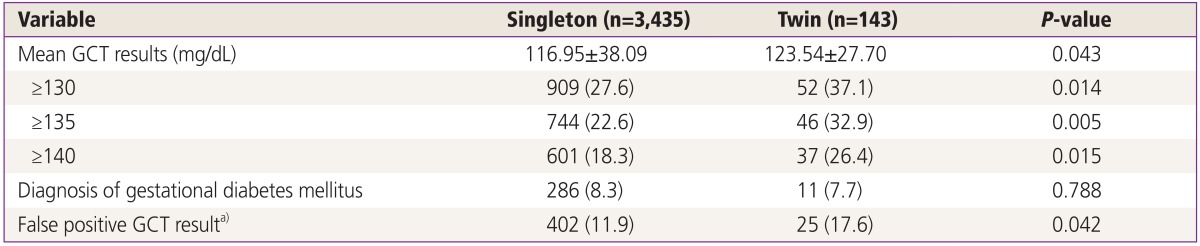
The characteristics of the GCT results in women with singleton and twin pregnancies are presented in Table 2. Twin pregnancies were associated with a significantly higher mean GCT result (123.5±27.7 vs. 117.0±38.1 mg/dL, P=0.043). The overall rate of GDM, diagnosed according to the standard set by ACOG, was similar between the twin and singleton pregnancies (7.7% vs. 8.3%, P=0.79). However, the false positive rate for GCT was considerably higher in the twin pregnancy group compared with the singleton pregnancy group, when the cutoff value was defined as 140 mg/dL.
Table 2. GCT results in twin and singleton pregnancies.
Values are presented as mean±standard deviation or number (%).
GCT, glucose challenge test.
a)Women with a GCT ≥140 mg/dL but normal oral glucose-tolerance test results (no abnormal values).
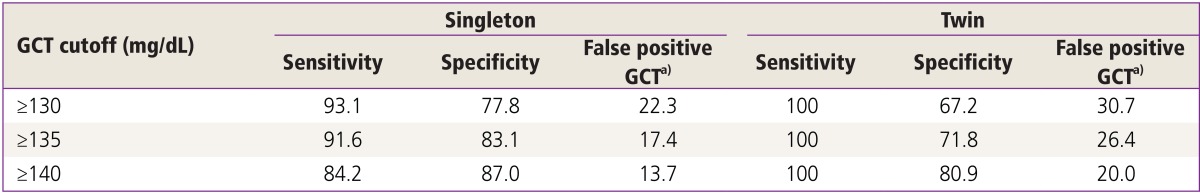
In addition to the overall result averages, GCT results were compared according to three different GCT cutoffs (≥130, ≥135, and ≥140 mg/dL) between the twin and singleton groups. Using a GCT cutoff of ≥130 mg/dL, 52 of 143 (37.1%) pregnancies were screened to be positive. However, using a GCT cutoff of ≥135 and ≥140 mg/dL, 46 (32.9%) and 37 (26.4%) pregnancies were screened to be positive, respectively.
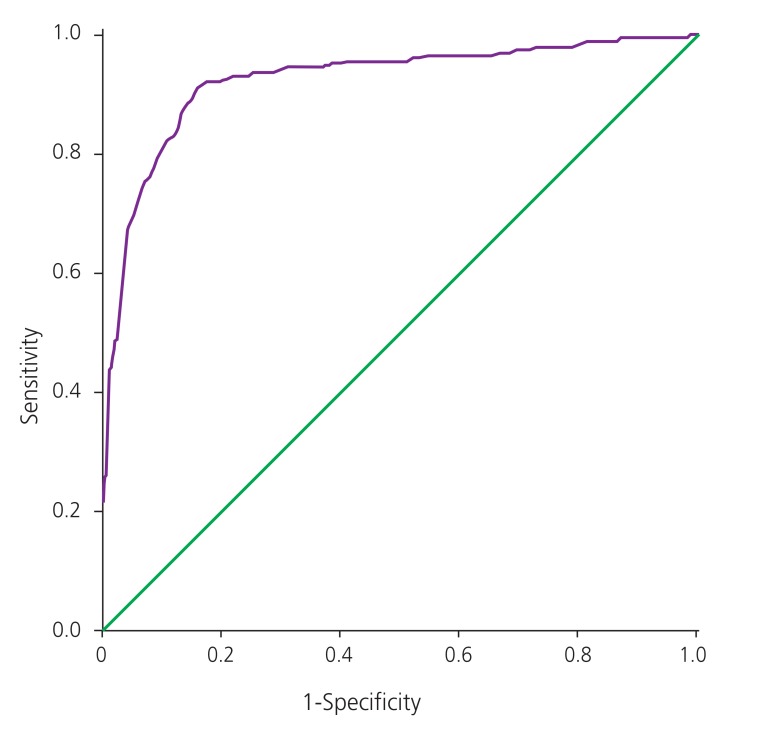
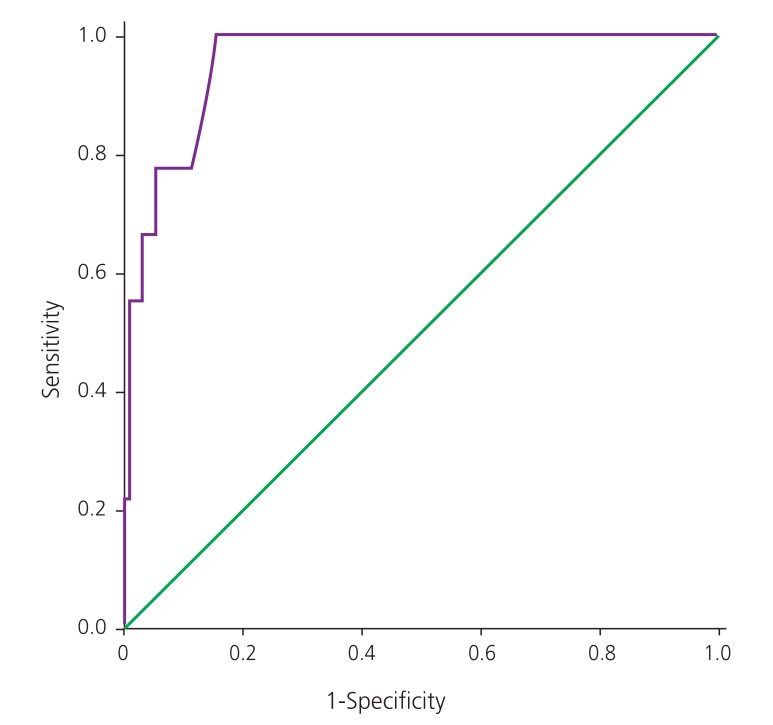
The diagnostic characteristics of the GCT based on the different cutoffs are shown in Table 3. All three GCT cutoffs in twin pregnancies had a high sensitivity. A GCT cutoff of ≥140 mg/dL had a higher specificity and had lower false positive rate for GCT. The receiver operating characteristic (ROC) curve considering singleton pregnancies is shown in Fig. 1. The area under ROC curve of GCT was 0.920 (95% confidence interval, 0.898 to 0.943; P<0.001). It was observed that a GCT cutoff value ≥139 mg/dL had a sensitivity of 87.1% and specificity of 86.3% in diagnosing GDM. The ROC curve reflecting twin pregnancies is shown in Fig. 2. The area under ROC curve of GCT in twin pregnancies was 0.958 (95% confidence interval, 0.917 to 0.999; P<0.001). It was observed that a GCT cutoff value ≥145 mg/dL had a sensitivity of 88.9% and specificity of 86.3% in diagnosing GDM.
Table 3. Diagnostic characteristics of GCT in singleton and twin pregnancies based on different cutoffs.
Values are presented as percent.
GCT, glucose challenge test.
a)Women with a GCT ≥140 mg/dL but normal oral glucose-tolerance test results (no abnormal values).
Fig. 1. Receiver operating characteristic curve for glucose challenge test in singleton pregnancies. This receiver operating characteristic shows the sensitivity and 1-specificity of diagnosis of gestational diabetes mellitus with singleton pregnancies undergoing glucose challenge test.
Fig. 2. Receiver operating characteristic curve for glucose challenge test in twin pregnancies. This receiver operating characteristic shows the sensitivity and 1-specificity of diagnosis of gestational diabetes mellitus for all patients with twin pregnancies undergoing glucose challenge test.
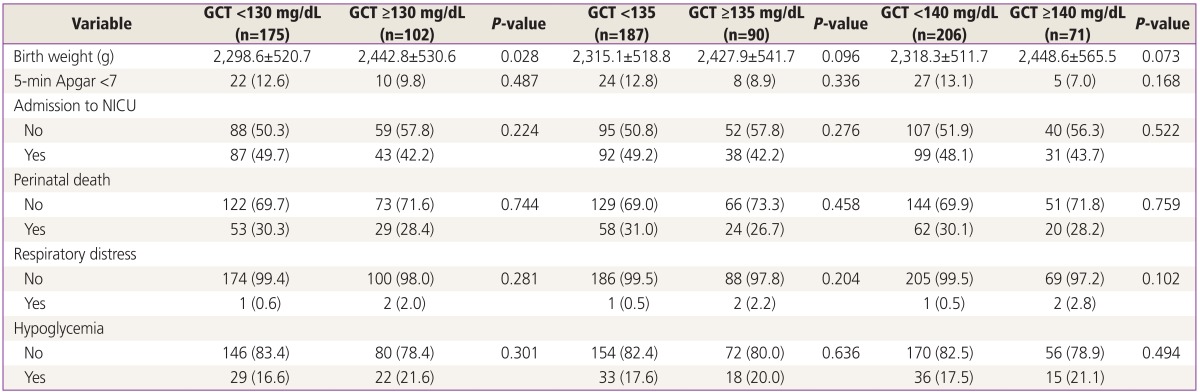
Neonatal outcomes for the twin group were compared according to the three different GCT cutoffs (≥130, ≥135, and ≥140 mg/dL). As shown in Table 4, there were no differences between the different GCT values with regard to the rate of low Apgar score at 5 minutes, respiratory morbidity, neonatal hypoglycemia, admission to the neonatal intensive-care unit, or perinatal death.
Table 4. Neonatal outcomes in twin pregnancies according to different levels of GCT.
Values are presented as mean±standard deviation or number (%).
GCT, glucose challenge test; NICU, neonatal intensive-care unit.
Discussion
Although correlations of twin pregnancy with GDM risk have been investigated in a number of studies, the results have been considered narrow and controversial. There have been reports concluding that GDM incidence in twin pregnancies does not differ from that in singleton pregnancies [9,12,13]. However, some studies demonstrated that multiple gestations have a higher incidence of GDM [10,14]. This discrepancy between studies could be explained by differences in the study design and the cutoff values for diagnosing GDM. Therefore, the Carpenter-Coustan criterion, which has high sensitivity in the diagnosis of GDM, was selected for our study [11].
Screening thresholds for GCT have varied from 130 to 140 mg/dL, with varying sensitivities and specificities reported. There are no randomized trials to support a clear benefit to one cutoff compared with others. In a recent review, sensitivity for a threshold of 140 mg/dL ranged from 75% to 83%. Sensitivity estimates for a GCT threshold of 135 mg/dL improved only slightly to 78 to 85 %. And, specificity dropped from a range of 72% to 85% for 140 mg/dL to 65% to 81% for a threshold of 135 mg/dL [15]. In other analysis, sensitivities were only marginally improved when using lower thresholds (130 and 135 mg/dL) [16]. ACOG recommends using either 135 or 140 mg/dL as the GCT threshold [1]. In the absence of clear evidence supporting a cutoff of 135 versus 140 mg/dL for the GCT, it is suggested that health care providers select one of these as a single consistent cutoff for their practice, with factors such as community prevalence rates of GDM considered in that decision. So, we compared the results for three different GCT threshold (130, 135, and 140 mg/dL) in singleton and twin pregnancies.
Women with twin pregnancy tend to gain more weight during pregnancy and tend to be older compared to women with singleton pregnancy. These two variables have been assessed as high risk factors for GDM in some studies [17,18,19,20]. Additionally, as mentioned previously, large placental mass is associated with high levels of hormone that influence insulin sensitivity [5,6]. This supports the hypothesis that twin pregnancy is associated with higher GDM risk. In our study, women with twin pregnancy had significantly greater gestational weight gain, but the proportion of women aged >35 years in twin pregnancies was not significantly different from that of women with singleton pregnancy. More women with multiple gestations delivered earlier than did women with singletons; however, this might be owing to a higher incidence of preterm labor in multiple gestations rather than owing to GDM [21].
In this study, twin pregnancies had relatively higher average GCT results than did singleton pregnancies. In addition, three different GCT cutoffs (≥130, ≥135, and ≥140 mg/dL) demonstrated greater GCT results exceeding the cutoffs in twin than in singleton pregnancies. However, the actual diagnosis of GDM with OGTT had no difference in the incidence of GDM between the two groups, with more false positives occurring in the twin group than in the singleton pregnancy group.
Yogev et al. [22] recently studied the characteristics of the GCT in twin versus singleton pregnancies. Although they found that the GCT results were significantly higher in twin pregnancies than in singleton pregnancies, their population of twin pregnancies had lower GCT values than did ours. For example, their mean GCT value was 104.7±28.5 mg/dL compared with 123.5±27.7 mg/dL in our study. In addition, the rates of GCT >130 (20.2% vs. 37.1%) and >140 mg/dL (13.8% vs. 26.4%) were lower in their cohort than in those of the present study. Moreover, these findings were independently associated with twin gestations even after adjusting for potential confounders. Their findings suggest that these physiological differences between singleton and twin pregnancies may lead to an only mild form of glucose intolerance that does not translate to a difference in the rate of GDM as defined by an abnormal OGTT. Another possible reason for the lack of difference in the rate of GDM in twin pregnancies may be that the diabetogenic effect by a twin gestation-related protective effect from GDM, which may be attributed to the increased demand of glucose due to the presence of multiple fetuses and to the higher basal metabolic rate in twin pregnancies [23].
As higher false positive GCT results were reported in singleton and twin pregnancies, investigations into the ideal cutoff value for GCT were performed. In our study, ROC curve analysis showed that the area under the ROC curve for GCT in twin pregnancy was 0.958 (P<0.001) with 145 mg/dL as the cutoff value. This resulted in 88.9% sensitivity and 86.3% specificity. Rebarber et al. [24] reported 100% sensitivity but only 28.6% test positive rate when they set the optimal GCT cutoff at ≥135 mg/dL. High GCT cutoff values in our studies, compared with other studies, are thought to be most likely due to our sample group consisting only of Koreans. While GDM is increasingly common worldwide, largely owing to the obesity epidemic, its frequency is relatively low in Korean women. These differences may be attributed to both genetic and environmental factors [25,26,27].
When a woman is diagnosed with GDM, maternal complications and neonatal outcomes should be monitored closely. Some reviews point out that the cases with false positive GCT results had poorer neonatal outcomes, such as glucose intolerance, than did the cases without false positive GCT results [28,29]. Comparative analysis of neonatal outcomes based on different GCT cutoff levels of 130, 135, and 140 mg/dL was done in the present study. The two groups below and above a GCT cutoff level of 130 mg/dL showed significant differences in birth weight; however, other complications were not noted. Comparing neonatal outcomes in twin pregnancies by GCT levels did not show any statistically significant results. This could be because we only included neonatal outcomes that are considered highly critical.
This is the first study comparing the performance of the GCT in twin versus singleton pregnancies and evaluating the ideal GCT cutoff value among Korean women. Our results could be utilized as references for comparisons with the results of other studies performed abroad. The study by Yogev et al. [22], which was performed in Israel, had only a 15% advanced maternal age rate, while another study by Rebarber et al. [24], which was performed in the US and mostly targeted Caucasians, reported an advanced maternal age rate at nearly 50%. This difference could be due to geographic discrepancies, because GDM is also associated with environmental factors, such as lifestyle and diet, and genetic factors, such as race.
In this study, the GCT cutoff value was above 139 mg/dL in singleton pregnancies which showed no difference compared to previous studies. But the GCT cutoff value was above 145 mg/dL in twin pregnancies which is higher than the currently accepted diagnosis value. As GDM is often asymptomatic, screening is necessary to identify women with GDM. High sensitivity is often warranted in screening tests, as a false-negative test result (in which disease remains undiscovered) is considered to be more harmful than a false-positive test result (in which a reference test is unnecessarily performed). Our study has a small sample size, with only 143 cases of twin pregnancy, which results in a low estimated accuracy. If the GCT cutoff value extrapolated from this study is used for diagnosis in twin pregnancies, there would be a higher false negative rate so diagnosis rates would fall. A study with a larger sample size is required in the future to establish a more accurate GCT cutoff value in Koreans. In addition, long-term neonatal outcomes should be investigated to obtain a more appropriate cutoff value.
In conclusion, our study suggests that the GCT is associated with a higher false positive rate in twin pregnancies than in singleton pregnancies. More research is needed in twin pregnancies to establish the optimal GDM screening and treatment paradigm in twin pregnancies.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Committee on Practice Bulletins: Obstetrics. Practice Bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 2.Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118:1379–1393. doi: 10.1097/AOG.0b013e31823974e2. [DOI] [PubMed] [Google Scholar]
- 3.Norwitz ER, Edusa V, Park JS. Maternal physiology and complications of multiple pregnancy. Semin Perinatol. 2005;29:338–348. doi: 10.1053/j.semperi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Spellacy WN, Buhi WC, Birk SA. Human placental lactogen levels in multiple pregnancies. Obstet Gynecol. 1978;52:210–212. [PubMed] [Google Scholar]
- 5.Kuhl C. Etiology and pathogenesis of gestational diabetes. Diabetes Care. 1998;21(Suppl 2):B19–B26. [PubMed] [Google Scholar]
- 6.Kazer RR, Cheng ER, Unterman TG, Glick RP. Maternal plasma concentrations of insulin-like growth factor-I (IGF-I) and human placental lactogen (hPL) in twin pregnancies. Acta Genet Med Gemellol (Roma) 1991;40(3-4):383–387. doi: 10.1017/s0001566000003573. [DOI] [PubMed] [Google Scholar]
- 7.Chu SY, D'Angelo DV. Gestational weight gain among US women who deliver twins, 2001-2006. Am J Obstet Gynecol. 2009;200:390.e1–390.e6. doi: 10.1016/j.ajog.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Fox NS, Rebarber A, Roman AS, Klauser CK, Peress D, Saltzman DH. Weight gain in twin pregnancies and adverse outcomes: examining the 2009 Institute of Medicine guidelines. Obstet Gynecol. 2010;116:100–106. doi: 10.1097/AOG.0b013e3181e24afc. [DOI] [PubMed] [Google Scholar]
- 9.Buhling KJ, Henrich W, Starr E, Lubke M, Bertram S, Siebert G, et al. Risk for gestational diabetes and hypertension for women with twin pregnancy compared to singleton pregnancy. Arch Gynecol Obstet. 2003;269:33–36. doi: 10.1007/s00404-003-0483-z. [DOI] [PubMed] [Google Scholar]
- 10.Rauh-Hain JA, Rana S, Tamez H, Wang A, Cohen B, Cohen A, et al. Risk for developing gestational diabetes in women with twin pregnancies. J Matern Fetal Neonatal Med. 2009;22:293–299. doi: 10.1080/14767050802663194. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 12.Spellacy WN, Handler A, Ferre CD. A case-control study of 1253 twin pregnancies from a 1982-1987 perinatal data base. Obstet Gynecol. 1990;75:168–171. [PubMed] [Google Scholar]
- 13.Simmons D, Yapa M. Association between twin pregnancy and hyperglycemia in a multiethnic community in New Zealand. Diabetes Care. 2002;25:934–935. doi: 10.2337/diacare.25.5.934. [DOI] [PubMed] [Google Scholar]
- 14.Wein P, Warwick MM, Beischer NA. Gestational diabetes in twin pregnancy: prevalence and long-term implications. Aust N Z J Obstet Gynaecol. 1992;32:325–327. doi: 10.1111/j.1479-828x.1992.tb02843.x. [DOI] [PubMed] [Google Scholar]
- 15.van Leeuwen M, Louwerse MD, Opmeer BC, Limpens J, Serlie MJ, Reitsma JB, et al. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. BJOG. 2012;119:393–401. doi: 10.1111/j.1471-0528.2011.03254.x. [DOI] [PubMed] [Google Scholar]
- 16.Esakoff TF, Cheng YW, Caughey AB. Screening for gestational diabetes: different cut-offs for different ethnicities? Am J Obstet Gynecol. 2005;193(3 Pt 2):1040–1044. doi: 10.1016/j.ajog.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 17.Leese B, Jomeen J, Denton J. Appropriate maternal weight gain in singleton and twin pregnancies: what is the evidence? Hum Fertil (Camb) 2012;15:194–199. doi: 10.3109/14647273.2012.723838. [DOI] [PubMed] [Google Scholar]
- 18.Beemsterboer SN, Homburg R, Gorter NA, Schats R, Hompes PG, Lambalk CB. The paradox of declining fertility but increasing twinning rates with advancing maternal age. Hum Reprod. 2006;21:1531–1532. doi: 10.1093/humrep/del009. [DOI] [PubMed] [Google Scholar]
- 19.Alshami HA, Kadasne AR, Khalfan M, Iqbal SZ, Mirghani HM. Pregnancy outcome in late maternal age in a high-income developing country. Arch Gynecol Obstet. 2011;284:1113–1116. doi: 10.1007/s00404-010-1821-6. [DOI] [PubMed] [Google Scholar]
- 20.Baci Y, Ustuner I, Keskin HL, Ersoy R, Avsar AF. Effect of maternal obesity and weight gain on gestational diabetes mellitus. Gynecol Endocrinol. 2013;29:133–136. doi: 10.3109/09513590.2012.730571. [DOI] [PubMed] [Google Scholar]
- 21.Elliott JP. Preterm labor in twins and high-order multiples. Clin Perinatol. 2007;34:599–609. doi: 10.1016/j.clp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Yogev Y, Eisner M, Hiersch L, Hod M, Wiznitzer A, Melamed N. The performance of the screening test for gestational diabetes in twin versus singleton pregnancies. J Matern Fetal Neonatal Med. 2014;27:57–61. doi: 10.3109/14767058.2013.799660. [DOI] [PubMed] [Google Scholar]
- 23.Shinagawa S, Suzuki S, Chihara H, Otsubo Y, Takeshita T, Araki T. Maternal basal metabolic rate in twin pregnancy. Gynecol Obstet Invest. 2005;60:145–148. doi: 10.1159/000086132. [DOI] [PubMed] [Google Scholar]
- 24.Rebarber A, Dolin C, Fields JC, Saltzman DH, Klauser CK, Gupta S, et al. Screening approach for gestational diabetes in twin pregnancies. Am J Obstet Gynecol. 2014;211:639.e1–639.e5. doi: 10.1016/j.ajog.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Kim C. Gestational diabetes mellitus in korean women: similarities and differences from other racial/ethnic groups. Diabetes Metab J. 2014;38:1–12. doi: 10.4093/dmj.2014.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao AK, Cheng YW, Caughey AB. Perinatal complications among different Asian-American subgroups. Am J Obstet Gynecol. 2006;194:e39–e41. doi: 10.1016/j.ajog.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Rao AK, Daniels K, El-Sayed YY, Moshesh MK, Caughey AB. Perinatal outcomes among Asian American and Pacific Islander women. Am J Obstet Gynecol. 2006;195:834–838. doi: 10.1016/j.ajog.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 28.Yogev Y, Langer O, Xenakis EM, Rosenn B. The association between glucose challenge test, obesity and pregnancy outcome in 6390 non-diabetic women. J Matern Fetal Neonatal Med. 2005;17:29–34. doi: 10.1080/14767050400028766. [DOI] [PubMed] [Google Scholar]
- 29.Melamed N, Hiersch L, Hod M, Chen R, Wiznitzer A, Yogev Y. Is abnormal 50-g glucose-challenge testing an independent predictor of adverse pregnancy outcome? J Matern Fetal Neonatal Med. 2012;25:2583–2587. doi: 10.3109/14767058.2012.718394. [DOI] [PubMed] [Google Scholar]