Abstract
Objectives:
The purpose of this experimental study was to compare the disinfection efficacy of sodium hypochlorite and peroxygenic acid (Virkon) solutions for dental stone casts contaminated with microbial strains.
Materials and Methods:
A total of 960 spherical stone beads with a diameter of 10 mm were prepared and used as carriers of bacterial inoculums. They were individually inoculated by soaking in broth culture media containing each of the four understudy microorganisms. Different concentrations of Virkon and hypochlorite solutions were prepared using distilled water and then were sprayed on the surfaces of dental casts contaminated with Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis and Candida albicans. The pour plate technique was used to evaluate the antimicrobial efficacy of each solution. Microbicidal effect (ME) was calculated according to the log10 of control colony counts minus the log10 of the remaining colony counts after the antimicrobial procedure. Statistical difference was assessed using the Kruskal Wallis and the Man Whitney U tests with a significance of 95%.
Results:
We observed different bactericidal effects of Virkon at various concentrations; 1% Virkon killed S. aureus, P aeruginosa, and Candida albicans, while 3% Virkon solution was required to kill B. subtilis. For S. aureus, P. aeruginosa, and C. albicans, no significant difference was observed between 1% Virkon and 0.525% sodium hypochlorite (P >0.05). For B. subtilis, the efficacy of 3% Virkon and 0.525% sodium hypochlorite was not significantly different (P >0.999).
Conclusion:
According to the obtained results for Virkon and based on its low toxicity and good environmental compatibility, it may be recommended as an antimicrobial disinfectant for dental stone casts as non-critical items.
Keywords: Infection control, Dental gypsum, Disinfectants, Sodium hypochlorite, Monoperoxysulfate
INTRODUCTION
Oral cavity is a potential source of infection as it harbors many microorganisms. Unsuccessful or inadequate cleaning, disinfection, or sterilization of dental instruments or impressions contaminated with pathogenic microorganisms may lead to cross-contamination. Cross-contamination refers to the transfer of pathogenic microorganisms, resulting in cross-infection [1,2].
Routine infection control and disinfection protocols have been developed in prosthodontics with particular emphasis on the disinfection of impressions and casts used for the fabrication of prostheses [3,4]. Leung and Schonfeld [5] indicated potential cross-infection between patients and dental personnel via contaminated dental casts. Porous structure and highly hydrophilic nature of dental casts enable deep penetration of microorganisms, rendering the surface disinfection techniques ineffective [6]. Cross-contamination via stone casts is possible due to the risk of transfer of infectious agents from blood and saliva to the casts via impressions, record bases, occlusion rims, and trial dentures [7, 8]. Despite effective disinfection of impressions, a cast from a properly disinfected impression may alternatively become contaminated by a technician or clinician [9]. According to Stern et al, it may be necessary to disinfect the definitive cast at least 7 times with disinfectants from the time of fabrication to delivery of complete or removable partial denture. There are studies searching for the best technique to disrupt this cycle [6, 8, 10].
Therefore, an efficient infection control protocol is necessary for dental offices and laboratories. The most frequently applied disinfection technique is via the use of chemicals. However, the criteria for an efficient disinfection are not often met. Mansfield and White [11] investigated the antimicrobial properties of sodium hypochlorite and reported that this agent reduced the bacterial count in experimental stone casts to that of negative controls in one hour. Sodium hypochlorite is known as the reference disinfectant for disinfection. However, it has several disadvantages including toxicity to humans and environment, difficult daily preparation of fresh solution, adaptation and resistance to biocides and inability to eliminate persistent pathogens in the oral cavity and environment, which is the most important drawback of this agent [10, 12, 13].
Virkon, a peroxygen-containing compound (Antec International, Sudbury, Suffolk, UK), is used as a disinfectant because of its wide spectrum of action against all major human pathogens, such as hepatitis B and HIV viruses; 1% Virkon solution is generally used to clean and disinfect medical equipment and remove blood and body fluid spillages from the skin. Virkon has been efficiently used for disinfection of impressions, burs and toothbrushes [1, 14].
It can be used in laboratories, dental offices and hospitals. Optimal properties of Virkon allow the thorough cold disinfection of medical and surgical instruments with this agent in a short time, without the risk of toxicity or other problems, no toxic vapor phase and no generation of chlorine, which is harmful for the technicians in direct contact with the waste materials. This solution is stable for 7 days, but should be discarded when the pink color fades. Other advantages of Virkon include pleasant odor and insignificant corrosive effect [14–16].
This experimental study aimed to test the hypothesis that type III stone casts contaminated with S. aureus, P. aeruginosa, B. subtilis and C. albicans can be efficiently disinfected with conventional chemical disinfectants. Hypochlorite sodium and Virkon solutions were used in different concentrations to disinfect the dental stones.
MATERIALS AND METHODS
Fabrication of samples:
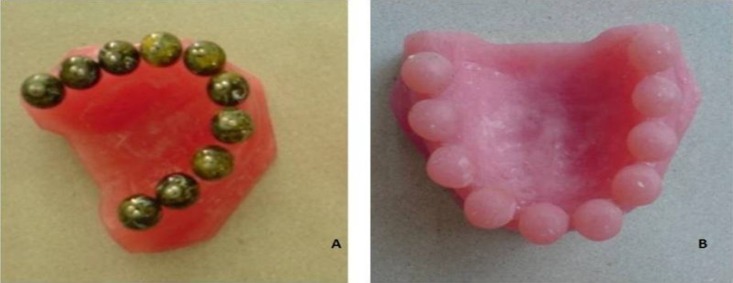
Acrylic dental arches were prepared with 10 unique spherical metal extensions 10 mm in diameter in the place of teeth (Figure 1). Then, stone casts were prepared by duplicating the acrylic models using irreversible hydrocolloid impression material (Tropicalgin, Zhermack, Italy) to make impressions and a Type III dental stone (Elite Model, Zhermack, Italy) to pour the casts, both mixed with sterile distilled water according to the manufacturer’s instructions.
Figu 1.
Dental arches with (a) metal and (b) acrylic spherical extensions 10 mm in diameter.
The casts were separated from the impressions 30 minutes after pouring and then left for 2 hours at ambient conditions. Then, the stone spherical extensions were separated from the casts by a sterile rongeur to be used as test and control samples. A total of 96 stone casts were prepared by duplicating the acrylic model according to the manufacturer’s instructions. Steel trays, bowl and other mixing tools were disinfected with 70° ethanol (Behvazan, Tehran, Iran) solution. The stone and irreversible hydrocolloid powder were not sterilized and dispensed from original manufacturing package. The acrylic models, trays, bowels, spatulas, and rongeurs were disinfected with 70° ethanol.
Bacterial strains:
The standard strains of Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Bacillus subtilis (9372) and Candida albicans (ATCC 10231) used in this project were obtained in lyophilized form (purchased from the Pasture Institute of Iran). These strains were activated by culturing in Luria Bertani (LB) agar medium (Hi Media, India) and incubated at 37°C for 24 hours. Next, 4 ml of LB medium was used to obtain individual colonies. Then, it was incubated over night at 37°C while being shaken at a speed of 200 rpm. The cells were separated using centrifugation at 4°C and at a rate of 3000 rpm for 15 minutes.
Consequently, they were cleaned by washing twice before re-suspending in Ringer’s solution to achieve bacterial concentrations ranging between 10 7–108 colony forming units (CFU)/mL.
Disinfectants:
Virkon (Antec International Ltd., Sudbury, Suffolk, UK) was obtained and various disinfectant solutions with different concentrations including 3, 2, 1, 0.5, 0.25 and 0.125% were prepared by dissolving appropriate amounts of Virkon in sterile distilled water. Sodium hypochlorite (NaOCl) (Pakshoo chemical & manufacturing Co. Iran) was obtained. The dilutions of 0.525% 0.262%, 0.131% 0.065%, 0.0325% and 0.016% were prepared using sterile distilled water [14,17].
Antimicrobial testing:
Susceptibility testing was performed according to the Association of Official Analytical Chemists (AOAC) Use Dilution Method [18]. Stone cast samples in the same shape and size used as the carriers of bacterial inoculum were inoculated by soaking in broth cultures containing 107–108 CFUs/mL of each bacterial strain for 15 minutes, yielding approximately 5 ×106 CFUs/mL.
The carriers were removed with a sterile hooked inoculating needle and left to dry for 40 minutes at 36 ± 1°C in a Petri dish matted with two filter paper sheets.
After drying, sodium hypochlorite and Virkon solutions were sprayed individually on the surfaces of the inoculated carriers and left at room temperature for 5 minutes to dry. Samples were then removed carefully and placed in sterile tubes containing 10 mL of neutralizing broth (Letheen Broth/DIFCO). After 20 minutes, each carrier was removed and placed in new sterile tubes containing the sterile nutrient broth culture medium. One-milliliter of nutrient broth from each tube was then transferred into 20 cm petri dishes and topped up to 20 mL with nutrient agar culture medium (pour plating method). After 24 hours of incubation at 37°C, the total number of colonies in each plate (duplicate counts) was counted and expressed as their mean in CFUs/mL.
All experiments were performed on three separate occasions. In each test, positive (containing microbial inoculum without exposure to the disinfectant) and negative samples (without microbial inoculums) were used as controls.
Overall, 16 dental cast samples were used for each disinfectant group (six different concentrations and two controls to be tested in duplicate) against each microorganism.
Results of the test were expressed as the microbicidal effect (ME), which is the log10 value of the counts after exposure to the test biocide subtracted from the log10 value of the counts without exposure to the disinfection procedure as the positive control.
The efficacy of the inactivation fluid was examined by performing parallel counts of positive controls [18, 19]. The comparisons were made based on the mean ME values. ME values equal to or more than 5 were considered as effective. Statistical assessments were performed by SPSS version 13. The Kruskal Wallis test was used to analyze the microbicidal effect of sodium hypochlorite and Virkon against the microbial strains. The Mann Whitney U test was used to compare the microbicidal effect of hypochlorite on Virkon. Statistical significance was set at P value < 0.05.
RESULT
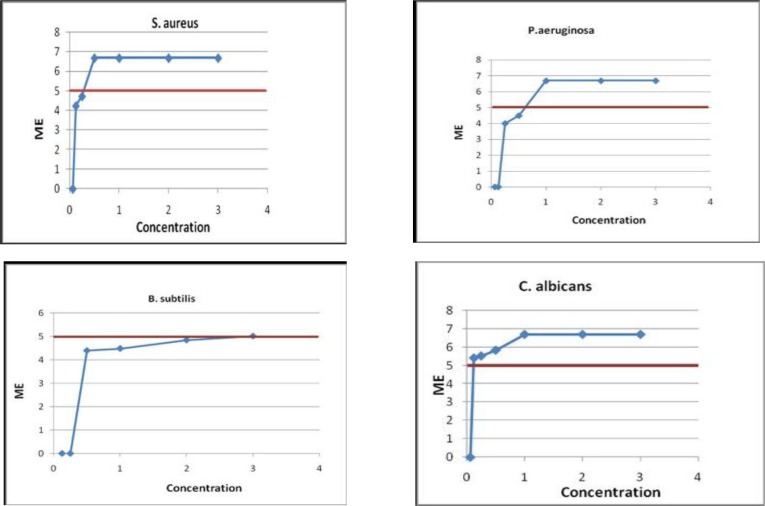
The microbicidal activity of Virkon in different concentrations against P. aeruginosa, S. aureus, B. subtilis and C. albicans is shown in Figure 2. The mean total number of microorganisms in inoculated casts was approximately 5 × 106 CFU/mL for all tested microorganisms. The corresponding counts before and after Virkon spray at different concentrations were also indicated in Table 1 for P. aeruginosa, S. aureus, B. subtilis and C. albicans.
Fig 2.
Microbicidal activity of Virkon with different concentrations against microbial strains
Table 1.
Microbicidal effect of Virkon against the microbial strains
| MO * | Untreated Mean Count | Mean counts before and after Virkon treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| ME ** 3% | Mean Count | ME 2% | Mean Count | ME 1% | Mean Count | ME 0.5% | Mean Count | ME 0.25% | Mean Count | ME 0.125% | Mean Count | ||
| B. subtilis | 5 × 10 6 | 5.02 | 4.6× 10 1 | 4.85 | 6.8× 10 1 | 4.49 | 1.55× 10 2 | 4.4 | 1.66× 10 2 | 0 | 5× 10 6 | 0 | 5× 10 6 |
| P. aeruginosa | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 6.69 | 0 | 4.49 | 1.56× 10 2 | 4 | 4.89× 10 2 | 0 | 5× 10 6 |
| S. aureus | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 6.69 | 0 | 6.69 | 9× 10 1 | 4.73 | 2.78× 0 2 | 4.24 | 5× 10 6 |
| C. albicans | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 6.69 | 0 | 5.83 | 0.7 × 10 1 | 5.52 | 1.4× 10 1 | 5.41 | 1.9× 10 1 |
Microorganism
Microbicidal effect
As seen in Table 1, spraying Virkon in the concentration of 0.125 % and above successfully disinfected the samples inoculated with C. albicans compared to untreated samples.
Virkon in the concentration of 1% caused complete disinfection of samples inoculated with S. aureus and P. aeruginosa. The concentration of 1% of Virkon did not have any effect on B. subtilis (var niger). The acceptable microbicidal effect with ME more than 5 was only observed in the concentration of 3%.
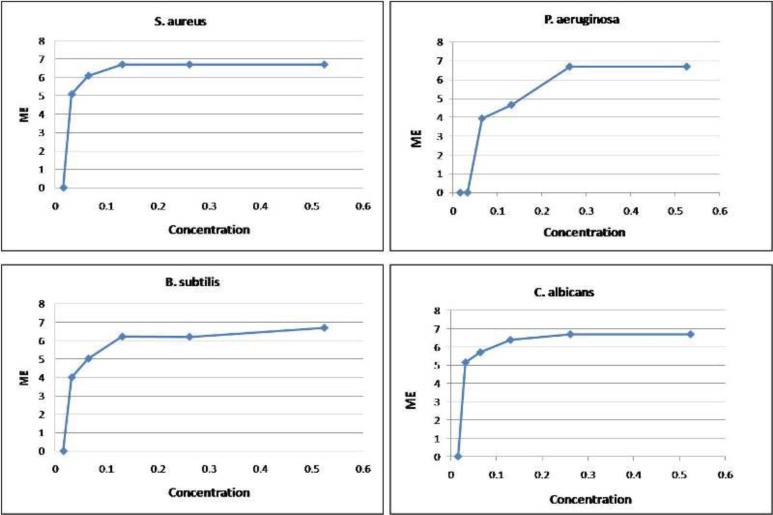
Figure 3 and Table 2 indicate the results of antimicrobial tests, which were carried out to determine the minimum microbicidal concentration of sodium hypochlorite against the selected bacterial strains.
Fig 3.
Microbicidal activity of sodium hypochlorite at different concentrations against microbial strains
Table 2.
Microbicidal effect of hypochlorite against the microbial strains
| MO * | Untreated Mean Count | Mean counts before and after hypochlorite treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| ME ** 0.525% | Mean Count | ME 0.262% | Mean Count | ME 0.131% | Mean Count | ME 0.065% | Mean Count | ME 0.0325% | Mean Count | ME 0.0162% | Mean Count | ||
| B. subtilis | 5 × 10 6 | 6.69 | 0 | 6.2 | 0.3× 10 1 | 6.1 | 0.4× 10 1 | 5.02 | 4.5× 10 1 | 0 | 5× 10 6 | 0 | 5× 10 6 |
| P. aeruginosa | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 4.66 | 1.1× 10 2 | 3.94 | 5.59× 10 2 | 0 | 5× 10 6 | 0 | 5× 10 6 |
| S. aureus | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 6.69 | 0 | 6.08 | 0.4× 10 1 | 5.08 | 4× 0 1 | 0 | 5× 10 6 |
| C. albicans | 5 × 10 6 | 6.69 | 0 | 6.69 | 0 | 6.38 | 0.2× 10 1 | 5.69 | 1 × 10 1 | 5.15 | 3.4× 10 1 | 0 | 5 × 10 6 |
Microorganism
Microbicidal effect
According to the results, aqueous 0.062% sodium hypochlorite spray disinfected all samples inoculated with P. aeruginosa, S. aureus, B. subtilis and C. albicans. Consistent with Virkon spray results, P. aeruginosa and B. subtilis were the most resistant species in the chemical disinfection procedure compared to S. aureus and C. albicans, as 5 log10 reduction in microbial counts of S. aureus and C. albicans was achieved even after exposure to 0.016 dilution; whereas this reduction just happened after exposure to 0.131% and 0.262 % for B. subtilis and P. aeruginosa, respectively. In order to precisely assess the results, we used several statistical tests.
Using the Kruskal Wallis test, we found that antibacterial efficacy of Virkon and hypochlorite disinfectants with different concentrations had significant differences for the four types of microbial strains.
For P. aeruginosa, antibacterial efficacy of 0.262 and 0.525% sodium hypochlorite was not significantly different (P>0.999); while significant differences were noted with concentrations less than 0.262% (P =0.01). For the same bacteria, antibacterial efficacy of 1, 2 and 3% Virkon was not significantly different (P>0.999); while the mentioned concentrations had significant differences with 0.25 and 0.5% Virkon in this regard (P<0.001). The same pattern as observed for P. aeruginosa was noted for the antibacterial activity of the disinfectants against C. albicans.
For S. aureus, 0.065, 0.131, 0.262 and 0.525% sodium hypochlorite were not significantly different in terms of antibacterial efficacy (P>0.999); while concentrations less than 0.065% showed a significantly different activity (P =0.04).
For Virkon, 0.5, 1, 2 and 3% concentrations did not have significantly different antibacterial efficacy (P>0.999); while concentrations less than 0.5% showed significantly different activity (P<0.001).
For B. subtilis, 0.525% sodium hypochlorite and 3% Virkon were the only disinfectants that exhibited antibacterial activity in each group and therefore were significantly different from other concentrations (P =0.01).
In addition, there was no significant difference between these two disinfectants at the above-mentioned concentrations (P>0.999).
DISCUSSION
Dental patients and dental health-care personnel may be exposed to a variety of microorganisms transmitted via blood or oral and respiratory secretions. Potential sources of microbial transmission are dental impressions and casts made from impressions [20]. Infections may be transmitted to dental clinicians via different routes including: (i) direct contact with blood, oral fluids, or other secretions and (ii) indirect contact with contaminated instruments, casts, equipment and so on [1]. The aim of this study was to determine the effectiveness of two disinfectants for disinfection of the gypsums contaminated with microbial strains. Both sodium hypochlorite and Virkon caused a great reduction in colony counts. However, the impact of sodium hypochlorite was slightly greater than that of Virkon. There were differences in bactericidal effect of Virkon at different concentrations. One percent concentration of Virkon killed S. aureus, P. aeruginosa, and C. albicans, but was not effective on B. subtilis. This particular bacterium was killed using 3% Virkon. The difference between sodium hypochlorite and Virkon was only on their effectiveness against the B. subtilis, which forms resistant spores [15].
As a rule of thumb, the antimicrobial efficacy is satisfactory when the tested chemical disinfectant causes a reduction of 105 or more CFUs/mL in viable counts (ME≥5). Evaluation of the antimicrobial effect of Virkon spray on P. aeruginosa showed no growth in 3, 2 and 1% concentrations. But ME was 4.49 in its 0.5% concentration.
Therefore, 1% Virkon was totally effective against P. aeruginosa. On the other hand, observations of the effectiveness of sodium hypochlorite indicated no growth in 0.525% and 0.262% concentrations of sodium hypochlorite after application on P. aeruginosa, but 0.131% sodium hypochlorite decreased ME to 4.66 in bacterial colonies.
Hernndez et al, [15] using the suspension tests showed that 1% Virkon was effective against S. aureus and P. aeruginosa in 5 minutes. These results were also seen in a study by Gasparini et al. They found that S. aureus was more susceptible to the disinfectant than P. aeruginosa. A reason for this can be the difference in the composition of cell wall of the Gram positive and Gram negative bacteria. T lipopolysaccharide layer in Gram negative bacteria such as P. aeruginosa confers a high resistance against chemical agents and toxins [15, 21].
In the current study, 0.525% sodium hypochlorite solution was observed to kill all the B. subtilis colonies. In 0.262, 0.131 and 0.062% concentrations of sodium hypochlorite, the colonies decreased to less than 5 log10. In 0.0325% concentartion, there was no decrease in the colony count; 3% Virkon caused a reduction of 105 log10. In 2, 1, 0.5, 0.25% concentrations, ME was less than 5. Therefore, 1% solution of Virkon is not effective against spores due to the same reason described above. Spores are highly resistant to the environmental fluctuations such as lack of humidity, toxic chemicals, radiations and high temperatures [15]. Previously, Hernandez et al. [15] showed remarkable efficacy of 1% Virkon on bacteria but not on spores [9]. These results were also observed by Gasparini et al [21]. In a study by Angellillo et al [22], Virkon was effective on spores after 18 hours. ME of hypochlorite on C. albicans in 0.262% and higher concentrations was greater than 5. Our data showed that ME of 1% and higher concentrations of Virkon was higher than 5. Thus the fungicidal effect of Virkon was acceptable. The above-mentioned results are convincing in view of the fact that the study was intentionally designed in such a way that maximum amounts of bacteria were transmitted to the casts. In the clinical setting, irrespective of the microbiological aspects, the impression is rinsed prior to eventual chemical disinfection which leads to a significant reduction in the bacterial count and consequently to an increase in the efficacy of subsequent disinfection [9]. The choice of a specific disinfectant depends on a number of factors that should be considered, including toxicity for patients and the staff, possible damage to equipments, costs, stability, the required magnitude of antimicrobial efficacy and the capability of quick removal of microorganisms. The disinfectant must have fast bactericidal activity in presence of blood and biological fluids [22]. For the clinical use, some differences may be considered with respect to the claimed disadvantages of sodium hypochlorite including its bleaching effect, denture and metal corrosion, and odor [23, 24]. Moslehifard et al. [25] evaluated the effect of 0.525% hypochlorite and 1% Virkon on hardness of dental gypsum casts. They found that the formation of micropores is responsible for the reduction of hardness, which is the least in the dental stones disinfected with Virkon. In another study, Moslehifard et al. [26] evaluated the effect of 0.525% hypochlorite and 1% Virkon on mechanical properties of dental gypsum casts such as compressive and tensile strengths. They found that Virkon disinfectant only slightly decreased the mechanical strength. Considering other advantages of Virkon as a disinfectant as well as minimal reduction in the mechanical strength of gypsum, it may be preferred for the disinfection of stone casts in the clinical and laboratory settings.
CONCLUSION
Antibacterial efficacy of Virkon and sodium hypochlorite was examined and compared for dental casts. Virkon at various concentrations showed different bactericidal effects, as 1% Virkon killed S. aureus, P. aeruginosa, and C. albicans, while 3% Virkon solution was required to kill B. subtilis. Also, 0.062% sodium hypochlorite spray disinfected all samples inoculated with P. aeruginosa, S. aureus, B. subtilis and C. albicans. Based on our results, Virkon can be considered as an appropriate disinfectant for dental casts, which have not been in direct contact with the saliva and are not considered critical dental items. Based on the low toxicity and good environmental compatibility of Virkon, it may be used as an antimicrobial agent for disinfection of dental stone casts as non-critical items.
ACKNOWLEDGMENTS
This article was written based on a dataset from two DDS theses entitled “Comparison between spray of 0.525% sodium hypochlorite and 1% virkon in disinfectation of the type III dental stone casts contaminated with microbial strains (Pseudomonasaeroginosa, Staphylococcus aureus, Candida albicans, Basillus subtilis)” registered at Tabriz University of Medical Sciences Faculty of Dentistry. The thesis was supported by the Vice Chancellor for Research at Tabriz University of Medical Sciences.
REFERENCES
- 1-. Polat Z, Tacir I, Değer Y, Özekinci T. Microbiological evaluation of dental airturbine handpieces after different disinfection prosedures. Biotechnol. & Biotechnol 2006; 20 ( 3): 160– 165. [Google Scholar]
- 2-. Pereira Rde P, Lucas MG, Spolidorio DM, Ariolio Filho JN. Antimicrobial activity of disinfectant agents incorporated into type IV dental stone. Gerodontology. 2012. June; 29 ( 2): e267– 74. [DOI] [PubMed] [Google Scholar]
- 3-. Bhat V, Shenoy K, Shetty S. Evaluation of efficacy of microwave oven irradiation in disinfection of patient derived dental cast. Int J Infect Cont 2012; 8: 1– 4. [Google Scholar]
- 4-. Anaraki RM, Moslehifard E, Aminifar S, Ghanati H. Effect of microwave disinfection on compressive and tensile strengths of dental stones. J Dent Res Dent Clin Dent Prospects. 2013. Winter; 7 ( 1): 42– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5-. Leung RL, Schonfeld SE. Gypsum casts as a potential source of microbial cross-contamination. J Prosthet Dent. 1983. February; 49 ( 2): 210– 1. [DOI] [PubMed] [Google Scholar]
- 6-. Breault LG, Paul JR, Hondrum SO, Christensen LC. Die stone disinfection: incorporation of sodium hypochlorite. J Prosthodont. 1998. March; 7 ( 1): 13– 6. [DOI] [PubMed] [Google Scholar]
- 7-. Stern MA, Johnson GH, Toolson LB. An evaluation of dental stones after repeated exposure to spray disinfectants. Part I: abrasion and compressive strength. J Prosthet Dent. 1991. May; 65 ( 5): 713– 8. [DOI] [PubMed] [Google Scholar]
- 8-. Abdullhh AM. Surface dental, compressive strength, and dimentional accuracy of gy psum casts after repeated immersion in hypochlorite. J Prosthet Dent. 2006. June; 95 ( 6): 462– 8. [DOI] [PubMed] [Google Scholar]
- 9-. Berg E, Nielson O, Skuag N. High-level microwave disinfection of dental casts. Int J Prosthodont. 2005. Nov-Dec; 18 ( 6): 520– 5. [PubMed] [Google Scholar]
- 10-. Shultz J. Limitations of Sodium Hypochlorite as a Disinfectant: AJKD 2007; 49 (4): B76. [Google Scholar]
- 11-. Mansfield SM, White AM. Antimicrobial effects from incorporation of disinfectants into gypsum casts. Int J Prosthodont. 1991. Mar-Apr; 4 ( 2): 180– 5. [PubMed] [Google Scholar]
- 12-. Egusa H, Watamoto T, Abe K, Kobayashi M, Kaneda Y, Ashida S, et al. An analysis of the persistent presence of opportunistic pathogens on patient-derived dental impressions and gypsum casts. Int J Prosthodont. 2008. Jan-Feb; 21 ( 1): 62– 8. [PubMed] [Google Scholar]
- 13-. kumar RN, Reddy SM, Karthigeyan S, Punithavathy R, Karthik KS, Manikandan R. The effect of repeated immersion of gypsum cast in sodium hypochlorite and glutaraldehyde on its physical properties: An in vitro study. J Pharm Bioallied Sci. 2012. August; 4 ( Suppl 2): S353– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14-. Fraise AP. Choosing disinfectant. J Hosp Infect. 1999. December; 43 ( 4): 255– 64. [DOI] [PubMed] [Google Scholar]
- 15-. Herńndez A, Martró E, Matas L, Martín M, Ausina V. Assessment of in vitro efficacy of 1% virkon against bacteria, fungi viruses and spores by means of AFNOR guidelines. J Hosp Infect. 2000. November; 46 ( 3): 203– 9. [DOI] [PubMed] [Google Scholar]
- 16-. McDonnell G, Burke P. Disinfection: is it time to reconsider Spaulding? J Hosp Infect. 2011. July; 78 ( 3): 163– 70. [DOI] [PubMed] [Google Scholar]
- 17-. Robati MA, Lotfipour F, Moslehifard E, Momtaheni A, Sigari P. Effect of different energy levels of microwave on disinfection of dental stone casts. J Dent Res Dent Clin Dent Prospects. 2013; 7 ( 3): 140– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18-. Guimarães MA, Tibana A, Nunes MP, Santos KR. Disinfectant and antibiotic activities: a comparative analysis in Brazilian hospitals bacterial isolates, Braz. J. Microbiol. 2000; 31 ( 3): 196– 199. [Google Scholar]
- 19-. Vizcaino-Alcaide MJ, Herruzo-Cabrera R, Fernandez-Acenäero MJ. Comparison of the disinfectant efficacy of Perasafe and 2% glutaraldehyde in in vitro tests. J Hosp Infect. 2003. February; 53 ( 2): 124– 8. [DOI] [PubMed] [Google Scholar]
- 20-. Davis DR, Curtis DA, White DM. Microwave irradiation of contaminated dental casts. Quintessence Int. 1989. August; 20 ( 8): 583– 5. [PubMed] [Google Scholar]
- 21-. Gasparini R, Pozzi T, Magnelli R, Fatighenti D, Giotti E, Poliseno G, et al. Evaluation of in vitro efficacy of the disinfectant Virkon. Eur J Epidemiol. 1995. April; 11 ( 2): 193– 7. [DOI] [PubMed] [Google Scholar]
- 22-. Angelillo IF, Bianco A, Nobile CG, Pavia M. Evaluation of the efficacy glutaraldeheyde and peroxygen for disinfection of dental instrument. Lett Appl Microbiol. 1998. November; 27 ( 5): 292– 6. [PubMed] [Google Scholar]
- 23-. Baysan A, Whiley R, Wright PS. Use of microwave energy to disinfect a long-term lining material contaminated with candida albicans or staphylococcus aureus. J Prosthet Dent. 1998. April; 79 ( 4): 454– 8. [DOI] [PubMed] [Google Scholar]
- 24-. Robati Anaraki M, Lotfipour F, Moslehifard E, Momtaheni A, Sigari P. Effect of different energy levels of microwave on disinfection of dental stone casts. J Dent Res Dent Clin Dent Prospects. 2013; 7 ( 3): 140– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25-. Moslehifard E, Nasirpouri F, Gasemzadeh S. Effect of disinfectants on the hardness of dental stones. J Islamic Dent Assoc Iran 2013; 25 ( 2): 127– 133. [Google Scholar]
- 26-. Moslehifard E, Nasirpouri F, Maboub F, Salehjou M, Ghasemzadeh S, Bahari M. Influence of chemical disinfection on mechanical and structural properties of type III and IV dental stones. Adv Appl Ceram 2012; 111 ( 8): 450– 458. [Google Scholar]