Abstract
Excessive alcohol consumption can lead to brain tissue damage and cognitive dysfunction. It has been shown that heavy drinking is associated with an earlier onset of neurodegenerative diseases such as Alzheimer's disease. Acetaldehyde, the most toxic metabolite of ethanol, is speculated to mediate the brain tissue damage and cognitive dysfunction induced by the chronic excessive consumption of alcohol. However, the exact mechanisms by which acetaldehyde induces neurotoxicity are not totally understood. In this study, we investigated the cytotoxic effects of acetaldehyde in SH-SY5Y cells and found that acetaldehyde induced apoptosis of SH-SY5Y cells by downregulating the expression of antiapoptotic Bcl-2 and Bcl-xL and upregulating the expression of proapoptotic Bax. Acetaldehyde treatment led to a significant decrease in the levels of activated Akt and cyclic AMP-responsive element binding protein (CREB). In addition, acetaldehyde induced the activation of p38 mitogen-activated protein kinase (MAPK) while inhibiting the activation of extracellular signal-regulated kinases (ERKs, p44/p42MAPK). Meanwhile, acetaldehyde treatment caused an increase in the production of reactive oxygen species and elevated the oxidative stress in SH-SY5Y cells. Therefore, acetaldehyde induces cytotoxicity of SH-SY5Y cells via promotion of apoptotic signaling, inhibition of cell survival pathway, and induction of oxidative stress.
1. Introduction
Excessive alcohol consumption can cause brain tissue damage and cognitive dysfunction [1]. It has been reported that heavy drinking is associated with an earlier onset of neurodegenerative diseases such as Alzheimer's disease (AD) [2]. Acetaldehyde, the most toxic metabolite of ethanol, is speculated to mediate the brain tissue damage and cognitive dysfunction induced by the chronic excessive consumption of alcohol. The major enzyme that generates acetaldehyde during ethanol metabolism in liver is alcohol dehydrogenase. Catalase and cytochrome p450 2E1 are additional enzymes that are important for the local formation of acetaldehyde in the brain [3]. Acetaldehyde can also be directly ingested from foods such as alcohol beverages, fruit juice, and yogurt or from tobacco smoke [4]. The principle enzyme responsible for the detoxification and metabolism of acetaldehyde into acetate is aldehyde dehydrogenase (ALDH) 2. Thus, the local accumulation of acetaldehyde depends on the amount of exposure and the rates of its formation and clearance. Indeed, it has been found that the levels of acetaldehyde in blood are much higher in individuals with defective ALDH2 in comparison with normal individuals after alcohol ingestion [5].
The generation and accumulation of acetaldehyde by local metabolism in brain may contribute to the synaptic dysfunction. It has been demonstrated that acetaldehyde mediates the acute inhibition of long-term potentiation by ethanol in the CA1 region of rat hippocampal slices [6]. The tissue damage caused by acetaldehyde is largely mediated by the direct cytotoxicity caused by acetaldehyde. In rat embryos, acetaldehyde treatment induces marked cell death in several tissues including neuroepithelium, correlating to the malformations seen in fetal alcohol syndrome (FAS) [7]. Similarly, exposure to acetaldehyde inhibits cell growth in primary cultures of rat astrocytes, presumably via apoptotic pathway [8]. Induction of mitochondria dysfunction and overproduction of reactive oxygen species (ROS) have been linked to the action of acetaldehyde [9, 10]. The redox imbalance induced by acetaldehyde is accompanied by a transient reduction in the protein content of mitochondrial superoxide dismutase SOD2 [11]. In rat cerebellar neuron cultures, acetaldehyde treatment causes decrease in cell viability while impairing mitochondrial function and significantly elevating markers of oxidative stress including 4-hydroxy-2-nonenal and 8-hydroxydeoxyguanosine [12]. In addition, it has been shown that acetaldehyde induces cytotoxic effects of neuronal cells by activating apoptotic signals such as cytochrome c release and caspase 3 activation [13].
Above evidence suggests acetaldehyde may cause neurotoxic effects by promoting oxidative stress and apoptotic signals. However, the exact molecular mechanisms of acetaldehyde-induced neurotoxicity are not totally understood. There are few studies on whether acetaldehyde affects pathways that are essential for cell survival such as the activation of Akt (a serine/threonine kinase or protein kinase, PKB) and cyclic AMP-responsive element binding protein (CREB). In this study, we investigated the cytotoxic effects of acetaldehyde in SH-SY5Y cells and found that acetaldehyde induced apoptosis of SH-SY5Y cells by downregulating the expression of antiapoptotic Bcl-2 and Bcl-xL and upregulating the expression of proapoptotic Bax. Acetaldehyde treatment led to a significant decrease in the levels of activated Akt and CREB. In addition, acetaldehyde induced the activation of p38 mitogen-activated protein kinase (MAPK) while inhibiting the activation of extracellular signal-regulated kinases (ERKs, p44/p42MAPK). Meanwhile, acetaldehyde treatment led to elevated production of ROS and oxidative stress in SH-SY5Y cells. Therefore, acetaldehyde induces cytotoxicity of SH-SY5Y cells via promotion of apoptotic signaling, inhibition of cell survival pathway, and induction of oxidative stress.
2. Materials and Methods
2.1. Materials
Fetal bovine serum (FBS), streptomycin, and penicillin were purchased from Thermo Scientific (Rockford, IL, USA). Dulbecco's modified Eagle's medium (DMEM), trypsin, and reverse-transcription reaction system were purchased from Invitrogen (Eugene, OR, USA). Caspase 3 activity kit, BCA protein assay kit, DMSO, BeyoECL plus Western blotting detection system, anti-Akt, and goat antirabbit IgG (H+L) antibodies were purchased from Beyotime Institute of Biotechnology (Haimen, China). MDA assay kit and GSH assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Brandford protein assay kit, anti-phospho-Akt (Ser 473) antibody, agarose, primers, and PCR reaction system were purchased from Sangon Biotech (Shanghai, China). 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA) was purchased from Sigma Chemical (St. Louis, MO, USA). Antibodies for CREB, phospho-CREB (Ser 133), p38MAPK, phospho-p38MAPK (Thr180/Tyr182), JNK, phospho-JNK (Thr183/Tyr185), p44/p42 MAPK, and phospho-p44/p42MAPK (Thr202/Tyr204) were purchased from Cell Signaling Technology (Beverly, MA, USA).
2.2. Cell Viability Assay
Human neuroblastoma SH-SY5Y cells were maintained in DMEM with 10% FBS and 1% streptomycin/penicillin in a CO2 incubator at 37°C, with 5% CO2 and 95% air. Cells were seeded in 12-well plates with a density of 4 × 104 per well. The viability of cells was checked by trypan blue exclusion assay.
2.3. Hoechst 33258 Nuclear Staining Assay
Apoptotic cells were detected by Hoechst 33258 nuclear staining assay. Cells were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature after 24 h treatment of acetaldehyde. The fixed cells were incubated with Hoechst 33258 for 5 min at room temperature and subsequently washed with PBS for three times. The fluorescence was examined using an Olympus BX53 fluorescence microscope.
2.4. Caspase 3 Activity
The activity of caspase 3 was determined using a caspase 3 activity kit according to the manufacturer's instructions. After treatments, cells were harvested by digesting with trypsin and the cell lysates were prepared. To evaluate the activity of caspase 3, cell lysates were incubated with Ac-DEVD-pNA and reaction buffer for 10 h at 37°C. Substrate cleavage was then measured using a spectrometer at 405 nm.
2.5. Measurement of Intracellular Oxidation Stress
Fluorescent probe DCFH-DA was used to determine the intracellular generation of ROS. After 1 h treatment with 10 mM acetaldehyde, cells were rinsed three times with PBS and incubated with 5 μM DCFH-DA at 37°C for 30 min. The fluorescence was examined using an Olympus BX53 fluorescence microscope. The concentrations of malondialdehyde (MDA) and glutathione (GSH) were determined using commercial available kits according to the manufacturer's instructions.
2.6. RT-PCR
Total RNA was isolated using RNAiso Plus reagent (Takara Biotechnology (Dalian) Co., Ltd.) and converted to cDNA using reverse-transcription reaction system. PCR was then performed to determine the expression of Bcl-2, Bcl-xL, and Bax. The housekeeping gene GAPDH was used as the internal control. The sequences of forward and reverse primers used were ACC ACA GTC CAT GCC ATC AC and ACC TTG CCC ACA GCC TTG for GAPDH; CCA GCT GCC TTG GAC TGT GT and GGT TTA TTA CCC CCT CAA GAC CAC for Bax; GGA GGA TTG TGG CCT TCT TG and GG TGC CGG TTC AGG TAC TCA for Bcl-2; and TTG GAC AAT GGA CTG GTT A and GTA TAG TGG ATG GTC AGT G for Bcl-xL. The PCR conditions were 95°C for 5 min followed by 35 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min followed by final extension at 72°C for 10 min for GAPDH; 94°C for 3 min followed by 25 cycles at 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min followed by final extension at 72°C for 5 min for Bcl-2; 95°C for 5 min followed by 40 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min followed by final extension at 72°C for 10 min for Bcl-xL; and 94°C for 5 min followed by 30 cycles at 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min followed by final extension at 72°C for 5 min for Bax. The PCR products were electrophoresed on 1.5% agarose gel, stained with ethidium bromide, and photographed under ultraviolet light.
2.7. Western Blot Analysis
After treatments, cells were washed twice with PBS and the cell lysates were prepared in cell lysis buffer (Tris 20 mM, NaCl 150 mM, EDTA 1 mM, sodium pyrophosphate 2.5 mM, NaF 20 mM, β-glycerophosphoric acid 1 mM, and sodium orthovanadate 1 mM). The supernatants were collected after centrifugation at 14000 ×g for 10 min at 4°C and the protein concentration of the supernatants was measured. 20 μg of protein extracts was resolved by SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membrane and subsequently incubated with specific primary antibodies. PVDF membrane was washed by Tris buffered saline (TBS) containing 0.1% Tween-20 for three times. For detection, the PVDF membrane was incubated with a horseradish peroxidase-coupled secondary antibody, followed by an enhanced chemiluminescence substrate reaction using BeyoECL plus Western blotting detection system.
2.8. Statistical Analysis
All the experiments were carried out in triplicates. Quantitative data are represented as the mean ± SD. Student's t-test was used to compare the difference between the control and treatment groups. P values of 0.05 were considered statistically significant.
3. Results
3.1. Acetaldehyde Induces Apoptosis and Affects the Gene Expression of Bcl-2 Family Proteins
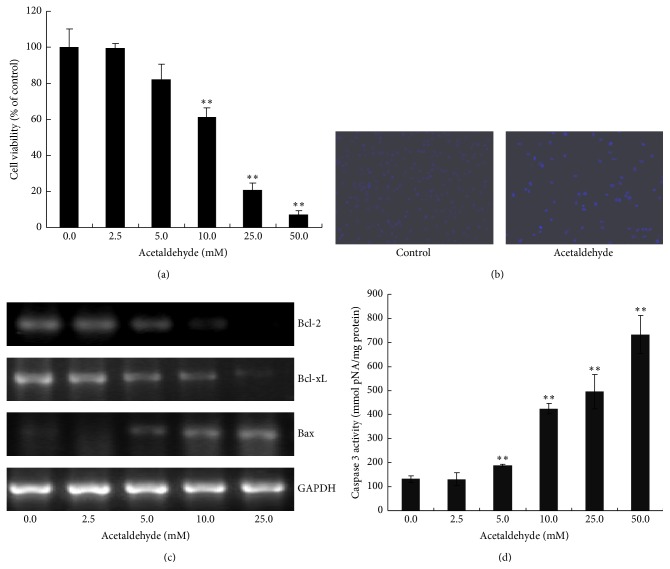
We first examined the effect of acetaldehyde on the cell viability of SH-SY5Y cells. As shown in Figure 1(a), acetaldehyde decreased the viability of SH-SY5Y cells significantly and in a concentration-dependent manner, suggesting that acetaldehyde induced cytotoxicity of SH-SY5Y cells. Hoechst 33528 staining of cells treated with 10 mM of acetaldehyde for 24 h demonstrated that acetaldehyde induced apoptosis of SH-SY5Y cells (Figure 1(b)).
Figure 1.
Effects of acetaldehyde on the cell viability and apoptosis of SH-SY5Y cells. Cells were incubated with various concentrations of acetaldehyde for 24 h. (a) Cell viability was determined with trypan blue assay. The number of cells in control group is set to 100%. (b) The cells were fixed and stained with Hoechst 33258. (c) SH-SY5Y cells were treated with different concentration of acetaldehyde for 24 h. Total RNA was isolated after the treatment and the expression of Bcl-2, Bcl-xL, and Bax genes was assessed by RT-PCR. The expression of GAPDH was used as an internal control. (d) Total lysates of cells were collected and the caspase 3 activities were determined. Data are expressed as the values of concentration of pNA. Values are means ± SD, n = 3. ∗∗, significantly different from untreated cells (P < 0.01).
Proapoptotic protein Bax and antiapoptotic proteins, Bcl-2 and Bcl-xL, are Bcl-2 family proteins that regulate apoptotic pathway via affecting the permeability of the mitochondrial outer membrane [14]. We next examined the effect of acetaldehyde on the expression of these Bcl-2 family proteins. As shown in Figure 1(c), exposure of SH-SY5Y cells to acetaldehyde for 24 h resulted in the decrease in Bcl-2 and Bcl-xL mRNA levels, which were concomitant with the increase in the level of Bax. Thus, acetaldehyde treatment might promote apoptosis by decreasing the expression of antiapoptotic proteins Bcl-2 and Bcl-xL and inducing the expression of proapoptic protein Bax. Caspase 3 is a hallmark of the late apoptotic events. As shown in Figure 1(d), acetaldehyde treatment significantly increased the activity of caspase 3 and the elevation of caspase 3 activities by acetaldehyde had a dose-response effect. These data indicated that acetaldehyde treatment induced cytotoxicity and apoptosis of SH-SY5Y cells.
3.2. Effect of Acetaldehyde on the Activation of Akt and CREB Pathway
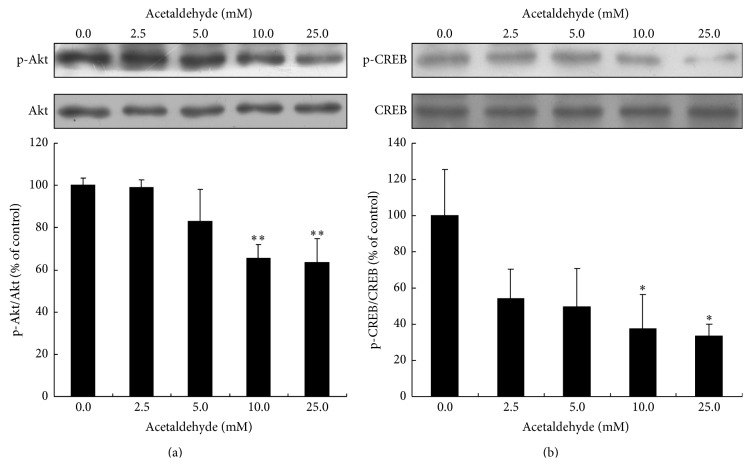
Akt/CREB pathway is important for the survival of neuronal cells [15]. To find out whether acetaldehyde affects cell survival pathway, the activation of Akt and CREB was examined by Western blot analysis. As shown in Figure 2(a), treatment of acetaldehyde (10 and 25 mM) for 24 h induced a significant decrease in the levels of activated Akt. Similarly, levels of activated CREB were also decreased by the treatment of acetaldehyde (Figure 2(b)). These data suggested that acetaldehyde may decrease cell viability and promote apoptosis by inhibiting the activation of Akt and CREB.
Figure 2.
Effects of acetaldehyde treatment on the activation of Akt and CREB in SH-SY5Y cells. SH-SY5Y cells were treated with different concentrations of acetaldehyde for 24 h. Total cell lysates were collected and the amount of Akt, phospho-Akt (Ser473) (a), CREB, and phospho-CREB (Ser 133) (b) was determined by Western blot analysis. The intensities of the bands were quantified by densitometric analyses and normalized by the amount of Akt or CREB. Values are means ± SD from three independent experiments. ∗, significantly different from control (P < 0.05); ∗∗, significantly different from control (P < 0.01).
3.3. Effect of Acetaldehyde on the Activation of MAPKs
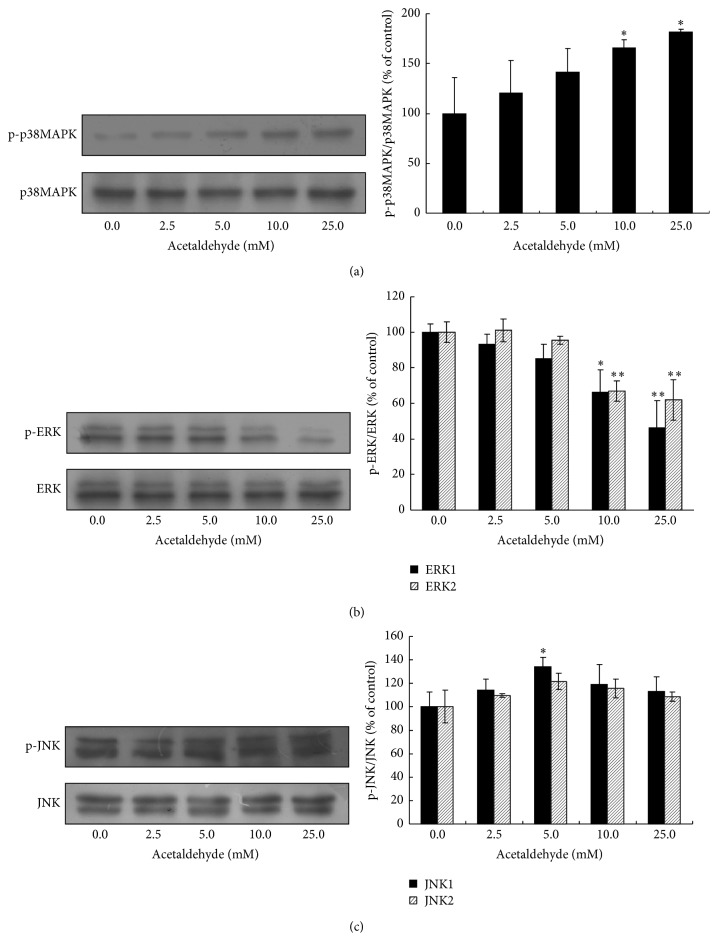
MAPKs have been shown to have important roles in promotion or inhibition of apoptosis. We next examined the effect of acetaldehyde on the activation of p38MAPK/ERK/JNK pathway by Western blot analysis. Treatment of acetaldehyde increased the levels of activated p38MAPK in a dose-dependent manner (Figure 3(a)). In contrast, acetaldehyde treatment caused a downregulation of the levels of activated ERK (Figure 3(b)). There was only a slight change in the activation of JNK after 24 h treatment of acetaldehyde (Figure 3(c)).
Figure 3.
Effects of acetaldehyde on the activation of p38MAPK/ERK/JNK in SH-SY5Y cells. SH-SY5Y cells were treated with different concentrations of acetaldehyde for 24 h. Total cell lysates were collected and the protein levels of p38MAPK, phosho-p38MAPK (Thr180/Tyr182) (a), ERK (p44/p42) MAPK and phospho-ERK (Thr202/Tyr204) (b), and JNK and phospho-JNK (Thr183/Tyr185) (c) were determined by Western blot analyses. The intensities of the bands were quantified by densitometric analyses and normalized by the amount of p38MAPK, JNK, or ERK. Values are means ± SD from three independent experiments. ∗, significantly different from control (P < 0.05); ∗∗, significantly different from control (P < 0.01).
3.4. Acetaldehyde Increases Oxidative Stress in SH-SY5Y Cells
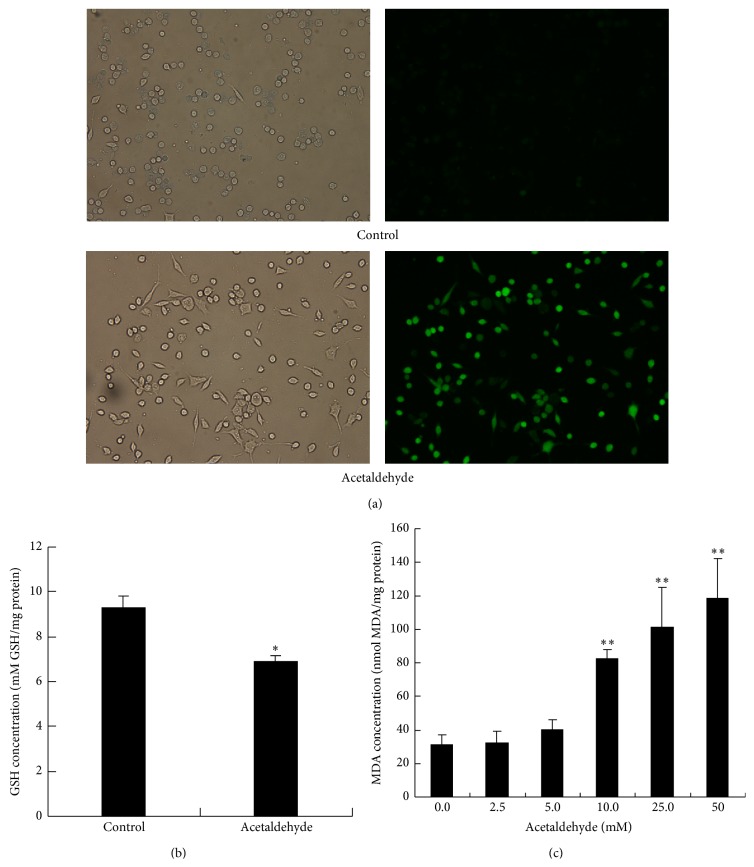
To further study the underlying mechanism of the cytotoxic effects of acetaldehyde, the effects of acetaldehyde on the redox status of the cells were studied. As shown in Figure 4(a), exposure of acetaldehyde caused a quick and dramatic increase in the production of ROS in SH-SY5Y cells. In addition, acetaldehyde treatment also induced a decrease in the concentration of tripeptide GSH (Figure 4(b)), which plays important function in the detoxification of ROS. MDA, which is a breakdown product of the oxidative degradation of cell membrane lipids, is generally considered as the marker of intracellular oxidative stress and an indicator of lipid peroxidation [16]. Thus, we next determined the concentration of MDA in acetaldehyde treated SH-SY5Y cells. As shown in Figure 4(c), MDA levels increased significantly by acetaldehyde treatment in a concentration-dependent manner. The increase of ROS and MDA levels and the decreased GSH concentration suggested that the oxidative stress was induced by the acetaldehyde treatment, which might subsequently lead to the cytotoxicity and apoptosis of SH-SY5Y cells.
Figure 4.
Acetaldehyde increases oxidative stress in SH-SY5Y cells. (a) SH-SY5Y cells were treated with 10 mM of acetaldehyde for 1 h. Cells were then stained with DCFH-DA to determine the production of ROS. Images on the left side are the phase images of the cells. (b) SH-SY5Y cells were treated with 5 mM of acetaldehyde for 2 h. The levels of reduced GSH were determined in the cell lysates. (c) SH-SY5Y cells were treated with different concentration of acetaldehyde for 24 h. MDA production was measured in the cell lysates. Values are means ± SD. ∗, significantly different from control cells (P < 0.05); ∗∗, significantly different from control cells (P < 0.01).
4. Discussion
Excessive accumulation of acetaldehyde in brain could lead to neurotoxicity and perhaps contribute to the acceleration of the development of neurodegenerative diseases; however, the exact molecular mechanisms of acetaldehyde-induced neurotoxicity are not totally understood. Here we studied the cytotoxic effects of acetaldehyde in SH-SY5Y cells and found that acetaldehyde induced cytotoxicity by promoting apoptosis and inhibiting cell survival pathways.
The role of mitochondria in the apoptotic process has been well established. Mitochondria apoptotic pathway is mainly regulated by Bcl-2 family proteins, including proapoptotic protein Bax and antiapoptotic proteins, Bcl-2 and Bcl-xL, via affecting the permeability of the mitochondrial outer membrane [14]. The balance between antiapoptotic and proapoptotic Bcl-2 family proteins determines the fate of cells including the survival of individual neurons [17–19]. Our results showed that the expression of Bcl-xL and Bcl-2 was decreased while the expression of Bax was upregulated by acetaldehyde treatment in SH-SY5Y cells. These results suggested that acetaldehyde induced apoptosis of SH-SY5Y cells though mitochondria apoptotic pathway.
The serine/threonine kinase Akt, also known as protein kinase B (PKB), is a key player in regulating cell signals that are important for cell death and survival [20]. Multiple apoptotic/survival regulating molecules are downstream substrates of Akt, for examples, Bcl-2-associated death protein (Bad) [21], caspase 9 [22], glycogen synthase kinase 3β (GSK3β) [23], and CREB [24]. The reduction of Akt activation by augmented acetaldehyde exposure has been shown in alcohol-induced myocardial dysfunction and hepatic apoptosis [25–27]. In this study, acetaldehyde treatment caused a dose-dependent inhibition of the activation of Akt in SH-SY5Y cells, suggesting that acetaldehyde might decrease the cell survival and promote apoptotic signaling by suppressing Akt activation. The phosphorylation of transcription factor CREB by Akt on Ser133 results in its transcriptional activation. Our data also showed that the levels of activated CREB were decreased by acetaldehyde treatment. CREB promotes cell survival via a transcription-dependent mechanism, upregulating the expression of antiapoptotic genes such as Bcl-2 [28, 29]. Thus, it is possible that the decrease of Bcl-2 gene expression by acetaldehyde treatment seen in our study resulted from the inhibition of acetaldehyde on Akt/CREB pathway.
MAPKs are important cell signals that are involved in both apoptosis and cell survival. It has been shown that the activation of p38MAPK and c-Jun N-terminal kinases (JNKs) promotes apoptosis [30, 31] while ERKs inhibit apoptosis [32]. There are few studies on how acetaldehyde affects the activation of MAPKs in neuronal cells. In this study, 24 h treatment of SH-SY5Y cells with acetaldehyde activated p38MAPK while inhibiting ERKs in a dose-dependent manner. The suppression of ERKs activation has been associated with the decrease of Bcl-2 expression or the ratio of Bcl-2/Bax that leads to the activation of caspase 3 [33, 34]. It is also reported that ERKs suppress the apoptosis of osteosarcoma cells induced by an acidic polysaccharide via activating Bcl-xL [35]. Thus, the inhibition of acetaldehyde on ERK activation may play a role in its modulation of the expression of Bcl-2 family proteins. As p38MAPK was shown to inhibit CREB activated Bcl-2 expression [36], the activation of p38MAPK by acetaldehyde may also contribute to the downregulation of Bcl-2 expression, potentially mediated by the inhibitory effect of p38MAPK on CREB activation. Furthermore, previous studies have shown that treating cells with ERKs inhibitor attenuates the activation of Akt [37, 38] while the activation of p38MAPK may be involved in the inactivation of PI3K/Akt signaling pathway [39]. Collectively, the evidence suggests that there may be a crosstalk between Akt-CREB and p38/ERKs pathways, and Akt/CREB may be located in the downstream of p38/ERK signaling pathway in acetaldehyde-induced apoptotic event, with p38MAPK playing a role as proapoptotic factor while ERKs acting as an antiapoptotic factor.
Activated JNKs have been shown to suppress the expression of Bcl-2, leading to the release of cytochrome c and triggering apoptosis [40]. In our study, we only found a slight increase in the activation of JNKs after 24 h exposure of acetaldehyde (5 mM). It is possible that acetaldehyde induces the activation of JNKs within short period after the treatment, as reported in the study of Lee and Shukla [41]. This is currently under investigation and the preliminary results showed that the elevation of activated JNK occurred after a short exposure (<1 h) of acetaldehyde (data not shown). And this short term activation of JNK could contribute to the downregulation of Bcl-2 expression and apoptosis induced by acetaldehyde. Overall, these data suggested that the modulation of MAPKs by acetaldehyde plays important roles in acetaldehyde-induced apoptosis in SH-SY5Y cells.
Oxidative stress has emerged as one of the important factors in neuronal cell death in neurodegenerative diseases such as AD. Accumulating evidence has shown that ethanol and acetaldehyde exposure could lead to elevated production of ROS and oxidative stress [42, 43]. Our results also showed that ROS production was significantly elevated in SH-SY5Y cells after a short time exposure of acetaldehyde. The induction of oxidative stress was also indicated by the decreased intracellular GSH content and the increased levels of MDA after acetaldehyde treatment. These data suggest that oxidative stress is one of the early events induced by acetaldehyde in the cell, and the cytotoxicity of acetaldehyde is at least partly caused by its induction of intracellular oxidative stress.
In summary, our results suggest that a complex crosstalk between signaling pathways, such as MAPKs and Akt/CREB, may act together in acetaldehyde-induced apoptotic event. And acetaldehyde induced cytotoxicity of SH-SY5Y cells via promotion of apoptotic signaling, inhibition of cell survival pathway, and induction of oxidative stress. The study also provides evidence that inhibition of oxidative stress by antioxidants may be beneficial for preventing neuronal damage associated with acetaldehyde-induced cytotoxicity which could be resulting from excessive alcohol consumption.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (31371082) and research fund from Harbin Institute of Technology at Weihai (HIT (WH) Y200902).
Abbreviations
- ALDH:
Aldehyde dehydrogenase
- AD:
Alzheimer's disease
- CREB:
Cyclic AMP-responsive element binding protein
- ERKs:
Extracellular signal-regulated kinases
- JNKs:
c-Jun N-terminal kinases
- MDA:
Malondialdehyde
- MAPK:
Mitogen-activated protein kinase
- ROS:
Reactive oxygen species.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Harper C. The neuropathology of alcohol-related brain damage. Alcohol and Alcoholism. 2009;44(2):136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 2.Harwood D. G., Kalechstein A., Barker W. W., et al. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer's disease. International Journal of Geriatric Psychiatry. 2010;25(5):511–518. doi: 10.1002/gps.2372. [DOI] [PubMed] [Google Scholar]
- 3.Crabb D. W., Liangpunsakul S. Acetaldehyde generating enzyme systems: roles of alcohol dehydrogenase, CYP2E1 and catalase, and speculations on the role of other enzymes and processes. Novartis Foundation Symposium. 2007;285:4–22. doi: 10.1002/9780470511848.ch2. [DOI] [PubMed] [Google Scholar]
- 4.Miyake T., Shibamoto T. Quantitative analysis of acetaldehyde in foods and beverages. Journal of Agricultural and Food Chemistry. 1993;41(11):1968–1970. doi: 10.1021/jf00035a028. [DOI] [Google Scholar]
- 5.Peng G.-S., Yin S.-J. Effect of the allelic variants of aldehyde dehydrogenase ALDH2∗2 and alcohol dehydrogenase ADH1B∗2 on blood acetaldehyde concentrations. Human Genomics. 2009;3(2):121–127. doi: 10.1186/1479-7364-3-2-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokuda K., Izumi Y., Zorumski C. F. Locally-generated acetaldehyde is involved in ethanol-mediated LTP inhibition in the hippocampus. Neuroscience Letters. 2013;537:40–43. doi: 10.1016/j.neulet.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menegola E., Broccia M. L., Di Renzo F., Giavini E. Acetaldehyde in vitro exposure and apoptosis: a possible mechanism of teratogenesis. Alcohol. 2001;23(1):35–39. doi: 10.1016/s0741-8329(00)00132-4. [DOI] [PubMed] [Google Scholar]
- 8.Holownia A., Ledig M., Braszko J. J., Ménez J.-F. Acetaldehyde cytotoxicity in cultured rat astrocytes. Brain Research. 1999;833(2):202–208. doi: 10.1016/S0006-8993(99)01529-2. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Quiroz L., Bucio L., Souza V., et al. Interleukin 8 response and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde or lipopolysaccharide. Hepatology Research. 2003;26(2):134–141. doi: 10.1016/S1386-6346(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 10.Farfán Labonne B. E., Gutiérrez M., Gómez-Quiroz L. E., et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biology and Toxicology. 2009;25(6):599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 11.Clavijo-Cornejo D., Gutiérrez-Carrera M., Palestino-Domínguez M., et al. Acetaldehyde targets superoxide dismutase 2 in liver cancer cells inducing transient enzyme impairment and a rapid transcriptional recovery. Food and Chemical Toxicology. 2014;69:102–108. doi: 10.1016/j.fct.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Tong M., Longato L., Nguyen Q.-G., Chen W. C., Spaisman A., De La Monte S. M. Acetaldehyde-mediated neurotoxicity: relevance to fetal alcohol spectrum disorders. Oxidative Medicine and Cellular Longevity. 2011;2011:13. doi: 10.1155/2011/213286.213286 [DOI] [Google Scholar]
- 13.Jung T. W., Lee J. Y., Shim W. S., et al. Adiponectin protects human neuroblastoma SH-SY5Y cells against acetaldehyde-induced cytotoxicity. Biochemical Pharmacology. 2006;72(5):616–623. doi: 10.1016/j.bcp.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Gross A., McDonnell J. M., Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes and Development. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 15.Tang M., Shi S., Guo Y., et al. GSK-3/CREB pathway involved in the gx-50's effect on Alzheimer's disease. Neuropharmacology. 2014;81:256–266. doi: 10.1016/j.neuropharm.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Finucane D. M., Bossy-Wetzel E., Waterhouse N. J., Cotter T. G., Green D. R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. The Journal of Biological Chemistry. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 18.Xu C., Wu A., Zhu H., et al. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomedicine & Pharmacotherapy. 2013;67(2):133–139. doi: 10.1016/j.biopha.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Yang D. Y., Liu X., Zhang R. F., et al. Increased apoptosis and different regulation of pro-apoptosis protein bax and anti-apoptosis protein bcl-2 in the olfactory bulb of a rat model of depression. Neuroscience Letters. 2011;504(1):18–22. doi: 10.1016/j.neulet.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 20.Hsu Y.-Y., Liu C.-M., Tsai H.-H., Jong Y.-J., Chen I.-J., Lo Y.-C. KMUP-1 attenuates serum deprivation-induced neurotoxicity in SH-SY5Y cells: roles of PKG, PI3K/Akt and Bcl-2/Bax pathways. Toxicology. 2010;268(1-2):46–54. doi: 10.1016/j.tox.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Yu F., Sugawara T., Maier C. M., Hsieh L. B., Chan P. H. Akt/Bad signaling and motor neuron survival after spinal cord injury. Neurobiology of Disease. 2005;20(2):491–499. doi: 10.1016/j.nbd.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Chuenkova M. V., Pereira M. A. PDNF, a human parasite-derived mimic of neurotrophic factors, prevents caspase activation, free radical formation, and death of dopaminergic cells exposed to the Parkinsonism-inducing neurotoxin MPP+ . Molecular Brain Research. 2003;119(1):50–61. doi: 10.1016/j.molbrainres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Stoica B. A., Movsesyan V. A., Lea P. M., IV, Faden A. I. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3β, and induction of the mitochondrial-dependent intrinsic caspase pathway. Molecular and Cellular Neuroscience. 2003;22(3):365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Zhao H., Zhang X., et al. Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Research Bulletin. 2013;96:45–53. doi: 10.1016/j.brainresbull.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Li S.-Y., Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. Journal of Molecular and Cellular Cardiology. 2008;44(6):992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Li S.-Y., Gilbert S. A. B., Li Q., Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. Journal of Molecular and Cellular Cardiology. 2009;47(2):247–255. doi: 10.1016/j.yjmcc.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo R., Zhong L., Ren J. Overexpression of aldehyde dehydrogenase-2 attenuates chronic alcohol exposure-induced apoptosis, change in akt and pim signalling in liver. Clinical and Experimental Pharmacology and Physiology. 2009;36(5-6):463–468. doi: 10.1111/j.1440-1681.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 28.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25(1):11–14. doi: 10.1016/S0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J., Ping F.-F., Lv W.-T., Feng J.-Y., Shang J. Interleukin-18 directly protects cortical neurons by activating PI3K/AKT/NF-κB/CREB pathways. Cytokine. 2014;69(1):29–38. doi: 10.1016/j.cyto.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Li F., Pan Z. Y., Wu Z. J., Wang Y. H., Cui Y. D. Activation of PI3K/Akt pathway limits JNK-mediated apoptosis during EV71 infection. Virus Research. 2014;192:74–84. doi: 10.1016/j.virusres.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Evans J., Ko Y., Mata W., et al. Arachidonic acid induces brain endothelial cell apoptosis via p38-MAPK and intracellular calcium signaling. Microvascular Research. 2015;98:145–158. doi: 10.1016/j.mvr.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang G. Y., Jin C. Y., Hou Y. Y., et al. Overexpression of Shp-2 attenuates apoptosis in neonatal rat cardiac myocytes through the ERK pathway. Experimental and Molecular Pathology. 2012;93(1):50–55. doi: 10.1016/j.yexmp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Pang W., Leng X., Lu H., et al. Depletion of intracellular zinc induces apoptosis of cultured hippocampal neurons through suppression of ERK signaling pathway and activation of caspase-3. Neuroscience Letters. 2013;552:140–145. doi: 10.1016/j.neulet.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 34.Yan J. P., Liu Q. B., Dou Y., et al. Activating glucocorticoid receptor-ERK signaling pathway contributes to ginsenoside Rg1 protection against beta-amyloid peptide-induced human endothelial cells apoptosis. Journal of Ethnopharmacology. 2013;147(2):456–466. doi: 10.1016/j.jep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z. Z., Zheng Y., Zhu R., et al. The ERK/eIF4F/Bcl-XL pathway mediates SGP-2 induced osteosarcoma cells apoptosis in vitro and in vivo. Cancer Letters. 2014;352(2):203–213. doi: 10.1016/j.canlet.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Hui K., Yang Y., Shi K., et al. The p38 MAPK-regulated PKD1/CREB/Bcl-2 pathway contributes to selenite-induced colorectal cancer cell apoptosis in vitro and in vivo. Cancer Letters. 2014;354(1):189–199. doi: 10.1016/j.canlet.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Liu J. Z., Hu J. X., et al. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radical Biology and Medicine. 2011;51(2):539–551. doi: 10.1016/j.freeradbiomed.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Guan X.-H., Fu Q.-C., Shi D., et al. Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Experimental Neurology. 2015;263:39–49. doi: 10.1016/j.expneurol.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Kim H. J., Oh J. E., Kim S. W., Chun Y. J., Kim M. Y. Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer Letters. 2008;260(1-2):88–95. doi: 10.1016/j.canlet.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Xu J., Qin X., Cai X., et al. Mitochondrial JNK activation triggers autophagy and apoptosis and aggravates myocardial injury following ischemia/reperfusion. Biochimica et Biophysica Acta. 2015;1852(2):262–270. doi: 10.1016/j.bbadis.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y. J., Shukla S. D. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. European Journal of Pharmacology. 2005;508(1–3):31–45. doi: 10.1016/j.ejphar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Gutiérrez-Ruiz M. C., Quiroz S. C., Souza V., et al. Cytokines, growth factors, and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde, and LPS. Toxicology. 1999;134(2-3):197–207. doi: 10.1016/S0300-483X(99)00044-X. [DOI] [PubMed] [Google Scholar]
- 43.Apte M. V., Phillips P. A., Fahmy R. G., et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118(4):780–794. doi: 10.1016/s0016-5085(00)70148-x. [DOI] [PubMed] [Google Scholar]