Abstract
Pancreatic ductal adenocarcinoma is the most common malignant tumor of the pancreas. The remaining pancreatic tumors are a diverse group of pancreatic neoplasms that comprises cystic pancreatic neoplasms, endocrine tumors and other uncommon pancreatic tumors. Due to the excellent soft tissue contrast resolution, magnetic resonance imaging (MRI) is frequently able to readily separate cystic from noncystic tumors. Cystic tumors are often easy to diagnose with MRI; however, noncystic non-adenocarcinoma tumors may show a wide spectrum of imaging features, which can potentially mimic ductal adenocarcinoma. MRI is a reliable technique for the characterization of pancreatic lesions. The implementation of novel motion-resistant pulse sequences and respiratory gating techniques, as well as the recognized benefits of MR cholangiopancreatography, make MRI a very accurate examination for the evaluation of pancreatic masses. MRI has the distinctive ability of non-invasive assessment of the pancreatic ducts, pancreatic parenchyma, neighbouring soft tissues, and vascular network in one examination. MRI can identify different characteristics of various solid pancreatic lesions, potentially allowing the differentiation of adenocarcinoma from other benign and malignant entities. In this review we describe the MRI protocols and MRI characteristics of various solid pancreatic lesions. Recognition of these characteristics may establish the right diagnosis or at least narrow the differential diagnosis, thus avoiding unnecessary tests or procedures and permitting better management.
Keywords: Pancreatic nodules, Malignant, Lymphoma, Benign, Magnetic resonance imaging, Adenocarcinoma, Pancreas, Neuroendocrine, Solid pseudopapillary tumor, Metastases
Core tip: In addition to pancreatic ductal adenocarcinoma other solid pancreatic lesions occur. Less common solid primary pancreatic tumors and non-neoplastic disease processes that may be diagnosed with relatively high specificity employing magnetic resonance imaging (MRI). The radiologist must be familiar with their MRI appearance to correctly diagnose them, or suggest them in the differential diagnosis when appropriate, since it may change substantially the approach, prognosis and patient management.
INTRODUCTION
Pancreatic ductal adenocarcinoma is the most common malignant tumor of the pancreas, accounting for 85%-90% of all malignant pancreatic tumors and is the 4th most common cause of cancer death worldwide[1]. The remaining 10%-15% of pancreatic tumors is a varied group of neoplasms that comprises cystic pancreatic neoplasms, endocrine tumors and other uncommon pancreatic tumors. Magnetic resonance imaging (MRI) is reliable for the characterization of solid and cystic pancreatic lesions. Due to the excellent soft-tissue contrast resolution, MRI is able to readily separate cystic from noncystic tumors. Cystic tumors are frequently easier to diagnose since they often possess typical imaging findings allowing an accurate and reliable prospective diagnosis. Noncystic noncarcinoma tumors may show a wide spectrum of imaging features, which most often are distinct from the features of ductal adenocarcinoma. Infrequently they may possess features that simulate carcinoma, and therefore clinical history and laboratory parameters are always important to be aware of. In this review we describe the typical MRI characteristics of various solid pancreatic lesions, which will aid in differentiation of adenocarcinoma from other benign and malignant entities. Recognition of these characteristics often may establish the right diagnosis or at least narrow the differential diagnosis, which will allow better patient management and avoid unnecessary tests or procedures.
We herein describe the typical MRI findings of ductal adenocarcinoma, of less common solid primary pancreatic tumors, and of non-neoplastic disease processes that may simulate ductal adenocarcinoma.
CLASSIFICATION OF PANCREATIC TUMORS
According to the World Health Organization classification, pancreatic tumors are classified depending on the cell lineage they arise from. The tumors may have an epithelial or nonepithelial origin.
Tumors with epithelial origin include the exocrine pancreas: (1) ductal cells, including ductal adenocarcinoma with its different histopathological variants, and mucinous and serous cystic tumors; (2) acinar cells, including acinar cell carcinoma (ACC) and mixed acinar-endocrine carcinoma; or (3) uncertain origin, including solid pseudopapillary tumor (SPT) and pancreatoblastoma or the endocrine pancreas (functioning and nonfunctioning tumors).
The nonepithelial tumors include neoplasms such as primary lymphoma and tumors of mesenchymal cell origin (hemangioma, lymphangioma, sarcoma, lipoma, etc.).
There are also nonpancreatic tumor lesions in origin that might involve the pancreas, including malignant lesions such as metastasis or secondary lymphoma, and benign lesions such as intrapancreatic splenule.
MRI EVALUATION OF THE PANCREAS
MRI is a reliable technique for the characterization of pancreatic lesions. The implementation of novel motion-resistant pulse sequences and respiratory gating techniques, as well as the recognized benefits of MR cholangiopancreatography, make MRI a very accurate examination for the evaluation of pancreatic masses. MRI has the distinctive ability of non-invasive assessment of the pancreatic ducts, pancreatic parenchyma, neighbouring soft tissues, and vascular network in one examination.
MRI of the pancreas should be performed with state of the art scanners using high field strength (1.5T or 3T) units[2-4] with phased-array torso coils and parallel imaging to maximize signal to noise ratio and permit for superior spatial resolution and faster acquisition times.
3T systems allow higher spatial resolution and provide the highest post-contrast imaging quality and temporal resolution of the pancreas, which is important when evaluating small focal pancreatic lesions[4], and looking for vascular involvement or encasement.
The typical MRI protocol for the assessment of the pancreas commonly includes coronal and transverse T2-weighted single-shot echo train spin echo (SS-ETSE), transverse T2-weighted fat suppressed fast spin echo or SS-ETSE, transverse in-phase and out-of-phase T1-weighted spoiled gradient echo (GRE), and transverse T1-weighted fat suppressed three-dimensional (3D) GRE images, obtained before and after contrast injection during the late arterial, portal-venous and interstitial phases. Magnetic resonance cholangiopancreatography (MRCP) is routinely added to abdominal protocols to assess ductal obstruction, dilatation or luminal outline. This sequence combination provides comprehensive evaluation of a full range of pancreatic disease processes.
T2-weighted SS-ETSE sequences such as half-Fourier acquisition snapshot turbo spin-echo offer anatomic display of the common bile duct (CBD) and pancreatic duct on coronal and transverse plane images. This sequence is important to evaluate fluid content, which most often allows clear separation of cystic and solid content lesions, as well as complications such as the assessment of the complexity of pancreatic fluid collections.
Pre- and post-contrast T1-weighted GRE sequences are typically obtained as a fat-suppressed 3D-GRE technique, allowing high-quality dynamic imaging of the pancreas. The main benefits of these sequences include the ability to obtain thinner slices (2-3 mm) and to perform multiplanar imaging, essential to assess and characterize focal pancreatic masses with < 1 cm in size and to evaluate diffuse pancreatic disease[3,5-10].
3D MRCP images are obtained in an oblique coronal projection following the plane of the main pancreatic duct, with the complementary benefit of being able to generate multiplanar maximum intensity projection and volume rendering imaging. This approach delineates longer segments of the pancreatic duct. MRCP depicts well the biliary and pancreatic ducts allowing optimal evaluation of ductal contour and dilatation, as well as abnormal duct pathways[7-9]. The combination of tissue imaging sequences and MRCP generate comprehensive information on pancreatic disease.
For the diagnosis of biliary disease, the secretin enhanced MRCP is routinely performed as part of the workup of patients with known or suspected pancreatic disease such as acute and chronic pancreatitis, congenital variants of the pancreaticoduodenal junction, and intraductal papillary mucinous neoplasms and follow-up of patients after pancreatectomy, in many centers. Secretin is well tolerated, and side effects are rarely seen[11-13].
MRI is a non-ionizing cross-sectional imaging method with a safer intra-venous contrast profile in comparison to computed tomography (CT). This is especially important in patients at higher risk of radiation injury (e.g., younger patients) especially those requiring repeated imaging follow-up.
Some gadolinium based contrast agents (GBCAs) are associated with nephrogenic systemic fibrosis. Avoidance of GBCAs exposure is the best approach for high-risk patients[14] including patients with acute or advanced chronic kidney disease. Also, is has been reported that the incidence of immediate hypersensitivity reactions to MR GBCAs was 0.079%, and the recurrence rate of hypersensitivity reactions was 30% in patients with prior reactions[15]. It is a very low percentage if we compare it to iodinate contrast media but however it should be considered.
New motion-resistant MRI techniques provide adequate images even in patients that are not able to suspend respiration[16]. Preliminary studies demonstrated that in patients who unable to suspend respiration, new imaging techniques for MRI, such as radial 3D-GRE acquired in a free breathing fashion, may be useful for pancreatic MR imaging and aid the radiologist in the detection and characterization of pancreatic focal lesions.
Normal pancreatic parenchyma displays high T1 signal intensity due to the presence of aqueous protein[3,4], which is accentuated on fat-suppressed GRE sequences. The normal pancreas typically shows uniform hyperintense enhancement on arterial phase images, fading overtime to become isointense to the liver on interstitial phase images (Figure 1)[6,17].
Figure 1.
Normal pancreas. Axial T2-weighted SS-ETSE (A), pre-contrast fat-suppressed T1-weighted (B) GRE and post-gadolinium fat-suppressed T1-weighted gradient echo images acquired in the arterial (C) and venous (D) phases of enhancement. The normal pancreas is high in signal intensity on T1-weighted images (B) due to the presence of aqueous protein in the pancreatic acini. A uniform capillary blush is apparent on the immediate post-gadolinium image (C). T2-weighted sequences allow the depiction of the pancreatic duct. SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
In the elderly, the high T1 signal intensity of the pancreas may be diminished and be lesser than that of the liver, reflecting fibrosis resultant from the aging process[5].
Pancreatic ductal adenocarcinoma
Ductal adenocarcinoma is the most common malignant pancreatic neoplasm and accounts for almost 90% of all malignant pancreatic tumors. Males are affected twice as often as women and the peak age of occurrence is in the 7th to 8th decades of life[18]. At clinical presentation, 2/3 of patients have an advanced tumor stage. This may justify why pancreatic adenocarcinoma shows a poor prognosis, with a 5-year survival rate of 5%[19]. Despite advances in patient management and new chemotherapy regimens, surgery remains the only curative treatment[19].
The appearance of the typical ductal adenocarcinoma is an irregular, small focal solid mass (2-3 cm) without necrosis or hemorrhage. It is a heterogeneous and poorly enhancing mass with a tendency for local infiltration, including vascular encasement.
Near 60%-70% of ductal adenocarcinomas of the pancreas involve the pancreatic head, 10%-20% are found in the body and 5%-10% in the tail. Diffuse pancreatic involvement occurs in 5% of the cases[20].
Spin-echo images are limited in the detection of pancreatic adenocarcinoma. On T2-weighted images, tumors are usually slightly hypointense relative to the background pancreatic parenchyma and consequently challenging to visualize. Ductal adenocarcinomas appear as low signal intensity lesions on noncontrast fat-suppressed T1-weighted images and usually well delineated from normal background pancreas, which is high in signal intensity[17,21-24].
Detection of adenocarcinoma is better performed on the arterial phase images, where the lesion will enhance to a lesser degree than the adjacent background pancreas (Figures 2 and 3), due to the abundant fibrous stroma and scarce tumor vascularity[23]. The increased volume of the extracellular space and the venous drainage of tumors compared to normal pancreatic tissue are responsible for the near isointense appearance of ductal adenocarcinoma on the interstitial phase[23].
Figure 2.
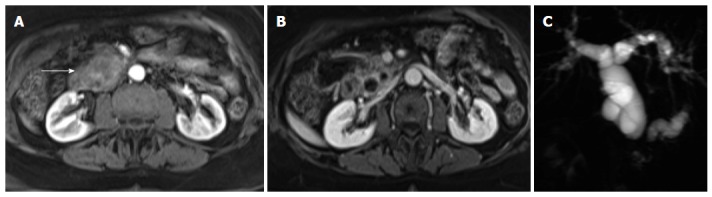
Pancreatic ductal adenocarcinoma. Axial post-gadolinium fat-suppressed T1-weighted images obtained in the arterial phase (A) and interstitial phase (B) image, and coronal maximum intensity projection image of MRCP (C). There is a hypovascular tumor arising in the pancreatic head (arrow, A) that obstructs the common bile duct and pancreatic duct (double duct sign). This sign is well depicted in the MRCP images (C). MRCP: Magnetic resonance cholangiopancreatography.
Figure 3.
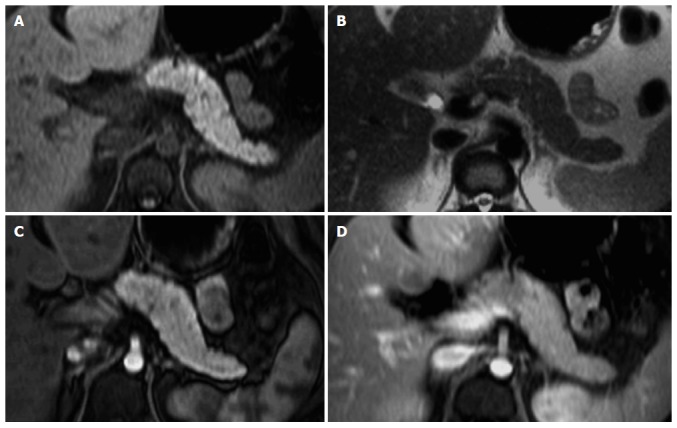
Pancreatic ductal adenocarcinoma. Axial T2-weighted single-shot echo train spin echo (A), pre-contrast fat-suppressed T1-weighted (B) gradient echo (GRE) and post-gadolinium fat-suppressed T1-weighted GRE images acquired in the arterial (C) and venous (D) phases of enhancement. There is a solid nodule in the head of the pancreas, which is more conspicuous in the pre-contrast and arterial phase T1-weighted images (B, arrow, C), consistent with ductal adenocarcinoma. Note that this lesion might be imperceptible on T2-weighted images (A) and venous phase images (D).
In general, large pancreatic tumors tend to persist low in signal intensity (Figure 2) on interstitial phase images, whereas the signal intensity of smaller tumors is more variable and may range from hypointense to minimally hyperintense on this phase (Figure 3).
Obstruction of the main pancreatic duct by the pancreatic adenocarcinoma, which may be seen even with small tumors, results in tumor-associated chronic pancreatitis. Many times, the pancreatic parenchyma distal to pancreatic adenocarcinoma is atrophic and low in signal intensity compared to normal pancreas, due to chronic inflammation, progressive fibrosis and diminished proteinaceous fluid of the gland[23,24]. In these cases, depiction of the tumor is poor on noncontrast T1-weighted fat-suppressed images; nevertheless, arterial phase images are very helpful in the discrimination of the size and extent of pancreatic ductal adenocarcinomas, as tumors almost always enhance less than the adjacent chronically inflamed pancreas[23,24]. These imaging characteristics along with substantial increase of CA 19.9 levels are unique to ductal adenocarcinoma and allow correct diagnosis with high accuracy.
Although it is non-specific, obstruction of the main pancreatic duct is one of the most important signs of ductal adenocarcinoma. The concurrent dilatation of the pancreatic and hepatic duct may frequently occurs when the pancreatic cancer develops in the cephalic region, thus depicting the so called “double duct sign”, in some cases this may be the only sign, if the pancreatic tumor is very small. Possible differential diagnosis in this case includes an acute or chronic papillitis[2,11] and the ampullary carcinoma[25].
Regarding tumor resectability, radiologists should evaluate certain MRI findings and describe them in the MRI report: (1) distant metastases, frequently to the liver, peritoneum, lung and paraortic lymph nodes; (2) infiltration of neighboring structures, including stomach, colon, spleen; (3) invasion of the peripancreatic arteries, including celiac trunk, hepatic artery, superior mesenteric artery; and (4) invasion of the peripancreatic veins, including portal and superior mesenteric vein[2,14,22,26].
State of the art MRI is suitable to detect and characterize focal ductal adenocarcinoma smaller than 1 cm[2,21,22,27], which tend to appear as small non-contour-deforming pancreatic lesions. Detection of this early manifestation of disease is difficult or impossible to identify with multiphasic current-generation CT[28,29].
Endoscopy ultrasound has been widely used in detection of clinically suspected pancreatic lesion; together with fine needle aspiration, it has been reported to be the best diagnostic method for small pancreatic neoplasms. Unfortunately this is an invasive and an operator-dependent-technique and is not yet widely used[30].
It is very important to differentiate adenocarcinoma from other benign and malignant entities, because the clinical management and prognosis varies according to the type of pathology[2,31]. Some lesions require surgery or imaging follow-up, whereas other lesions are clinically irrelevant, not requiring further evaluation and/or treatment.
BENIGN LESIONS THAT MAY SIMULATE DUCTAL ADENOCARCINOMA
Pancreatic lipomatosis
Pancreatic lipomatosis is a condition related to fatty infiltration/replacement of the pancreatic parenchyma, and is commonly seen in the elder, especially in obese patients. Involvement is normally diffuse but occasionally it may simulate a neoplastic lesion. The anterior aspect of the head of the pancreas is the most common location for pancreatic lipomatosis (Figure 4).
Figure 4.
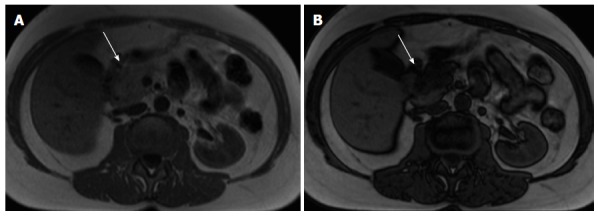
Pancreatic lipomatosis. Axial in-phase (A) and out-of-phase (B) T1-weighted GRE images. There is a focal fat infiltration in the region of the pancreatic head (arrows), only noticeable in the out-of-phase images, which is diagnostic for this entity. Focal fat infiltration is a benign condition that can simulate pancreatic adenocarcinoma especially on CT. GRE: Gradient echo; CT: Computed tomography.
The absences of mass effect, ductal or vascular displacement are important findings to establish the correct diagnosis.
MRI is highly specific for the detection of fat[32]. A moderate to marked signal loss in the out-of-phase relative to the in-phase images is distinctive of this condition (Figure 4)[33]. Macroscopic fatty replacement of the pancreas will show high T1 and T2 signal intensity, and marked signal loss on fat-suppressed sequences[32-34].
Acute pancreatitis
Acute pancreatitis is defined as an acute inflammatory condition typically presenting with abdominal pain and elevation in pancreatic enzymes secondary mainly to alcoholism or cholelithiasis.
MRI is more sensitive than CT, supporting the use of MRI in the evaluation of patients with a nonconclusive CT or to differentiate pure inflammatory condition from neoplastic lesion of the pancreas.
The acutely inflamed pancreas shows either focal or diffuse enlargement of the parenchyma, with signal intensity comparable to that of normal pancreatic tissue in non-complicated mild to moderate pancreatitis. Peripancreatic fluid is an important sign visualized in acute pancreatitis, best displayed on fat-suppressed T2-weighted sequences, seen as high signal in a background of intermediate to low-signal pancreas and fat (Figure 5)[2,35,36].
Figure 5.
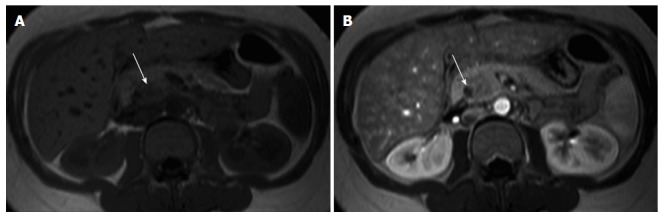
Acute pancreatitis. Axial in-phase (A) T1-weighted GRE image and arterial phase (B) fat-suppressed T1-weighted GRE image (B) in a patient with elevated amylase. There is a nodular area in the uncinate process showing mild T1-weighted hypointensity due to edema, however showing arterial enhancement (arrow, B) and with preservation of marbled pancreatic texture. This was compatible with focal acute pancreatitis. GRE: Gradient echo.
Chronic pancreatitis
Chronic pancreatitis is defined as a continuous or relapsing, chronic, inflammatory process of the pancreas, characterized by permanent morphologic changes and typically leads to diminishing of function. Distinction between focal pancreatitis and adenocarcinoma may be challenging because both entities may result in focal enlargement of the pancreatic head, atrophy of the tail of the pancreas and obliteration of the fat plane around the superior mesenteric artery. Ductal adenocarcinoma arising in the pancreatic head may cause obstruction of the CBD and pancreatic duct, with the MRCP appearance of a “double duct sign”. This sign can be also appreciated, although less commonly, in patients with focal pancreatitis.
Focal chronic pancreatitis and adenocarcinoma may display comparable signal intensity changes of the enlarged region of pancreas on noncontrast T1- and T2-weighted images, including mild hypointensity on T1-weighted images and heterogeneous and mild hyperintensity on T2-weighted images.
On arterial phase images, focal chronic pancreatitis usually displays heterogeneous enhancement and may show signal voids from cysts and/or calcifications with no evidence of a definable mass. In this setting, the focally enlarged region of the pancreas preserve the glandular feathery texture, comparable to the remaining pancreas[37]. Conversely, in ductal adenocarcinoma, the focally enlarged region of the pancreas loses its usual anatomic detail.
Diffuse low T1 signal intensity and hypovascularity of the entire pancreas, including the region of focal enlargement, are distinctive for chronic pancreatitis. In the setting of ductal adenocarcinoma, the tumor enhances less than the adjacent chronically inflamed pancreatic parenchyma[38].
Several features favor a diagnosis of focal pancreatitis, including: (1) non-dilated or smoothly tapering pancreatic and bile ducts coursing through the mass (“duct penetrating sign”)[38]; (2) parenchymal calcifications (seen as signal voids); (3) pancreatic duct beading and varying caliber; and (4) side branch dilatation. Instead, abrupt interruption of a smoothly dilated main pancreatic duct, minimal side-branch dilatation, upstream pancreatic atrophy and a high ratio of duct caliber to the pancreatic gland width are features typically observed in adenocarcinoma (Figure 2)[39].
A previous investigation evaluated the accuracy of MRI in the differentiation between ductal adenocarcinoma and chronic pancreatitis, in patients with focal pancreatic mass[37]; in this study, MR technique with the use of fat-suppressed T1-weighted 3D-GRE sequence was able to differentiate ductal adenocarcinoma from chronic pancreatitis with a sensitivity and specificity of 93% and 75%, respectively. The most critical finding for ductal adenocarcinoma of the pancreas was a relative delineation of the mass compared to the background pancreatic parenchyma. On the other hand, the most critical finding of chronic pancreatitis was an imprecise delineation with a mildly increased signal intensity and enhancement compared with the background pancreatic parenchyma on portal-venous phase images. These features reflect a more progressive enhancement of inflammatory tissue compared to ductal adenocarcinoma from arterial phase to portal-venous images. A further useful imaging feature is effacement of the fine, lobular architectural pattern of the pancreas in pancreatic adenocarcinoma[37].
The encasement of the celiac axis, superior mesenteric artery, lymphadenopathy and liver metastases establish the diagnosis of ductal adenocarcinoma[26,40], with liver metastases representing the definitive distinction.
OTHER SOLID PANCREATIC TUMORS WITH EPITHELIAL AND ENDOCRINE ORIGIN THAT MIGHT SIMULATE DUCTAL ADENOCARCINOMA
ACC
ACC is a rare primary tumor of the exocrine gland of the pancreas, and although acinar cells comprise most of the pancreatic parenchyma, ACC represents only 1% of all exocrine pancreatic cancers. Tumors generally occur between the fifth and seventh decades[2]. It is defined as a carcinoma exhibiting pancreatic enzyme secretion by neoplastic exocrine cells, and its clinical presentation is usually related to either the local effects of the tumor or to metastases[41]. Presenting symptoms are frequently nonspecific. “Lipase hypersecretion syndrome” is related to hypersecretion of lipase by the ACC, which may result in subcutaneous fat necrosis, bone infarcts, and polyarthritis[42,43]. Hypoglycemia as a presenting symptom has also been observed in some patients.
These cancers are generally exophytic, oval or round, well marginated, and hypovascular. Small tumors are usually solid, whereas larger tumors almost invariably contain cystic areas representing regions of necrosis, hemorrhage, and occasionally amorphous intratumoral calcifications, seen as signal voids[38,39].
These tumors are frequently uniformly or partially well-defined, with thin, enhancing capsules, and enhance less than the adjacent normal background pancreas[38,39]. These tumors are predominantly low in signal intensity on T1-weighted images and iso- to moderately hyperintense on T2-weighted images.
ACC should always be considered when a large pancreatic mass with typical imaging is found a solid mass with variably sized central cystic areas or cystic masses[2,43-45]. The tumor marker CA 19.9, which is generally increased in pancreatic adenocarcinoma, is rarely elevated in ACC.
UNCERTAIN ORIGIN
SPT
SPT of the pancreas is an uncommon, low-grade epithelial malignancy of the exocrine pancreas that most often appears in young female patients[2,20,46], and accounts for about 1% to 2% of all pancreatic tumors[47].
This tumor is typically benign and is found mainly in young women between the 2nd and 3rd decades of life. This age presentation is rarely seen in ductal adenocarcinoma. This tumor shows a predilection for African-American and Asian women, despite rare cases having been reported in children and men. Malignant degeneration may occur, however most SPTs show benign behavior[48,49]. Complete surgical removal is the treatment of choice. Metastasis is rare but local recurrence has been described. The prognosis is excellent after resection.
The mass occurs most frequently in the head or tail. SPT is often discovered incidentally and appears as a large well-demarcated and encapsulated pancreatic mass, surrounded by a marginal thick capsule and with variable relative amounts of intralesional solid, cystic and hemorrhagic components. The peripheral capsule is often present and seen on MRI, with an incidence of 95%-100%[48]. Vascular encasement is exclusively seen in malignant types.
MR imaging characteristically shows a well-defined lesion with heterogeneous T1- and T2-weighted signal intensity, reflecting the complex nature of the mass. Regions of high T1 and low or inhomogeneous T2 signal intensity may be seen and are related with blood products and may help to differentiate SPTs from endocrine tumors, whose cystic components generally are not hemorrhagic and therefore not typically possessing moderately increased T1 signal intensity. Furthermore, the peripheral areas of SPTs are not hypervascular, which is characteristically observed in islet cell tumors[2,46,48,49] (Figure 6).
Figure 6.
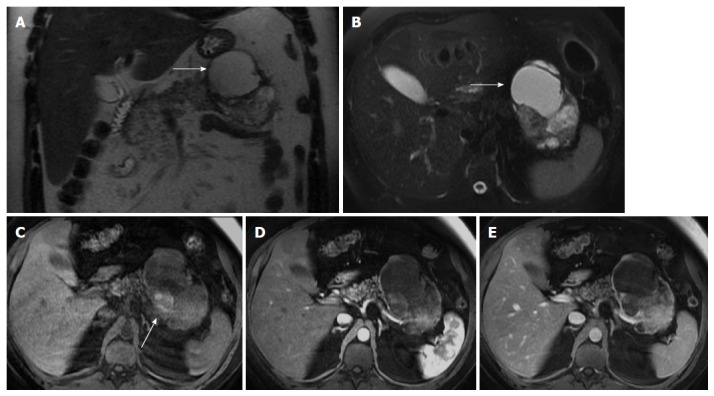
Solid pseudopapillary tumor. Coronal (A) and axial fat-suppressed (B) T2-weighted SS-ETSE, pre-contrast fat-suppressed T1-weighted (C) GRE and post-gadolinium fat-suppressed T1-weighted GRE images acquired in the arterial (D) and venous (E) phases of enhancement. A large well-demarcated and encapsulated mass is located in the tail and body of the pancreas. The mass shows heterogeneous signal intensity, with cystic (arrows, A and B) and hemorrhagic areas (arrow, C). The solid component of the lesion shows progressive enhancement over time. A mass with these characteristics, appearing in a young patient is most likely related with solid pseudopapillary tumor. SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
PANCREATIC NEUROENDOCRINE TUMORS
Pancreatic neuroendocrine tumors (NET) were previously called islet cell tumor, as it was believed that they derived from the islets of Langerhans. Recent evidence suggests that these tumors arise from pluripotential stem cells in the ductal epithelium[50]. They account for 1%-2% of all pancreatic neoplasms. The majority NETs are sporadic, but association with syndromes such as with Wermer syndrome, neurofibromatosis type 1, von Hippel-Lindau syndrome, and tuberous sclerosis have been reported. NETs are classified into functioning and non-functioning tumors. Functioning tumors may clinically present with an endocrine malfunction subsequent to hormone secretion[51]. The diagnosis of functioning NETs is almost always established biochemically, and the role of imaging is to depict the precise location of the tumor. Insulinomas and gastrinomas are the most common pancreatic NETs, followed by non-functional or untyped tumors.
Non-functional tumors account for 15%-20% of pancreatic NETs and tend to be symptomatic due to large tumor mass or metastatic disease. Functioning tumors manifest early in the course of disease when they are small, due to the clinical manifestations of excessive hormone secretion.
Malignancy cannot be determined based on the histological appearance of pancreatic NETs, instead it is established by the coexistence of metastases or local invasion. The liver is the most affected organ for metastatic spread.
Insulinomas are usually benign tumors, whereas gastrinomas are malignant in nearly 60% of cases. Nonfunctioning tumors are malignant in most cases.
Tumor morphology is variable. Small tumors are usually solid and homogeneous, whereas larger tumors are usually heterogeneous with cystic degeneration and calcifications.
On MRI, NETs are moderately low T1 and intermediate to high signal intensity on T2-weighted fat-suppressed images[52]. These tumors are typically highly hypervascular and therefore they enhance intensely on arterial/pancreatic phase after contrast administration. This distinctive feature must be interpreted cautiously, as although they may enhance more rapidly and avidly than the background pancreas, they may also appear iso-intense during the arterial phase, as the normal pancreatic parenchyma is also highly vascularized (Figure 7).
Figure 7.
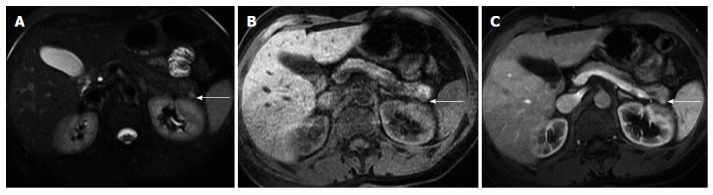
Pancreatic neuroendocrine tumor. Axial fat-suppressed T2-weighted SS-ETSE (A), pre-contrast fat-suppressed T1-weighted (B) GRE and arterial phase fat-suppressed T1-weighted GRE image (C). There is a small solid lesion in the tail of the pancreas (arrow, A-C), showing moderate signal intensity on T2-weighted images (arrow, A) and demonstrates marked enhancement in the arterial phase of enhancement (arrow, C). This was an insulinoma. SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
Insulinomas are usually seen as small tumors (< 2 cm), with intense and homogeneous enhancement on arterial phase images, whereas gastrinomas most commonly are larger lesions (3-4 cm approximately), with peripheral ring-like enhancement on arterial phase images[2,53]. Gastrinomas generally occur in a distinctive location, termed the gastrinoma triangle, bordered superiorly by the confluence of the cystic and CBDs; inferiorly, by the second and third portions of the duodenum; and medially, by the neck and body of the pancreas.
The likelihood of malignancy rises in parallel with tumor size, and tumors larger than 5 cm are frequently malignant. Even when malignant, these tumors are slow-growing and the prognosis is better than for ductal adenocarcinoma[18,54]. Metastases to lymph nodes and solid organs may have an enhancement pattern similar to that of the primary tumor (Figure 8).
Figure 8.
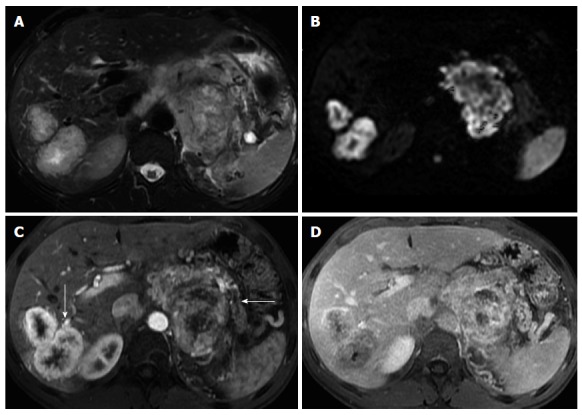
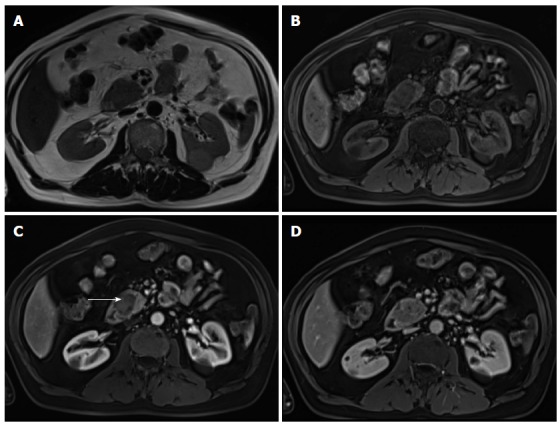
Pancreatic neuroendocrine tumor and liver metastases. Axial T2-weighted SS-ETSE (A), diffusion weighted images (b = 500) (B) and post-gadolinium fat-suppressed T1-weighted GRE images acquired in the arterial (C) and venous (D) phase of enhancement. A large mass is seen arising in the body and tail of the pancreas, showing moderate signal intensity on T2-weighted images (A) and restriction to diffusion (B). This lesion shows hypervascular characteristics (C). This mass was diagnosed as poorly differentiated neuroendocrine tumor. Note that the liver metastases show similar signal characteristics on T2- and diffusion-weighted images and demonstrate the characteristic arterial wash-in (arrow, C) and late washout (D) seen with neuroendocrine tumor metastases. SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
It is essential to distinguish pancreatic NETs from other neoplasms, especially from ductal adenocarcinoma, as the prognosis and treatment options are usually substantially different for these two entities.
Features that discriminate most pancreatic NETs from pancreatic adenocarcinoma include the high T2 signal, increased homogeneous enhancement on arterial phase images, hypervascular liver metastases, and absence of pancreatic duct obstruction or vascular encasement[53]. On the other hand, venous thrombosis, peritoneal, and regional node enlargement are distinguishing features of pancreatic ductal adenocarcinoma, usually not seen in NETs.
MESENCHYMAL TUMORS
Mesenchymal neoplasms of the pancreas are rare, accounting for 1% to 2% of all pancreatic tumors[55,56]. They derive from various connective tissue components and are classified according to their histologic origin.
Primary pancreatic lymphoma, although unusual, is the most common malignant mesenchymal tumor appearing in the pancreas. Benign mesenchymal adipose tissue tumors, such as lipomas or teratomas are extremely rare and show diagnostic features on MRI, with homogeneous encapsulated mature fat or with fat-fluid levels, respectively[55,56]. Other mesenchymal tumors, such as lymphangiomas, leiomyoma, leiomyosarcoma, schwannoma, hemangioma, or hemangioendothelioma, have also been reported; however, they are exceedingly rare, appearing described only in the form of isolated case reports.
Pancreatic lymphoma
Non-Hodgkin lymphoma may involve peripancreatic lymph nodes or may directly infiltrate the pancreas. Peripancreatic lymph nodes show low to intermediate signal intensity on T1-weighted fat-suppressed images, which permit to be distinguished from the normal pancreas that shows high signal intensity[57].
Primary pancreatic lymphoma is a rare entity, accounting for less than 2% of extranodal lymphomas and 0.5% of pancreatic tumors[58].
Pancreatic lymphoma has a better prognosis than pancreatic ductal adenocarcinoma, as first-line treatment with chemotherapy is normally effective, allowing long-term disease regression or remission. Surgery is usually not required.
Two morphologic patterns are recognized: focal and diffuse form[59]. The focal form occurs in the pancreatic head in 80% of the cases and may mimic adenocarcinoma.
On MRI, lymphoma shows low T1 and intermediate T2 signal intensity. Several features may help discriminate pancreatic lymphoma from ductal adenocarcinoma such as the presence of a bulky confined tumor in the pancreatic head with absent or minimal main pancreatic duct dilatation, enlarged lymph nodes below the level of the renal vein, and a tendency to noninvasive tumor growth, which are characteristic for pancreatic lymphoma and atypical for pancreatic ductal adenocarcinoma (Figure 9). Vascular invasion is also less commonly seen in lymphoma[2]. Additionally, CA 19.9 levels are usually not elevated in primary or secondary pancreatic lymphoma.
Figure 9.
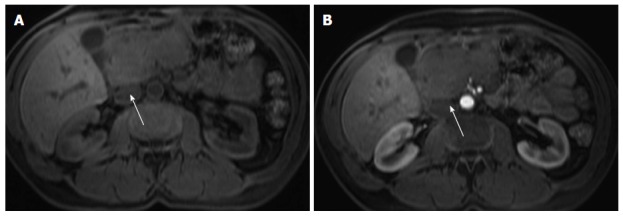
Pancreatic lymphoma. Pre-contrast fat-suppressed axial T1-weighted gradient - echo images (A) and acquired in the arterial phase after gadolinium injection (B). There is a large hypointence and hypovascular mass (arrows) localized in the pancreatic head, however showing no significant duct dilatation. There was evidence of enlarged lymph nodes. This mass was diagnosed as pancreatic lymphoma.
NONPANCREATIC TUMOR LESION
Metastases
Metastases to the pancreas may be the result of direct invasion or hematogenous spread. Direct invasion from stomach and transverse colon carcinoma and GIST tumors are rare, but the most common forms of direct extension.
Metastases derive most frequently from renal cell carcinoma and lung cancer followed by breast, colon, prostate and malignant melanoma.
Three morphological patterns of metastatic involvement of the pancreas have been described: solitary lesion (50%-70% of cases), multifocal (5%-10%), and diffuse (15%-44%)[59].
Metastases generally show low T1 and mildly high T2 signal intensity. The enhancement of the majority of metastases follows a ring pattern, with a variable degree of enhancement depending on the angiogenic properties of the primary neoplasm (Figure 10)[60].
Figure 10.
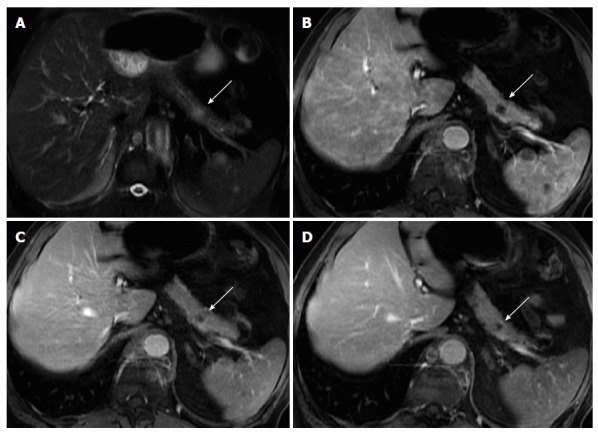
Solitary pancreatic metastasis. Axial T2-weighted SS-ETSE (A) and post-gadolinium fat-suppressed T1-weighted GRE images acquired in the arterial (B), venous (C) and interstitial (D) phases of enhancement. There is a nodular lesion in the pancreatic body showing moderate signal intensity on T2-weighted images (arrow, A) and showing hypovascular characteristics, with no pancreatic duct dilatation. These are typical features of a solitary pancreatic metastasis (arrows). Note additional splenic metastases (lung cancer). SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
Renal cancer metastases resemble the appearance of NETs. Melanocytic melanoma metastases may display high T1 signal due to the paramagnetic properties of melanin pigment.
Ductal obstruction is uncommon, even with larger tumors, which is an important feature distinguishing from pancreatic ductal adenocarcinoma[2,59].
INTRAPANCREATIC SPLENULE
The presence of accessory splenules may arise within the parenchyma of solid organs, notably the pancreas. Intrapancreatic splenule is a somewhat uncommon location for splenules. These lesions typically are < 2 cm in size and are located within 3 cm of the tip of the pancreatic tail[55]. The presence of a well marginated rounded mass located in the distal tail of the pancreas with signal intensity features comparable to those of the spleen on all MR sequences suggests the diagnosis of intrapancreatic accessory spleen (Figure 11). A distinctive feature of these masses is that when greater than 2 cm they may exhibit serpiginous enhancement on arterial phase images, as typically seen in the spleen[61]. Other entities may simulate the signal intensity and post-gadolinium enhancement features of intrapancreatic splenules, and DWI and SPIO-enhanced MRI can be used to characterize the lesion and to establish the definite diagnosis[61,62].
Figure 11.
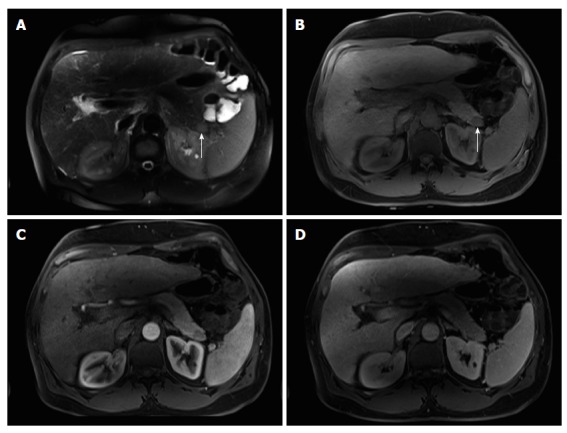
Intra-pancreatic splenule. Axial T2-weighted SS-ETSE (A), pre-contrast fat-suppressed T1-weighted (B) GRE and post-gadolinium fat-suppressed T1-weighted GRE images acquired in the arterial (C) and venous (D) phases of enhancement. There is a well-marginated and lobulated round intra-pancreatic splenule (arrows, A and B) that shows isointense signal to the pancreas on T2- and T1-weighted images (A and B). This nodule is hypointense compared to the background pancreas on the precontrast T1-weighted image (B) and demonstrates lesser enhancement relative to the pancreas on the arterial phase (C) and hepatic venous phase (D). The enhancement pattern was homogenous on the postgadolinium images and similar to that of the pancreas. SS-ETSE: Single-shot echo train spin echo; GRE: Gradient echo.
CONCLUSION
In addition to pancreatic ductal adenocarcinoma other solid pancreatic lesions occur. Many of the above-described tumors may be diagnosed with relatively high specificity employing MRI. The radiologist must be familiar with their MRI appearance to correctly diagnose them, or suggest them in the differential diagnosis when appropriate, since it may change substantially the approach, prognosis and patient management. We have described specific features that may aid in the discrimination from ductal adenocarcinoma and establish the correct diagnosis.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other co-authors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 28, 2015
First decision: August 4, 2015
Article in press: October 8, 2015
P- Reviewer: Aktas S S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Altun E, Elias Jr J, Armao D, Busakorn V, Semelka RC. Pancreas. 535-676. In: Abdominal-Pelvic MRI, editor. 3rd edition. Semelka RC. editor. New Jersey: Wiley-Blackwell; 2010. [Google Scholar]
- 3.Semelka RC, Ascher SM. MR imaging of the pancreas. Radiology. 1993;188:593–602. doi: 10.1148/radiology.188.3.8351317. [DOI] [PubMed] [Google Scholar]
- 4.Zapparoli M, Semelka RC, Altun E, Tsurusaki M, Pamuklar E, Dale BM, Gasparetto EL, Elias J. 3.0-T MRI evaluation of patients with chronic liver diseases: initial observations. Magn Reson Imaging. 2008;26:650–660. doi: 10.1016/j.mri.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DG, Vinitski S, Saponaro S, Tasciyan T, Burk DL, Rifkin MD. Liver and pancreas: improved spin-echo T1 contrast by shorter echo time and fat suppression at 1.5 T. Radiology. 1991;178:67–71. doi: 10.1148/radiology.178.1.1984328. [DOI] [PubMed] [Google Scholar]
- 6.Winston CB, Mitchell DG, Outwater EK, Ehrlich SM. Pancreatic signal intensity on T1-weighted fat saturation MR images: clinical correlation. J Magn Reson Imaging. 1995;5:267–271. doi: 10.1002/jmri.1880050307. [DOI] [PubMed] [Google Scholar]
- 7.Takehara Y, Ichijo K, Tooyama N, Kodaira N, Yamamoto H, Tatami M, Saito M, Watahiki H, Takahashi M. Breath-hold MR cholangiopancreatography with a long-echo-train fast spin-echo sequence and a surface coil in chronic pancreatitis. Radiology. 1994;192:73–78. doi: 10.1148/radiology.192.1.8208969. [DOI] [PubMed] [Google Scholar]
- 8.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
- 9.Soto JA, Barish MA, Yucel EK, Clarke P, Siegenberg D, Chuttani R, Ferrucci JT. Pancreatic duct: MR cholangiopancreatography with a three-dimensional fast spin-echo technique. Radiology. 1995;196:459–464. doi: 10.1148/radiology.196.2.7617861. [DOI] [PubMed] [Google Scholar]
- 10.Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20:3–9. doi: 10.1097/RMR.0b013e3181b48392. [DOI] [PubMed] [Google Scholar]
- 11.Maccioni F, Martinelli M, Al Ansari N, Kagarmanova A, De Marco V, Zippi M, Marini M. Magnetic resonance cholangiography: past, present and future: a review. Eur Rev Med Pharmacol Sci. 2010;14:721–725. [PubMed] [Google Scholar]
- 12.Bian Y, Wang L, Chen C, Lu JP, Fan JB, Chen SY, Zhao BH. Quantification of pancreatic exocrine function of chronic pancreatitis with secretin-enhanced MRCP. World J Gastroenterol. 2013;19:7177–7182. doi: 10.3748/wjg.v19.i41.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirkes T, Sandrasegaran K, Sanyal R, Sherman S, Schmidt CM, Cote GA, Akisik F. Secretin-enhanced MR cholangiopancreatography: spectrum of findings. Radiographics. 2013;33:1889–1906. doi: 10.1148/rg.337125014. [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4:461–469. doi: 10.2215/CJN.06011108. [DOI] [PubMed] [Google Scholar]
- 15.Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264:414–422. doi: 10.1148/radiol.12112025. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo RM, de Campos RO, Ramalho M, Herédia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650–657. doi: 10.2214/AJR.10.5881. [DOI] [PubMed] [Google Scholar]
- 17.Semelka RC, Kroeker MA, Shoenut JP, Kroeker R, Yaffe CS, Micflikier AB. Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1991;181:785–791. doi: 10.1148/radiology.181.3.1947098. [DOI] [PubMed] [Google Scholar]
- 18.Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997;17:281–301. doi: 10.1148/radiographics.17.2.9084072. [DOI] [PubMed] [Google Scholar]
- 19.Ros PR, Mortelé KJ. Imaging features of pancreatic neoplasms. JBR-BTR. 2001;84:239–249. [PubMed] [Google Scholar]
- 20.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993–1015. doi: 10.1148/rg.314105731. [DOI] [PubMed] [Google Scholar]
- 21.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864–7877. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, Mazzeo F. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Gabata T, Matsui O, Kadoya M, Yoshikawa J, Miyayama S, Takashima T, Nagakawa T, Kayahara M, Nonomura A. Small pancreatic adenocarcinomas: efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology. 1994;193:683–688. doi: 10.1148/radiology.193.3.7972808. [DOI] [PubMed] [Google Scholar]
- 24.Semelka RC, Kelekis NL, Molina PL, Sharp TJ, Calvo B. Pancreatic masses with inconclusive findings on spiral CT: is there a role for MRI? J Magn Reson Imaging. 1996;6:585–588. doi: 10.1002/jmri.1880060405. [DOI] [PubMed] [Google Scholar]
- 25.Ahualli J. The double duct sign. Radiology. 2007;244:314–315. doi: 10.1148/radiol.2441041978. [DOI] [PubMed] [Google Scholar]
- 26.Clark LR, Jaffe MH, Choyke PL, Grant EG, Zeman RK. Pancreatic imaging. Radiol Clin North Am. 1985;23:489–501. [PubMed] [Google Scholar]
- 27.Morgan KA, Adams DB. Solid tumors of the body and tail of the pancreas. Surg Clin North Am. 2010;90:287–307. doi: 10.1016/j.suc.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Saisho H, Yamaguchi T. Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas. 2004;28:273–278. doi: 10.1097/00006676-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Vellet AD, Romano W, Bach DB, Passi RB, Taves DH, Munk PL. Adenocarcinoma of the pancreatic ducts: comparative evaluation with CT and MR imaging at 1.5 T. Radiology. 1992;183:87–95. doi: 10.1148/radiology.183.1.1312736. [DOI] [PubMed] [Google Scholar]
- 30.Wang XY, Yang F, Jin C, Fu DL. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol. 2014;20:15580–15589. doi: 10.3748/wjg.v20.i42.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adsay NV, Basturk O, Klimstra DS, Klöppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004;21:260–267. doi: 10.1053/j.semdp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Byun JH, Park SH, Shin YM, Kim PN, Ha HK, Lee MG. Focal fatty replacement of the pancreas: usefulness of chemical shift MRI. AJR Am J Roentgenol. 2007;188:429–432. doi: 10.2214/AJR.05.1095. [DOI] [PubMed] [Google Scholar]
- 33.Isserow JA, Siegelman ES, Mammone J. Focal fatty infiltration of the pancreas: MR characterization with chemical shift imaging. AJR Am J Roentgenol. 1999;173:1263–1265. doi: 10.2214/ajr.173.5.10541101. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto S, Siegelman SS, Bluemke DA, Hruban RH, Fishman EK. Focal fatty infiltration in the head of the pancreas: evaluation with multidetector computed tomography with multiplanar reformation imaging. J Comput Assist Tomogr. 2009;33:90–95. doi: 10.1097/RCT.0b013e31815cff0d. [DOI] [PubMed] [Google Scholar]
- 35.Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19–37. [PubMed] [Google Scholar]
- 36.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–116. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 37.Kim JK, Altun E, Elias J, Pamuklar E, Rivero H, Semelka RC. Focal pancreatic mass: distinction of pancreatic cancer from chronic pancreatitis using gadolinium-enhanced 3D-gradient-echo MRI. J Magn Reson Imaging. 2007;26:313–322. doi: 10.1002/jmri.21010. [DOI] [PubMed] [Google Scholar]
- 38.Semelka RC, Shoenut JP, Kroeker MA, Micflikier AB. Chronic pancreatitis: MR imaging features before and after administration of gadopentetate dimeglumine. J Magn Reson Imaging. 1993;3:79–82. doi: 10.1002/jmri.1880030114. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqi AJ, Miller F. Chronic pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:384–394. doi: 10.1053/j.sult.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Wittenberg J, Simeone JF, Ferrucci JT, Mueller PR, vanSonnenberg E, Neff CC. Non-focal enlargement in pancreatic carcinoma. Radiology. 1982;144:131–135. doi: 10.1148/radiology.144.1.7089244. [DOI] [PubMed] [Google Scholar]
- 41.Chiou YY, Chiang JH, Hwang JI, Yen CH, Tsay SH, Chang CY. Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr. 2004;28:180–186. doi: 10.1097/00004728-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Khalili M, Wax BN, Reed WP, Schuss A, Drexler S, Weston SR, Katz DS. Radiology-pathology conference. Acinar cell carcinoma of the pancreas. Clin Imaging. 2006;30:343–346. doi: 10.1016/j.clinimag.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 43.Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, Silverman SG. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005;184:511–519. doi: 10.2214/ajr.184.2.01840511. [DOI] [PubMed] [Google Scholar]
- 44.Hsu MY, Pan KT, Chu SY, Hung CF, Wu RC, Tseng JH. CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin Radiol. 2010;65:223–229. doi: 10.1016/j.crad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Hu S, Hu S, Wang M, Wu Z, Miao F. Clinical and CT imaging features of pancreatic acinar cell carcinoma. Radiol Med. 2013;118:723–731. doi: 10.1007/s11547-012-0908-5. [DOI] [PubMed] [Google Scholar]
- 46.Guerrache Y, Soyer P, Dohan A, Faraoun SA, Laurent V, Tasu JP, Aubé C, Cazejust J, Boudiaf M, Hoeffel C. Solid-pseudopapillary tumor of the pancreas: MR imaging findings in 21 patients. Clin Imaging. 2014;38:475–482. doi: 10.1016/j.clinimag.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Ng KH, Tan PH, Thng CH, Ooi LL. Solid pseudopapillary tumour of the pancreas. ANZ J Surg. 2003;73:410–415. doi: 10.1046/j.1445-2197.2003.t01-1-02634.x. [DOI] [PubMed] [Google Scholar]
- 48.Cooper JA. Solid pseudopapillary tumor of the pancreas. Radiographics. 2006;26:1210. doi: 10.1148/radiographics.26.4.0261210. [DOI] [PubMed] [Google Scholar]
- 49.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749–1765. doi: 10.1148/rg.296095506. [DOI] [PubMed] [Google Scholar]
- 50.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Mozell E, Stenzel P, Woltering EA, Rösch J, O’Dorisio TM. Functional endocrine tumors of the pancreas: clinical presentation, diagnosis, and treatment. Curr Probl Surg. 1990;27:301–386. doi: 10.1016/0011-3840(90)90025-z. [DOI] [PubMed] [Google Scholar]
- 52.Semelka RC, Custodio CM, Cem Balci N, Woosley JT. Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J Magn Reson Imaging. 2000;11:141–148. doi: 10.1002/(sici)1522-2586(200002)11:2<141::aid-jmri10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 53.Semelka RC, Cumming MJ, Shoenut JP, Magro CM, Yaffe CS, Kroeker MA, Greenberg HM. Islet cell tumors: comparison of dynamic contrast-enhanced CT and MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1993;186:799–802. doi: 10.1148/radiology.186.3.8381551. [DOI] [PubMed] [Google Scholar]
- 54.Sheth S, Hruban RK, Fishman EK. Helical CT of islet cell tumors of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2002;179:725–730. doi: 10.2214/ajr.179.3.1790725. [DOI] [PubMed] [Google Scholar]
- 55.Ferrozzi F, Zuccoli G, Bova D, Calculli L. Mesenchymal tumors of the pancreas: CT findings. J Comput Assist Tomogr. 2000;24:622–627. doi: 10.1097/00004728-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Megibow AJ, Francis IR. Unusual Pancreatic Neoplasms: Imaging. In: Imaging of the Pancreas Cystic and Rare Tumors. Carlo Procacci C, Megibow AJ, editors. Springer Berlin Heidelberg; 2003. pp. 249–265. [Google Scholar]
- 57.Zeman RK, Schiebler M, Clark LR, Jaffe MH, Paushter DM, Grant EG, Choyke PL. The clinical and imaging spectrum of pancreaticoduodenal lymph node enlargement. AJR Am J Roentgenol. 1985;144:1223–1227. doi: 10.2214/ajr.144.6.1223. [DOI] [PubMed] [Google Scholar]
- 58.Zucca E, Roggero E, Bertoni F, Cavalli F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8:727–737. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 59.Tsitouridis I, Diamantopoulou A, Michaelides M, Arvanity M, Papaioannou S. Pancreatic metastases: CT and MRI findings. Diagn Interv Radiol. 2010;16:45–51. doi: 10.4261/1305-3825.DIR.1996-08.1. [DOI] [PubMed] [Google Scholar]
- 60.Al Ansari N, Kim BS, Srirattanapong S, Semelka CT, Ramalho M, Altun E, Woosley JT, Calvo B, Semelka RC. Mass-forming cholangiocarcinoma and adenocarcinoma of unknown primary: can they be distinguished on liver MRI? Abdom Imaging. 2014;39:1228–1240. doi: 10.1007/s00261-014-0172-3. [DOI] [PubMed] [Google Scholar]
- 61.Herédia V, Altun E, Bilaj F, Ramalho M, Hyslop BW, Semelka RC. Gadolinium- and superparamagnetic-iron-oxide-enhanced MR findings of intrapancreatic accessory spleen in five patients. Magn Reson Imaging. 2008;26:1273–1278. doi: 10.1016/j.mri.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Jang KM, Kim SH, Lee SJ, Park MJ, Lee MH, Choi D. Differentiation of an intrapancreatic accessory spleen from a small (&lt; 3-cm) solid pancreatic tumor: value of diffusion-weighted MR imaging. Radiology. 2013;266:159–167. doi: 10.1148/radiol.12112765. [DOI] [PubMed] [Google Scholar]


