Abstract
18-fluorodeoxygluocose positron emission tomography/computed tomography (18FDG-PET/CT) provides significant information in multiple settings in the management of head and neck cancers (HNC). This article seeks to define the additional benefit of PET/CT as related to radiation treatment planning for squamous cell carcinomas (SCCs) of the head and neck through a review of relevant literature. By helping further define both primary and nodal volumes, radiation treatment planning can be improved using PET/CT. Special attention is paid to the independent benefit of PET/CT in targeting mucosal primaries as well as in detecting nodal metastases. The utility of PET/CT is also explored for treatment planning in the setting of SCC of unknown primary as PET/CT may help define a mucosal target volume by guiding biopsies for examination under anesthesia thus changing the treatment paradigm and limiting the extent of therapy. Implications of the use of PET/CT for proper target delineation in patients with artifact from dental procedures are discussed and the impact of dental artifact on CT-based PET attenuation correction is assessed. Finally, comment is made upon the role of PET/CT in the high-risk post-operative setting, particularly in the context of radiation dose escalation. Real case examples are used in these settings to elucidate the practical benefits of PET/CT as related to radiation treatment planning in HNCs.
Keywords: Head and neck cancer, Radiation treatment planning, Computed tomography, Fluorodeoxygluocose positron emission tomography, Imaging
Core tip: The 18-fluorodeoxygluocose positron emission tomography (18FDG-PET) scan has increasing clinical importance in the management of head and neck cancers. It has also proven useful in treatment planning for radiation therapy. PET scans have utility in tumor volume delineation, the identification of metastatic lymph nodes, the management of carcinoma of unknown primary, dental artifact reduction and high-risk postoperative radiation therapy. Many of these applications of 18FDG-PET scans are still in the preliminary stages of development and active investigations are ongoing to standardize these processes.
INTRODUCTION
Head and neck cancer (HNC) is a significant cause of cancer morbidity and mortality worldwide. Approximately 650000 new HNC are diagnosed annually worldwide with approximately half of this number resulting in deaths[1]. The majority of HNCs worldwide are squamous cell carcinomas (SCCs) related to tobacco abuse, however the incidence of head and neck SCCs resulting from infection with the human papilloma virus (HPV) is rising. The prognosis for HPV-negative (tobacco-related) cancers is inferior to that of HPV-positive cancers. Undoubtedly, radiation therapy plays a central role in the management of the majority of HNC patients and meticulous radiation treatment planning is required to ensure high rates of cure as well as in limiting toxicity. This is particularly important for HPV-positive oropharyngeal cancer patients as these patients are generally younger and with few co-morbidities and thus are expected to live longer with long-term radiation complications. Most HNCs present in a locally advanced stage and primary management takes one of the two courses: surgical resection with or without adjuvant radiation based upon pathologic features or definitive radiation therapy with or without concurrent chemotherapy saving surgery as a salvage option.
Modern radiation therapy is delivered using highly conformal technologies including three-dimensional conformal radiotherapy, intensity modulated radiation therapy (IMRT) and most recently IMRT with image guidance. Target delineation on treatment planning computed tomography (CT) scans is a central part of the treatment process in both cure and limiting toxicity. This is particularly important in the era of IMRT, in which steep radiation dose falloff is achievable within millimeters.
A risk-stratified approach is taken in radiation treatment planning in HNCs. Areas of high-risk of disease spread and gross disease receive a high dose of radiation (usually 66-70 Gy). Other areas, including those of intermediate-risk of disease spread and grossly uninvolved nodal regions in which tumor recurrence may occur, receive lower doses of radiation (56-60 Gy and 50-56 Gy, respectively). Clinical data from physical examinations and diagnostic laryngoscopies, as well as pathologic data and multimodality imaging using CT, magnetic resonance imaging (MRI), ultrasound and positron emission tomography (PET) all contribute to the target delineation and treatment planning process. This article seeks to define the additional benefit of the utility of 18-fluorodeoxyglucose (18FDG) PET/CT scans in radiation treatment planning particularly as related to five areas: (1) Primary gross tumor and nodal volume (GTV) delineation; (2) Identification of involved metastatic lymph nodes; (3) Management of SCCs of unknown primary in the head and neck; (4) Utility in accounting for dental artifact; and (5) High-risk post-operative radiation (PORT).
DELINEATION OF THE PRIMARY AND NODAL GTV
18FDG-PET can help clarify the extent of primary tumor and eliminate unnecessary treatment volumes that may appear as abnormal on CT or MRI imaging. Significant variations have been noted in both primary tumor and lymph nodal volumes based on the diagnostic imaging modality(ies) used to define these. Iodinated-contrast-enhanced CT scans remain the imaging modality of choice due to their widespread availability in radiation oncology departments worldwide. Additionally, CT scans have a short image acquisition time and thus do not suffer from the image quality degradation that may result from longer acquisition times and normal breathing or swallowing motion. They provide sufficient anatomical details of the gross tumor and involved lymph nodes, provide electron density information for attenuation correction for treatment planning purposes, and require less end-user training for image interpretation relative to other modalities. Many reports have investigated the impact of additional PET imaging on primary and lymph nodal volume definition for radiation treatment planning purposes. Most of these reports have found major discrepancies between CT-based and PET-based volumes resulting in clinically significant implications for radiation therapy.
Studies assessing the addition of PET to CT-based planning
A number of studies have assessed the impact of the addition of PET in the manual segmentation of HNC GTVs. In an analysis of 12 HNC patients comparing CT based GTV (GTV-CT) and PET based GTV (GTV-PET), the GTV increased or decreased by 25% or more because of PET in 17% and 33% of cases, respectively[2]. In this report, the primary and nodal volumes were not assessed separately.
In another study, Heron et al[3] did analyze the changes in primary and nodal volumes separately and noted a change in GTV delineation in 80% of cases with the use of PET scans. For the primary site, the GTV was larger in 14% and smaller in 66% of cases based on PET scans. Interestingly, for the abnormal lymph nodes, the volume was larger in 33% of cases and smaller in 14% of cases. The average ratio of GTVs for the CT-defined and PET-defined volumes was 3.1 (range, 0.3-23.6), whereas for abnormal nodes was 0.7 (range, 0-4). Hence, tumor volumes for the primaries were significantly larger as delineated on CT than on PET but not for nodal regions suggesting a larger benefit in delineating the primary tumor with the addition of PET.
A similar discrepancy between CT-GTV and PET-GTV was noted by Guido et al[4]. Thirty-eight consecutive HNC patients underwent treatment planning CTs with intravenous contrast enhancement. A radiation oncologist defined all GTVs using both the PET/CT and CT scans. The CT-GTV was larger than PET-GTV in 92% cases. Unlike the previous study, no statistically significant difference was seen between these volumes for primary and nodal sites.
Variations in target delineation with the addition of PET/CT may also affect treatment planning. In a study of a group of 40 patients, Paulino et al[5] noted changes in the PET-GTV in 37 cases (30 smaller, 7 larger). IMRT plans were generated based on the CT-based volumes and dosimetric analysis was performed to examine the adequacy of coverage for the PET-based volume in these IMRT plans. The volume of PET-GTV receiving at least 95% of the prescribed dose was 100% and 95%-99% in 20 and 10 cases, respectively. Thus, inadequate coverage (< 95% of the PET-GTV receiving the prescribed dose) was seen in 25% of cases.
A major reason for these variations in study results is the subjectivity associated with 18FDG-PET image interpretation and consequent user dependence on how the GTV is defined using PET images. The potential impact of interobserver and intraobserver variation was studied by Breen et al[6] Eight experienced observers (6 head and neck oncologists and 2 neuroradiologists) outlined the primary GTV for 10 patients. There was a very high agreement between and within observers on GTVs derived from contrast-enhanced CT scans. However, there was less reliability noted when PET/CT scans were used for outlining the GTV. In another similar analysis[7], 4 physicians (2 neuroradiologists and 2 radiation oncologists) contoured GTVs in 16 patients on the basis of the CT alone, and then on PET/CT fusion. A high degree of variation was noted across physicians for the CT volumes (P = 0.09) and significant variation was seen for the PET/CT volumes (P = 0.0002). Observer variation in lymph nodal volume outlining was not assessed in either of these studies.
Automated techniques of PET-GTV delineation
Due to limitations in how different experienced physicians define the GTVs for various cases it is very difficult to perform interinstitutional comparative studies and arrive at any meaningful conclusions on the utility of PET scans for head and neck treatment planning. To overcome these interobserver and intraobserver variations, many attempts have been made to automate the process of volume definition using PET scans. Various groups have attempted to describe “thresholding” or “segmentation” techniques to make the process more objective and eliminate or reduce the subjectivity associated with the use of PET imaging data.
Schinagl et al[8] evaluated PET based GTVs derived using 5 different segmentation techniques: visual interpretation, applying an isocontour of a standardized uptake value (SUV) of 2.5, using a fixed threshold of 40% and 50% of the maximum signal intensity, and applying an adaptive threshold based on the signal-to-background ratio. Seventy-eight patients with Stages II-IV SCC of the head and neck were studied. The primary tumor was delineated on CT. The GTV method of applying an isocontour of a SUV of 2.5 failed to provide successful delineation in 45% of cases. However, the other segmentation methods resulted in PET-GTVs that were smaller than that seen on the CT scan. Additionally, the PET scans frequently showed tumor extension outside that seen on the CT scans. The authors concluded that none of the segmentation methods provided a satisfactory result.
In a subsequent publication, the authors used the same data set of 78 patients to assess whether PET scans could be used for target volume definition for metastatic lymph nodes in the head and neck region[9]. On the CT scans, lymph nodes measuring 7-10 mm were labeled as “marginally enlarged” and those > 10 mm were “enlarged”. Eight different PET segmentation methods were used to identify these nodes: visual interpretation, applying fixed thresholds at SUV of 2.5 and at 40% and 50% of the maximum signal intensity of the primary tumor and applying a variable threshold based on the signal-to-background ratio. Additionally, these same thresholds were acquired using the signal of the lymph node as the threshold reference. Based on the CT scan imaging, 208 lymph nodes were > 7 mm while 108 were >10 mm. A large percentage of these lymph nodes were not identified by the segmentation methods when normalized to the primary tumor PET-SUV. The results were better when the thresholds were set based on the lymph node SUV values. Due to these limitations, the authors concluded that until proper validation of 18FDG-PET based segmentation tools is done it should not be recommended for target volume definition of metastatic lymph nodes in routine clinical practice.
Another recent prospective study in 19 patients with 39 lesions used the signal-to-background ratio thresholding method to define tumor volumes. It concluded that methods that rely mainly on SUVmax for thresholding are very sensitive to partial volume effects and may provide unreliable results when applied on small lesions[10]. Thus, automated thresholding and segmentation methods have not yet yielded promising results in PET-GTV delineation.
Pathologic correlation of PET-GTV volumes
Investigators have also evaluated the pathologic correlates to PET imaging findings in HNC patients. Daisne et al[11] compared delineation of tumor volumes on MRI, CT and PET in multiple patients with HNCs. Average PET-GTVs (20.3 cc) were smaller than those derived from MR (27.9 cc) or CT (32 cc). Additionally, PET-GTVs contained additional volume not delineated on either primary MR or CT images. For 9 patients with laryngeal cancer who underwent total laryngectomy after multi-modality imaging in this cohort, pathologic tumor volumes were significantly smaller than the estimated image volumes on all modalities studied. The average surgical specimen tumor volume measured 12.6 cc while tumor volume was measured as 16.3 cc, 20.8 cc and 23.8 cc on PET, CT and MRI images, respectively.
In a similar radio-pathologic correlative study, Schinagl et al[12] identified 28 lymph nodes in 12 HNC patients and looked at the ability of various PET segmentation methods to predict pathologic size after lymph node dissection. Nodal volumes on CT scans and visual interpretation of PET scans showed good correlations with the pathological volume. The authors noted that 18FDG-PET scans are valuable for detection of lymph nodes for staging purposes but provide no additional information over CT scans for outlining radiotherapy target volumes.
Guidelines for the utility of PET/CT for GTV delineation
There is currently no consensus on the methods of auto-segmentation, volume definition and the overall utility of 18FDG-PET scans in RT of HNCs. This remains an active area of research and development. Currently, we use 18FDG-PET co-registered with the simulation CT images to identify the tumor, contour the GTV and subsequently modify the GTV volume with the CT images, especially with contrast-enhancing CT images. 18FDG-PET is especially helpful when it is difficult to separate the tumor from surrounding soft tissue and muscle in CT imaging, such as tumor in the oral tongue or oropharynx. Figure 1 shows a patient with oral tongue cancer, comparing CT vs PET. The GTV based on CT (Figure 1C) is much larger than that based on 18FDG-PET (Figure 1D).
Figure 1.
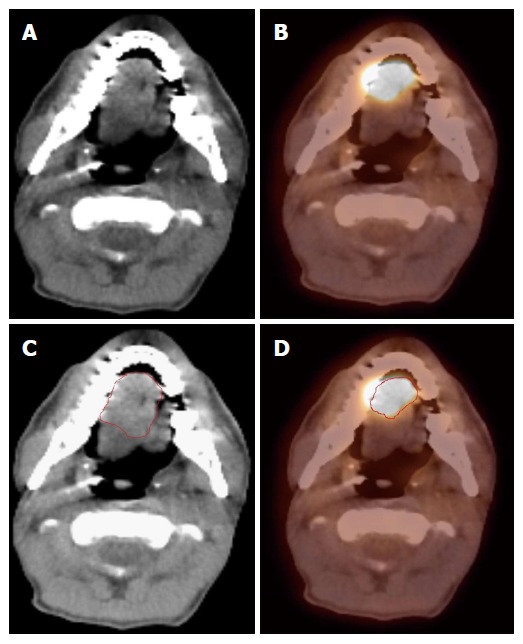
Positron emission tomography/computed tomography improves gross tumor and nodal volume delineation in a patient with oral tongue cancer. A: In the CT image, the tumor is difficult to separate from the soft tissues of the tongue; B: In the PET image, there is sharp demarcation of the primary tumor; C: The GTV is outlined in red based on CT scan; D: The GTV outlined in red based on 18FDG PET/CT is much smaller. 18FDG-PET/CT: 18-fluorodeoxygluocose positron emission tomography/computed tomography; GTV: Gross tumor and nodal volume.
18FDG-PET is also very helpful in identifying tumor extent that is not detectable on either CT or MR images. In particular, HNC patients often have synchronous second primaries. 18FDG-PET can help detect additional primary disease to be included in the high dose radiation field. Figure 2 shows a patient who presented with a right lateral oral tongue cancer. PET/CT revealed additional primary cancers in the soft palate and in the cervical esophagus (Figure 2A). Thus, the IMRT plan delivered 70 Gy to all of these primary tumors (Figure 2B-D).
Figure 2.
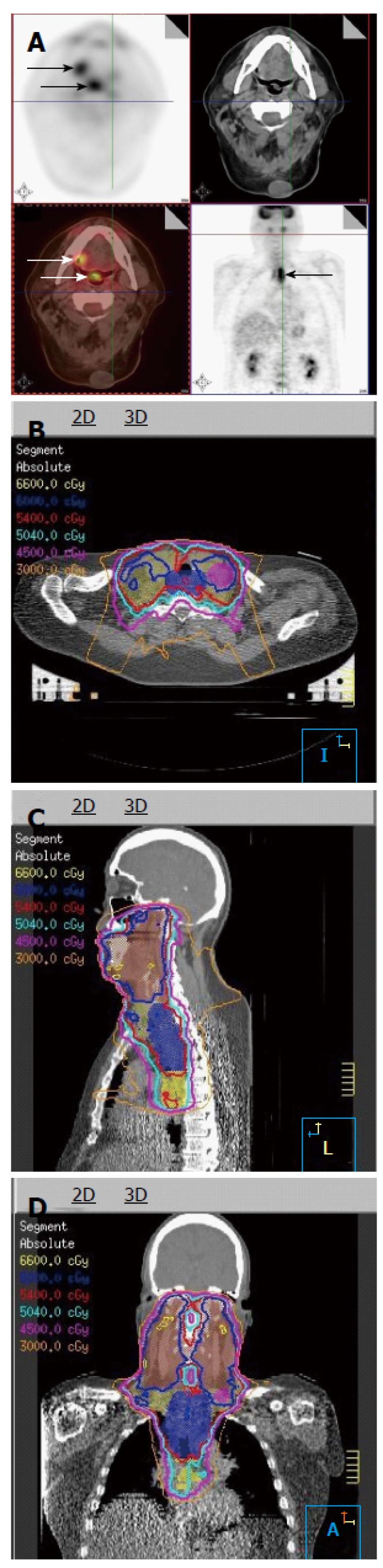
Positron emission tomography/computed tomography discovers multiple primaries in a patient with a right lateral tongue cancer. A: PET/CT images revealed that in addition to an oral tongue tumor, this patient had a primary tumor of the soft palate (inferior white arrow) as well as the cervical esophagus (black arrow); B-D: The IMRT plan for this patient demonstrates all three tumors covered in the high dose CTV. PET/CT: Positron emission tomography/computed tomography; IMRT: Intensity modulated radiation therapy.
IDENTIFICATION OF INVOLVED LYMPH NODES THAT ARE MISSED ON CT/MR
In addition to helping better delineate primary tumors, 18FDG-PET can clarify the involvement of metastatic lymph nodes in HNC patients. Standard CT and MR criteria for malignant cervical lymph nodes include size, shape, margins, and internal architecture, with size as the main criteria[13]. These criteria for CT and MR may under- or over-diagnose lymph node involvement and nodal stage in patients, resulting in 20%-30% false positive and false negative results[13]. The consequence of such errors may have a significant impact on treatment planning in HNC; in particular, small malignant lymph nodes may be under-dosed using CT or MRI criteria alone.
PET sensitivity and specificity for predicting involved nodes has been estimated at over 90%[14]. Heron et al[3] reported that the addition of 18FDG-PET to CT-based treatment planning increased the number of abnormal nodes contoured in 5 of 21 (24%) patients in a small institutional study. In another study, Koshy et al[15] reported significant changes in TNM staging affecting tumor volume delineation with the addition of 18FDG-PET to CT alone. Amongst a group of 36 patients in this study, 6 patients had a change in N-stage with the use of PET/CT relative to CT alone: 3 patients were upstaged including one to bilateral disease, while 3 other patients were downstaged to N0 disease. These changes in nodal staging are not trivial, as grossly involved nodal disease will receive radiation doses of up to 70 Gy, while node-negative disease may receive an elective radiation dose of 50-56 Gy or no radiation therapy at all.
In a prospective study by Schwartz et al[16] evaluating the feasibility of PET/CT based treatment planning, 63 patients underwent PET/CT simulation and 20 patients underwent neck dissection after a PET/CT simulation. 18FDG-PET correctly identified all 17 diseased heminecks and 9 negative heminecks. Additionally, 26/27 pathologically involved nodal levels were identified with positive predictive value (PPV) and negative predictive values (NPV) for nodal staging at 98.5% and 96% respectively. This affirms the high level of accuracy by which 18FDG-PET can be used to assess nodal disease. Further, in comparing CT-based and PET/CT-based treatment planning, PET/CT-based planning directly improved parotid and laryngeal doses, thus theoretically sparing patients from long-term toxicities of xerostomia and dysphagia.
Figure 3 illustrates a patient with nasopharyngeal cancer. Initial staging by CT and MRI was T1N0, but an 18FDG-PET scan revealed a hypermetabolic level II lymph node which did not meet size criteria for malignancy by CT/MRI (Figure 3A-D). Fine needle aspiration (FNA) of this node confirmed metastatic disease. Thus, this lymph node was treated to a high dose of radiation (Figure 3E-H) and concurrent chemotherapy was indicated.
Figure 3.
Positron emission tomography/computed tomography upstages a T1 nasopharyngeal cancer patient from N0 to N1. A-D: PET/CT images reveal that in addition to the T1 nasopharyngeal primary, there is increased FDG uptake in a level II node which was 1.0 cm in size (arrows). FNA of this node confirmed metastatic carcinoma. Thus, the patient was upstaged as T1N1 and treated with concurrent chemotherapy with IMRT; E-H: The IMRT plan for this patient treated the right level II node to a dose of 70 Gy. IMRT: Intensity modulated radiation therapy; PET/CT: Positron emission tomography/computed tomography; FDG: Fluorodeoxygluocose.
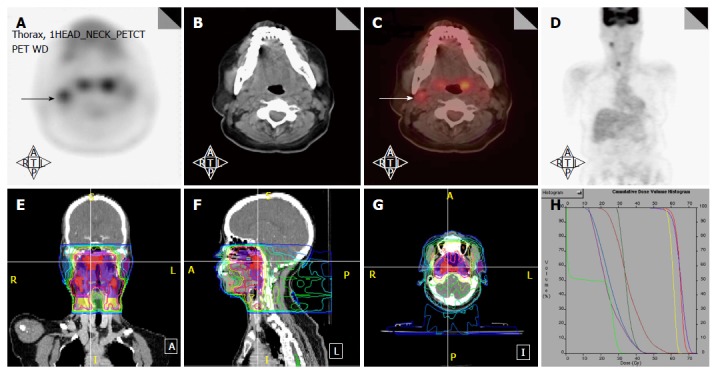
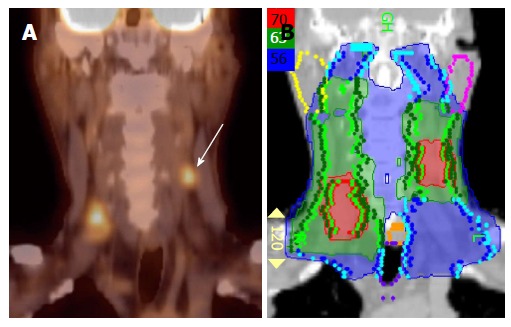
PET/CT has especially been helpful in detecting lymph nodes at a far distance from the primary tumor or in the contralateral neck, specifically when the lymph node has not reached size criteria by CT/MRI (Figure 4). 18FDG-PET is also very helpful in detecting involved lymph nodes in the lower neck in which CT visualization is hindered by complex muscular and vascular structures (Figure 5). Thus, additional highly suspicious nodes could be included in the high-dose dose field with the addition of 18FDG-PET.
Figure 4.
Positron emission tomography/computed tomography extends the high dose CTV contralaterally in a patient with a T2N2B base of tongue cancer. A: PET/CT reveals a contralateral level III node (white arrow), and thus, the patient was upstaged as T2N2C; B: An IMRT plan of this patient showing that this node was treated to 70 Gy and contralateral levels II and III were treated to 63 Gy. IMRT: Intensity modulated radiation therapy; PET/CT: Positron emission tomography/computed tomography.
Figure 5.
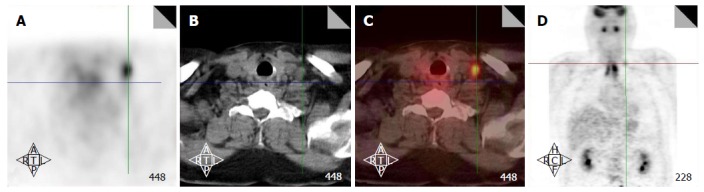
Positron emission tomography/computed tomography detects a low neck lymph node involved by metastasis. Increased FDG uptake in the low neck reveals a metastatic lymph node which would otherwise be difficult to detect because of the presence of muscular and vascular structures in this region of the neck (A-D). FDG: Fluorodeoxygluocose.
Finally, well-lateralized tonsil cancer is often treated to the ipsilateral side to reduce toxicities. However, before subjecting the patient to ipsilateral radiation, it is prudent to rule out any contralateral suspicious nodes. Because of the highly sensitivity of 18FDG-PET to detect metastatic lymph nodes, it should be the best modality in selecting patients for this treatment (Figure 6).
Figure 6.
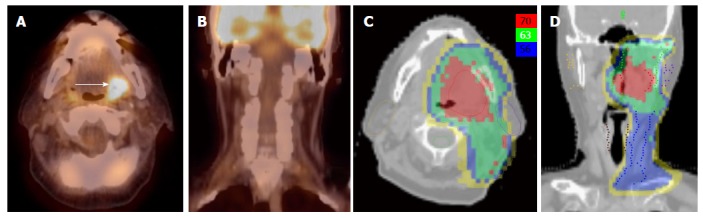
Positron emission tomography/computed tomography allows for unilateral tonsil cancer treatment in a patient with a T2N0 left tonsil cancer. A, B: PET/CT shows no evidence of lymph node metastasis in the contralateral (right) neck; C, D: An IMRT plan of the radiation treatment plan showing effective contralateral sparing. IMRT: Intensity modulated radiation therapy; PET/CT: Positron emission tomography/computed tomography.
UNKNOWN PRIMARY OF THE HEAD AND NECK
Between 2% and 9% of patients with HNC present with an enlarged cervical node without a definitive site of origin of the primary tumor by clinical examination and routine imaging studies[17]. This entity is called HNC of unknown primary (HNCUP). As most HNCUPs are SCCs[18], patients presenting with this entity undergo an examination under anesthesia (EUA) of the mucosal surface of the upper aerodigestive tract as well as directed biopsies of the nasopharynx and oropharynx if no suspicious lesion is noted during EUA. Additionally, ipsilateral or bilateral tonsillectomies may be performed for diagnostic purposes. Approximately 50% of primaries are detected in this manner[19].
PET/CT has been shown to be valuable in the work up and in identifying the primary tumor in patients with unknown primary. A systematic review of 16 studies of 302 patients by Rusthoven et al[20] validated the benefit of 18FDG-PET in HNCUP. The sensitivity, specificity and accuracy of 18FDG-PET in detecting unknown primary tumors were 88.3%, 74.9% and 78.8%. Additionally, 18FDG-PET detected 24.5% of primary tumors not apparent after traditional workup. The authors also noted that 18FDG-PET has a similar benefit in detecting additional occult nodal disease and metastases in the HNCUP population with a 15.9% and 11.2% detection rate, respectively.
In a prospective study, 20 patients with HNCUP underwent conventional workup prior to EUA[21]. PET/CT was performed, and the surgeons performing EUA were blinded to the PET results. EUAs and traditional random biopsies were performed prior to the surgeon viewing the PET/CT. After EUA and biopsies, the surgeon was shown the PET/CT intraoperatively and further biopsies were obtained according to the 18FDG-PET results. PET/CT increased the detection of the primary site from 25% to 55%. This suggests that PET/CT directed biopsy is superior to traditional random biopsy in detecting a primary tumor.
Radiation treatment is the main treatment modality in HNCUP, but radiation volumes vary drastically between institutions and no standard has been defined. Radiation treatment may be directed to either the ipsilateral involved neck or to the bilateral neck, and may include pan-mucosal irradiation to areas that may harbor a microscopic primary tumor including the nasopharynx, oropharynx, larynx, and hypopharynx. Identifying the primary tumor is therefore critical and may allow tailoring the radiation volume according to the primary disease, thus avoiding high dose radiation to unnecessary areas and reducing toxicities of the treatment.
Figure 7 illustrates a patient who presented with multiple left neck nodes with FNA confirming SCC. Conventional workup including EUA and traditional biopsies failed to identify the primary tumor. PET/CT showed a hypermetabolic focus in the left base of tongue corresponding to the primary tumor. This patient was treated as a base of tongue cancer and thus other mucosal areas including the larynx and hypopharynx were spared from high dose radiation.
Figure 7.
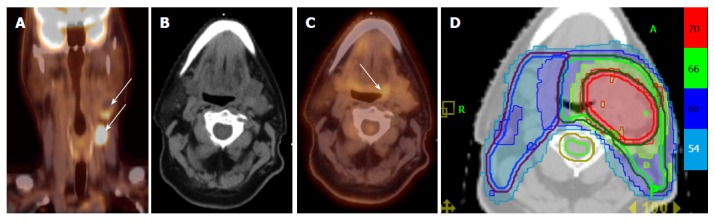
Positron emission tomography/computed tomography allows for detection for occult primary in a patient with multiple involved left neck nodes. A: PET/CT showing the initial presentation with multiple enlarged left neck nodes (white arrows); B: A conventional CT scan does not reveal a source of primary cancer; C: PET/CT demonstrated increased FDG uptake in the left base of tongue (white arrow) and a directed biopsy of this area revealed this as the primary site; D: IMRT treatment plan for this patient showing that the left base of tongue was included in the high-dose (70 Gy) volume while sparing uninvolved mucosal areas. IMRT: Intensity modulated radiation therapy; PET/CT: Positron emission tomography/computed tomography.
ACCOUNTING FOR DENTAL ARTIFACTS
Artifact from amalgam-based fillings and other dental procedures may significantly distort and hinder CT-based target delineation for primary tumors in HNCs. Though artifact reduction techniques exist and have been used to improve the quality of CT-based target delineation and radiation treatment planning, many facilities may not have access to software to reduce dental artifacts on head and neck treatment planning CTs[22].
CT-based attenuation correction is used to improve spatial resolution from PET imaging. Gamma rays produced after positron emission are affected by tissue heterogeneity, and utilizing CT data in addition to raw PET data improves spatial and quantitative accuracy of PET imaging[23]. Multiple authors have addressed the issue of attenuation correction in the context of metallic dental artifact. A study by Kamel et al[24] compared CT-based attenuation correction with Ge-68 PET-based attenuation correction in patients with metallic artifact and demonstrated quantitative value differences in regions of dental artifact raising the question of the impact of dental artifact on CT-based attenuation correction. Goerres et al[25] confirmed that 18FDG-PET artifacts are indeed generated adjacent to dental artifact using CT-based correction using a similar methodology of Ge-68 PET-based attenuation correction. However, they also found that these artifacts demonstrated significantly lower 18FDG-uptake when compared to primary tumor, mitigating the clinical significance of these artifacts in target delineation. Others have also demonstrated that irrespective of artifact reduction techniques, CT-based PET attenuation correction is robust as metallic artifacts do not propagate into the attenuation correction using CT imaging in HNC patients[26].
As 18FDG-PET resolution is not significantly modified by dental artifact, it is practical to use 18FDG-PET to improve target delineation in this context. A study out of Korea compared tumor staging between CT alone, MRI alone, and PET/CT in 37 patients with dental artifact on CT and MRI[27]. PET/CT had improved staging regardless of the presence of dental artifact, and had better specificity in ruling out involvement of the sublingual gland and floor of mouth. Comparing MRI-delineated primary tumor volume and PET-delineated primary tumor volume using an SUV cutoff of 2.5 with post-operative pathologic samples, demonstrated that MRI inferiorly predicted pathologic tumor size relative to PET/CT with an SUV cutoff of 2.5. Thus, PET/CT improved target definition in patients with dental artifact. A previous study also investigated the utility of PET/CT scans in oral cavity cancers comparing 69 patients with dental artifacts and 40 patients without such artifacts[28]. The PET/CT scans detected more tumors as compared to CT scans (95% vs 75%). A regression equation was developed equating the pathologic volume of the tumors with the PET volume as defined by a SUV = 3.5.
Recently more algorithms have been developed to use PET/CT imaging in combination with MRI images to reduce the impact of the dental artifacts[29-31].
UTILITY IN HIGH-RISK PORT
PORT for HNC can improve locoregional control and overall survival in patients with adverse pathologic features, including positive or close margins, extracapsular extension, perineural invasion, lymphovascular invasion, advanced tumor stage (T4), and advanced nodal stage (N2B or higher). Indications for PORT were validated in work by Peters et al[32] and further stratified by Ang et al[33] at the MD Anderson Cancer Center. Randomized clinical trials from the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer have shown that concurrent chemotherapy and radiation is significantly better than radiation alone in patients with high risk pathologic features; particularly those with extracapsular nodal extension or positive surgical margins. This was further validated by a pooled analysis of individual patient data from both trials[34-36]. PORT should include the entire postoperative area to a dose between 57.6 and 60 Gy in 30 to 33 fractions. High-risk areas including areas of close or positive post-operative margins or extracapsular nodal extension may also benefit from radiation dose escalation in addition to the radio-sensitizing effect of concurrent chemotherapy[37]. Radiation treatment to these high-risk areas is often escalated to 66 Gy.
Defining the radiation treatment targets is very challenging in the post-operative setting due to the anatomical changes after surgical resection and reconstruction especially in patients who have free flap reconstruction. Information including pre-operative imaging, pre-operative physical examination or endoscopy, surgical and pathologic findings, and post-operative imaging should be incorporated in target delineation in PORT and often requires a multidisciplinary approach with coordination between radiation oncologists, surgeons, radiologists, and pathologists. For patients who have pre-operative 18FDG-PET scans, registration of these PET images to postoperative simulation CT images can help to define the tumor beds which are often the high risk areas. Pre-operative PET/CT may be registered to the simulation CT using either rigid or deformable algorithms to provide guidance in assessing areas of high risk of recurrence[38]. Through image registration, the radiation oncologist is able to directly correlate the pre-treatment disease volumes to the post-operative CT imaging, and delineate high-risk areas to be included in the high radiation dose field.
PORT is often delivered 4 to 6 wk after surgery when the surgical wound is fully healed. Unfortunately, some patients with high-risk features may have recurrences even before starting PORT. Because of the anatomical distortion and fibrotic changes after surgery, and flap reconstruction, these recurrences are difficult to detect by physical examination and CT imaging. 18FDG-PET is an ideal imaging modality in this setting. Shintani et al[39] examined the utility of early post-operative pre-radiation PET/CT in HNCs. Among a cohort of 91 HNC patients, post-operative pre-radiation PET/CT performed at a median time of 28 d after surgery led to the discovery of 27 patients with suspicious findings on PET/CT. Of these, 24 patients (29% of the total cohort) underwent biopsy of these sites, with 11 biopsies positive for cancer. Treatment was changed in 14 patients (15.4%) with the addition of post-operative PET/CT: 4 underwent palliative care only, 6 had treatment to an extended volume, 1 received treatment to a higher dose, 2 underwent additional surgery and 2 received concurrent chemotherapy. Liao et al[40] also reported 29 patients who had a 18FDG-PET scan obtained before PORT. They found 7 patients with positive PET studies, 3 with distant metastases and 4 with local regional recurrences. For those who had locoregional disease detected by 18FDG-PET, the radiation volumes and radiation dose have to be changed, with higher doses delivered to the recurrent tumor. Thus, for patients with high-risk features or for those who have a prolonged interval from surgery to radiation, a post-surgery and pre-radiation 18FDG-PET will be valuable in treatment decision and radiation treatment planning.
Figure 8 illustrates a patient with initial stage T4AN2B right buccal mucosal cancer. He had surgery and radial forearm flap reconstruction. Due to the patient’s non-compliance, he did not have radiation treatment planning until 50 d after surgery. A PET/CT was obtained at simulation that revealed tumor recurrence in the right masticator space and right infratemporal fossa region (Figure 8A and B). The recurrent tumor was not resectable and thus this was treated to 70 Gy rather than a traditional post-operative dose of 60 Gy. Figure 8C and D represents the IMRT plan for this patient.
Figure 8.
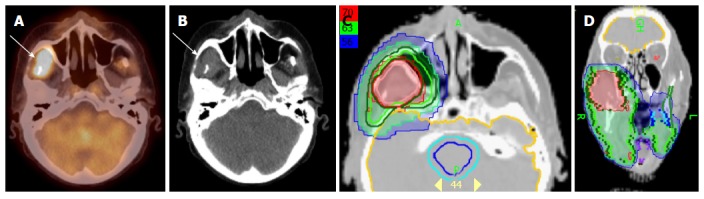
Positron emission tomography/computed tomography demonstrates tumor recurrence in a patient with a resected T4aN2b right buccal mucosa cancer prior to postoperative radiation. A-B: PET-CT obtained 50 d after surgery before postoperative radiation shows recurrent tumor (white arrow) in the infratemporal fossa; C-D: An IMRT treatment plan for this patient showing the recurrent tumor treated to a definitive radiation dose of 70 Gy rather a typical postoperative radiation dose of 60 Gy. IMRT: Intensity modulated radiation therapy; PET/CT: Positron emission tomography/computed tomography.
Both pre-operative and post-radiation PET/CT may also assist in predicting the likelihood of disease-free survival and locoregional recurrence after PORT. Kim et al[41] correlated multiple PET/CT derived imaging factors with areas of high likelihood of recurrence. Examining 100 patients with both pre-operative and post-operative post-radiation PET/CT, the authors found that a metabolic tumor volume defined as a pre-operative autosegmentation of SUV > 2.5 of more than 41 cc predicted for poorer disease-free survival. Additionally, post-radiation treatment SUVmax predicted for areas of locoregional recurrence. The authors suggested a post-treatment cutoff SUV value of 5.38 yielding a 93.7% NPV and a 66.7% positive predictive value. This may be used to select patients after postoperative radiation for further treatment interventions.
FUTURE DIRECTIONS
In addition to the routinely clinically available 18FDG substrate, many newer radioisotopes and radiotracers are being developed to image further functional characteristics of tumors including hypoxia, tumor proliferation, amino acid metabolism and presence of EGFR on tumor cells[42]. Hypoxia is commonly noted in head and neck tumors including in the primary site and metastatic lymph nodes. This is commonly seen in HPV-positive cancers; tumors which often present with small primaries and large necrotic and hypoxic neck nodes. Identification of these hypoxic areas may allow for radiation dose escalation to hypoxic sub-regions of tumors. Hypoxia poses a major radiobiologic disadvantage and confers radioresistance to the tumor. Hypoxic cells are not killed in response to radiation therapy and may be responsible for treatment failure, either locally or as distant metastasis. A commonly used dose-prescription and dose-delivery technique is the “simultaneous integrated boost” method in which doses of 70 Gy are delivered to areas of gross disease while areas of intermediate-risk and low-risk of involvement by disease simultaneously receive 59.4 Gy to 63 Gy and 56 Gy, respectively. Using the same technique it may be possible to further escalate the dose to the radio-resistant hypoxic regions in the same number of fractions while still meeting the dose constraints for surrounding normal tissue. Examples of radiopharmaceuticals being used to image the hypoxic portion of the tumor include 18F-fluoromisonidazole, copper-diacetyl-bis (N4-methylthiosamicarbazone) (Cu-ATSM) and 18F-fluoroazomycin arabinoside. The use of these agents has been described in literature[43,44]. Another interesting possibility is the use of 3’-deoxy-3’-[18F]-fluorothymidine, a PET tracer to noninvasively image tumor cell proliferation, and deliver higher doses to areas of the tumor showing higher degree of tumor growth[45,46].
In addition to these newer substrates, there has also been an evolution in the imaging modalities with the development of simultaneous PET/MR imagers. These offer the advantages of high-quality soft tissue imaging from MR with whole-body and functional imaging from the PET component[47-49]. The use of PET/MR in HNC patients has been recently described[50,51].
Finally, improved acquisition technologies including time-of-flight PET (TOF-PET) and four-dimensional PET (4D-PET) are emerging. TOF-PET improves the signal-to-noise ratio in acquisition as well as reduces scanning time leading to improved image resolution[52]. This may further improve the benefit of PET in target delineation of HNCs by reducing PET-GTVs. 4D-PET has primarily found clinical utility in lung cancers[53], an entity in which tumor motion is more prominent than in HNCs. Though small relative to lung motion, organ motion does exist in the head and neck and an improvement in resolution and target delineation in HNCs by reducing artifacts may be expected with 4D-PET.
CONCLUSION
The widespread availability of PET imagers and clinical experience has increased considerably in the recent years. Although methodologies of how to use PET information with either 18FDG or new substrates in radiation therapy planning for HNCs are still under development, PET scans have changed our daily practice in management of these patients. Integrating tumor biology obtained from these images with advanced delivery techniques using IMRT and image-guided radiation therapy has the potential to significantly impact outcomes in HNCs.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to disclose related to the publication of this work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: June 18, 2015
Article in press: September 18, 2015
P- Reviewer: Chang Z, Gao BL S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2002;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, Lütolf UM, Steinert HC, Von Schulthess GK. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–863. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 3.Heron DE, Andrade RS, Flickinger J, Johnson J, Agarwala SS, Wu A, Kalnicki S, Avril N. Hybrid PET-CT simulation for radiation treatment planning in head-and-neck cancers: a brief technical report. Int J Radiat Oncol Biol Phys. 2004;60:1419–1424. doi: 10.1016/j.ijrobp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Guido A, Fuccio L, Rombi B, Castellucci P, Cecconi A, Bunkheila F, Fuccio C, Spezi E, Angelini AL, Barbieri E. Combined 18F-FDG-PET/CT imaging in radiotherapy target delineation for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;73:759–763. doi: 10.1016/j.ijrobp.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 5.Paulino AC, Koshy M, Howell R, Schuster D, Davis LW. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:1385–1392. doi: 10.1016/j.ijrobp.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Breen SL, Publicover J, De Silva S, Pond G, Brock K, O’Sullivan B, Cummings B, Dawson L, Keller A, Kim J, et al. Intraobserver and interobserver variability in GTV delineation on FDG-PET-CT images of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;68:763–770. doi: 10.1016/j.ijrobp.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CM, Sun W, Buatti JM, Maley JE, Policeni B, Mott SL, Bayouth JE. Interobserver and intermodality variability in GTV delineation on simulation CT, FDG-PET, and MR Images of Head and Neck Cancer. Jacobs J Radiat Oncol. 2014;1:006. [PMC free article] [PubMed] [Google Scholar]
- 8.Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:1282–1289. doi: 10.1016/j.ijrobp.2007.07.2333. [DOI] [PubMed] [Google Scholar]
- 9.Schinagl DA, Hoffmann AL, Vogel WV, van Dalen JA, Verstappen SM, Oyen WJ, Kaanders JH. Can FDG-PET assist in radiotherapy target volume definition of metastatic lymph nodes in head-and-neck cancer? Radiother Oncol. 2009;91:95–100. doi: 10.1016/j.radonc.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Romasanta LA, Bellon-Guardia M, Torres-Donaire J, Lozano-Martin E, Sanz-Martin M, Velasco-Jimenez J. Tumor volume delineation in head and neck cancer with 18-fluor-fluorodeoxiglucose positron emission tomography: adaptive thresholding method applied to primary tumors and metastatic lymph nodes. Clin Transl Oncol. 2013;15:283–293. doi: 10.1007/s12094-012-0914-z. [DOI] [PubMed] [Google Scholar]
- 11.Daisne JF, Sibomana M, Bol A, Cosnard G, Lonneux M, Grégoire V. Evaluation of a multimodality image (CT, MRI and PET) coregistration procedure on phantom and head and neck cancer patients: accuracy, reproducibility and consistency. Radiother Oncol. 2003;69:237–245. doi: 10.1016/j.radonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Schinagl DA, Span PN, van den Hoogen FJ, Merkx MA, Slootweg PJ, Oyen WJ, Kaanders JH. Pathology-based validation of FDG PET segmentation tools for volume assessment of lymph node metastases from head and neck cancer. Eur J Nucl Med Mol Imaging. 2013;40:1828–1835. doi: 10.1007/s00259-013-2513-9. [DOI] [PubMed] [Google Scholar]
- 13.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–969. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 14.Adams S, Baum RP, Stuckensen T, Bitter K, Hör G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998;25:1255–1260. doi: 10.1007/s002590050293. [DOI] [PubMed] [Google Scholar]
- 15.Koshy M, Paulino AC, Howell R, Schuster D, Halkar R, Davis LW. F-18 FDG PET-CT fusion in radiotherapy treatment planning for head and neck cancer. Head Neck. 2005;27:494–502. doi: 10.1002/hed.20179. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz DL, Ford EC, Rajendran J, Yueh B, Coltrera MD, Virgin J, Anzai Y, Haynor D, Lewellen B, Mattes D, et al. FDG-PET/CT-guided intensity modulated head and neck radiotherapy: a pilot investigation. Head Neck. 2005;27:478–487. doi: 10.1002/hed.20177. [DOI] [PubMed] [Google Scholar]
- 17.Jereczek-Fossa BA, Jassem J, Orecchia R. Cervical lymph node metastases of squamous cell carcinoma from an unknown primary. Cancer Treat Rev. 2004;30:153–164. doi: 10.1016/j.ctrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Grau C, Johansen LV, Jakobsen J, Geertsen P, Andersen E, Jensen BB. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother Oncol. 2000;55:121–129. doi: 10.1016/s0167-8140(00)00172-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee DJ, Rostock RA, Harris A, Kashima H, Johns M. Clinical evaluation of patients with metastatic squamous carcinoma of the neck with occult primary tumor. South Med J. 1986;79:979–983. doi: 10.1097/00007611-198608000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 21.Rudmik L, Lau HY, Matthews TW, Bosch JD, Kloiber R, Molnar CP, Dort JC. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011;33:935–940. doi: 10.1002/hed.21566. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Noel C, Chen H, Harold Li H, Low D, Moore K, Klahr P, Michalski J, Gay HA, Thorstad W, et al. Clinical evaluation of a commercial orthopedic metal artifact reduction tool for CT simulations in radiation therapy. Med Phys. 2012;39:7507–7517. doi: 10.1118/1.4762814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998;25:2046–2053. doi: 10.1118/1.598392. [DOI] [PubMed] [Google Scholar]
- 24.Kamel EM, Burger C, Buck A, von Schulthess GK, Goerres GW. Impact of metallic dental implants on CT-based attenuation correction in a combined PET/CT scanner. Eur Radiol. 2003;13:724–728. doi: 10.1007/s00330-002-1564-2. [DOI] [PubMed] [Google Scholar]
- 25.Goerres GW, Schmid DT, Eyrich GK. Do hardware artefacts influence the performance of head and neck PET scans in patients with oral cavity squamous cell cancer? Dentomaxillofac Radiol. 2003;32:365–371. doi: 10.1259/dmfr/77741718. [DOI] [PubMed] [Google Scholar]
- 26.Nahmias C, Lemmens C, Faul D, Carlson E, Long M, Blodgett T, Nuyts J, Townsend D. Does reducing CT artifacts from dental implants influence the PET interpretation in PET/CT studies of oral cancer and head and neck cancer? J Nucl Med. 2008;49:1047–1052. doi: 10.2967/jnumed.107.049858. [DOI] [PubMed] [Google Scholar]
- 27.Hong HR, Jin S, Koo HJ, Roh JL, Kim JS, Cho KJ, Choi SH, Nam SY, Kim SY. Clinical values of (18) F-FDG PET/CT in oral cavity cancer with dental artifacts on CT or MRI. J Surg Oncol. 2014;110:696–701. doi: 10.1002/jso.23691. [DOI] [PubMed] [Google Scholar]
- 28.Baek CH, Chung MK, Son YI, Choi JY, Kim HJ, Yim YJ, Ko YH, Choi J, Cho JK, Jeong HS. Tumor volume assessment by 18F-FDG PET/CT in patients with oral cavity cancer with dental artifacts on CT or MR images. J Nucl Med. 2008;49:1422–1428. doi: 10.2967/jnumed.108.051649. [DOI] [PubMed] [Google Scholar]
- 29.Buchbender C, Hartung-Knemeyer V, Forsting M, Antoch G, Heusner TA. Positron emission tomography (PET) attenuation correction artefacts in PET/CT and PET/MRI. Br J Radiol. 2013;86:20120570. doi: 10.1259/bjr.20120570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delso G, Wollenweber S, Lonn A, Wiesinger F, Veit-Haibach P. MR-driven metal artifact reduction in PET/CT. Phys Med Biol. 2013;58:2267–2280. doi: 10.1088/0031-9155/58/7/2267. [DOI] [PubMed] [Google Scholar]
- 31.Burger IA, Wurnig MC, Becker AS, Kenkel D, Delso G, Veit-Haibach P, Boss A. Hybrid PET/MR imaging: an algorithm to reduce metal artifacts from dental implants in Dixon-based attenuation map generation using a multiacquisition variable-resonance image combination sequence. J Nucl Med. 2015;56:93–97. doi: 10.2967/jnumed.114.145862. [DOI] [PubMed] [Google Scholar]
- 32.Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, Morrison WH, Weber RS, Garden AS, Frankenthaler RA. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3–11. doi: 10.1016/0360-3016(93)90167-t. [DOI] [PubMed] [Google Scholar]
- 33.Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, Geara FB, Klotch DW, Goepfert H, Peters LJ. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 34.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 35.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 36.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 37.Grégoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Kovalchuk N, Jalisi S, Subramaniam RM, Truong MT. Deformable registration of preoperative PET/CT with postoperative radiation therapy planning CT in head and neck cancer. Radiographics. 2012;32:1329–1341. doi: 10.1148/rg.325125008. [DOI] [PubMed] [Google Scholar]
- 39.Shintani SA, Foote RL, Lowe VJ, Brown PD, Garces YI, Kasperbauer JL. Utility of PET/CT imaging performed early after surgical resection in the adjuvant treatment planning for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:322–329. doi: 10.1016/j.ijrobp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 40.Liao CT, Fan KH, Lin CY, Wang HM, Huang SF, Chen IH, Kang CJ, Ng SH, Hsueh C, Lee LY, et al. Impact of a second FDG PET scan before adjuvant therapy for the early detection of residual/relapsing tumours in high-risk patients with oral cavity cancer and pathological extracapsular spread. Eur J Nucl Med Mol Imaging. 2012;39:944–955. doi: 10.1007/s00259-012-2103-2. [DOI] [PubMed] [Google Scholar]
- 41.Kim G, Kim YS, Han EJ, Yoo IeR, Song JH, Lee SN, Lee JH, Choi BO, Jang HS, Yoon SC. FDG-PET/CT as prognostic factor and surveillance tool for postoperative radiation recurrence in locally advanced head and neck cancer. Radiat Oncol J. 2011;29:243–251. doi: 10.3857/roj.2011.29.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang TJC, Menda Y, Cheng SK, Wu CC, Lee NY. New Tracers PET in head and neck squamous cell carcinoma. PET Clin. 2012;7:431–441. doi: 10.1016/j.cpet.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, Dempsey JF, Perez CA, Purdy JA, Welch MJ. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–1182. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 44.Servagi-Vernat S, Differding S, Hanin FX, Labar D, Bol A, Lee JA, Grégoire V. A prospective clinical study of 18F-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur J Nucl Med Mol Imaging. 2014;41:1544–1552. doi: 10.1007/s00259-014-2730-x. [DOI] [PubMed] [Google Scholar]
- 45.Arens AI, Troost EG, Hoeben BA, Grootjans W, Lee JA, Grégoire V, Hatt M, Visvikis D, Bussink J, Oyen WJ, et al. Semiautomatic methods for segmentation of the proliferative tumour volume on sequential FLT PET/CT images in head and neck carcinomas and their relation to clinical outcome. Eur J Nucl Med Mol Imaging. 2014;41:915–924. doi: 10.1007/s00259-013-2651-0. [DOI] [PubMed] [Google Scholar]
- 46.Troost EG, Bussink J, Hoffmann AL, Boerman OC, Oyen WJ, Kaanders JH. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med. 2010;51:866–874. doi: 10.2967/jnumed.109.069310. [DOI] [PubMed] [Google Scholar]
- 47.Berker Y, Franke J, Salomon A, Palmowski M, Donker HC, Temur Y, Mottaghy FM, Kuhl C, Izquierdo-Garcia D, Fayad ZA, et al. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med. 2012;53:796–804. doi: 10.2967/jnumed.111.092577. [DOI] [PubMed] [Google Scholar]
- 48.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, Nekolla SG, Ziegler S, Ganter C, Rummeny EJ, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 49.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med. 2010;51:812–818. doi: 10.2967/jnumed.109.065425. [DOI] [PubMed] [Google Scholar]
- 50.Covello M, Cavaliere C, Aiello M, Cianelli MS, Mesolella M, Iorio B, Rossi A, Nicolai E. Simultaneous PET/MR head-neck cancer imaging: Preliminary clinical experience and multiparametric evaluation. Eur J Radiol. 2015;84:1269–1276. doi: 10.1016/j.ejrad.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Queiroz MA, Huellner MW. PET/MR in cancers of the head and neck. Semin Nucl Med. 2015;45:248–265. doi: 10.1053/j.semnuclmed.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Surti S. Update on time-of-flight PET imaging. J Nucl Med. 2015;56:98–105. doi: 10.2967/jnumed.114.145029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chirindel A, Adebahr S, Schuster D, Schimek-Jasch T, Schanne DH, Nemer U, Mix M, Meyer P, Grosu AL, Brunner T, et al. Impact of 4D-(18)FDG-PET/CT imaging on target volume delineation in SBRT patients with central versus peripheral lung tumors. Multi-reader comparative study. Radiother Oncol. 2015;115:335–341. doi: 10.1016/j.radonc.2015.05.019. [DOI] [PubMed] [Google Scholar]


