Abstract
In 2014, there were an estimated 136800 new cases of colorectal cancer, making it the most common gastrointestinal malignancy. It is the second leading cause of cancer death in both men and women in the United States and over one-third of newly diagnosed patients have stage III (node-positive) disease. For stage II and III colorectal cancer patients, the mainstay of curative therapy is neoadjuvant therapy, followed by radical surgical resection of the rectum. However, the consequences of a proctectomy, either by low anterior resection or abdominoperineal resection, can lead to very extensive comorbidities, such as the need for a permanent colostomy, fecal incontinence, sexual and urinary dysfunction, and even mortality. Recently, trends of complete regression of the rectal cancer after neoadjuvant chemoradiation therapy have been confirmed by clinical and radiographic evaluation-this is known as complete clinical response (cCR). The “watch and wait” approach was first proposed by Dr. Angelita Habr-Gama in Brazil in 2009. Those patients with cCR are followed with close surveillance physical examinations, endoscopy, and imaging. Here, we review management of rectal cancer, the development of the “watch and wait” approach and its outcomes.
Keywords: Rectal cancer, Watch and wait approach, Neoadjuvant chemotherapy rectal cancer, Nonoperative management rectal cancer
Core tip: Standard treatment for stage II and IIIrectal cancer includes neoadjuvant chemoradiation followed by radical surgical resection. Recent studies have demonstrated that a select population of patients will achieve a pathological complete response with the absence of residual cancer present after surgical resection. Preliminary attempts to identify those rectal cancer patients with a clinical complete response to neoadjuvant therapy, through various diagnostic modalities, may prevent future patients from having to undergo a very morbid operation.
INTRODUCTION
Colorectal cancer is the most common gastrointestinal malignancy with an estimated 136800 new cases diagnosed in 2014 in the United States[1]. Over one third of colorectal cancers consist of Stage III node-positive disease and rectal cancer accounts for approximately a third of these cases. Proctectomy has been the cornerstone of therapy to achieve long-term oncological results either via low anterior resection or abdominoperineal resection. Standard surgical technique involves total mesorectal excision as proposed by Heald et al[2] to achieve the lowest rates of regional recurrences with reported morbidity and mortality rates of 35% and 4%-5%, respectively and over a third of patients report some degree of urologic and sexual dysfunction, and fecal incontinence[3].
Additionally, landmark studies, like the Dutch trial and German trial CAO/ARO/AIO-94, have proven the beneficial effects of preoperative chemoradiation therapy (CRT)[4,5]. Locoregional failure rates are reported as < 10% and thus, neoadjuvant CRT plus radical surgical resection have become the standard of care for rectal cancer. Long-term results with this approach show stage-specific 5-year survival rates between 63% and 77.4%[6-8].
Despite excellent oncologic outcomes with neoadjuvant CRT followed by radical surgery, contemporary data is shifting the current paradigm of rectal cancer management towards nonoperative therapy. Multiple studies have shown an absence of viable malignant cells in surgical resection specimens after CRT, termed pathological complete response (pCR) in 18.1%-26% of cases[9]. Thus questions arise in colorectal surgery: Do patients benefit from radical surgery after an “adequate” response to CRT? How does one define an “adequate” response to neoadjuvant therapy? Do these patients achieve equivalent oncological long-term outcomes with reduced morbidity and mortality?
This paper reviews the non-operative treatment algorithm known as the “Watch and Wait” protocol, first proposed by Habr-Gama et al[10] in Brazil. Indications, treatment algorithms, outcomes, and areas of uncertainty are assessed from a worldwide perspective.
The utilization of an inaccurate staging system: Treating with uncertainty
According to the American joint committee on cancer, tumor depth is denoted by T; N is nodal metastasis, and M is distant metastasis - for evaluation of TNM cancer staging. Nodal positivity or a ≥ T3 tumor (stages II and III disease) qualifies a patient for neoadjuvant CRT prior to surgical resection[11,12]. Digital rectal examination (DRE) combined with imaging modalities including endorectal ultrasound (ERUS), magnetic resonance imaging (MRI) and/or positron emission tomography - computed tomography are utilized to determine TNM status. Staging determines prognosis and guides therapy.
The depth of tumor invasion can be determined with acceptable accuracy rates of > 90% with either ERUS or MRI, whereas lymph node (N) status is much less reliable with these imaging modalities. Accuracy rates have been determined to be between 60%-80%[13,14]. The evaluation of lymph node status is limited by the shortcomings of current diagnostic methods available in rectal cancer staging. Failure to identify up to 25% of malignant lymph nodes because of their size being less than 3 mm counters conventional beliefs that lymph node size must exceed 1 cm in order to be deemed positive for metastasis[15,16]. In other words, our current diagnostic imaging modalities understage N status. Furthermore, tumor response may not correlate with lymph node status in patients after CRT. Previous studies have shown that between 16.3%-28% of patients with complete clinical response (cCR) harbor nodal disease and its incidence is associated with initial T stage[17,18].
Defining response after CRT: Clinical complete response vs pathological complete response
pCR has been defined as the absence of neoplastic cells in the surgical resection specimen after neoadjuvant CRT and resection. Fifteen to forty percent of patients who receive neoadjuvant chemotherapy will have a pCR[19-21]. Tumor response is considered a marker of tumor biology. Patients with complete tumor response after neoadjuvant CRT have improved disease-free survival (DFS) and distant metastatic rates of 89.5% and 7%-10.5%, respectively, when compared to poor responders of neoadjuvant therapy (65% and 26%-31%, respectively)[9,22]. Variables such as sex, age and tumor location are not predictors of tumor response, whereas lymph node status is significantly associated with the risk of locoregional recurrence and subsequent distant metastases.
At present, no predictive factors exist to determine which patients will respond to CRT based on preoperative data. However, pCR is not an appropriate primary endpoint to guide clinical decision-making because it depends on the pathological results after radical surgery. Habr-Gama et al[10,23] developed the “watch and wait” protocol by creating a new endpoint: cCR. Based on a strict surveillance protocol, patients are determined to be responders once they have no evidence of tumor on: (1) DRE; (2) endoscopic assessment; and (3) imaging. When irregularities of the rectal wall (including mass, ulceration, or stenosis) are palpated on digital rectal examination, it is concerning for residual cancer. Endoscopic assessment not only confirms DRE but identifies ulceration or mucosal irregularity that may have been missed during DRE. During flexible or rigid proctoscopy, the procurement of biopsies is helpful in verifying a cCR. MRI evaluates for mixed signal intensity of the rectal wall, in addition to malignant mesorectal lymph node involvement (Figures 1 and 2). Finally, carcinoembryonic antigen (CEA) levels are obtained pre- and post-neoadjuvant CRT. If abnormal CEA levels persist after CRT, this suggests an incomplete response to neoadjuvant therapy and/or distant metastatic disease.
Figure 1.
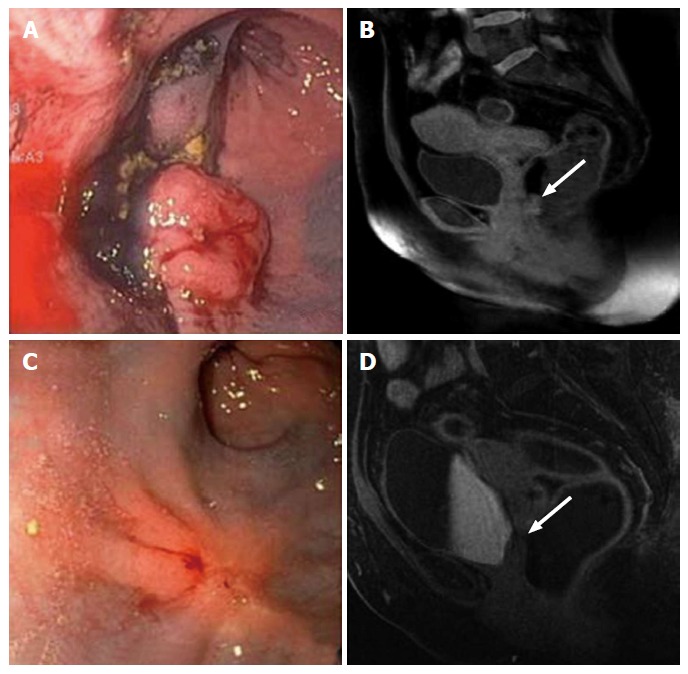
Clinical incomplete response. Evaluation of the rectal cancer prior to the initiation of neoadjuvant chemoradiation therapy by flexible sigmoidoscopy (A) and MRI (B, white arrow: Tumor). Evaluation of 7 wk after completion of neoadjuvant chemoradiation therapy. The tumor has decreased in size; however, it continues to be present as evidenced by flexible sigmoidoscopy (C) and MRI (D, white arrow: Tumor). MRI: Magnetic resonance imaging.
Figure 2.
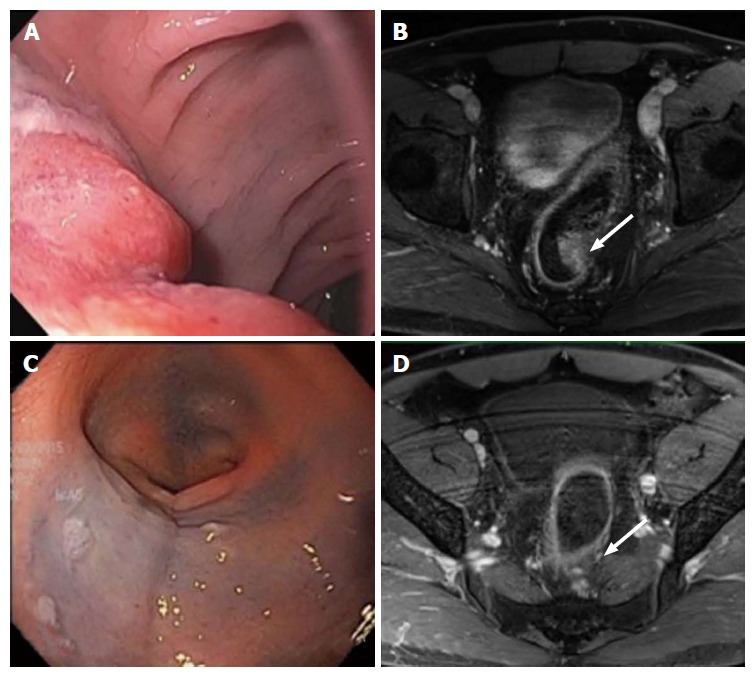
Complete clinical response. Evaluation of the rectal cancer prior to the initiation of neoadjuvant chemoradiation therapy by flexible sigmoidoscopy (A) and MRI (B, white arrow: Tumor). Evaluation of 7 wk after completion of neoadjuvant chemoradiation therapy showed no evidence of tumor by flexible sigmoidoscopy (C) and MRI (D, white arrow: Tumor). MRI: Magnetic resonance imaging.
As previously discussed, lymph node status is the most important prognostic factor in rectal cancer. The challenge of a nonoperative approach is determining whether contemporary imaging modalities adequately evaluates lymph node status in these patients; thus yielding an inferior oncological outcome compared to that of conventional operative management.
This is the basis of uncertainty and the main criticism to the “watch and wait” protocol. Deciding not to offer radical surgery based on inaccurate diagnostic tools that could potentially understage neoplastic process has been a deterrent to the acceptance of the “watch and wait” protocol in the United States. Studies are ongoing to determine whether this protocol is acceptable as standard of care.
Outcomes with watch and wait protocol: Brazil, Netherlands, United Kingdom, and United States
The “watch and wait” approach was first proposed by Habr-Gama[1] in Brazil in 2009. The current protocol by Habr-Gama[1], includes radiation therapy of 54 Gy with combination 5-fluorouracil and leucovorin chemotherapy, which extends for an additional 3 cycles beyond the neoadjuvant radiation period for a duration of 9 wk. At 10 wk, patients undergo an initial assessment with DRE, flexible sigmoidoscopy, and imaging for cCR. The patients then enroll in a vigorous surveillance program: DRE, CEA, and endoscopic assessment every 1-2 mo in the first year, every 3 mo in the second year, and every 6 mo in the third year and beyond. If the initial radiologic assessment shows cCR, then serial imaging may be performed every 6 mo (Table 1)[10]. Habr-Gama[1] prospectively studied 70 patients, of which one died due to cardiac complications from chemotherapy. On initial assessment, 68% of patients had cCR. After follow-up of 12 mo, 56% of patients had sustained cCR. For those who initially had cCR, the 3-year overall survival was 90% and DFS was 72%[24].
Table 1.
Watch and wait protocol surveillance schedule (adapted from Habr-Gama et al[10])
| Assessment of complete response | Initial assessment | First year | Second year | Third year and after |
| DRE | 10 wk | Every 1-2 mo | Every 3 mo | Every 6 mo |
| CEA | 10 wk | Every 1-2 mo | Every 3 mo | Every 6 mo |
| Endoscopic assessment | 10 wk | Every 1-2 mo | Every 3 mo | Every 6 mo |
| MRI | 10 wk | If 1st assessment normal with cCR, then every 6 mo | Every 6 mo | Every 6 mo |
DRE: Digital rectal examination; CEA: Carcinoembryonic antigen; MRI: Magnetic resonance imaging; cCR: Clinical complete response.
Another study from the Netherlands prospectively followed 192 patients with locally advanced rectal cancer who were treated with CRT[4,25]. Twenty-one patients had cCR and were followed for 25 ± 19 mo. The control cohort consisted of 20 patients who had a pCR after chemoradiation followed by surgical resection. Out of the twenty-one patients in the watch and wait protocol group, one patient developed a small endoluminal local recurrence without nodal recurrence at 22 mo follow-up. The remaining 20 patients neither had local nor distant recurrence of disease. DFS and overall survival did not statistically differ between both the watch and wait and control groups.
There are two retrospective studies from the United States and the United Kingdom which are concordant with the aforementioned prospective studies. Disease-free and overall survival rates are similar in patients with cCR, who undergo the watch and wait protocol vs conventional neoadjuvant chemoradiation therapy, followed by surgery (Table 2)[24-28].
Table 2.
Studies evaluating the watch and wait protocol
| Study | Patients (n) |
Neoadjuvant therapy |
Details | Outcomes/endpoints | |
| Radiation | Chemotherapy | ||||
| Prospective | |||||
| Habr-Gama et al[24] (Brazil) | 69 | 54 Gy radiation (45-Gy delivered as 3-field approach with daily doses of 1.8 Gy on weekdays to pelvis, followed by 9-Gy boost to the primary tumor and perirectal tissue) | 3 cycles of 5-FU (450 mg/m2) bolus and a fixed dose of 50 mg leucovorin for 3 consecutive days every 3 wk. After completion of radiation, patients received 3 additional identical cycles of chemotherapy every 3 mo | Assessment after CRT: 10 wk; assessment for sustained cCR: From 10 wk to 12 mo after CRT; patients with local recurrences after sustained cCR classified as LR | 3-yr OS for patients with initial cCR = 3-yr DFS for patients with initial cCR = 72% |
| Lambregts et al[25] and Maas et al[26] (Netherlands) | 21 | 28 fractions of 1.8 Gy = 50.4 Gy | IV oxaliplatin and capecitabine | Assessment after CRT: 6-8 wk; evaluation for cCR: MRI and endoscopy; operative management with CRT and resection (control group): 20 patients with pCR after surgery | Nonoperative management group; 1 patient developed LR and had surgery as salvage treatment; 20 patients are alive without disease; no difference in 2-yr DFS and OS between the watch and wait and the CRT and resection groups |
| Smith et al[27] (United States) | 32 | External beam radiation over 5-6 wk, median dose 50.4 Gy (range 45-56 Gy) | 5-FU or capecitabine | Assessment after CRT: 4-10 wk; evaluation for cCR: DRE, endoscopy ± biopsy; evaluation for cCR at 1-yr: DRE, flexible sigmoidoscopy every 3 mo; evaluation for cCR subsequent years: DRE, flexible sigmoidoscopy every 4-6 mo; operative management (control group): 256 patients, 57 (22%) with pCR; median follow up: 28 mo | Nonoperative management group had a higher rate of LR (21% vs 0%, P = 0.001): 6 recurred locally (median 11 mo), 3 had concurrent DR; 2-yr DR (8% vs 2%, P = 0.30), DFS (88% vs 98%, P = 0.27), and OS (97% vs 100%, P = 0.56) were similar for nonoperative management and rectal resection/pCR groups |
| Dalton et al[28] (United Kingdom) | 12 | 45 Gy in 25 fractions over 5 wk | Concurrent capecitabine | Assessment after CRT: 8 wk; evaluation for cCR: MRI complemented with EUA/biopsy and PET/CT if tumor regression is suspected; cCR patients are followed with repeat EUA at 3 mo and 12 mo, and 6-monthly PET/CT and MRI; median follow up 25.5 mo | cCR in 12/49 (24.4%); 6/12 patients with cCR without evidence of disease |
LR: Local recurrence; DR: Distant recurrence; DFS: Disease-free survival; OS: Overall survival; Gy: Gray; CRT: Chemoradiation therapy; DRE: Digital rectal examination; EUA: Examination under anesthesia; 5-FU: 5-fluorouracil; cCR: Clinical complete response; pCR: Pathological complete response; PET/CT: Positron emission test/computerized tomography; MRI: Magnetic resonance imaging.
In conclusion, advances in chemoradiation therapy for rectal cancer have delineated a select population of patients who have a pCR after surgical resection. Observation of this pCR led to the conception of the watch and wait protocol by Habr-Gama et al[24], in Brazil. Patients are identified as having a cCR and followed with close surveillance by physical examination, endoscopic assessment, and imaging studies. Thus far, they have followed prospectively, a highly selected patient population. This study has been confirmed by a study in the Netherlands[26,27].
However, the watch and wait protocol has not been widely accepted as standard of care. There are limitations for current data in the literature. First, only two prospective cohort studies exist with small sample sizes. No randomized controlled trials exist, comparing the watch and wait protocol with standard neoadjuvant chemoradiation therapy followed by surgery. Enrollment into these studies is biased by patient selection due to the lack of randomization. Despite close surveillance, no studies have delineated patient characteristics or predictive factors that predict tumor response to chemoradiation therapy. Though patients undergo a very strict surveillance protocol, the ultimate question arises as to whether cancer remains in the rectum and whether they exist in the lymph nodes. The inaccuracies of current imaging modalities limit the accurate staging of rectal cancer. Further precision in rectal cancer staging would require innovative advances in diagnostic technologies in order to avoid radical surgery.
The uncertainty of outcomes of a cCR after chemoradiation therapy for rectal cancer continues to exist. Further randomized controlled trials are required to validate the watch and wait protocol. As nonoperative management for rectal cancer advances, we predict that the evolution of rectal cancer treatment will mimic that of anal cancer. Prior to the 1970’s anal cancer management was purely surgical. However, with the ground-breaking work of Nigro et al[29], the anal cancer treatment paradigm has shifted to a nonsurgical approach with primary treatment consisting of multimodality therapy with chemotherapy and radiation. Further changes in the standard of care to nonoperative management will be dependent on the identification of patient factors that can predict a pCR. The introduction of molecular techniques that allow the identification of high-risk patients could play a substantial role in the creation of a genetic profile that would funnel a highly selected group of rectal cancer patients into the watch and wait protocol.
Footnotes
Conflict-of-interest statement: There are no conflicting interests to report from both authors of this paper.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 29, 2015
First decision: August 16, 2015
Article in press: September 30, 2015
P- Reviewer: Lin Y, Vallicelli C S- Editor: Kong JX
L- Editor: A E- Editor: Li D
References
- 1.Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis. 2006;8 Suppl 3:21–24. doi: 10.1111/j.1463-1318.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 2.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 3.Schneider EB, Hyder O, Brooke BS, Efron J, Cameron JL, Edil BH, Schulick RD, Choti MA, Wolfgang CL, Pawlik TM. Patient readmission and mortality after colorectal surgery for colon cancer: impact of length of stay relative to other clinical factors. J Am Coll Surg. 2012;214:390–398; discussion 398-399. doi: 10.1016/j.jamcollsurg.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W, Tschmelitsch J, Sabitzer H, Karstens JH, Becker H, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5:406–415. doi: 10.1046/j.1463-1318.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 6.Marks JH, Huang R, McKeever D, Greenfield M. Outcomes in 132 patients following laparoscopic total mesorectal excision (TME) for rectal cancer with greater than 5-year follow-up. Surg Endosc. 2015:April 24; Epub ahead of print. doi: 10.1007/s00464-015-4210-1. [DOI] [PubMed] [Google Scholar]
- 7.Joern F, Gunter H, Thomas J, Erik P, Jörg Z, Dorothea B, Thomas K, Helmut W, Sigmar S. Outcome for stage II and III rectal and colon cancer equally good after treatment improvement over three decades. Int J Colorectal Dis. 2015;30:797–806. doi: 10.1007/s00384-015-2219-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhou ZX, Zhao LY, Lin T, Liu H, Deng HJ, Zhu HL, Yan J, Li GX. Long-term oncologic outcomes of laparoscopic vs open surgery for stages II and III rectal cancer: A retrospective cohort study. World J Gastroenterol. 2015;21:5505–5512. doi: 10.3748/wjg.v21.i18.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habr-Gama A, São Julião GP, Perez RO. Nonoperative management of rectal cancer: identifying the ideal patients. Hematol Oncol Clin North Am. 2015;29:135–151. doi: 10.1016/j.hoc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Mohiuddin M, Winter K, Mitchell E, Hanna N, Yuen A, Nichols C, Shane R, Hayostek C, Willett C. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 12.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A AJCC Cancer Staging Man-ual. Vol 649. 7th ed. New York: Springer [Google Scholar]
- 13.Kosinski L, Habr-Gama A, Ludwig K, Perez R. Shifting concepts in rectal cancer management: a review of contemporary primary rectal cancer treatment strategies. CA Cancer J Clin. 2012;62:173–202. doi: 10.3322/caac.21138. [DOI] [PubMed] [Google Scholar]
- 14.Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s–6884s. doi: 10.1158/1078-0432.CCR-07-1137. [DOI] [PubMed] [Google Scholar]
- 15.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 16.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. doi: 10.1080/02841860701697720. [DOI] [PubMed] [Google Scholar]
- 17.Swellengrebel HA, Bosch SL, Cats A, Vincent AD, Dewit LG, Verwaal VJ, Nagtegaal ID, Marijnen CA. Tumour regression grading after chemoradiotherapy for locally advanced rectal cancer: a near pathologic complete response does not translate into good clinical outcome. Radiother Oncol. 2014;112:44–51. doi: 10.1016/j.radonc.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Feig B, Nguyen S, Hu CY, Chang GJ. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56:135–141. doi: 10.1097/DCR.0b013e318278ff8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Aguilar J, Shi Q, Thomas CR, Chan E, Cataldo P, Marcet J, Medich D, Pigazzi A, Oommen S, Posner MC. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–391. doi: 10.1245/s10434-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319–1328; discussion 1328-1329. doi: 10.1016/j.gassur.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–1562. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]
- 23.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 24.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, Nadalin W, Perez RO. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e. [DOI] [PubMed] [Google Scholar]
- 25.Lambregts DM, Maas M, Bakers FC, Cappendijk VC, Lammering G, Beets GL, Beets-Tan RG. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum. 2011;54:1521–1528. doi: 10.1097/DCR.0b013e318232da89. [DOI] [PubMed] [Google Scholar]
- 26.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 27.Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965–972. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 28.Dalton RS, Velineni R, Osborne ME, Thomas R, Harries S, Gee AS, Daniels IR. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567–571. doi: 10.1111/j.1463-1318.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 29.Nigro ND, Vaitkevicius VK, Considine B. Combined therapy for cancer of the anal canal: a preliminary report 1974. Dis Colon Rectum. 1993;36:709–711. doi: 10.1007/BF02238600. [DOI] [PubMed] [Google Scholar]


