Abstract
AIM: To compare the surgical outcomes between laparoscopic liver resection (LLR) and open liver resection (OLR) as a curative treatment in patients with hepatocellular carcinoma (HCC).
METHODS: A PubMed database search was performed systematically to identify comparative studies of LLR vs OLR for HCC from 2000 to 2014. An extensive text word search was conducted, using combinations of search headings such as “laparoscopy”, “hepatectomy”, and “hepatocellular carcinoma”. A comparative study was also performed in our institution where we analysed surgical outcomes of 152 patients who underwent liver resection between January 2005 to December 2012, of which 42 underwent laparoscopic or hand-assisted laparoscopic resection and 110 underwent open resection.
RESULTS: Analysis of our own series and a review of 17 high-quality studies showed that LLR was superior to OLR in terms of short-term outcomes, as patients in the laparoscopic arm were found to have less intraoperative blood loss, less blood transfusions, and a shorter length of hospital stay. In our own series, both LLR and OLR groups were found to have similar overall survival (OS) rates, but disease-free survival (DFS) rates were higher in the laparoscopic arm.
CONCLUSION: LLR is associated with better short-term outcomes compared to OLR as a curative treatment for HCC. Long-term oncologic outcomes with regards to OS and DFS rates were found to be comparable in both groups. LLR is hence a safe and viable option for curative resection of HCC.
Keywords: Hepatocellular carcinoma, Laparoscopy, Open liver resection, Hepatectomy, Surgery
Core tip: Surgical resection is the standard treatment for hepatocellular carcinoma (HCC), and provides the best outcomes for patients eligible for resection. Laparoscopic liver resection (LLR) is a relatively new advancement in treatment of HCC and has raised concerns on its feasibility and safety. We reviewed 17 studies and performed our own comparative study on surgical outcomes of LLR vs open liver resection for the curative treatment of HCC. We showed that LLR resulted in more desirable short-term outcomes, whereas long-term oncologic outcomes were comparable. Hence, LLR is a safe and feasible option in the surgical treatment of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most frequent cause of cancer-related death, with about 750000 new cases diagnosed and approximately 700000 deaths worldwide each year[1,2]. Potentially curative treatment options for HCC include surgical resection[3], liver transplantation[4], and local ablation[5]. Surgical resection remains the standard treatment, and provides the best outcomes, for candidates who are eligible for resection[6]. In 1991, Reich et al[7] performed the first laparoscopic hepatic resection (LLR) for a benign liver tumour; subsequently, Hashizume et al[8] reported the first LLR performed for HCC. However, many barriers have hindered the popularity of LLR, including concerns of uncontrollable bleeding, resection margins, tumour seeding, and port-site metastases. LLR may also be perceived as a challenge especially in cirrhotic patients, who are at increased risk of complications related to underlying synthetic and metabolic dysfunction[9]. Nevertheless, over the past 2 decades, it has become a widely accepted mode of curative resection for HCC by being established as both safe and feasible. It has also evolved to encompass more difficult anatomic resections.
A number of comparative studies have been published on the surgical outcomes of LLR vs open liver resection (OLR) as a curative treatment for HCC, and most suggest that while LLR and OLR both have similar overall survival (OS) and disease-free survival (DFS) rates, LLR confers the additional advantages of shorter duration of hospitalization and lower complication rates. To our knowledge, there has been so far no prospective, randomized controlled study done on this subject.
In this review article, we systematically reviewed 17 comparative studies from 2001 to 2014 to look at the surgical outcomes of LLR vs OLR for curative resection of HCC. We also conducted our own comparative study by analysing data from 152 patients who underwent surgical resection of HCC from 2005 to 2012 at the National University Hospital (Singapore) and compared our results to those of the 17 comparative studies.
MATERIALS AND METHODS
A PubMed database search was performed systematically to identify comparative studies of LLR vs OLR for HCC from 2000 to 2014. An extensive text word search was conducted, using combinations of search headings such as “laparoscopy”, “laparoscopic”, “minimally invasive surgery”, “hepatectomy”, “hepatic resection”, “hepatic lobectomy”, “liver resection”, “hepatocellular carcinoma”, “HCC”, and “primary liver cancer”. The search was restricted to comparative studies and human studies only. All studies identified for screening were manually reviewed. References from these articles were also searched for relevant studies. The most recent search was conducted on 6 June 2014 (Figure 1).
Figure 1.
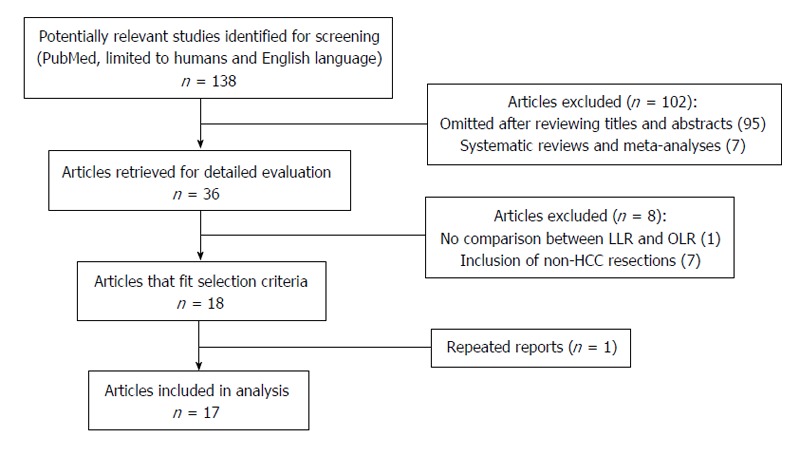
Flow chart illustrating the screening and selection process. LLR: Laparoscopic liver resection; OLR: Open liver resection; HCC: Hepatocellular carcinoma.
Studies were included in the analysis if they: (1) were comparative studies on humans and in the English language; (2) focused on outcomes of LLR vs OLR for HCC; (3) had more than 10 patients in each group included in the study; and (4) if multiple studies were reported by the same institution or authors, the most recent publication was included. Studies were excluded from the analysis if they: (1) were reviews lacking original data, abstracts, editorials, or expert opinions; (2) did not show clear comparisons between outcomes on LLR vs OLR; and (3) included resections of benign tumours or metastatic lesions other than HCC. Our own series was analysed together with the selected studies.
National University Hospital series
Case records of 152 patients who underwent curative resection for HCC at the National University Hospital (NUH) in Singapore from January 2005 to December 2012 were prospectively retrieved and manually culled for clinical data. Of the patients included in our study, 42 underwent laparoscopic or hand-assisted laparoscopic resection and 110 underwent open resection. All patients were followed up for recurrence at least 3-mo for the 1st year, 4-mo for the 2nd year, then every 6 mo subsequently. Patients were stratified according to the type of operation they underwent (OLR vs LLR). Vital status and the death date for subjects were obtained from the National Death Registry Database, and death data was supplemented with data from hospital records. For long-term oncologic outcomes, the study endpoints analyzed were OS and DFS. OS was calculated from the date of operation to the date of death. DFS was calculated from the date of operation to the date of 1st recurrence or HCC-related death.
Statistical analysis
The clinical characteristics of patients and postoperative results were expressed as means with standard deviations. The χ2 or Fisher’s exact test was used to compare categorical variables and the Mann-Whitney U test was used to compare continuous variables. Survival analysis was performed using the time of disease-free survival vs recurrence of a tumor or death. Survival curves were computed using the Kaplan-Meier method and compared between open and laparoscopic groups by the log-rank test. A P value of < 0.05 was considered as being statistically significant. All statistical calculations were performed using SPSS version 21.0.
RESULTS
NUH series
One hundred and fifty-two patients undergoing liver resection for HCC were retrospectively reviewed at the National University Hospital in Singapore, from January 2005 to December 2012. Of the patients included in our study, 42 underwent laparoscopic or hand-assisted laparoscopic resection and 110 underwent open resection. All patients were followed up for recurrence at least 3-mo for the 1st year, 4-mo for the 2nd year, then every 6 mo subsequently.
Demographics
The demographic data and clinical characteristics of both groups are shown in Table 1. Both groups did not differ in terms of age, gender, Child-Pugh score, pre-operative laboratory investigations, and tumour locations; however, there was a significant difference in the ASA score (P = 0.045), number of co-morbidities (mean 3 vs 2.32, P = 0.028), and tumour size (mean 3.85 cm vs 7.15 cm, P < 0.001).
Table 1.
Preoperative characteristics n (%)
| Variable | LLR (n = 42) | OLR (n = 110) | P value |
| Age | 61.07 (11.91) | 59.45 (11.15) | 0.400 |
| Gender | 0.359 | ||
| Male | 32 (76.2) | 91 (82.7) | |
| Female | 10 (23.8) | 19 (17.3) | |
| Child-Pugh score | 0.094 | ||
| A | 42 (100.0) | 103 (93.6) | |
| B | 0 (0.0) | 7 (6.4) | |
| No. of comorbidities | 3 ± 1.86 | 2.32 ± 1.64 | 0.028 |
| HBsAg | 6 (42.9) | 25 (55.6) | 0.406 |
| Anti-HCV | 1 (7.1) | 1 (2.7) | 0.466 |
| Alpha-fetoprotein | 734.33 ± 2978.62 | 2126.96 ± 8456.88 | 0.654 |
| ALT | 40.64 ± 28.86 | 46.57 ± 35.59 | 0.408 |
| AST | 46.83 ± 36.03 | 56.93 ± 50.26 | 0.280 |
| ALP | 90.26 ± 35.51 | 102.29 ± 44.24 | 0.099 |
| Total bilirubin | 13.12 ± 7.18 | 12.91 ± 14.26 | 0.367 |
| PT | 13.71 ± 0.94 | 13.61 ± 1.64 | 0.176 |
| ASA class | 0.045 | ||
| I | 3 (7.1) | 9 (8.2) | |
| II | 24 (57.1) | 62 (56.4) | |
| III | 15 (35.7) | 39 (35.5) | |
| No. of tumours | 0.469 | ||
| Solitary | 37 (88.1) | 91 (82.7) | |
| Multiple | 5 (11.9) | 19 (17.3) | |
| Size of largest tumour (cm) | 3.85 ± 2.60 | 7.15 ± 4.88 | < 0.001 |
| Tumour location | 0.256 | ||
| Left lobe | 15 (35.7) | 27 (24.5) | |
| Right lobe | 21 (50.0) | 71 (64.5) | |
| Bilobar | 6 (14.3) | 12 (10.9) |
Data are mean ± SD or n (%) unless otherwise indicated. HBsAg: Hepatitis B virus surface antigen; Anti-HCV: Anti-hepatitis C virus antibody; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; PT: Prothrombin time; ASA: American Society of Anaesthesiologists; LLR: Laparoscopic liver resection; OLR: Open liver resection.
Intraoperative results
Table 2 shows the intraoperative results of the two groups. In the LLR group, conversion from LLR to OLR occurred in 5 patients (11.9%). The duration of operation in the LLR group was significantly shorter compared to the OLR group (mean 250.43 min vs 349.90 min, P < 0.001). The intraoperative blood loss was significantly lower in the LLR group (495.83 mL vs 1085.00 mL, P < 0.001), as was the requirement for blood transfusion (9.5% vs 39.1%, P < 0.001). However, there was no difference in the amount of blood transfused in patients who required transfusion in both groups.
Table 2.
Perioperative data n (%)
| Variable | LLR (n = 42) | OLR (n = 110) | P value |
| Type of resection | < 0.001 | ||
| Right hepatectomy | 4 (9.5) | 33 (30.0) | |
| Left hepatectomy | 4 (9.5) | 11 (10.0) | |
| Extended right hepatectomy | 0 (0.0) | 9 (8.2) | |
| Extended left hepatectomy | 0 (0.0) | 6 (5.5) | |
| Right anterior sectionectomy | 0 (0.0) | 2 (1.8) | |
| Right posterior sectionectomy | 6 (14.3) | 2 (1.8) | |
| Left lateral sectionectomy | 8 (19.0) | 3 (2.7) | |
| Wedge resection | 6 (14.3) | 10 (9.1) | |
| Segmentectomy | 14 (33.3) | 31 (28.2) | |
| Others | 0 (0.0) | 3 (2.7) | |
| Conversion from LLR to OLR | 5 (11.9) | - | - |
| Duration of operation (min), means ± SD | 250.43 ± 98.85 | 349.90 ± 132.29 | < 0.001 |
| Intra-operative blood loss (mL), mean ± SD | 495.83 ± 501.94 | 1085.00 ± 943.55 | < 0.001 |
| Blood transfusion | 4 (9.5) | 43 (39.1) | < 0.001 |
| Amount transfused (mL), mean ± SD | 709.25 ± 726.18 | 1349.30 ± 1532.32 | 0.269 |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Pathologic results
As for the pathologic results shown in Table 3, there was no difference in the condition of the surrounding liver parenchyma in both groups, except for a larger proportion of patients with cirrhosis in the LLR group (59.5% vs 35.5%, P = 0.007). Microscopic vascular invasion occurred more often in the OLR group (14.3% vs 30.9%, P = 0.037). There was no difference between both groups in the histological grade of the tumours as well as the number of patients with local tumour invasion and positive resection margins.
Table 3.
Pathologic results n (%)
| Variable | LLR (n = 42) | OLR (n = 110) | P value |
| Condition of non-tumourous liver | |||
| Normal | 8 (19.0) | 35 (31.8) | 0.118 |
| Chronic hepatitis | 12 (28.6) | 31 (28.2) | 0.962 |
| Cirrhosis | 25 (59.5) | 39 (35.5) | 0.007 |
| Steatosis | 16 (38.1) | 29 (26.4) | 0.157 |
| Others | 2 (4.8) | 5 (4.5) | 0.955 |
| Microscopic vascular invasion | 6 (14.3) | 34 (30.9) | 0.037 |
| Invasion into adjacent organs | 0 (0.00) | 2 (1.8) | 0.379 |
| Histological grade | 0.077 | ||
| Well differentiated | 9 (21.4) | 20 (18.2) | |
| Moderately differentiated | 30 (71.4) | 61 (55.5) | |
| Poorly differentiated | 3 (7.1) | 28 (25.5) | |
| Undifferentiated | 0 (0.0) | 1 (0.9) | |
| Positive resection margin | 1 (2.4) | 8 (7.3) | 0.253 |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Post-operative outcomes
With regards to post-operative outcomes (Table 4), there was no difference in the overall complications rate as well as the specific complications (cardiac, pulmonary, gastrointestinal, wound infections, bleeding, prolonged ascites, intra-abdominal sepsis, liver failure) among the LLR and OLR groups. There was no difference in postoperative mortality as well. The total length of hospital stay was significantly shorter in the LLR group (7.55 d vs 11.42 d, P < 0.001).
Table 4.
Postoperative outcomes n (%)
| Variable | LLR (n = 42) | OLR (n = 110) | P value |
| Patients with complications | 16 (38.1) | 50 (45.5) | 0.413 |
| Bleeding | 1 (2.4) | 1 (0.9) | 0.476 |
| Prolonged ascites | 1 (2.4) | 4 (3.6) | 0.698 |
| Intra-abdominal sepsis | 0 (0.0) | 3 (2.7) | 0.280 |
| Liver failure | 2 (4.8) | 1 (0.9) | 0.127 |
| Cardiac | 3 (7.1) | 10 (9.1) | 0.701 |
| Pulmonary | 8 (19.0) | 15 (13.6) | 0.405 |
| Gastrointestinal | 1 (2.4) | 9 (8.2) | 0.197 |
| Wound infections | 0 (0.0) | 5 (3.3) | 0.160 |
| Postoperative mortality | 1 (2.4) | 3 (2.7) | 0.905 |
| Length of hospital stay (d), means ± SD | 7.55 ± 11.74 | 11.42 ± 9.35 | < 0.001 |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Long-term oncologic outcomes
Table 5 shows the long-term oncologic outcomes of the two groups. In the LLR group, the 5-year overall survival was 80.5%. In the OLR group, the 5-year overall survival was 83.8% (P = 0.949) (Figure 2). For disease-free survival rates, the LLR group had a survival rate of 52.5% whereas their counterparts in the OLR group had a survival rate of 38.2% (P = 0.035) (Figure 3). Hence, there was a significant difference in the disease-free survival rates between both groups but not in overall survival rates.
Table 5.
Oncological outcomes
| Variable | LLR (n = 42) | OLR (n = 110) | P value |
| Overall survival time (mo), mean ± SD | 71.25 ± 6.59 | 76.42 ± 4.468 | 0.949 |
| Disease-free survival time (mo), mean ± SD | 46.81 ± 7.132 | 34.390 ± 4.254 | 0.035 |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Figure 2.
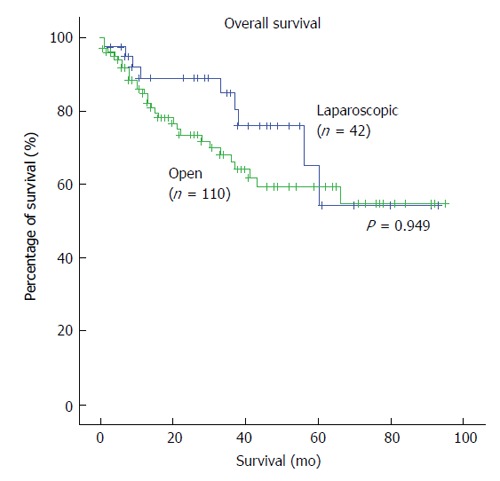
Kaplan-Meier survival curves of overall survival in laparoscopic and open liver resection.
Figure 3.
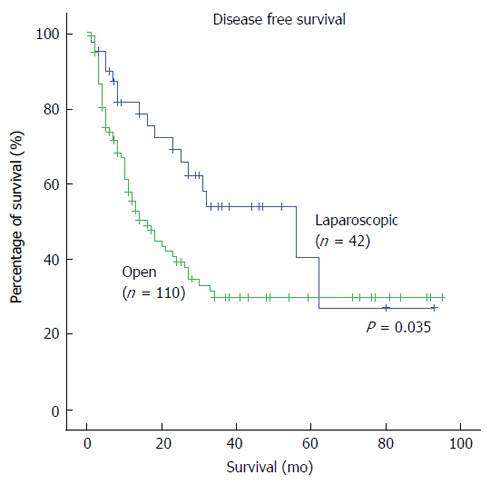
Kaplan-Meier survival curves of disease-free survival in laparoscopic and open liver resection.
Systematic review
After an extensive literature search and screening, a total of 138 references were identified. The flow of reference selection is depicted in Figure 1. A total of 17 studies published between 2001 and 2014 were identified as eligible for analysis[10-26].
Our review of the above selected articles, as well as the results of our own comparative study, showed that post-operative outcomes in the OLR cohort were significantly and consistently poorer compared to the LLR cohort. The characteristics of the selected articles are summarised in Table 6, and some of the post-operative outcomes analysed using Forest plots (Figures 4-6).
Table 6.
Characteristics of included studies
| Ref. |
No. of patients |
Child-Pugh score A |
Solitary tumour |
Mean tumour size (cm) |
|||||||
| LLR | OLR | LLR n (%) | OLR n (%) | P value | LLR n (%) | OLR n (%) | P value | LLR | OLR | P value | |
| Shimada et al[10] | 17 | 38 | - | - | - | - | - | - | 2.6 ± 0.9 | 2.5 ± 1.0 | 0.89 |
| Laurent et al[11] | 13 | 14 | 13 (100.0) | 14 (100.0) | 0.49 | - | - | - | 3.35 ± 0.89 | 3.43 ± 1.05 | 0.48 |
| Kaneko et al[12] | 30 | 28 | 22 (73.3) | 22 (57.9) | NS | - | - | - | 3.0 ± 0.8 | 3.1 ± 0.9 | NS |
| Sarpel et al[13] | 20 | 56 | - | - | - | - | - | - | 4.3 ± 2.1 | 4.3 ± 2.2 | 0.876 |
| Lai et al[14] | 25 | 33 | 23 (92.0) | 31 (93.9) | 0.90 | ||||||
| Belli et al[15] | 54 | 125 | 49 (90.7) | 117 (93.6) | 0.499 | 44 (81.5) | 96 (76.8) | 0.486 | 3.8 ± 1.3 | 6.0 ± 2.3 | 0.006 |
| Endo et al[16] | 10 | 11 | 10 (100.0) | 7 (63.6) | NS | 9 (90.0) | 10 (90.9) | NS | 3.0 ± 1.5 | 4.1 ± 0.8 | NS |
| Aldrighetti et al[17] | 16 | 16 | 9 (56.2) | 9 (56.2) | NS | - | - | - | 4 ± 2.2 | 4.6 ± 2.5 | NS |
| Tranchart et al[18] | 42 | 42 | 30 (71.4) | 33 (78.6) | - | - | - | - | 3.58 ± 1.75 | 3.68 ± 2.09 | 0.95 |
| Ker et al[19] | 116 | 209 | 98 (84.5) | 197 (94.3) | 0.08 | - | - | - | 2.5 ± 1.2 | 5.4 ± 3.5 | 0.001 |
| Kim et al[20] | 26 | 29 | - | - | - | - | - | - | - | - | - |
| Truant et al[21] | 36 | 53 | 32 (88.9) | 47 (88.7) | 1 | 34 (94.4) | 44 (83.0) | 0.2 | 2.9 ± 1.2 | 3.1 ± 1.2 | 0.5 |
| Hu et al[22] | 30 | 30 | 29 (96.7) | 24 (80.0) | NS | - | - | - | 6.7 ± 3.1 | 8.7 ± 2.3 | NS |
| Lee et al[23] | 33 | 50 | 33 (100.0) | 50 (100.0) | NS | 31 (93.9) | 41 (82.0) | 0.186 | - | - | - |
| Cheung et al[24] | 32 | 64 | 32 (100.0) | 60 (93.8) | 0.367 | - | - | - | - | - | - |
| Kim et al[25] | 70 | 76 | - | - | - | - | - | - | 2.58 ± 1.44 | 2.45 ± 1.27 | 0.550 |
| Kim et al[26] | 29 | 29 | 28 (96.6) | 29 (100.0) | 0.317 | 24 (82.8) | 28 (96.6) | 0.103 | 3.59 ± 2.17 | 4.28 ± 2.55 | 0.278 |
| Our reports | 42 | 110 | 42 (100.0) | 103 (93.6) | 0.094 | 37 (88.1) | 91 (82.7) | 0.469 | 3.85 ± 2.60 | 7.15 ± 4.88 | < 0.001 |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Figure 4.
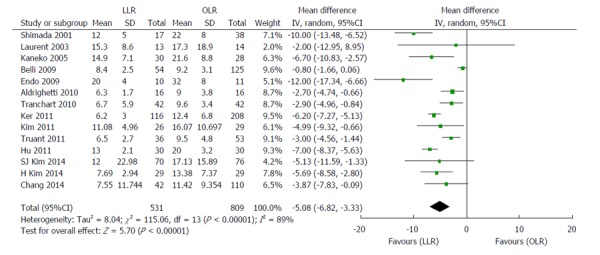
Forest plots depicting length of hospital stay in the included studies. LLR: Laparoscopic liver resection; OLR: Open liver resection.
Figure 6.
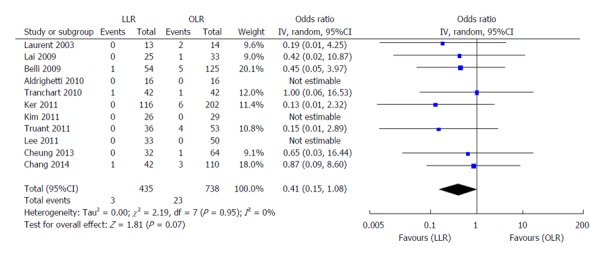
Forest plots depicting postoperative mortality in the included studies. LLR: Laparoscopic liver resection; OLR: Open liver resection.
Fourteen high-quality studies (including the NUH series) reported on length of hospital stay (Figure 4); pooled outcome measure favored LLR [patients 1340; WMD: -5.08; 95%CI: -6.82-(-3.33); P < 0.00001]. The results of 18 studies on post-operative complications (Figure 5) showed that patients who underwent LLR experienced significantly fewer complications than their counterparts who underwent OLR (patients: 1653; WMD: 0.40; 95%CI: 0.30-0.54; P < 0.0001). No significant differences were observed between LLR and OLR with regards to post-operative mortality in the 11 studies analysed, as shown in Figure 6 (patients: 1173; WMD: 0.41; 95%CI: 0.14-1.08; P = 0.07).
Figure 5.
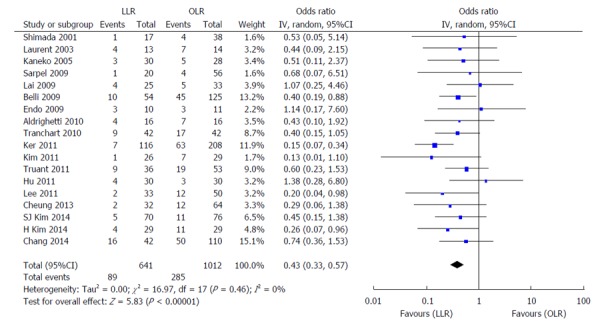
Forest plots depicting postoperative complications in the included studies. LLR: Laparoscopic liver resection; OLR: Open liver resection.
DISCUSSION
Intraoperative bleeding is a significant problem faced during liver resection, and is frequently the most common reason for conversion from laparoscopic to open hepatectomy[27,28]. The number of transfusions required intraoperatively has also been shown to be an independent risk factor for a worse post-operative prognosis[29,30]. The worldwide acceptance of LLR was delayed due to concerns of the technical difficulties of controlling hemorrhage and obtaining hemostasis. However, our study showed that intraoperative blood loss and the number of patients requiring transfusion were significantly lower in the laparoscopic arm. Reasons for this include image magnification during LLR, usage of intra-operative ultrasonography to visualize the tumour and surrounding intrahepatic vessels and equipment such as ultrasonic laparoscopic coagulation shears and argon beam coagulators to provide rapid hemostasis in the event of hepatic hemorrhage. The pneumoperitoneum in LLR results in increased intra-abdominal pressure, which also reduces visceral blood flow, in turn decreasing blood loss[31,32].
Another major concern regarding LLR for malignant lesions is difficulty assessing resection margins, due to the lack of tactile sensation and distance perception in laparoscopic resection. However, our study showed that there was no difference in resection margins in both series. We are able to make up for the lack of palpation in LLR and hence achieve the intended margins laparoscopically, with pre-operative surgical planning using a variety of imaging techniques and the use of intra-operative ultrasonography to demarcate surgical margins.
Our analysis of the 17 studies showed that the rates of postoperative complications were significantly lower in patients who underwent LLR. Possible reasons for this include less mobilization and manipulation of the liver and other intra-abdominal organs, avoidance of long incisions and division of the abdominal muscles hence minimizing disruption to the abdominal wall collateral circulation, less severe pain, earlier ambulation and oral food intake, and more post-operative cough and expectoration. However, the findings in our comparative study were not significant. Nevertheless, it is worthy to note that even though there was a significantly higher number of co-morbidities in patients in the LLR group, and a significantly greater number of patients found to have cirrhosis in the LLR group, the LLR cohort experienced fewer postoperative complications, though this result was not statistically significant.
Liver resection in HCC patients with chronic liver disease (CLD) or cirrhosis has been a major issue due to the high rates of postoperative morbidity from decompensation due to their underlying liver disease. In these patients, portal hypertension is a major risk factor for development of postoperative decompensation[33,34]. The studies we analysed which were specific to HCC patients with underlying CLD or cirrhosis showed that LLR resulted in fewer postoperative complications compared to OLR. Belli et al[15] showed that a significantly decreased postoperative morbidity rate in the laparoscopic group. The studies by Laurent et al[11] and Truant et al[21] showed lower rates of post-operative ascites and liver failure in the LLR group as well. Overall, fewer complications in the LLR group result in a shorter length of hospital stay. Furthermore, from our own comparative study, the rates of prolonged ascites and liver failure in both groups were not significantly different despite a significantly larger number of patients with cirrhosis in the LLR group.
Laparoscopic hepatectomy has not been shown to increase the risk of tumor recurrence and affect the oncologic outcomes (in terms of overall survival and disease-free survival). However, in our study, there was a significant increase in disease-free survival rates in the LLR group; this could be attributed to the higher incidence of microscopic vascular invasion found on histology in the OLR group, which is a significant underlying risk factor for tumour recurrence.
Although LLR has been shown to be superior to OLR in terms of surgical outcomes, the clinical significance of these results should be interpreted keeping in mind that they were based on selected patients who fulfill certain criteria. The size and location of the tumour are important considerations that influence a surgeon’s decision to perform an open or a laparoscopic resection. As a general rule, small (< 5 cm) tumours, in superficial or peripheral locations, far away from major vessels, are considered for LLR. Large tumours and cases requiring vascular or biliary reconstruction are usually indications for open resection. Nevertheless, with improvement of the laparoscopic technique and new advances in technology over the past 2 decades, LLR is being performed more frequently and for more complex cases with tumours in difficult anatomical locations[22,35,36].
Strengths and limitations
Our systematic review has some limitations which warrant discussion and should be considered when interpreting the results. Firstly, all comparative studies including our own are non-randomized controlled studies that are retrospective or retrospective matched. To our knowledge, no randomized control trial has been published on this subject. Also, as mentioned above, selection of patients in both the LLR and OLR groups followed certain criteria based on the pre-operative clinicopathologic characteristics of each case, as well as according to the experience and expertise of the surgeons. This tends to increase the risk of selection bias. However, many of the studies we analysed performed case-matched analysis and matched patients in both groups based on similar characteristics, such as tumour size, tumour location, and presence of CLD or cirrhosis[12-14,17,18,20-22,24,26]. This minimized the degree of selection bias to some extent.
The strengths of our review are, firstly, a substantial number of studies analysed from various centres around the world, in addition to our own. Also, strict inclusion and exclusion criteria were implemented to select the highest quality and most recent studies after an extensive literature search.
In conclusion, our systematic review and comparative study show that as a curative treatment for HCC, LLR provides better short-term outcomes than OLR in terms of intraoperative blood loss, blood transfusions, and length of hospital stay, while both LLR and OLR provide similar long-term oncologic outcomes. Further research should be undertaken in the form of prospective randomized control trials to substantiate our findings even further.
COMMENTS
Background
For hepatocellular carcinoma (HCC), surgical resection is the standard treatment and provides the best outcomes for candidates who are eligible for resection. With advances in technology, laparoscopic liver resection (LLR) is becoming more widely accepted as a safe and effective approach to the management of HCC. Studies comparing various outcomes of the open vs laparoscopic approach to surgical resection of HCC have reported that LLR results in better short-term outcomes, both methods of resection give rise to similar long-term oncologic results.
Research frontiers
Since LLR for HCC was first reported in 1995, it has been constantly evolving to encompass more difficult anatomic resections, including larger tumours, and tumours located in the posterosuperior segments of the liver, which were previously traditionally done via the open method.
Innovations and breakthroughs
In this study, the authors analysed a substantial number of studies from various established and reputable centres all around the world, including the authors’ own. Strict inclusion and exclusion criteria were implemented to select the highest quality and most recent studies after an extensive literature search.
Applications
The study results suggest that LLR is associated with better short-term outcomes compared to open liver resection as a curative treatment for HCC, with comparable long-term oncologic outcomes between both groups. LLR is hence a safe and viable option for curative resection of HCC.
Peer-review
This is an excellent paper dealing with comparison between laparoscopic and open liver resection in the treatment of HCC. The manuscript is well written and provides important clinical information that is potentially useful to readers.
Footnotes
P- Reviewer: Ding MX, He ST, Ikuta S S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Data sharing statement: Technical appendix, statistical code, and the dataset are available from the corresponding author at cfscky@nus.edu.sg. As this study comprises a review in literature and a retrospective study on patient’s data in our own hospital, informed consent from patients was not taken. The presented data are anonymized and risk of identification is low. All data generated during the project will be made available via the National University Hospital (Singapore)’s research data repository. There is no security, licensing or ethical issues related to the data, and all data used in the project was generated directly as a result of the project, without any pre-existing data being used.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 26, 2015
First decision: May 13, 2015
Article in press: October 27, 2015
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52 Suppl 3:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 5.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–958. [PubMed] [Google Scholar]
- 8.Hashizume M, Takenaka K, Yanaga K, Ohta M, Kajiyama K, Shirabe K, Itasaka H, Nishizaki T, Sugimachi K. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc. 1995;9:1289–1291. doi: 10.1007/BF00190161. [DOI] [PubMed] [Google Scholar]
- 9.Nagasue N, Kohno H, Tachibana M, Yamanoi A, Ohmori H, El-Assal ON. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child-Turcotte class B and C cirrhosis. Ann Surg. 1999;229:84–90. doi: 10.1097/00000658-199901000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada M, Hashizume M, Maehara S, Tsujita E, Rikimaru T, Yamashita Y, Tanaka S, Adachi E, Sugimachi K. Laparoscopic hepatectomy for hepatocellular carcinoma. Surg Endosc. 2001;15:541–544. doi: 10.1007/s004640080099. [DOI] [PubMed] [Google Scholar]
- 11.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769; discussion 769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–194. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Sarpel U, Hefti MM, Wisnievsky JP, Roayaie S, Schwartz ME, Labow DM. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: case-matched analysis. Ann Surg Oncol. 2009;16:1572–1577. doi: 10.1245/s10434-009-0414-8. [DOI] [PubMed] [Google Scholar]
- 14.Lai EC, Tang CN, Ha JP, Li MK. Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg. 2009;144:143–147; discussion 148. doi: 10.1001/archsurg.2008.536. [DOI] [PubMed] [Google Scholar]
- 15.Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–1048. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]
- 16.Endo Y, Ohta M, Sasaki A, Kai S, Eguchi H, Iwaki K, Shibata K, Kitano S. A comparative study of the long-term outcomes after laparoscopy-assisted and open left lateral hepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2009;19:e171–e174. doi: 10.1097/SLE.0b013e3181bc4091. [DOI] [PubMed] [Google Scholar]
- 17.Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, Ferla G. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. 2010;102:82–86. doi: 10.1002/jso.21541. [DOI] [PubMed] [Google Scholar]
- 18.Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 19.Ker CG, Chen JS, Kuo KK, Chuang SC, Wang SJ, Chang WC, Lee KT, Chen HY, Juan CC. Liver Surgery for Hepatocellular Carcinoma: Laparoscopic versus Open Approach. Int J Hepatol. 2011;2011:596792. doi: 10.4061/2011/596792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HH, Park EK, Seoung JS, Hur YH, Koh YS, Kim JC, Cho CK, Kim HJ. Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc. 2011;80:412–419. doi: 10.4174/jkss.2011.80.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668–3677. doi: 10.1007/s00464-011-1775-1. [DOI] [PubMed] [Google Scholar]
- 22.Hu BS, Chen K, Tan HM, Ding XM, Tan JW. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4725–4728. doi: 10.3748/wjg.v17.i42.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg. 2011;35:2268–2274. doi: 10.1007/s00268-011-1212-6. [DOI] [PubMed] [Google Scholar]
- 24.Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–511. doi: 10.1097/SLA.0b013e31827b947a. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Jung HK, Lee DS, Yun SS, Kim HJ. The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res. 2014;86:61–67. doi: 10.4174/astr.2014.86.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28:950–960. doi: 10.1007/s00464-013-3254-3. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015;45:142–151. doi: 10.1111/hepr.12388. [DOI] [PubMed] [Google Scholar]
- 28.Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One. 2013;8:e72328. doi: 10.1371/journal.pone.0072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva JM, Cezario TA, Toledo DO, Magalhães DD, Pinto MA, Victoria LG. Complications and prognosis of intraoperative blood transfusion. Rev Bras Anestesiol. 2008;58:454–461, 447-454. [PubMed] [Google Scholar]
- 30.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869-870. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava A, Niranjan A. Secrets of safe laparoscopic surgery: Anaesthetic and surgical considerations. J Minim Access Surg. 2010;6:91–94. doi: 10.4103/0972-9941.72593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papp A, Vereczkei J, Horváth OP. The effect of different levels of peritoneal CO2 pressure on bleeding time of spleen capsule injury. Surg Endosc. 2003;17:1125–1128. doi: 10.1007/s00464-002-9204-0. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 34.Powell-Jackson P, Greenway B, Williams R. Adverse effects of exploratory laparotomy in patients with unsuspected liver disease. Br J Surg. 1982;69:449–451. doi: 10.1002/bjs.1800690805. [DOI] [PubMed] [Google Scholar]
- 35.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–97. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santambrogio R, Aldrighetti L, Barabino M, Pulitanò C, Costa M, Montorsi M, Ferla G, Opocher E. Laparoscopic liver resections for hepatocellular carcinoma. Is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg. 2009;394:255–264. doi: 10.1007/s00423-008-0349-8. [DOI] [PubMed] [Google Scholar]


