Abstract
Objectives
To assess and explore over 1 year poststroke (1) the societal costs, (2) changes in costs and quality of life (QoL) and (3) the relation between costs and QoL.
Design
The current study is a burden of disease study focusing on the cost-of-illness (in Euros) and QoL (in utilities) after stroke.
Setting
Adult patients with stroke were recruited from stroke units in hospitals and followed for 1 year.
Participants
Data were collected from 395 patients with stroke.
Main outcome measures
Costs and QoL expressed in utilities.
Methods
Cost categories were identified through a bottom-up method. The Dutch 3-level 5-dimensional EuroQol (EQ-5D-3L) was used to calculate utilities. Non-parametric bootstrapping was applied to test for statistical differences in costs. Subgroup analyses were performed to identify predictors for costs and QoL. Robustness of results was tested via sensitivity analyses.
Results
The total societal costs for 1 year poststroke were €29 484 (n=352) of which 74% were in the first 6 months. QoL remained stable over time. The discharge location was a significant predictor for cost and QoL; men had a significantly higher QoL than women and younger patients (<65) had significantly more costs than older patients (>65). Ceiling effects appear on all dimension of the EQ-5D-3L. Costs and QoL show a weak correlation (r=−0.29). Sensitivity analyses showed robustness of results.
Conclusions
We found lower patient costs and higher QoL than expected. This may be explained by the good state of health of our study population and by change in the Dutch healthcare system, which has led to considerable shorter hospitalisation poststroke. Future research must question the use of the EQ-5D-3L in a similar population due to ceiling effects.
Trial registration number
NTR3051.
Keywords: HEALTH ECONOMICS
Strengths and limitations of this study.
We conducted a multicentre study enabling the inclusion of a wider population, hence increasing the generalisability of study results.
The current study included a large group of patients with stroke in which quality of life and costs after stroke were investigated from a societal perspective.
To estimate costs, we used a bottom-up approach which is considered preferable when investigating chronic patients.
We used self-reported measurements to collect data—a method which might be subject to recall bias and missing items. However, literature review showed that questionnaire design and respondent motivation have more influence on recall bias than period of recall, few missing records or items reported, and the validity of self-reported trials was confirmed in a large trial.
Introduction
In 2011, 1.03% of the Dutch population suffered a stroke and faced decreased physical, social and psychological functioning.1 Innovations and major improvements in acute stroke care have been raising poststroke survival rates. Accordingly, more people experience long-term difficulties in terms of quality of life (QoL),2 social reintegration,3 life satisfaction,4 and emotional functioning, including depression and anxiety.5 In addition, stroke creates considerable social and economic burdens for individuals and society.
In cost studies, QoL is usually expressed in utilities which is a score between zero (death) and one (full health). In previous research, a utility of 0.68,6 0.637 and 0.688 was found for moderate stroke survivors. Other research showed a significantly lower utility for women 3 months poststroke, in comparison with men.9 Research on the economic burden of stroke found that stroke induces considerable costs: significant cost categories in the first year poststroke were informal care,10–12 rehabilitation,10 11 13 and hospital costs.10 11 14 According to a Dutch report, an estimated 1.5 billion Euros was spent on stroke in the Netherlands in 2005; this is over 2.2% of total Dutch healthcare costs.15
Previous research is subject to several limitations such as small sample size, narrow perspective, top-down methods of costing, and outdated study results particularly in the Dutch context. The decentralised healthcare system and changes in stroke care, such as more patients being directly admitted to the stroke unit, early start of rehabilitation, and early discharge strengthen the need for new up-to-date evidence.16
The current study is a multicentre, prospective, bottom-up burden of disease (BoD) study conducted from a societal perspective. The aims of the study were to estimate and explore over 1 year poststroke: (1) the societal costs, (2) changes in costs and QoL and (3) the relation between costs and QoL.
Methods
Study design
This BoD study focuses on the cost-of-illness (in Euros) and QoL (in utilities) of a disease. This study is embedded in the Restore4Stroke Cohort study. Further information can be found elsewhere.17 18
Setting and participants
Patients were recruited from stroke units in six general hospitals in the Netherlands between March 2011 and March 2013. Eligible patients with stroke had suffered a clinically confirmed stroke (both first ever and recurrent) within the last 7 days. All participants had to be at least 18 years old. Patients were excluded if they: (1) had serious other conditions whereby an interference with the study outcomes might be expected; (2) were already dependent regarding the activities of daily living before their stroke as defined by a Barthel Index (BI) score of 17 or lower; (3) had insufficient command of the Dutch language to understand and complete the questionnaires (based on clinical judgement) or (4) were already suffering from cognitive decline before their stroke, as defined by a score of 1 or higher on the Heteroanamnesis list Cognition. The medical ethics committees of all participating hospitals approved the Restore4Stroke Cohort study and informed consent was obtained from all included patients.
Procedure
Participants were informed regarding the nature of the study by the nurse practitioner or the trial nurse of the participating hospital. After informed consent, the first assessment (T1) was conducted during the patient's hospital stay. Cost measurements were conducted at 2 months (T2), 6 months (T3) and 12 months (T4) poststroke. Questions were asked about the previous period: 2 months at T2, 4 months at T3 and 6 months at T4. At T2 and T3, a research assistant visited patients at home or at the institution the patients were residing in at that time. At T3 and T4, patients could choose to fill in an online form or paper questionnaire. Previous research had found no differences between online questionnaires and questionnaires administered on paper.19 All questions administered on paper were checked by the research assistant to prevent missing data. All patients were contacted several times in case of delay or non-response.
Measures
The primary measures of this study were costs (Euros) and QoL expressed in utilities. Cost information was retrieved through a specially designed 14-item cost questionnaire with open answers that was based on an existing questionnaire used in previous research.20 The questionnaire focused on healthcare resource use (eg, number of hospital days/nights, medication) and non-healthcare resource use (eg, paid and unpaid help, and absence from work).
QoL was assessed with the Dutch three-level five-dimensional EuroQoL (EQ-5D-3L) consisting of the dimensions mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension was scored on a three-point scale representing ‘no problems’, ‘some problems’ and ‘extreme problems’. The EQ-5D-3L has shown reasonable validity and reliability, with more limited responsiveness in German patients with stroke.21 Utilities were derived from the EQ-5D-3L using Dutch tariffs.22
Cost analysis and valuation
A bottom-up costing approach was used, meaning that cost data were obtained from individual patients with stroke who were included in a large cohort. The valuation of healthcare costs was based on the updated Dutch Manual for Cost Analysis in Health Care Research.23 Medication costs were based on the price per dosage of the drug in the Netherlands, and the price of over-the-counter drugs was based on their market prices (including 6% tax). Productivity costs were estimated with the friction cost method, calculating production losses confined to the period needed to replace a sick employee (160 days).23 In case of uncertainty, we used a conservative estimate (eg, lowest cost price). Informal care was valued by using standard cost prices based on the average hourly wages of healthcare professionals doing the same tasks (eg, domestic help). Costs were calculated and if necessary, indexed for the year 2012. Further information can be found in online supplementary table I.
Statistical methods
Missing data
Patients were excluded from the analyses if they missed two or more complete assessments of either one of the primary outcomes. The last observation carried forward was used to replace missing items at T2 and T4, using T3 data as reference. Missing items at T3 were replaced with mean data from T2 and T4. We assumed that this method resulted in the best estimation and diminished the risk of overestimation and/or underestimation. Deceased patients were included in the analysis; they induced no costs from their death onward for the remaining of the follow-up period, and we estimated the lowest possible utility score for these patients.
Statistics
All statistical analyses were performed using the SPSS V.21. Cost data was skewed; therefore, non-parametric bootstrapping (1000 replications) was used to test for statistical differences (with the 95% CI based on the 2.5th and 97.5th percentile) in costs between the first 6 months poststroke and the subsequent 6 months. Estimates, such as mean, median, SD and CI, were extracted.
Changes in utility scores were reported and interpreted according to minimal important change (MIC). Since no evidence exists on the MIC of the EQ-5D-3L conducted in a stroke population, we chose MIC values used in cancer research by Pickard et al.24 This approach suggests that 0.5 * SD is a good estimate for MIC on the EQ-5D-3L. Floor and ceiling effects over 15% were considered critical.25
To further analyse the changes in utilities, patients were divided into three groups: improved, diminished or equal utility score over time. Six subgroup analyses were conducted to identify predictors for high/low costs and utilities. Subgroups were based on gender, age (>65 or <65 years), stroke type (infarction or haemorrhage), recurrent stroke (yes or no), education (high or low), and discharge location (home; yes or no). The non-parametric Mann-Whitney test was used to test for significance. Furthermore, we explored the correlation between total costs and utility scores expressed in a Pearson’s correlation coefficient, where a correlation score >(−)0.35 is considered strong and a score between 0 and (−)0.35 weak.26 The critical p value was set at 0.05.
Sensitivity analyses
We performed five one-way sensitivity analyses to check the potential influence of base case assumptions on our study findings. The method of imputation and choices of cost prices may have led to differences in results; therefore, we analysed base case imputation method versus mean imputation, and figured the costs of day treatment for rehabilitation as equal to the costs of a hospital day (€266.53) versus the costs of a rehabilitation treatment (€116.81), and the friction cost method versus the human capital approach to estimate productivity costs.23 The choice of perspective is an ambivalent subject,27 so we analysed total societal costs versus total healthcare costs. As different sets of tariffs exist to calculate utilities, we compared Dutch tariffs versus UK tariffs.
Results
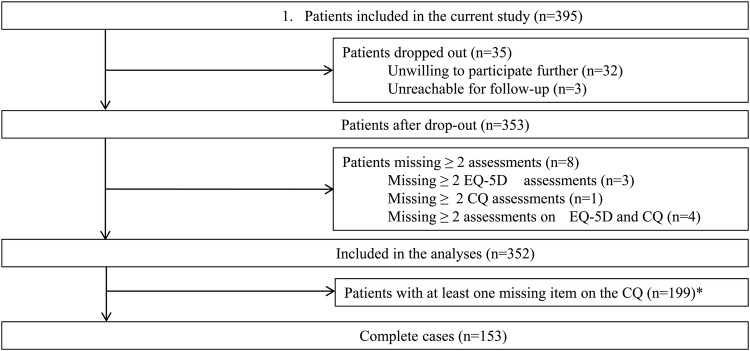
Data were collected from 395 patients with stroke (figure 1 and online supplementary material). Forty-three patients dropped out (11%) as they were unwilling to participate (n=32), unreachable (n=3), or missed assessments (n=8). Therefore, the data of 352 patients were available for analysis, of which 153 were complete cases (no missing values). The seven deceased patients were included in the analyses.
Figure 1.
Inclusion of patients.
The majority of patients were male (64.8%) and the mean age of patients was 66.8 years (SD 12.27). The majority of patients (n=200) suffered from a minor stroke and had a mean score of 2.57 (SD 2.96) on the National Institutes of Health Stroke Scale (NIHSS). Seventy-one per cent of patients went home after hospital discharge, 14% went to a rehabilitation clinic and 15% to geriatric rehabilitation. Ninety-three per cent of the patients suffered an ischaemic stroke. Demographic and stroke-related characteristics were similar for the subsample of complete cases (table 1 and online supplementary material).
Table 1.
Patient characteristics
| Patient characteristics (n=352) | N | SD* | |
|---|---|---|---|
| Demographic characteristics | |||
| Male gender, % | 228 | 64.8 | |
| Age in years, mean | 66.8 | 12.27 | |
| Marital status, % (living together) | 243 | 69 | |
| High education level†, % (n=349) | 94 | 27 | |
| Stroke-related characteristics | |||
| Ischaemic stroke, % | 327 | 92.9 | |
| Left hemisphere, % | 133 | 38.2 | |
| Severity of stroke, mean | 2.6 | 2.96 | |
| No stroke symptoms (NIHSS 0), % | 87 | 24.7 | |
| Minor stroke symptoms (NIHSS 1-4), % | 200 | 56.8 | |
| Moderate stroke symptoms (NIHSS 5-12), % | 60 | 17.2 | |
| Moderate to severe stroke symptoms (NIHSS≥13), % | 5 | 1.3 | |
| ADL four days post stroke, mean | 16.9 | 4.64 | |
| ADL dependent (BI≤17), % | 117 | 33.2 | |
| Residence after discharge,% | |||
| Home | 251 | 71.3 | |
| Rehabilitation center | 50 | 14.2 | |
| Geriatric rehabilitation | 51 | 14.5 |
*SD: standard deviation
† High education was categorized as ‘attended at least the school of higher general secondary education
Total resource use and total societal costs
Patients stayed on average 7.8 nights in a hospital and 11.5 nights in a rehabilitation clinic, and had 20.0 consults with allied health professionals (eg, physiotherapist, social worker). Informal care was provided 10.9 h/week, and patients were unable to perform unpaid labour for 28.2 days and paid labour for 34 days. After discharge, 94% of stroke survivors used general practitioner services and 95.3% had specialist contacts. Five patients (1.4%) spent night(s) in a psychiatric hospital, 28 patients (8.0%) spent night(s) in a nursing home, and 75 patients (21.3%) spent nights in a rehabilitation clinic.
Total societal costs were on average €29 484 (SD €3867; median €29 316) per patient (table 2). Healthcare costs were 61% (€18 068) and non-healthcare costs accounted for 39% (€11 416) of total societal costs. Healthcare costs were for the largest part costs of rehabilitation treatment days (€6179) and nights spent in a hospital (€3794) or a rehabilitation clinic (€4172). Categories within non-healthcare costs were evenly distributed between 7% and 12% of societal costs with paid home care being largest (€3384) and informal care, the smallest (€2029).
Table 2.
Total resource use and costs during 12 months poststroke (bootstrapped)
| Users |
Resource use per patient |
Costs per patient |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Unit | N | Per cent | Mean | SD | Mean | SD | Median | Per cent | |
| Healthcare costs | |||||||||
| General practitioner | Contact | 331 | 94.0 | 7.2 | 5.75 | 214.2 | 10.32 | 214.0 | 0.73 |
| Specialist | Contact | 332 | 95.3 | 8.1 | 8.08 | 1021.3 | 58.25 | 1021.3 | 3.46 |
| Allied health professionals | Contact | 232 | 65.9 | 20.9 | 30.56 | 682.3 | 56.66 | 681.5 | 2.31 |
| Mental healthcare professionals | Contact | 63 | 17.9 | 1.5 | 8.51 | 138.8 | 44.98 | 134.5 | 0.47 |
| Rehabilitation treatment | Day | 230 | 65.3 | 23.2 | 32.95 | 6178.7 | 489.09 | 6173.5 | 20.95 |
| Hospital | Night | 285 | 81.0 | 7.8 | 8.90 | 3793.9 | 245.51 | 3785.9 | 12.87 |
| Rehabilitation clinic | Night | 75 | 21.3 | 11.5 | 36.06 | 4172.0 | 751.45 | 4127.7 | 14.15 |
| Nursing home | Night | 28 | 8.0 | 5.2 | 29.00 | 1345.4 | 423.94 | 1325.3 | 4.56 |
| Psychiatric clinic | Night | 5 | 1.4 | 0.1 | 0.67 | 14.7 | 9.76 | 13.2 | 0.05 |
| Medication | Various | 345 | 98.0 | 506.9 | 26.32 | 506.3 | 1.72 | ||
| Total healthcare costs | 18 068.2 | 2116.28 | 17 983.2 | 61.28 | |||||
| Non-healthcare costs | |||||||||
| Paid home care | h/week | 108 | 30.7 | 5.2 | 16.80 | 3383.6 | 767.68 | 3329.8 | 11.5 |
| Informal care | h/week | 190 | 54.0 | 10.9 | 24.46 | 2029.3 | 293.70 | 2022.4 | 6.88 |
| Inability to do unpaid labour | Day | 177 | 50.3 | 28.2 | 58.15 | 3000.0 | 355.43 | 2996.2 | 10.17 |
| Production losses | Day | 102 | 29.0 | 34.0 | 72.17 | 3003.1 | 333.48 | 2984.1 | 10.18 |
| Total non-healthcare costs | 11 416.0 | 1750.30 | 11 332.5 | 38.72 | |||||
| Total societal costs | 29 484.2 | 3866.58 | 29 315.7 | 100.00 | |||||
Changes in societal costs and QoL
In the first 6 months (period T1–T3) poststroke, 74% (on average €21 731 per patient) of the yearly total societal costs occur and in the subsequent 6 months (period T3–T4) 26% (€7711). In period T1–T3, 66% of the total costs were healthcare costs and 34% non-healthcare costs, whereas period T3–T4 showed an equal distribution of 50%. The costs of rehabilitation treatment days were a major category in periods T1–T3 (22%) and T3–T4 (19%), and show a significant decrease over time (from €4760 to €1451). The costs of hospital and rehabilitation clinic stays also decreased significantly (from €3426 to €380 and from €3487 to €676, respectively). Production losses of €3026 accounted for the majority of non-healthcare costs (13.9%) in period T1–T3.On average, paid home care increases over time from €1327 up to €2041, and informal care decreases from €1252 to €762 on average.
Total healthcare costs were not significantly different between periods T1–T3 and T3–T4. Costs due to the inability to do unpaid labour and due to production losses decrease significantly. Non-healthcare costs and total societal costs show significant differences between both periods (table 3).
Table 3.
Division of cost per patient during 12 month follow-up (bootstrapped)
| Stroke—6 months |
6–12 months |
Difference |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Unit | Mean (€) | SD | Per cent | Mean (€) | SD | Per cent | Mean | CI* | |
| Healthcare costs | |||||||||
| General Practitioner | Contact | 133.7 | 6.94 | 0.61 | 81.3 | 4.79 | 1.06 | −52 | (−69 to −36)† |
| Specialist | Contact | 751.0 | 42.84 | 3.45 | 266.0 | 25.54 | 3.46 | −485 | (−584 to −288)† |
| Allied health professionals | Contact | 414.5 | 36.11 | 1.90 | 264.7 | 31.84 | 3.44 | −150 | (−243 to −57)† |
| Mental healthcare professionals | Contact | 83.6 | 30.30 | 0.38 | 54.3 | 18.63 | 0.71 | −29 | (−110 to 30) |
| Rehabilitation treatment | Day | 4760.3 | 380.15 | 21.86 | 1451.3 | 270.02 | 18.87 | −3309 | (−4196 to −2365)† |
| Hospital | Night | 3426.4 | 232.7 | 15.74 | 379.8 | 89.04 | 4.94 | −3047 | (−3553 to −2563)† |
| Rehabilitation clinic | Night | 3487.7 | 534.54 | 16.02 | 675.5 | 298.12 | 8.78 | −2812 | (−4030 to −1638)† |
| Nursing home | Night | 957.7 | 246.15 | 4.40 | 364.8 | 218.31 | 4.74 | −593 | (−1209 to 73) |
| Psychiatric clinic | Night | 7.5 | 8.3 | 0.03 | 7.1 | 4.40 | 0.09 | 0 | (−19 to 15) |
| Medication | Various | 258.8 | 13.88 | 1.19 | 248.9 | 14.80 | 3.24 | −10 | (−51 to 29) |
| Total healthcare costs | 14 281.2 | 1531.91 | 65.59 | 3793.7 | 975.49 | 49.33 | −10 450 | (−12 713 to −8243) | |
| Non-healthcare costs | |||||||||
| Paid home care | h/week | 1326.8 | 239.40 | 6.09 | 2040.9 | 690.20 | 26.54 | 714 | (−525 to 2305) |
| Informal care | h/week | 1252.0 | 188.60 | 5.75 | 761.5 | 200.50 | 9.90 | −491 | (−1000 to 100) |
| Inability to do unpaid labour | Days | 1886.7 | 223.56 | 8.67 | 1093.9 | 229.59 | 14.24 | −792 | (−1417 to −145)† |
| Production losses* | Days | 3026.2 | 343.25 | 13.90 | 0 | 0 | 0.00 | −3026 | (−3714 to −2380)† |
| Total non-healthcare costs | 7491.4 | 584.52 | 34.41 | 3903.9 | 773.89 | 50.67 | −3587 | (−5261 to 1660)‡ | |
| Total societal costs (100%) | 21 730.5 (74%) | 1161.99 | 100 | 7710.8 (26%) | 1057.06 | 100 | −14 020 | (−17 252 to −10 807)† | |
*CI (2.5th centile, 97.5th percentile).
†Statistical significant difference (p<0.05).
At T2, the average utility was 0.73 (SD 0.24), in comparison with 0.74 (SD 0.25) at T3 and 0.74 (SD 0.27) at T4. No average MIC was found, but 19% of patients between T2 and T3 and 16% of patients between T3 and T4 improved with a MIC or more. Utility improved in 36% (n=128) of all patients between T2 and T3, decreased in 29% (n=105), and was equal in 33% (n=119). Between T3 and T4, 33% (n=118) showed improved utility scores, 29% (n=104) had diminished utility scores, and 37% (n=130) were equal in utility score. Ceiling effects in all dimensions of the EQ-5D-3L were in the range of 36.6–77.8%, and no floor effects were found. Details are given in table 4 and online supplementary table III.
Table 4.
Quality of life group analysis
| Group | N | Utility 2 months poststroke (SD) | Utility 6 months poststroke (SD) | Utility 12 months poststroke (SD) | Utility difference |
|---|---|---|---|---|---|
| Increased utility 2–6 months poststroke | 128 | 0.62 (0.24) | 0.81 (0.20) | +0.19 | |
| Decreased utility 2–6 months poststroke | 105 | 0.79 (0.18) | 0.60 (0.26) | −0.19 | |
| Equal utility 2–6 months poststroke | 119 | 0.78 (0.25) | 0.78 (0.25) | 0 | |
| Increased utility 6–12 months poststroke | 118 | 0.62 (0.25) | 0.80 (0.17) | +0.18 | |
| Decreased utility 6–12 months poststroke | 104 | 0.76 (0.21) | 0.58 (0.31) | −0.18 | |
| Equal utility 6–12 months poststroke | 130 | 0.83 (0.23) | 0.83 (0.23) | 0 |
Relation between total societal costs and QoL
A correlation score was calculated between total costs and utilities at 12 months poststroke, resulting in a weak Pearson's correlation coefficient of −0.29 (p=0.00), which convinced us not to explore the relation between total costs and QoL further.
Subgroup analyses
Male gender (p value of 0.024, 0.006 and 0.005 at T2, T3 and T4, respectively) and home discharge (p value of 0.004, 0.000 and 0.000, respectively) were significant positive predictors for utilities (table 5). Furthermore, significant positive predictors of costs were male gender (p=0.034 at T4), old age (>65 years) (p value of 0.025, 0.048 and 0.007 at T2, T3 and T4, respectively) and so was discharge to a rehabilitation clinic or nursing home (p value of 0.000, 0.000 and 0.000, respectively). The total costs between >65 and <65 years age categories were significantly different (−€1628.6, p=0.15), and a significant difference was also found between home discharge and other postdischarge destinations (−€39 391.1, p=0.000). The other clinical variables showed no significant difference in the prediction of either QoL or costs.
Table 5.
Sensitivity analyses
| Base case analysis* |
Sensitivity analysis |
|||||
|---|---|---|---|---|---|---|
| Imputation and costs | Total costs € | SD | Total costs € | SD | Mean difference | CI† |
| Method of imputation, mean € | 29 429.7 | 1977.41 | 28 524.4 | 1666.51 | −905 | (−5963 to 3999) |
| Rehabilitation treatment day, mean € | 29 363.3 | 1864.74 | 2689.3 | 1674.97 | −3274 | (−8053 to 1840) |
| Production losses, mean € | 29 356.0 | 1858.82 | 31 521.3 | 2099.90 | 2165 | (−3065 to 7896) |
| Perspective, mean € | 29 490.6 | 1929.14 | 18 047.6 | 1335.40 | −11 443 | (−15 978 to −6796) |
| Quality of life | Utility | SD | Utility | SD | Mean difference | p Value‡ |
| Average utility score 2 months poststroke | 0.73 | 0.24 | 0.67 | 0.28 | −0.049 | 0.000 |
| Average utility score 6 months poststroke | 0.74 | 0.25 | 0.69 | 0.29 | −0.049 | 0.000 |
| Average utility score 12 months poststroke | 0.74 | 0.24 | 0.70 | 0.32 | −0.037 | 0.000 |
*Base case analysis uses the original method of imputation, values a rehabilitation day as a hospital treatment day, calculates production losses by means of the friction cost method, uses the societal perspective to calculate total costs and calculates utilities with a Dutch tariff. Sensitivity analyses uses mean imputation to calculate total costs, values a rehabilitation treatment day as a rehabilitation contact, calculates production losses with the human capital approach, estimates of total healthcare costs and utilities are calculated with a UK tariff.
†If CI includes 0, no significant difference is found.
‡Significant differences estimated by means of the Wilcoxon signed-rank test; Critical p=0.005.
Sensitivity analyses
No significant differences in total costs were found in testing the method of imputation (−€905; CI −€5963 to €3999), the rehabilitation treatment day price estimate (−€3274; CI −€8053 to €1640) or the calculation of production costs (€2165; CI −€3065 to €7896). A significant difference was found between total societal costs and total healthcare costs (−€11 443, CI −€15 978 to −€6796). Finally, all utility scores were significantly lower when calculated by means of UK tariffs instead of using the Dutch tariffs (table 6).
Table 6.
Subgroup analyses
| Subgroup analysis quality of life | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 months poststroke |
6 months poststroke |
12 months poststroke |
|||||||
| Characteristics | Group | Mean difference (utility) | p Value | Mean difference (utility) | p Value | Mean difference (utility) | p Value | ||
| Gender | Male–female | 0.053 | 0.024* | 0.057 | 0.006* | 0.0458 | 0.005* | ||
| Age (years) | 65+ to 65− | −0.014 | 0.953 | −0.009 | 0.806 | −0.0599 | 0.088 | ||
| Stroke type | Infarction–haemorrhage | 0.016 | 0.988 | 0.007 | 0.600 | −0.0555 | 0.610 | ||
| Recurrent stroke | Yes–no | 0.053 | 0.244 | 0.016 | 0.896 | 0.0052 | 0.643 | ||
| Education | High–low | 0.025 | 0.487 | 0.053 | 0.144 | −0.0133 | 0.955 | ||
| Home discharge | Yes–no | 0.088 | 0.004* | 0.112 | 0.000* | 0.125 | 0.000* | ||
| Subgroup analysis costs | |||||||||
| Characteristics | Group | Mean difference (€) | p Value | Mean difference (€) | p Value | Mean difference (€) | p Value | Mean difference (€) | Total costs |
| Gender | Male–female | −1344.7 | 0.434 | 941.4 | 0.508 | −1145.4 | 0.034* | −1548.8 | 0.298 |
| Age (years) | 65+ to 65− | −1602.1 | 0.025* | −2740.9 | 0.048* | 5970.8 | 0.007* | −1628.6 | 0.015* |
| Stroke type | Infarction–haemorrhage | −2553.2 | 0.437 | 1139.7 | 0.735 | −4146.6 | 0.384 | −5560.1 | 0.657 |
| Recurrent stroke | Yes–no | −2193.4 | 0.366 | −2263.0 | 0.798 | 379.1 | 0.177 | −4077.2 | 0.704 |
| Education | High–low | 48.8 | 0.720 | 573.1 | 0.762 | −1666.5 | 0.391 | −1044.6 | 0.899 |
| Home discharge | Yes–no | −17 371.7 | 0.000* | 12 001.1 | 0.000* | −10 018.3 | 0.000* | −39 391.1 | 0.000* |
*Statistically significant (p<0.05).
Discussion
We found that the societal cost for each stroke survivor in the first year poststroke was €29 484 per patient. Seventy-four per cent of total costs are induced in the first 6 months, mainly due to hospital, rehabilitation and productivity costs. The majority of cost categories decrease significantly over time where only the costs of paid home care increases. Younger patients incur higher costs than older patients; age was a significant predictor of costs on all measurement points due to productivity costs being a major cost category for the <65 years age group and zero for the >65 years age group. Patients who were sent home after hospital discharge had significantly fewer costs. Gender was a significant predictor for QoL on all measurement points, with men scoring significantly higher than women. QoL increased slightly over time and did not result in MIC. Patients who were sent home after discharge showed significantly higher utility scores on all measurement points compared with patients discharged to (geriatric) rehabilitation. Ceiling effects were reported for all domains of the EQ-5D-3L. The sensitivity analyses showed an overall robustness of the results; however, significant differences were found while calculating costs from a societal and from a healthcare perspective, and between utilities calculated with Dutch or with UK tariffs. Differences in costs due to the perspective were expected, since fewer cost categories were considered from the healthcare perspective. Despite the sensitivity analyses, an overestimation of rehabilitation treatments is still a factor in the current study, mainly due to difficulties with interpreting questions on the cost questionnaire.
It is difficult to compare these results with other studies due to the large variety in patient populations, methodology, data collection, follow-up period and perspective. Fattore et al11 estimated the 1-year poststroke societal costs in Italy at €20 000 per patient. They conclude that the costs of informal care (33.4%) and costs due to rehabilitation (20%) were major cost components during the follow-up period. Our findings show an even larger cost component for rehabilitation (45%), but fewer costs due to informal care (7%). A recent incidence-based Swedish study found that 9% of total costs were due to production losses and 6% due to informal care.13 Our results are consistent with these findings as we found that productivity losses accounted for over 12% of total costs and informal care 7% of total costs. According to our findings of €29.484 per stroke patient and an incidence of 26 200 Dutch patients with stroke,1 stroke costs the Dutch society almost 775 million Euros annually. This is significantly less than findings from 2005,15 possibly related to the relative good health of our study population, but also due to changes in stroke care which has led to more patients being directly admitted to the stroke unit, early start of rehabilitation and early hospital discharge.16 Our QoL results are in line with previous research6–9 in which utility scores 1 year poststroke remained stable. The rather high, constant utility score obtained in this study might be explained by the nature of the study population, since high scores were also found on other outcome measures. High ceiling effects may have influenced minor changes in QoL, raising the question of whether the EQ-5D is the most valid instrument for measuring QoL in patients poststroke.
Strengths and limitations
The current study has several strengths. First, a large group of patients with stroke was included in this study. Second, the current study is a multicentre study enabling the inclusion of a wider population, and therefore, increasing the generalisability of study findings. Third, a societal perspective was used. Fourth, a bottom-up approach was used for costing, which is considered preferable in terms of estimating costs for chronic patients.28 29 Finally, we consider it a strength that our imputation method proved to be robust in the sensitivity analysis.
Our study was subject to several limitations as well. First, we used a self-reported questionnaire to estimate healthcare consumption. This may cause recall bias, although an extensive literature review showed that questionnaire design and respondent motivation have more influence on recall bias than period of recall.30 Second, we used self-reported measurements to collect data causing possible missing items. However, few missing records or items were reported and the validity of self-reported trials was confirmed in a large trial.31 Third, the majority of patients were recruited from general instead of academic hospitals, which may have influenced study findings. Finally, the choice to use the EQ-5D-3L to measure QoL may have been a limitation. As mentioned before, due to the high proportion of ceiling effects conceivable improvement in QoL was not possible. Therefore, for future research, we recommend the possibility of using other questionnaires for measuring generic health-related QoL in stroke populations.
Conclusion
The societal costs incurred by a Dutch stroke patient are on average €29 484 in the first year poststroke; 74% of these costs can be accounted for within the first 6 months. These costs are lower than previously reported. This may be explained by our study population's good state of health and by changes in the Dutch healthcare system, which have led to considerable fewer inpatients days poststroke. Costs decreased over time, although some cost categories (eg, rehabilitation) remained a major part of total costs. Male gender was a significant predictor for fewer costs, low age for better QoL, and discharge location for both. Sensitivity analyses proved that the imputation method, rehabilitation day treatment, and productivity cost calculation had no influence on total costs. Choice of tariff and perspective did have a significant influence on total costs. Future research must question the use of the EQ-5D-3L in a similar population due to ceiling effects.
Acknowledgments
The authors appreciate Barbara Greenberg's language review of this study. This study is embedded in the Restore4Stroke research programme. Restore4Stroke is coordinated by ZonMw (Dutch Organisation for Health Research and Development).
Footnotes
Contributors: MvE is the primary author. MvE and GAPGvM were responsible for the design, data analysis and interpretation, and reporting of study results. SMAAE and CvH acted as main supervisors, and were responsible for the study aim and formulating the initial research questions. They thoroughly supervised the entire research process. MvM and JMAV-M conducted a different study based on the same data, and supervised the current manuscript in its final stages, making sure that all references to their study were correct. All the authors confirm that they contributed to the design of the study, and analyses and interpretation of data; and also approved the final version of this manuscript.
Funding: Restore4Stroke is funded through the VSBfonds (the Dutch organisation for supporting Dutch society with money, knowledge and networks), project 60-61300-98-022.
Competing interests: None declared.
Ethics approval: Ethical approval was obtained through the Medical Ethics Committee of the University Medical Center Utrecht and the ethics committees of participating institutes.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Kompas N. Prevalence and incidence of stroke. 2011. http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/hartvaatstelsel/beroerte/omvang/
- 2.Carod-Artal J, Egido JA, Gonzalez JL et al. . Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke 2000;31:2995–3000. 10.1161/01.STR.31.12.2995 [DOI] [PubMed] [Google Scholar]
- 3.Hommel M, Trabucco-Miguel S, Joray S et al. . Social dysfunctioning after mild to moderate first-ever stroke at vocational age. J Neurol Neurosurg Psychiatry 2009;80:371–5. 10.1136/jnnp.2008.157875 [DOI] [PubMed] [Google Scholar]
- 4.Ostwald SK, Godwin KM, Cron SG. Predictors of life satisfaction in stroke survivors and spousal caregivers after inpatient rehabilitation. Rehabil Nurs 2009;34:160–7, 174; discussion 174 10.1002/j.2048-7940.2009.tb00272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergersen H, Frøslie KF, Stibrant Sunnerhagen K et al. . Anxiety, depression, and psychological well-being 2 to 5 years poststroke. J Stroke Cerebrovasc Dis 2010;19:364–9. 10.1016/j.jstrokecerebrovasdis.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003;21:191–200. 10.2165/00019053-200321030-00004 [DOI] [PubMed] [Google Scholar]
- 7.Lindgren P, Glader EL, Jönsson B. Utility loss and indirect costs after stroke in Sweden. Eur J Cardiovasc Prev Rehabil 2008;15:230–3. 10.1097/HJR.0b013e3282f37a22 [DOI] [PubMed] [Google Scholar]
- 8.Haacke C, Althaus A, Spottke A et al. . Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193–8. 10.1161/01.STR.0000196990.69412.fb [DOI] [PubMed] [Google Scholar]
- 9.Bushnell CD, Reeves MJ, Zhao X et al. . Sex differences in quality of life after ischemic stroke. Neurology 2014;82:922–31. 10.1212/WNL.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewey HM, Thrift AG, Mihalopoulos C et al. . Cost of stroke in Australia from a societal perspective: results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2001;32:2409–16. 10.1161/hs1001.097222 [DOI] [PubMed] [Google Scholar]
- 11.Fattore G, Torbica A, Susi A et al. . The social and economic burden of stroke survivors in Italy: a prospective, incidence-based, multi-centre cost of illness study. BMC Neurol 2012;12:137 10.1186/1471-2377-12-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27–32. 10.1093/ageing/afn281 [DOI] [PubMed] [Google Scholar]
- 13.Persson J, Ferraz-Nunes J, Karlberg I. Economic burden of stroke in a large county in Sweden. BMC Health Serv Res 2012;12:341 10.1186/1472-6963-12-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evers SM, Engel GL, Ament AJ. Cost of stroke in the Netherlands from a societal perspective. Stroke 1997;28:1375–81. 10.1161/01.STR.28.7.1375 [DOI] [PubMed] [Google Scholar]
- 15. Poos MJJC, et al. (2008). Cost of Illness in the Netherlands 2005, Rijksinstituut voor Volksgezondheid en Mileu.
- 16.Heijnen R, Limburg M, Evers S et al. . Towards a better integrated stroke care: the development of integrated stroke care in the southern part of the Netherlands during the last 15 years (special 10th anniversary edition paper). Int J Integr Care 2012;12:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Eeden M, van Heugten CM, Evers SM. The economic impact of stroke in the Netherlands: the euro-restore4stroke study. BMC Public Health 2012;12:122 10.1186/1471-2458-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Mierlo ML, van Heugten CM, Post MWM et al. . A longitudinal cohort study on quality of life in stroke patients and their partners: restore4stroke cohort. Int J Stroke 2014;9:148–54. 10.1111/j.1747-4949.2012.00882.x [DOI] [PubMed] [Google Scholar]
- 19.Ritter P, Lorig K, Laurent D et al. . Internet versus mailed questionnaires: a randomized comparison. J Med Internet Res 2004;6:e29 10.2196/jmir.6.3.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schram MT, Sep SJ, van der Kallen CJ et al. . The Maastricht study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol 2014;29:439–51. 10.1007/s10654-014-9889-0 [DOI] [PubMed] [Google Scholar]
- 21.Hunger M, Sabariego C, Stollenwerk B et al. . Validity, reliability and responsiveness of the EQ-5D in German stroke patients undergoing rehabilitation. Qual Life Res 2012;21:1205–16. 10.1007/s11136-011-0024-3 [DOI] [PubMed] [Google Scholar]
- 22.Lamers LM, Stalmeier PF, McDonnell J et al. . [Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff]. Ned Tijdschr Geneeskd 2005;149:1574–8. [PubMed] [Google Scholar]
- 23.Oostenbrink JB, Koopmanschap MA, Rutten FFH. Handleiding voor kostenonderzoek. Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Diemen, College voor zorgverzekeringen, 2010.
- 24.Pickard AS, Neary M, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4:293–307. 10.1007/BF01593882 [DOI] [PubMed] [Google Scholar]
- 26.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr 1990;6:35–9. 10.1177/875647939000600106 [DOI] [Google Scholar]
- 27.Neumann PJ. Costing and perspective in published cost-effectiveness analysis. Med Care 2009;47:S28–32. 10.1097/MLR.0b013e31819bc09d [DOI] [PubMed] [Google Scholar]
- 28.Tan SS, Rutten FF, van Ineveld BM et al. . Comparing methodologies for the cost estimation of hospital services. Eur J Health Econ 2009;10:39–45. 10.1007/s10198-008-0101-x [DOI] [PubMed] [Google Scholar]
- 29.Wordsworth S, Ludbrook A. Comparing costing results in across country economic evaluations: the use of technology specific purchasing power parities. Health Econ 2005;14:93–9. 10.1002/hec.913 [DOI] [PubMed] [Google Scholar]
- 30.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol 1990;43:87–91. 10.1016/0895-4356(90)90060-3 [DOI] [PubMed] [Google Scholar]
- 31.van den Brink M, van den Hout WB, Stiggelbout AM et al. . Cost measurement in economic evaluations of health care: whom to ask? Med Care 2004;42:740–6. 10.1097/01.mlr.0000132351.78009.a1 [DOI] [PubMed] [Google Scholar]