Abstract
Objectives
We aimed to assess the association between long-term use of inhaled corticosteroids (ICS) and bone adverse effects in patients with asthma.
Design
Systematic review and meta-analysis of fracture risk and changes in bone mineral density with long-term ICS use in asthma.
Methods
We initially searched MEDLINE and EMBASE in July 2013, and performed an updated PubMed search in December 2014. We selected randomised controlled trials (RCTs) and controlled observational studies of any ICS (duration at least 12 months) compared to non-ICS use in patients with asthma. We conducted meta-analysis of ORs for fractures, and mean differences in bone mineral density. Heterogeneity was assessed using the I2 statistic.
Results
We included 18 studies (7 RCTs and 11 observational studies) in the systematic review. Meta-analysis of observational studies did not demonstrate any significant association between ICS and fractures in children (pooled OR 1.02, 95% CI 0.94 to 1.10, two studies), or adults (pooled OR 1.09, 95% CI 0.45 to 2.62, four studies). Three RCTs and three observational studies in children reported on bone mineral density at the lumbar spine, and our meta-analysis did not show significant reductions with ICS use. Three RCTs and four observational studies in adults reported on ICS use and bone mineral density at the lumbar spine and femur, with no significant reductions found in the meta-analysis compared to control.
Conclusions
ICS use for ≥12 months in adults or children with asthma was not significantly associated with harmful effects on fractures or bone mineral density.
Keywords: CLINICAL PHARMACOLOGY
Strengths and limitations of this study.
Comprehensive search of two databases with independent study selection and data extraction.
Included both observational and randomised studies in adults and/or children with asthma.
Heterogenous nature of studies and the outcome measures which were available for analysis.
Inability to properly assess differences between drugs, type of inhaler device or dose-responsiveness.
Introduction
Asthma is a chronic inflammatory condition that affects both adults and children. There is a substantial body of evidence that suggest inhaled corticosteroids (ICS) are effective at controlling symptoms, improving lung function and reducing acute exacerbations.1 They are therefore considered the gold standard first-line preventative therapy and are widely recommended in national and international guidelines.2 3
However, long-term ICS use may be associated with adverse effects such as cataract, osteoporosis, fractures and reduction in growth velocity in children.4 Concerns surrounding these potential harms may have a negative effect on ICS adherence, thus exposing patients to poorer asthma control and a potentially higher risk of needing oral corticosteroids for acute exacerbations.4 Certain age groups, such as children or postmenopausal women may be particularly susceptible to adverse effects on bone metabolism and formation, and this therefore remains an area of concern for these patients.
The existing meta-analyses of ICS and bone adverse effects have usually included data from participants with chronic obstructive pulmonary disease (COPD)5–7 and to date, there has been less focus on the effects in asthma alone. Patients with asthma may not share the same susceptibilities to osteoporosis as the patient with COPD because of differences in risk factors such as cigarette consumption, multimorbidity and nutritional problems that are prevalent in patients with COPD.8 9 It therefore remains unclear whether patients with asthma have a greater or lesser risk of bone adverse effects than those with COPD and a further review is necessary to clarify these risks for patients with asthma alone.
Hence we aimed to analyse the effects of long-term (≥12 months) ICS use in patients with asthma alone, concentrating on fracture and bone mineral density (BMD) outcomes.
Methods
Study selection criteria
We aimed to focus on long-term, important but infrequent adverse effects on bone and as such, eligible studies had to have >20 users of each ICS formulation, with follow-up of at least 12 months in duration.
Our inclusion criteria for RCTs were (1) parallel-group RCT; (2) participants with asthma of any severity; (3) ICS as the intervention versus a control treatment, where the comparison groups consisted of ICS versus other asthma therapy (or placebo), or ICS in combination with long-acting β-agonist (LABA) versus a LABA alone; and (4) stated aim to evaluate fractures or BMD.
We also evaluated controlled observational studies (case control, prospective cohort or retrospective cohort) reporting on risk of fractures or change in BMD with any ICS exposure compared to those without ICS exposure.
Exclusion criteria
We excluded studies that recruited mixed groups of participants (asthma/COPD) if the outcomes were not separately reported according to specific disease condition. We excluded crossover trials and studies that considered only oral corticosteroid use without reporting the effects of inhaled corticosteroids.
Search strategy
We initially searched MEDLINE and EMBASE in June 2013 using a broad strategy for a wide range of adverse effects potentially associated with ICS use, and we subsequently updated this through a more focused PubMed search in December 2014 (see eAppendix 1 for search terms and restrictions). We also manually looked through the bibliographies of included studies as well as existing systematic reviews for any other articles that may be potentially suitable.
Study selection
Two reviewers (MT and PB) independently, and in duplicate scanned all titles and abstracts and excluded articles that clearly were not RCTs or observational studies of ICS in patients with asthma. We proceeded to assess full-text versions of potentially relevant articles and conducted more detailed checks against our eligibility criteria, focusing on bone and fracture adverse effects. A third researcher (YKL or AMW) evaluated the decision on inclusion or exclusion in discussion with the two reviewers.
Study characteristics and data extraction
We used preformatted tables to record study design and participant characteristics, definition of asthma, pharmacological agent (dose, device and frequency), and duration of follow-up. Two reviewers independently extracted data (MT and PB) on relevant outcomes, where we prespecified fracture risk of primary interest, and BMD at the lumbar spine or the femur as secondary end points. Any discrepancies were resolved through the involvement of a third reviewer (DG or YKL or AMW) after rechecking the source papers.
Risk of bias assessment
Two reviewers independently assessed the reporting of blinding of participants and personnel, randomisation sequence, allocation concealment, withdrawals and the loss to follow-up in RCTs. In order to assess validity of the associations between adverse effects and ICS use, we extracted information on participant selection, ascertainment of exposure and outcomes, and methods of addressing confounding in observational studies.10
We aimed to use a funnel plot and asymmetry testing to assess publication bias provided that there were more than 10 studies in the meta-analysis, and the absence of significant heterogeneity.11
Statistical analysis
We pooled trial data using Review Manager (RevMan) V.5.3.2 (Nordic Cochrane Center, Copenhagen, Denmark). We used the inverse variance method to pool ORs for fracture events, and mean differences for BMD (g/cm2). In accordance with the recommendations of the Cochrane Handbook, we derived any SDs from 95% CIs or p values.12 We assessed statistical heterogeneity using the I2 statistic with I2>50% indicating a substantial level of heterogeneity.
If a trial had more than one group of non-ICS users as controls, we analysed data for ICS versus placebo (if available) in preference to data from active comparators such as ICS versus nedocromil, montelukast or disodium cromoglycate. If combination formulations were evaluated in the trial, we chose unconfounded comparisons based on ICS used together with the other drug versus other drug alone.
If a trial had several arms involving different ICS doses, we combined all the ICS arms together as recommended by the Cochrane Handbook.13
We did not have a pre-registered protocol.
Results
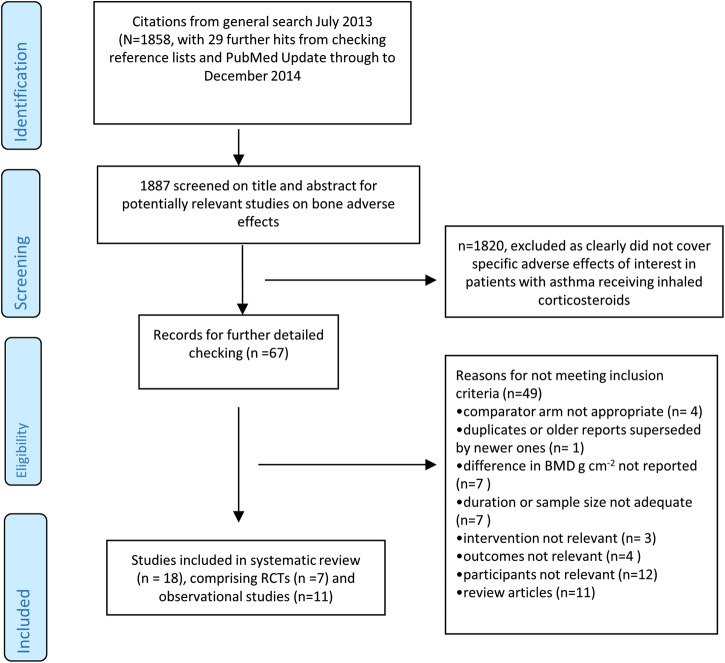
We screened 1887 potentially relevant articles, and finally included 18 studies in our systematic review (comprising 7 RCTs,14–20and 11 observational studies).21–31 The process of study selection is shown in figure 1.
Figure 1.
Flow diagram of study selection. BMD, bone mineral density; RCT, randomised controlled trial.
Table 1A, B show the characteristics of the included RCTs, and the observational studies, respectively. Tables 2 and 3 report on study validity and outcomes in adult and children, respectively.
Table 1.
Characteristics of included trials and observational studies
| (A) Randomised controlled trials | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Location | Treatment duration | Asthma criteria | Drug and inhaler device | Male % |
Mean age (years) | Mean % predicted FEV1 | Prior ICS use (%) |
| CAMP/Kelly et al15 32 | Multicentre US | >208 weeks | Mild-to-moderate asthma defined by symptoms or by use of inhaled bronchodilator ≥ twice weekly or daily medication for asthma. Airway methacholine challenge test | BUD 200 µg bd (n=311) | 58.2 | 9.0 | 93.6 | 40.5 |
| Nedocromil 8 mg daily (n=312) | 66.0 | 8.8 | 93.4 | 36.5 | ||||
| Placebo (n=412) | 56.0 | 9.0 | 94.2 | 35.9 | ||||
| Ferguson et al 200714 | Multicentre—35 centres in 11 countries | 52 weeks | Age 6–9 years persistent asthma ≥6 months; FEV1 ≥ 60% predicted; ↑PEFR of ≥15% after salbutamol. Exclusions: oral corticosteroids on >2 occasions or >12 days or >210 mg prednisolone past 6 months; known growth disorder or glaucoma/cataracts | FP 100 μg bd (n=114) Diskus (dry powder inhaler) |
68 | 7.2 | 90.2 | 25% oral steroids past 6 months |
| BUD 200 μg bd (n=119) Turbuhaler |
70 | 7.4 | 92.3 | 21% oral steroids past 6months | ||||
| Kemp et al16 | Multicentre US | 104 weeks | 6-month history of mild asthma (FEV1 82–85% predicted) able to be managed without steroids for 2 years | FP 88 µg bd (n=55) Metered dose inhaler |
60 | 31.6 | 83 | 0 |
| FP 440 µg bd (n=51) Metered dose inhaler |
59 | 29.0 | 82 | 0 | ||||
| Placebo (n=54) | 59 | 28.4 | 85 | 0 | ||||
| Li et al17 | Multicentre US | 104 weeks | At least 6-month history with diagnosis using American Thoracic Society definition. FEV1 of ≥60% predicted, and limited previous corticosteroid therapy | FP 500 μg bd (n=32) Diskhaler |
91 | 28.0 | 91 | Not reported |
| Placebo bd (n=32) Diskhaler |
81 | 31.1 | 91 | Not reported | ||||
| Maspero et al18 | 50 centres worldwide | 52 weeks | Adults with >3 months history of asthma, and not using ICS past 3 months. FEV1 between 60 and 90% predicted. Must have DEXA scan, and no evidence of low vitamin D | Mometasone 400 µg daily (n=137) | 34 | 30 | 76.5 | 7 |
| Mometasone 200 µg daily (n=140) | 35 | 30 | 74.7 | 7 | ||||
| FP 250 µg bd (n=147) | 39 | 28 | 75.3 | 6 | ||||
| Montelukast 10 mg (n=142) | 38 | 28 | 76.9 | 10 | ||||
| Roux et al19 | 52 respiratory specialist clinics in France | 104 weeks | Exacerbations ≥1×/week but <1× daily; or chronic symptoms requiring daily treatment. Fulfilling: (1) FEV1 or PEFR ≥80% predicted; (2) reversibility ≥15%; (3) daily variability PEFR 20%–30% ≥2 days, or salbutamol use >3 times previous week, or nocturnal symptoms ≥2× during run-in | FP 100 μg bd (n=87) Diskus/Accuhaler dry powder inhaler |
64 | 9.1 | 88.9 | Not reported |
| Nedocromil 4 mg bd (n=87) MDI |
66 | 9.4 | 88.5 | Not reported | ||||
| Turpeinen et al20 | Helsinki University Hospital, Finland | 72 weeks | ‘Newly detected mild asthma’ Excluded if history of inhaled, nasal or oral corticosteroid use in the previous 2 months before enrolment |
Continuous BUD (n=50) Turbuhaler BUD 400 μg bd for 1 month, then 200 μg bd for 2nd–6th months, then 100 μg bd for final 12 months. |
60 | 6.9 | Not reported | Not reported |
| BUD/placebo (n=44) Turbuhaler BUD 400 μg bd for 1st month, then 200 μg bd for 2nd to 6th months, then placebo for final 12 months |
66 | 6.7 | Not reported | Not reported | ||||
| Sodium cromoglicate—10 mg tds for 18 months (unblinded) (n=42) MDI |
50 | 7.0 | Not reported | Not reported | ||||
Bd, two times a day; BUD, budesonide; DEXA, dual-energy X-ray absorptiometry; FEV1, forced expiratory volume in 1 s; FP, fluticasone propionate; ICS, inhaled corticosteroids; MDI, metered dose inhaler; PEFR, peak expiratory flow rate.
Table 2.
Study validity and outcomes (bone mineral density and fractures) in children
| (A) RCTs of inhaled corticosteroids—children | |||||||
|---|---|---|---|---|---|---|---|
| Source | Sequence generation | Allocation concealment | Blinding of participants and personnel | AE monitoring | Adverse events | Discontinued, number (%) | Loss to follow-up, number (%) |
| CAMP/Kelly et al15 32 | Permuted blocks, stratified | Adequate | Adequate | Height recorded at every visit; BMD once every year | Fracture rate (adjusted for age, ethnic group, sex, clinic, base line duration, skin-test reactivity and asthma severity): BUD: 5.7 per 100 person-years Placebo: 5.1 per 100 person-years p=0.59 Mean difference in BMD (ICS vs placebo): Females: −0.001 (derived SE 0.0016) Male:−0.003 (derived SE 0.0014) |
11% | 5% |
| Ferguson et al 200714 | Not reported | Remote computerised allocation | Adequate | Lumbar-spine BMD assessed at beginning and end of treatment with DEXA scan | Mean difference in lumbar spine BMD for FP vs BUD: 0.0075 (95% CI −0.033 to 0.048) | 90% patients received >40 weeks | 26% did not reach 51 weeks |
| Roux et al19 | Central Block randomisation with gender stratification | . | Largely open. Analysis of DEXA scans blinded | Lumber spine and femoral neck BMD (DEXA) during run-in and 6, 12 and 24 months. Adjusted for age, height, weight, baseline BMD, gender and measuring device | Mean difference in lumbar spine BMD for FP vs control: 0.012 (SE 0.0073); values calculated from % change in manuscript | 23% | 4% |
| Turpeinen et al20 | Block | Unclear | Blinded for budesonide and placebo arms | BMD of L1–4 measured by radiologist using DEXA at baseline and at 18 months | Mean change in lumbar spine BMD: Budesonide for 12 months 0.023 (SD 0.022) Placebo for 12 months 0.029 (SD 0.022) DSCG: 0.034 (SD 0.022) |
20% | 3% |
BUD, budesonide; DEXA, dual-energy X-ray absorptiometry; DSCG, disodium cromoglicate; FP, fluticasone propionate.
Table 3.
Study validity and outcomes (bone mineral density and fractures) in adults
| (a) RCTs of inhaled corticosteroids—adults | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Sequence generation | Allocation concealment | Blinding of participants and personnel | AE monitoring | Drug (n) | Mean change in BMD g/cm2 | Dis-continued, number (%) | Loss to follow-up, number (%) |
| Kemp et al16 | Random code with blinded labels | Adequate | Adequate | DEXA scan every 6 months at lumbar spine (L1-L4). analysed by central osteoporosis research facility for quality assurance Adjusted for baseline value, investigator, sex, age |
FP 88 µg bd | At week 104 1) Lumbar spine: 0.008, SE 0.006 2) Proximal femur: −0.009, SE 0.009 |
17 (31) | 6 (11) |
| FP 440 µg bd | At week 104 1) lumbar spine: -0.003, SE 0.008 2) Proximal femur: −-0.020, SE 0.009 |
18 (35) | 7 (14) | |||||
| Placebo bd | At week 104 1) Lumbar spine: 0.001, SE 0.005 2) Proximal femur: −0.007, SE 0.007 |
10 (19) | 4 (7) | |||||
| Li et al17 | Unclear | Unclear | Adequate | DEXA at L1-L4 of lumbar spine. Measured at screening and 6-month intervals | FP | At week 104, lumbar spine: −0.006, SE 0.008 | 9 (28) | 2 (6) |
| Placebo | At week 104, lumbar spine: −0.007, SE 0.010 | 8 (25) | 7 (22) | |||||
| Maspero et al18 | Centrally administered through interactive voice response system | Adequate | Adequate | DEXA at L1-L4 of lumbar spine. Follow-up at 26 and 52 weeks | Mometasone 400 µg | 1) Lumbar spine: 0.0092) Femur: 0.004 | 34 (25) | 5 (3) |
| Mometasone 200 µg daily | 1) Lumbar spine: 0.0082) Proximal femur: 0.004 | 35 (25) | 7 (4) | |||||
| FP 250 µg bd | 1) Lumbar spine: 0.0122) Femur: −-0.005 | 38 (26) | 4 (3) | |||||
| Combined estimate for all ICS users | 1) Lumbar spine: 0.0092) Femur: 0.0008 | 107 (25) | 16 (4) | |||||
| Montelukast 10 mg daily | 1) Lumbar spine: 0.0132) Femur: −0.002 | 31 (22) | 3 (3) | |||||
AE, adverse event; bd, two times a day; DEXA, dual-energy X-ray absorptiometry; FP, fluticasone propionate; ICS, inhaled corticosteroids; RCT, randomised controlled trial.
| (B) Observational studies | |||||
|---|---|---|---|---|---|
| Study | Design | Adverse effects measured | Data source and number of patients | Selection of patients: asthma definition and patient characteristics (or selection of cases and controls) | Type of ICS |
| Agertoft and Pedersen21 | Cross-sectional study | BMD | Outpatient paediatric clinic, Kolding Hospital, Denmark 157 cases, 111 controls |
Selection of cases: Children with persistent asthma and no other chronic disease, on ICS continuously for ≥3 years. Mean age: 10.3 years, male 69%, %FEV1 predicted: 97 Selection of controls: asthmatic children, who have never taken inhaled/systemic corticosteroids for >2 weeks per year. Mean age: 9.9 years, male 55%, %FEV1 predicted: 81 |
BUD |
| Allen et al22 | Prospective | BMD | Department of Paediatrics, Royal North Shore Hospital, Sydney, Australia 48 cases, 9 controls |
Selection of cases: prepubertal asthmatic children requiring >3 courses oral corticosteroids within study period Mean age: 7.8 years, male 63% Selection of controls: children not using corticosteroids. Mean age: 8.4 years, male 78% |
BDP, BUD |
| Bahceciler et al23 | Cross-sectional study | BMD | Outpatient Allergy Clinic of Marmara University Hospital, Istanbul, Turkey 52 cases, 22 controls |
Asthma definition: mild intermittent plus persistent mild to moderate asthma Selection of cases: Children treated for ≥6 months. Mean age: 6.4 years, male 42% Characteristics of high-dose ICS group: Mean age: 3 years Mean duration of disease: 50.4 months Characteristics of low dose ICS group: Mean age: 5.8 years Mean duration of disease: 38.3 months Selection of controls: Age-matched asthmatic children who have never received ICS Mean age: 6.8 years, male 45% |
BUD |
| El et al24 | Observational | BMD | Outpatients, Dokuz Eylul University, Balcova, Izmir, Turkey 45 cases, 46 controls |
Asthma severity defined according to Global Initiative for Asthma guideline Selection of cases: patients with mild or moderate asthma and regular ICS use. Mean age: 44.04 years, male 0%, %FEV1: 89.71 Controls : Mean age: 44.43 years, male 0% |
Not specified |
| Harris et al25 | Cross-sectional study | BMD | Outpatient clinics of Sydney Children's Hospital, Randwick, New South Wales and Monash Medical Centre, Clayton, Victoria, Australia. 76 subjects |
Selection of subjects: Prepubertal asthmatic children stratified into groups according to corticosteroid treatment received in the past 6 months 1) no inhaled corticosteroid, mean age: 8.2 years, male 70% 2) moderate dose inhaled corticosteroid (400–800 μg/day), mean age: 7.4 years, male 56% 3) high-dose inhaled corticosteroid (>800 µg/day), mean age: 8.9 years, male 75%, |
BDP, BUD, FP |
| Johannes et al26 | Nested case-control study | Risk of non-vertebral fracture | Ingenix Epidemiology—Research database of United Healthcare members, 17 states in the USA. 1722 cases, 17 220 controls |
Adults ≥40 years age, in health plan for ≥12 continuous months Jan 1997 to Jun 2001, with ICD-9 code for asthma, or COPD Selection of cases: Non-vertebral fractures by ICD-9 codes, with claim for treatment (including inpatient hip fractures) Mean age 52.9 years, male 29.4% Selection of controls: Sampled from person-time of respiratory cohort by two-tiered random sampling with replacement Mean age 52.2 years, male 41.1% |
BDP, BUD, FP flunisolone, triamcinolone |
| Schlienger et al27 | Retrospective Population-based nested case-control analysis | Fracture risk | UK General Practice Research Database 3744 cases, 21 757 controls |
Aged 5–79 years with ICD code for asthma or COPD with ≥1 prescription for ICS and/or OCS; or with no exposure to corticosteroids From there 65 779 individuals aged 5–17 years identified to form base population for study Selection of cases: Patients with 1st-time diagnosis ICD-8 bone fracture; male 65.6% Selection of controls: Up to 6 control subjects selected per case, matched on age, gender, general practice attended, calendar time and years of history in GPRD; male 64.9% |
76.2% BDP 21.7% BUD 2.1% FP |
| Sosa et al28 | Cross-sectional study | BMD; Fracture risk | Canary Islands, Spain 105 cases; 133 controls |
Selection of cases: Women suffering from stable bronchial asthma, treated with ICS ≥1 year, and who did not receive oral or parenteral steroids. Mean age: 53.0 years, number of menopausal subjects n (%): 65 (61.9) Selection of controls: weight-matched women, no asthma and no steroids. Controls were usually friends or neighbours of the patients. Mean age: 49.7 years, number of menopausal subjects n (%): 74 (57.8) |
ICS formulations not specified |
| Van Staa et al29 | Population-based cohort study/nested case-control analysis | Fracture risk | UK General Practice Research Database (GPRD) Cohort: ICS users: 97 387 Bronchodilators only: 70 984 Controls: 345 758 Fracture cases: 23 984; Controls: 23 984 |
Children aged 4–17 years old, on ICS. 3 study groups: selection of cases: non-vertebral fracture. Male 61%, 8856 (36.9%) aged 4–9 years, 8496 (35.4%) aged 10–13 years, 6632 (27.7%) aged 14–17 years Selection of controls: for each fracture case, one control patient randomly selected, matched by age, sex, GP practice and calendar time. Male 61%, 8861 (36.9%) aged 4–9 years, 8497 (35.4%) aged 10–13 years, 6626 (27.6%) aged 14–17 years |
BDP, BUD, FP |
| Wisniewski et al30 | Cross-sectional study | BMD | Asthma register and local general practices in Nottingham, UK 47 cases; 34 controls |
Selection of cases: aged 20–40 years with documented history of asthma: Group 1: asthmatics using inhaled β2-agonist only. Males 56%, mean age: men 30.3 years; women 25.6 years, mean FEV1 (litres): men 3.87; women 3.13 Group 2: ICS use ≥5 years with no systemic steroids in the past 6 months. Males 40%, mean age: men 32.3 years; women 32 years, mean FEV1 (litres): men 3.40; women 2.83 |
BDP, BUD |
| Yanik et al31 | Observational | BMD | Pulmonology outpatient clinic at Fatih University Faculty of Medicine, Ankara, Turkey 46 cases, 60 controls |
Selection of cases: Regular ICS use ≥12 months) as defined by The Global Initiative for Asthma (GINA) criteria Mean age: 62.5 years, male 0%, %FEV1 predicted: 83.1, All cases were postmenopausal Selection of controls: healthy postmenopausal females. Mean age: 63 years |
BDP, BUD, FP |
BDP, beclomethasone dipropionate; BUD, budesonide; COPD, chronic obstructive pulmonary diseases; FEV1, forced expiratory volume in 1 s; FP, fluticasone propionate; GP, general practitioner; ICD, International Classification of Diseases; ICS, inhaled corticosteroids.
| (B) Observational studies of bone mineral density and fractures—children | ||||||
|---|---|---|---|---|---|---|
| Study | Ascertainment of BMD | Ascertainment of exposure | Definition of ICS use | Adjustments | ICS exposure | BMD (g/cm) |
| Agertoft and Pedersen21 | DEXA scan at one visit, performed by same investigator blinded to treatment group | Compliance checked: Good Duration: Mean 1603 days |
Asthmatic children with ICS use continuously for ≥3 years Type of inhaler: MDI; Turbuhaler Type of steroid: BUD |
Log of accumulated dose of BUD; gender; age | Mean ICS BUD dose 504 μg (daily) | Mean BMD: BUD group: 0.92 Control group: 0.92 |
| Allen et al22 | DEXA scan at baseline and again at 9–20 months later. Value for 12-month time point calculated with all outcomes | Compliance checked: Adequate Duration of follow-up: 9–20 months |
Type of inhaler: Spacer, Turbohaler Type of steroid: BDP, BUD |
Age; height; weight; dose of inhaled corticosteroid | Mean ICS dose 0.67±0.48 mg/m2/day | Change in mean vertebral BMD (SD) over 12 months: ICS group (n=47): 0.03±0.03 Control group (n=9): 0.06±0.04 p:<0.025 |
| Bahceciler et al23 | Anteroposterior (AP) spine (L2–4) by DEXA scan | Compliance: not reported Follow-up: 13.0±9.8 months |
Use of BUD as MDI ≥6 months | None | ICS Mean daily dose (SD): 419±154 μg | Mean lumbar spine BMD: ICS group: 0.593 (SD 0.122) |
| Control | Mean lumbar spine BMD: 0.579 (SD 0.156) | |||||
| Harris et al25 | Lumbar spine by DEXA | Compliance checked: not reported Duration of follow-up: 3.5±2.4 years |
Stratified by treatment in past 6 months Type of inhaler: Spacer device Type of steroid: BDP,BUD, FP |
Weight | 0 μg/day | Mean lumbar spine BMD (SD) 0.68 (0.07) |
| 400–800 μg/day | Mean lumbar spine BMD (SD) 0.70 (0.08) |
|||||
| >800 μg/day | Mean lumbar spine BMD (SD) 0.67 (0.08) |
|||||
| Studies reporting on fracture risk |
Fracture outcomes | |||||
|---|---|---|---|---|---|---|
| Schlienger et al27 | Identified by ICD-8 codes 800.x −829.x, from computerised records Cases=1st-time diagnosis of bone fracture Controls—no fracture |
Compliance checked: not reported Duration: Median number of prescriptions: 26, corresponds to >7 years of continuous exposure |
ICS use in UK General Practice Research Database Type of inhaler: not reported Type of steroid: BDP, BUD, FP |
Matched for age, gender, general practice, calendar time, years in GPRD Adjusted for comorbidities: chronic renal failure, hyperthyroidism, hyperparathyroidism, inflammatory bowel disease, malnutrition, malabsorption Medications: asthma drugs, psychotropic drugs, antihypertensives, calcium, fluoride, vitamin D |
1–9 prescriptions Cases: n=332 Controls: n=2017 |
Adjusted OR: 0.97 (0.85 to 1.11) |
| 10–19 prescriptions Cases: n=124 Controls: n=682 |
Adjusted OR: 1.08 (0.87 to 1.33) | |||||
| ≥20 prescriptions Cases: n=88 Controls: n=422 |
Adjusted OR: 1.15 (0.89 to 1.48) | |||||
| All ICS users combined | Adjusted OR: 1.01 (0.90 to 1.13) | |||||
| Van Staa et al29 | Ascertained from diagnoses within computer records | Compliance not reported Start of follow-up:1987 onwards or from age 4 years End: December 1997 or age 18 years |
Current users of ICS Type of inhaler: not reported Type of inhaled steroid: BDP, BUD, FP |
History of seizures; use of non-steroidal anti-inflammatory drugs or bronchodilators; hospitalisation for asthma past 2 years; number of prescriptions in past year. Age; sex | 200 μg | Adjusted OR: 0.96 (0.83 to 1.12) |
| 201–400 μg | Adjusted OR: 1.07 (0.93 to 1.24) | |||||
| >400 μg | Adjusted OR: 1.17 (0.93 to 1.45) | |||||
| All ICS users | Adjusted OR: 1.03 (0.93 to 1.15) | |||||
BDP, beclometasone diproprionate; BUD, budesonide; DEXA, dual-energy X-ray absorptiometry; FP, fluticasone propionate; ICD, International Classification of Disease; ICS, inhaled corticosteroids; MDI, metered dose inhaler.
| Observational studies of bone mineral density and fractures—adults | ||||||
|---|---|---|---|---|---|---|
| Study | Ascertainment of BMD/fracture | Ascertainment of ICS exposure | Definition of ICS use | Adjustments | ICS exposure | Results of BMD (g/cm2) and fractures |
| El et al24 | DEXA lumbar spine (L1–4) and femoral neck | Compliance checked: poor Duration: mean duration (SD) (years): 2.79±1.77 |
Regular ICS >6 months Type of inhaler: not reported Type of ICS: not reported |
Age | Cases Mean daily ICS dose 326.43 µg |
Mean lumbar: 0.925, SD 0.211 Mean femoral neck: 0.746, SD 0.127 |
| Controls (no exposure) | Mean lumbar: BMD: 0.927, SD 0.229 Mean femoral neck: 0.792, SD 0.097 |
|||||
| Johannes et al26 | Non-vertebral identified by ICD-9 codes and insurance claim for fracture treatment within 2 weeks | Compliance checked: not reported Duration: 1 Year ICS exposure |
ICS use from pharmacy claims in the 365 days before index date. Type of inhaler: not reported Type of steroid: BDP, BUD, FP, flunisolone, triamcinolone |
Demographics—age, sex, region, time and season Comorbidities—wide range of cardiovascular, endocrine, metabolic and musculoskeletal conditions Medications—oral corticosteroids, bisphosphonates, statins, anticonvulsants, oestrogen, raloxifene, calcitonin Healthcare utilisation for underlying respiratory disease |
1–167 μg | OR 1.00 95% CI 0.84 to 1.18 |
| 168–504 μg | OR: 1.02 95% CI 0.83 to 1.26 |
|||||
| 505–840 μg | OR: 1.14 95% CI 0.80 to 1.62 |
|||||
| >840 μg | 0.99 95% CI 0.66 to 1.50 |
|||||
| Sosa et al28 | DEXA lumbar spine (L2-L4) and proximal femur |
Compliance: not reported Duration of follow up: Median treatment with ICS: 10 years |
ICS for >1 year Type of inhaler: not reported Type of ICS: not reported |
Age | Cases (dose not reported) | Lumbar spine: 0.960; 95% CI 0.925 to 0.995 Femoral neck:0.776; 95% CI 0.750 to 0.802 Fractures: 22/105 (21.0%) |
| Controls | Lumbar spine: 0.991; 95% CI 0.960 to 1.022 Femoral neck: 0.780; 95% CI 0.758 to 0.803 Fractures: 9/133 (7.0%) |
|||||
| Wisniewski et al30 | Posterior-anterior spine (L2–4), lateral spine (body of L3) measured by DEXA once. All scans by same radiographer (blinded) | Compliance checked: Adequate Duration: Median duration of use of ICS (years) Men: 9.00 Women: 6.29 |
ICS for > 5 years Type of inhaler: Metered dose inhaler—36 patients; dry powder inhaler—11 patients Type of ICS: BDP, BUD |
age; weight; smoking; alcohol; activity grade; asthma severity; age at menarche; lifetime total dose of oestrogen and progesterone; prednisolone use | Cases | Lumbar spine±SD Men : 1.28±0.13; Women: 1.04±0.14 Femoral neck±SD: Men : 1.17±0.18; Women: 1.09±0.14 Vertebral fractures overall: 2/47 |
| Controls (No exposure) | Lumbar spine±SD Men:1.21±0.17; Women: 1.25±0.12 Femoral neck±SD: Men : 1.04±0.14; Women: 1.10±0.14 Vertebral fractures overall: 6/34 |
|||||
| Yanik et al31 | DEXA lumbar spine and hip (femoral neck and trochanter). Patient-reported history of fractures |
Compliance checked: Adequate Duration of Follow-up: 4.3±2.6 years |
Regular ICS >12 months Type of inhaler: Not reported Type of ICS: BDP, BUD, FP |
None | Cases (total) Mean daily ICS dose (μg) (SD): 324.9±121.8 |
Lumbar spine± SD 0.95±0.29 Femoral neck± SD 0.83±0.12 Atraumatic vertebral fractures: 4 (8.6%) |
| Controls | Lumbar spine±SD 0.88±0.14 Femoral neck±SD 0.74±0.23 Atraumatic vertebral fracture: 6 (10%) |
|||||
BDP, beclometasone diproprionate; BUD, budesonide; DEXA, dual-energy X-ray absorptiometry; FP, fluticasone propionate; ICD, International Classification of Disease; ICS, inhaled corticosteroids.
Four of the RCTs focused solely on children,14 15 19 20 while the remaining three were in adults.16–18 Treatment duration was up to 4 years in one study,15 while the remaining six trials had ICS therapy for between 52 and 104 weeks. Intervention arms of the trials included fluticasone (5 trials), budesonide (3 trials) and mometasone (one trial). Fluticasone and mometasone were the ICS used in the intervention arms of one trial, and in this trial, we evaluated the results of all ICS users combined against montelukast.18
Five of the observational studies focused solely on children,21–23 25 29 while the remainder looked at adults or a mixture of age groups. The observational studies looked at wider range of ICS than the RCTs, with the inclusion of beclometasone, flunisolide and triamcinolone users.
Study validity
Validity assessment of the included studies is reported in tables 2 and 3.
Randomised controlled trials (n=7)
Overall, four of the RCTs reported an appropriate method of sequence generation, while five provided details on how concealment of allocation was achieved. With regards to blinding, five trials reported the use of double-blinding. Ascertainment of BMD was consistently performed through dual-energy X-ray absorptiometry (DEXA) scans, but the trials did not state how and when fracture diagnoses were confirmed. One major limitation that affected all the trials stemmed from discontinuations and substantial losses to follow-up for measurement of BMD outcomes at final time-points.
Observational studies (n=11)
We felt that only four studies took account of a good range of variables when tackling baseline confounding.26 27 29 30 Assessment of compliance or adherence to ICS use was reported in four studies.21 22 30 31 Fracture events were typically recorded through administrative codes while one study relied on patient self-report. Ascertainment of BMD was through DEXA scans. Overall, we felt that most of the studies were at moderate to high risk of bias due to the above limitations, with four studies possibly of slightly better methodological quality because of adequate outcome ascertainment and adjustment for confounders.26 27 29 30
Fractures with ICS
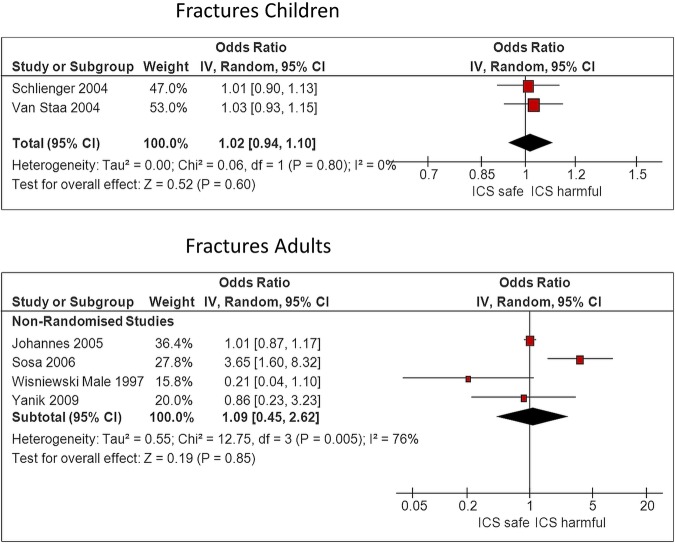
We identified one large long-term RCT in children that reported adjusted fracture rate of 5.7 per 100 patient years with budesonide as compared to 5.1 per 100 patient years with placebo (p=0.53).32 Similarly, there was no significant increase in likelihood of fracture in a meta-analysis of two observational studies in children, (OR 1.02, 95% CI 0.94 to 1.10, I2=0%)27 29 as shown in figure 2. The point estimates of fracture risk was not significantly elevated at higher dose levels, with one study demonstrating an OR of 1.15 (0.89 to 1.48) for children with ≥20 prescriptions,27 and the other study reporting an OR of 1.17 (0.93 to 1.45) for children using a daily dose of >400 μg beclomethasone dipropionate equivalents.29
Figure 2.
Fracture risk, ICS use versus non-use. ICS, inhaled corticosteroids.
No consistent association between ICS use and fracture risk in adults was seen in the pooled estimate from four observational studies (overall OR 1.09, 95% CI 0.45 to 2.62; figure 2).26 28 30 31 There was substantial heterogeneity in this meta-analysis (I2=76%), with Sosa's study reporting significantly increased fracture risk,28 while the others did not.
However, we judged a study by Sosa et al28 to be at high risk of bias because the control group consisted of relatives and neighbours of patients, the type of ICS was not reported and there were no statistical adjustments for confounders. In this data set, Johannes et al26 was the only study reporting fractures according to dose, but this did not demonstrate any consistent trend towards elevated risk at higher doses.
Lumbar spine BMD
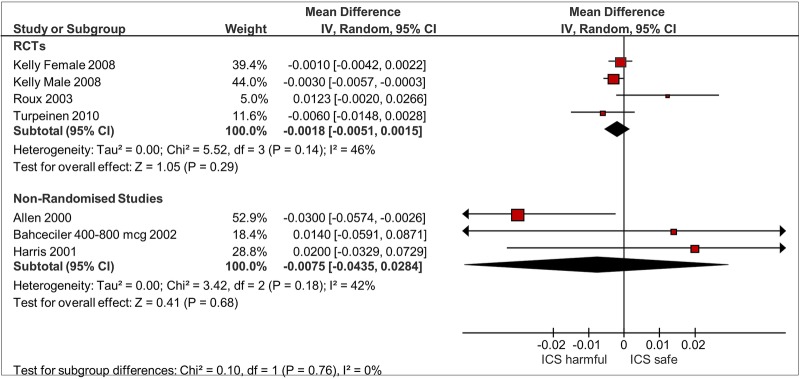
Three RCTs and three observational studies reported on comparative change at the lumbar spine in children.15 19 20 22 23 25 (figure 3) ICS use was not associated with significant reductions in BMD as compared to controls in RCTs (mean difference −0.0018 g/cm2; 95% CI −0.0051 to 0.0015 g/cm2; I2=46%) or observational studies (mean difference −0.0075 g/cm2; 95% CI −0.044 to 0.028 g/cm2; I2=42%). There was no clear signal of dose responsiveness in one observational study that separated participants into different dose levels,25 whereas one RCT suggested that longer term users of budesonide with greater cumulative doses had lower BMD compared to those who received lower cumulative doses.20
Figure 3.
BMD in lumbar spine children, ICS use versus non-use. BMD, bone mineral density; ICS, inhaled corticosteroids.
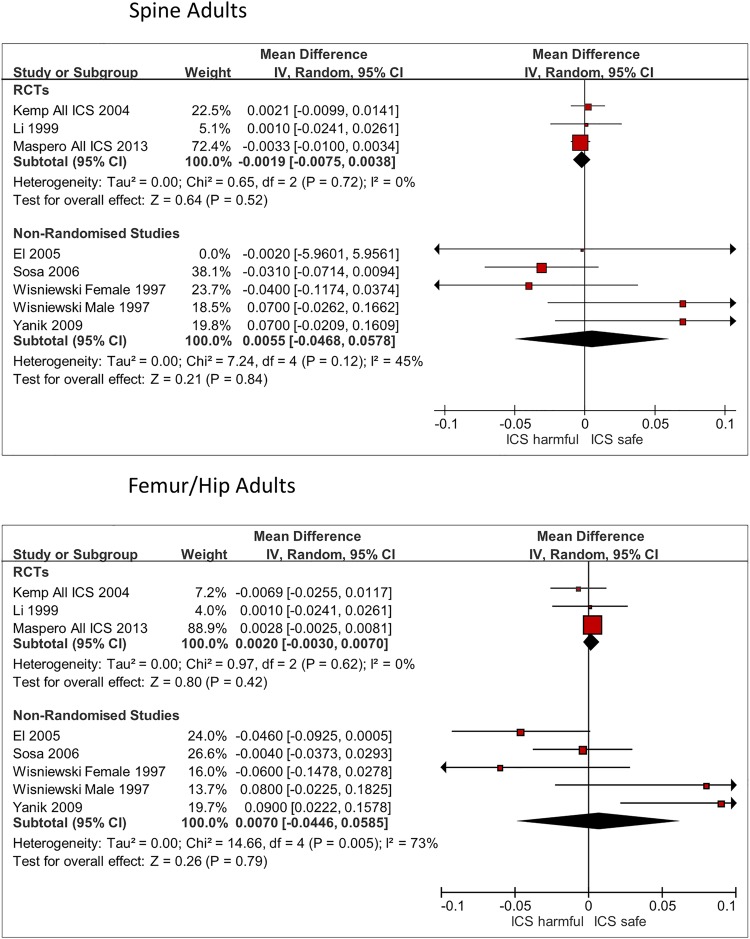
Three RCTs and four observational studies reported on comparative change in BMD at the lumbar spine in adults (figure 4).16–18 24 28 30 31 ICS use was not associated with significant reductions in BMD as compared to controls in RCTs (mean difference −0.0019 g/cm2; 95% CI −0.0075 to 0.0038 g/cm2; I2=0%) or observational studies (mean difference −0.0055 g/cm2; 95% CI −0.047 to 0.058 g/cm2; I2=45%).
Figure 4.
BMD in adults, ICS use versus non-use. BMD, bone mineral density; ICS, inhaled corticosteroids; RCT, randomised controlled trial.
Femur/hip BMD for adults
There were three RCTs and four observational studies reporting comparative change in BMD at the femur or hip in adults (figure 4).16–18 24 28 30 31 ICS use was not associated with significant reductions in BMD as compared to controls in RCTs (mean difference 0.0020 g/cm2; 95% CI −0.0030 to 0.0070 g/cm2; I2=0%) or observational studies (mean difference 0.0070 g/cm2; 95% CI −0.045 to 0.059 g/cm2; I2=73%).
There was sparse data comparing different ICS molecules head to head. Ferguson et al14 measured lumbar spine BMD and reported a non-significant finding between children randomised to fluticasone propionate 100 μg twice daily as compared to budesonide, mean difference 0.0075 g/cm2 (95% CI −0.033 to 0.048 g/cm2). Maspero conducted a five arm trial that included mometasone and fluticasone propionate in adults. There were no significant differences in lumbar spine and femur BMD between the two compounds at the end of the trial.18
We did not proceed to constructing a funnel plot for detection of publication bias because we had less than 10 studies in the meta-analysis of each outcome, and there was substantial heterogeneity.
Discussion
We focused our systematic review of RCTs and observational studies on skeletal adverse effects of ICS in patients with asthma. We did not find convincing evidence of increased fracture risk with ICS use in adults or children. Equally, there was no consistent evidence of any significant detrimental relationship between ICS use and BMD at the lumbar spine (in adults and children) or femur (in adults). There was insufficient data for us to detect any dose–response relationship, or to judge any potential differences between the available ICS molecules.
Our findings should be contrasted with those of other recent published reviews. There have been at least four systematic reviews evaluating fractures or BMD in ICS users, with two earlier reviews demonstrating a significant reduction in BMD but no definite impact on fractures.5 33 The most recent meta-analyses have identified a small but statistically significant dose-related increase in risk of fracture associated with ICS use in patients with COPD.6 7 Our findings differ from these other reviews as we have specifically focused on ICS use in patients with asthma. Here, we used very rigid selection criteria in an attempt to exclude patients with COPD from our meta-analysis.
The deleterious effects of ICS on BMD seen in previous meta-analyses could be explained in part by the higher prevalence of smoking in patients with COPD as previous studies have shown that smoking has a harmful effect on BMD, and increasing fracture risk.8 In addition, as a group, patients with asthma are likely to be younger and to have fewer comorbidities than those with COPD which may impact on BMD and fracture risk. Recent research indicates that multimorbidity (including cachexia and low-grade systemic inflammation) is often seen in patients with COPD,9 and it is conceivable that these factors may have a further negative impact on bone formation that accentuate the risks of ICS in COPD.
ICS therapy may have a positive impact on bone density through reduction of chronic inflammation and avoidance of need for acute short courses of oral corticosteroids during exacerbations. In addition, ICS may allow better control of asthma in patients such that they become more active, thereby slowing or preventing steroid-induced osteoporosis through the beneficial effects of physical activity on BMD. Bone mass can also be influenced by a wide range of other factors (such as nutrition, genetic make-up, endocrine status and amount of physical exercise),1 and ICS may therefore not be the most important influence on bone density in patients with asthma.
There are a number of limitations to our systematic review. Our search was limited to English language articles. Although, studies have attempted to assess skeletal adverse effects in many different ways, we have limited our review to clinically meaningful outcomes such as BMD in g/cm2 at lumbar spine and femur, and fractures. We did not have sufficient data from the primary studies for us to conduct meaningful analyses on different combinations of drug compounds, inhaler devices and dosage regimens. Some of the included studies were published more than a decade ago, and advances in asthma care may have made their findings less applicable to current-day patients. We recognise that there is potential for risk of bias (stemming from substantial loss to follow-up for BMD measurements) within this data set. Hence, we are unable to interpret the effects of ICS in very long-term use of ICS over a decade or more.
Our systematic review demonstrates that there is no consistent evidence of serious skeletal harm from use of ICS. Although there are intrinsic limitations to the evidence, we believe that our systematic review provides some reassurance to patients and prescribers of ICS. Our findings enables ICS users to judge the benefits and harms of their medication in a more accurate manner and helps to address concerns and uncertainty surrounding the exact risk of skeletal adverse effects.
Footnotes
Contributors: YKL and AMW conceptualised the review and obtained funding. YKL, DG, MT, PB and AMW selected studies and abstracted the data. YKL carried out the synthesis of the data and wrote the manuscript with critical input from all authors. YKL acts as guarantor for the paper.
Funding: This manuscript presents a systematic review commissioned by Asthma UK (AUK-PG-2012-181), and we are grateful to the Asthma UK Research team for their guidance. The views expressed in this paper are those of the authors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Ricciardolo FLM. The treatment of asthma in children: inhaled corticosteroids. Pulm Pharmacol Ther 2007;20:473–82. 10.1016/j.pupt.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 2.British-Thoracic-Society/Scottish-Intercollegiate-Guidelines-Network. British Guideline on the Management of Asthma. Thorax 2008;63(Suppl 4):iv1–121. 10.1136/thx.2008.097741 [DOI] [PubMed] [Google Scholar]
- 3.National-Asthma-Education-and-Prevention-Program. Expert Panel Report 3 (EPR-3): guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(5 Suppl):S94–138. 10.1016/j.jaci.2007.09.043 [DOI] [PubMed] [Google Scholar]
- 4.Rossi GA, Cerasoli F, Cazzola M. Safety of inhaled corticosteroids: room for improvement. Pulm Pharmacol Ther 2007;20:23–35. 10.1016/j.pupt.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 5.Jones A, Fay JK, Burr M et al. . Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002;(1):CD003537 10.1002/14651858.CD003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax 2011;66:699–708. 10.1136/thx.2011.160028 [DOI] [PubMed] [Google Scholar]
- 7.Weatherall M, James K, Clay J et al. . Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy 2008;38:1451–8. 10.1111/j.1365-2222.2008.03029.x [DOI] [PubMed] [Google Scholar]
- 8.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 1997;315:841–6. 10.1136/bmj.315.7112.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanfleteren LE, Spruit MA, Groenen M et al. . Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728–35. 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 10.Loke YK, Golder SP, Vandenbroucke JP. Comprehensive evaluations of the adverse effects of drugs: importance of appropriate study selection and data sources. Ther Adv Drug Saf 2011;2:59–68. 10.1177/2042098611401129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. 10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, 2008. Chapter 16, pp 481–529. [Google Scholar]
- 13.Higgins JPT, Deeks JJ, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, 2008. Chapter 9, pp 243–296. [Google Scholar]
- 14.Ferguson AC, Van Bever HP, Teper AM et al. . A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respir Med 2007;101:118–29. 10.1016/j.rmed.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 15.Kelly HW, Van Natta ML, Covar RA et al. . Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics 2008;122:e53–61. 10.1542/peds.2007-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp JP, Osur S, Shrewsbury SB et al. . Potential effects of fluticasone propionate on bone mineral density in patients with asthma: a 2-year randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 2004;79:458–66. 10.4065/79.4.458 [DOI] [PubMed] [Google Scholar]
- 17.Li JTC, Ford LB, Chervinsky P et al. . Fluticasone propionate powder and lack of clinically significant effects on hypothalamic-pituitary-adrenal axis and bone mineral density over 2 years in adults with mild asthma. J Allergy Clin Immunol 1999;103:1062–8. 10.1016/S0091-6749(99)70180-6 [DOI] [PubMed] [Google Scholar]
- 18.Maspero J, Backer V, Yao R et al. . Effects of mometasone, fluticasone, and montelukast on bone mineral density in adults with asthma. J Allergy Clin Immunol Pract 2013;1:649–55.e1. 10.1016/j.jaip.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 19.Roux C, Kolta S, Desfougeres JL et al. . Long-term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics 2003;111(6 Pt 1):e706–13. 10.1542/peds.111.6.e706 [DOI] [PubMed] [Google Scholar]
- 20.Turpeinen M, Pelkonen AS, Nikander K et al. . Bone mineral density in children treated with daily or periodical inhaled budesonide: the Helsinki early intervention childhood asthma study. Pediatr Res 2010;68:169–73. 10.1203/PDR.0b013e3181e69e36 [DOI] [PubMed] [Google Scholar]
- 21.Agertoft L, Pedersen S. Bone mineral density in children with asthma receiving long-term treatment with inhaled budesonide. Am J Respir Crit Care Med 1998;157:178–83. 10.1164/ajrccm.157.1.9707072 [DOI] [PubMed] [Google Scholar]
- 22.Allen HDW, Thong IG, Clifton-Bligh P et al. . Effects of high-dose inhaled corticosteroids on bone metabolism in prepubertal children with asthma. Pediatr Pulmonol 2000;29:188–93. [DOI] [PubMed] [Google Scholar]
- 23.Bahceciler NN, Sezgin G, Nursoy MA et al. . Inhaled corticosteroids and bone density of children with asthma. J Asthma 2002;39:151–7. 10.1081/JAS-120002196 [DOI] [PubMed] [Google Scholar]
- 24.El O, Gulbahar S, Ceylan E et al. . Bone mineral density in asthmatic patients using low dose inhaled glucocorticosteroids. J Investig Allergol Clin Immunol 2005;15:57–62. [PubMed] [Google Scholar]
- 25.Harris M, Hauser S, Nguyen TV et al. . Bone mineral density in prepubertal asthmatics receiving corticosteroid treatment. J Paediatr Child Health 2001;37:67–71. 10.1046/j.1440-1754.2001.00628.x [DOI] [PubMed] [Google Scholar]
- 26.Johannes CB, Schneider GA, Dube TJ et al. . The risk of nonvertebral fracture related to inhaled corticosteroid exposure among adults with chronic respiratory disease. Chest 2005;127:89–97. 10.1378/chest.127.1.89 [DOI] [PubMed] [Google Scholar]
- 27.Schlienger RG, Jick SS, Meier CR. Inhaled corticosteroids and the risk of fractures in children and adolescents. Pediatrics 2004;114:469–73. 10.1542/peds.114.2.469 [DOI] [PubMed] [Google Scholar]
- 28.Sosa M, Saavedra P, Valero C et al. . Inhaled steroids do not decrease bone mineral density but increase risk of fractures: data from the GIUMO study group. J Clin Densitom 2006;9:154–8. 10.1016/j.jocd.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 29.Van Staa TP, Bishop N, Leufkens HGM et al. . Are inhaled corticosteroids associated with an increased risk of fracture in children? Osteoporos Int 2004;15:785–91. 10.1007/s00198-004-1606-5 [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski AF, Lewis SA, Green DJ et al. . Cross sectional investigation of the effects of inhaled corticosteroids on bone density and bone metabolism in patients with asthma. Thorax 1997;52:853–60. 10.1136/thx.52.10.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanik B, Ayrim A, Ozol D et al. . Influence of obesity on bone mineral density in postmenopausal asthma patients undergoing treatment with inhaled corticosteroids. Clinics 2009;64: 313–18. 10.1590/S1807-59322009000400008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–63. 10.1056/NEJM200010123431501 [DOI] [PubMed] [Google Scholar]
- 33.Richy F, Bousquet J, Ehrlich GE et al. . Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int 2003;14:179–90. 10.1007/s00198-003-1398-z [DOI] [PubMed] [Google Scholar]