Abstract
Purpose
Autophagy is a cell survival mechanism that plays a critical role in pancreatic carcinogenesis. Murine studies have previously demonstrated that treatment with the late-autophagy inhibitor chloroquine in combination with chemotherapy limited tumor growth.
Methods
In this Phase I/II trial we examined treatment with hydroxychloroquine (HCQ) with gemcitabine for patients with pancreatic adenocarcinoma. The primary endpoints were the safety and tolerability, evaluated with Storer's dose escalation design. Secondary endpoints were CA 19-9 biomarker response, R0 resection rates, survival and correlative studies of autophagy.
Results
Thirty-five patients were enrolled. There were no dose-limiting toxicities and no Grade 4/5 events related to treatment. 19 patients (61%) had a decrease in CA19-9 following treatment. 29 patients (94%) underwent surgical resection as scheduled with a 77% R0 resection rate. Median overall survival was 34.8 months (95% CI: (11.57 months, not reached)). Patients who had more than a 51% increase in the autophagy marker LC3-II in circulating peripheral blood mononuclear cells had improvement in disease-free (15.03 vs. 6.9 months, p<0.05) and overall survival (34.83 vs. 10.83 months, p<0.05). No outcome differences were demonstrated in the 81% of patients with abnormal p53 expression assessed by immunohistochemistry in the resected specimens.
Conclusion
Pre-operative autophagy inhibition with HCQ plus gemcitabine is safe and well-tolerated. Surrogate biomarker responses (CA19-9) and surgical oncologic outcomes were enouraging. p53 status was not associated with adverse outcomes.
Introduction
Autophagy is a pathway of cell survival in response to stress in which damaged organelles, proteins and materials are recycled to enhance energy production (1-3). Autophagy is crucial to late pancreatic carcinogenesis, allowing cancer cells to survive nutrient deprivation and hypoxia and survive treatment with chemotherapy, leading to chemoresistance (4-9,10, 11). Levels of autophagy have been linked to prognosis in pancreatic ductal adenocarcinoma (PDA) (12). Inhibition of autophagy results in increased apoptosis and therefore represents a potential mechanism to induce cell death in established PDA (13).
Hydroxychloroquine (HCQ) is an inexpensive drug that blocks acidification of the lysosome, inhibiting the final step of autophagy (14). Although originally developed to treat patients with malaria, HCQ has been utilized for treatment of systemic lupus erythematosus and rheumatoid arthritis (15, 16). The oral bioavailability, safety profile and low cost of HCQ make it a suitable candidate to test the concept of autophagy inhibition in cancer clinical trials (17).
Herein we report a Phase I/II prospective clinical trial, in which pre-operative autophagy inhibition with HCQ in combination with gemcitabine was evaluated as a novel treatment strategy in PDA.
Methods
Patient Population
Prior to initiation of the study, Institutional Review Board approval was obtained from the University of Pittsburgh (PRO10010028) and the trial was registered with the NCI (NCT01128296). All patients signed informed consent. Study eligibility was limited to those patients with biopsy-proven PDA predicted to have limited survival advantage from surgical resection based on a previously published study from our institution (21). This model defined patients as “high risk” for inability to achieve an R0 resection if they had 1)evidence of vascular involvement by CT scan, 2)were endoscopic ultrasound (EUS) ≥stage 2B and 3)had primary tumor ≥2.6 cm on EUS.
Trial Design
This was a Phase I/II prospective clinical trial evaluating pre-operative gemcitabine plus hydroxychloroquine. Two doses of fixed-dose gemcitabine (1500mg/m2) were administered (study days 3 and 17) in combination with oral hydroxychloroquine taken for 31 consecutive days until the day of surgery. A maximum dose of 1200 mg/day of HCQ was established based on pharmacologic studies (15). A Storer BD design was utilized to escalate the HCQ dose from 200mg/day to 1200mg/day (52).
Study Outcomes
The primary outcome was safety and tolerability. Toxicity was graded by the NCI Common Toxicity Criteria version 3.0. Patient outcomes were evaluated only if they completed more than 80% of the intended dose of HCQ. Secondary outcomes included clinical response assessed by CA 19-9 response, R0 resection rate, and survival.
Data Analysis
Survival was characterized by Kaplan-Meier estimates with 95% Borgan-Liestøl confidence regions. Survival functions were compared by means of the log-rank test. The change in CA19-9 after treatment was evaluated by analysis of covariance on the log-transformed values. A CA19-9 response was determined based on increase or decrease of the value following treatment. Statistical analyses were performed using R3.1 software.
Additional methods for the clinical trial and correlative studies are listed in the supplemental resources.
Results
Safety of Gemcitabine/Hydroxychloroquine (HCQ)
The safety of autophagy inhibition with HCQ was evaluated in a dose-escalation clinical trial in pancreatic cancer patients. Subjects were at elevated risk for failure to achieve an R0 resection based on criteria previously defined at our institution (18). Thirty-five of 48 patients (73%) were enrolled in the clinical trial (CONSORT diagram, Figure 1a). Two patients withdrew study consent prior to initiation of treatment. One patient was taken off protocol following the first dose of gemcitabine after suffering a cerebrovascular accident judged to be unrelated to study medications. A second patient was removed from the protocol due to the development of an allergic rash likely related to gemcitabine treatment, resulting in 31 patients (89% of the 35 enrolled) completing treatment. Per study protocol, outcomes were reported only for those patients who completed at least 80% of the hydroxcyhloroquine dose (n=31). Patient demographics and pathologic characteristics are reported in Table 1. There were no dose-limiting toxicities or treatment delays attributed to HCQ, allowing treatment of one patient at each of the lower dose cohorts and proceeding to the maximum dose of 1200 mg/day for the subsequent 26 patients (Figure 1b). Toxicity to treatment is listed in Figure 1c. The single Grade 4 event occurred in a patient with carotid atherosclerosis who developed a cerebrovascular accident, judged to be unrelated to the study drugs.
Figure 1. Patient enrollment and treatment outcomes.
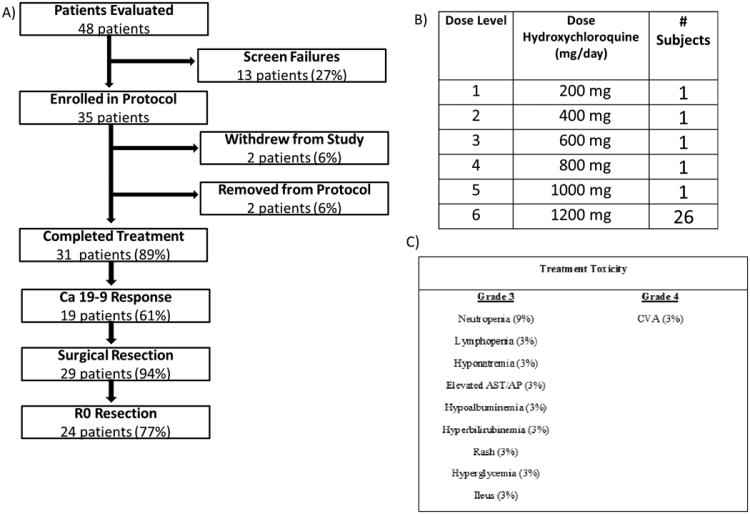
Thirty-five patients were enrolled in the trial (a). Two patients withdrew from the study after signing consent but prior to receiving treatment to pursue alternative treatment regimens. Two patients were removed from the protocol; one developed a cerebrovascular accident thought to be unrelated to study drugs and another had an allergic rash to gemcitabine. b) Storer up and down design and dose escalation schema was used to enable rapid dose escalation of HCQ in individual patients. There were no dose limiting toxicities to treatment, allowing a single patient to be treated at each of the lower dose cohorts, resulting in 26 patients receiving the maximum tolerated dose. c) Grade 3-5 toxicities as assessed by the NCI CTCAE are reported in the table. Preoperative treatment with Gemcitabine/HCQ was well tolerated with no dose limiting toxicities and no surgical treatment delays.
Table 1. Clinical and pathologic characteristics of patients treated with Preoperative Gemcitabine/Hydroxychloroquine.
| Patient Demographics | |
| Age | 64 ± 10 |
| Male gender | 17 (55%) |
| Pre-treatment Ca 19-9 | 356 (35 -10,643) |
| Pancreatic head tumor | 25 (81%) |
| Pathologic Characteristics | |
| Tumor size, cm | 4 (0.3-7) |
| N1 disease | 20 (65%) |
| Lymph node ratio | 0.13 (0-1) |
| Angiolymphatic invasion | 21 (68%) |
| Perineural invasion | 24 (77%) |
| Peri-Operative Outcomes | |
| Robotic resection | 12 (39%) |
| Estimated blood loss, mL | 250 (50-2,500) |
| R0 Resection | 24 (77%) |
| Positive margin | 5 (16%) |
| Retroperitoneal | 4 (13%) |
| Portal Vein | 1 (3.2%) |
| Mortality | 1 (3.2%) |
| Length of stay, days | 8 (4-17) |
| Readmission | 7 (23%) |
| Reoperation | 1 (3%) |
n=31, Data reported as mean ± SD, median (range) or n(%).
Twenty-four patients (77%) required pancreaticoduodenectomy for resection with 7 patients (23%) requiring venous resection. Although the study was not powered to detect changes in post-operative morbidity, complications were observed in 58% of patients. A majority of these were Clavien-Dindo Grade I or II (72%) (19). A Grade IV event occurred in a patient who developed a gastric leak following an Appleby procedure, requiring care in the intensive care unit. This patient made a full recovery and is still alive with disease more than 36 months following treatment. There was one peri-operative mortality (3%) in a patient who developed superior mesenteric vein thrombosis due to technical complications with the venous anastomosis resulting in ischemic bowel and multi-system organ failure. Pancreatic leak affected four patients (13%) with two Grade A and 2 Grade B leaks. 24 of the 29 patients resected (83%) were treated with adjuvant gemcitabine. Two patients received postoperative stereotactic body radiation therapy without adjuvant chemotherapy.
Surrogate Oncologic Outcomes of Gemcitabine/Hydroxychloroquine (HCQ)
Twenty-six patients (84%) had an elevated CA 19-9 (>33 U/mL) prior to initiation of treatment. The CA 19-9 response of each patient is shown in Figure 2a. Nineteen patients (73% of those with baseline elevation) demonstrated a CA 19-9 response following completion of preoperative therapy. Thirteen patients (50%) had a decrease in CA 19-9 of ≥ 50%. Twenty-nine patients (94%) underwent surgical resection following treatment. Two patients were deemed unresectable at exploration due to liver metastases. Twenty-four patients (77%) underwent an R0 resection, defined as absence of tumor at the surgical inked margin. R1 resections resulted from a positive retroperitoneal margin (n=4) and a positive portal vein margin (n=1). There were no complete pathologic responses(20).
Figure 2. Clinical outcomes following treatment with pre-operative Gemcitabine/HCQ.
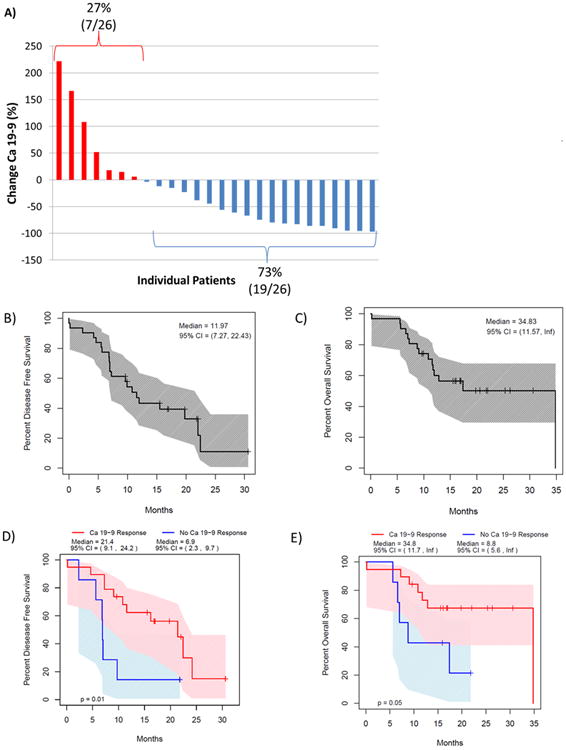
Waterfall plot demonstrates the change in CA 19-9 with treatment of each individual patient with an elevated baseline CA 19-9 (a). 73% of patients had a decrease in CA 19-9 versus only 27% with an increase. Median disease free survival was 12 months (b) and median overall survival was 34.8 months for the entire cohort (c). CA 19-9 response to treatment predicted disease free and overall survival, suggesting biologic activity of gemcitabine/HCQ (d&e).
Study patients had a median disease-free survival of 12.0 months (95% CI: (7.3, 22.4)) and overall survival of 34.8 months (95% CI: (11.57, not reached); Figures 2b & c). Patients who had a CA 19-9 response to treatment had an improvement in disease-free survival (21.4 vs. 6.9 months, p=0.01, Figure 2d) and overall survival (34.8 vs.8.8 months, p<0.05, Figure 2e) compared to those without a change in CA19-9.
Comparison to Previous Institutional Cohort
To put these results into perspective, outcomes were compared to those from the previously established cohort of patients at our institution that defined eligibility for this trial (21). Demographics and criteria were not different between patients from the prior series and the current trial (Supplemental Table 1). The resection rate for patients treated with gemcitabine/HCQ was 94% compared to 52% in the prior series (p<0.001) with an R0 resection achieved in 77% versus only 34% (p<0.01). Treatment with gemcitabine/HCQ resulted in improved overall survival compared with the prior cohort (Supplemental Figure 1, 34.83 vs. 12.27 months, p=0.03).
Correlative Studies
Peripheral blood samples and the resected pancreatic specimen were evaluated for autophagic markers (Supplemental Table 2 & 3). Peripheral blood mononuclear cells (PBMCs) were harvested from whole blood before and following treatment. PBMCs were stained for the autophagic marker LC3-II (Figure 3a). 65% of patients (11/17) had an increase in LC3 staining with treatment, consistent with autophagy inhibition. Patients who had > 51% increase in their LC3 staining were classified as responders to HCQ. HCQ responders had improved disease-free survival (15.03 months vs. 6.9, p=0.05) and overall survival (34.83 months vs. 10.83, p<0.05) compared with patients who had minimal LC3-II response (Figure 3b & c). There were trends toward HCQ responders having decreases in Ca 19-9 that did not reach statistical significance. There was no correlation between autophagy markers evaluated in the resected tumor and outcomes, including CA 19-9 response, resection rate, or survival.
Figure 3. Autophagic response to treatment predicts improved outcome following pre-operative Gemcitabine/HCQ.
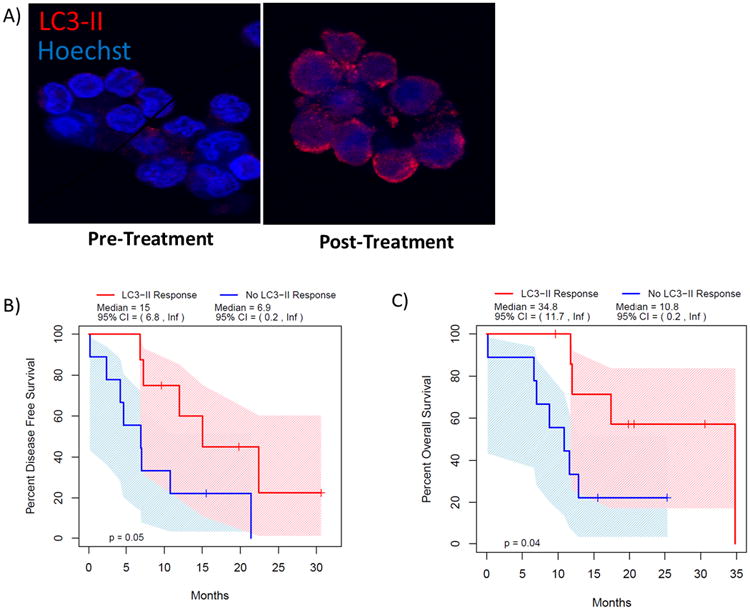
LC3 punctate staining was evaluated by immunoflourescence in circulating peripheral blood mononuclear cells (PBMCs) before and after treatment (a). Patients with a greater than 51% increase in LC3 puncta (LC3-II Response) had improved disease free (b) and overall survival (c).
p53 status was evaluated by immunohistochemical staining of the resected tumor as outlined in the supplemental methods. Of the 24 patients assessed, 20 had biologic alteration in p53 (81%), which was defined either as positive staining in >50% of tumor cell nuclei (Figure 4a) or total absence of nuclear staining (Figure 4b). These alterations have been associated with p53 mutation (22). p53 status did not influence Ca 19-9 response (100% vs. 67%, p=0.53), ability to achieve an R0 resection (75% vs. 80%, p=1.0), or survival (Figure 4d & e).
Figure 4. Response to autophagy inhibition is independent of p53.
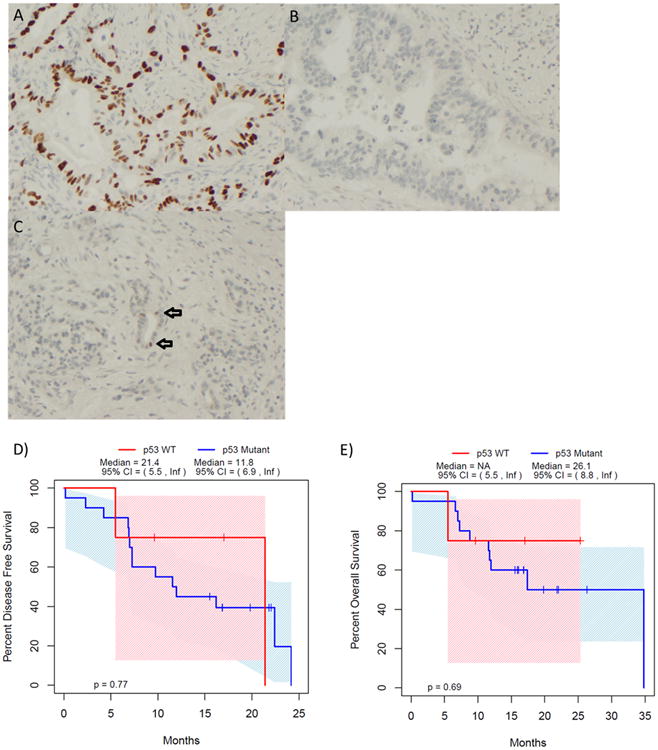
81% of patients demonstrated a p53 alteration evidenced by either high levels (a) or absence (b) of p53 immunohistochemical staining, which has been associated with p53 mutation. Apparently unaltered p53 patients are demonstrated in (c) with the arrows identifying cells with normal staining. Patient outcomes following treatment with Gemcitabine/HCQ were not impacted by p53 status, with no difference in disease-free (d) and overall survival (e) for p53 wild type (WT) patients as defined by immunohistochemistry.
Discussion
Despite recent advances in developing more effective chemotherapeutic regimens (23, 24), pancreatic cancer remains highly lethal. Inhibition of autophagy represents a novel treatment strategy in pancreatic cancer at a time when new solutions are desperately needed (8, 14). In this Phase I/II clinical trial, we establish that preoperative treatment with the autophagy inhibitor hydroxychloroquine in combination with chemotherapy is safe and tolerable.
Early biomarker responses in this trial were greater than what would be expected with a single cycle of gemcitabine. 73% of patients with elevated baseline CA 19-9 had a CA 19-9 response, with 50% of patients demonstrating a reduction of ≥ 50%. Hammad et al evaluated CA 19-9 response following treatment with one cycle of gemcitabine, resulteing in only 25% of patients demonstrating a decrease in CA 19-9 of ≥ 50% (25). Moreover, patients who had a decrease in CA 19-9 with treatment had improvements in disease-free and overall survival, suggesting that the study regimen translated into meaningful improvement in oncologic outcomes. This may not be surprising as early administration of effective chemotherapy has been suggested to be the single most important factor in improved survival in pancreatic cancer (26). Thus, this surrogate marker of clinical response is encouraging (28, 29), especially after only a single cycle of gemcitabine and 30 days of autophagy inhibition. Greater responses may be demonstrated with longer duration of treatment.
The ability to achieve margin negative, R0 resection in PDA is closely linked to survival (30). We previously defined a population at high risk for a nontherapeutic operation (unresectable disease or an R1 resection (31)). In this cohort of patients from our own institution who received no pre-operative treatment, we demonstrated a resection rate of 53% and an R0 resection rate of 33% with an overall survival of 12.27 months. Patients meeting the same high-risk criteria defined the eligibility for the current study. The current study demonstrates improvement in surgical resectability and R0 resection rate, suggesting that this regimen is having significant biologic impact. Although the trial was not designed nor powered to analyze survival differences, we did observe an improvement in overall survival following treatment with gemcitabine/HCQ compared to the historical cohort. This improvement may be confounded by introduction of FOLFIRINOX and gemcitabine/nab-paclitaxel, which was utilized as treatment of metastatic disease in some subjects in this cohort but not the historical studies. However, given the observed differences in survival in CA 19-9 responders in this trial we cannot discount that the brief, early introduction of an effective chemotherapy regimen played a role in improving survival. Clearly, these results must be interpreted with some caution as this Phase I/II trial design lacks a prospective control group for direct outcome comparison. The comparison to a historical cohort has several limitations which limit conclusions regarding the efficacy of the treatment and serves to put our data into perspective rather than as a direct comparison.
Linking the observed clinical outcomes to autophagy inhibition requires correlation with markers of autophagy. Hydroxychloroquine functions as a late inhibitor of autophagy by blocking acidification of the lysosome. As a consequence, autophagosomes accumulate, resulting in an increase of LC3 when autophagy is inhibited (32). Recently, an increase in greater than 50% in LC3 in peripheral blood has been suggested to be a marker of successful autophagy inhibition with HCQ in a murine model and human subjects (33). In our study, we measured the change in LC3 expression in PBMCs before and after treatment. Patients with greater than 51% increase in LC3 had significantly better disease-free and overall survival, suggesting that autophagy inhibition played a role in observed clinical outcomes. Evaluation of PBMCs allowed for pre and post treatment comparisons. Unfortunately, similar studies were not possible in the resected pancreatic tumor; as we have not yet validated autophagy measures in cell blocks obtained by fine needle aspirate when compared to paraffin embedded surgical specimens. All surgically resected specimens were evaluated for autophagic markers and there were no significant outcome correlations. It may be that the change from baseline autophagic levels is more predictive than measurement of autophagy at a single time point post treatment. Identifying patients most likely to respond to autophagy inhibition is an important goal. Although no assays to identify these patients are currently available, this is an area of ongoing investigation in our laboratory.
Recently, p53 status has been implicated in determining the role of autophagy in preclinical murine models (34). Rosenfeldt et al found that blocking autophagy prevented pancreatic carcinogenesis in mice with an oncogenic Kras allele. However, in Kras mutant/p53 knockout mice, inhibition of autophagy actually promoted tumor growth. This work has garnered substantial attention throughout the biomedical community, given several ongoing trials utilizing autophagy inhibition in various malignancies (35, 36). The role of autophagy in established tumors may be much different than that observed during carcinogenesis. The murine models evaluated by Rosenfeldt et al only examined the impact of autophagy inhibition during carcinogenesis. Additionally, these studies did not use chemotherapy in combination with autophagy inhibitors, which is known to further promote the survival pathway of autophagy (37-39). We and others have not observed promotion of tumor growth either in vitro or in vivo with autophagy inhibition (5, 7, 8, 40, 41). In the current clinical study, there was no evidence to conclude that clinical outcomes following autophagy inhibition were related to p53 status. However, because only 19% of the patients assessed had no apparent p53 alterations, this study was underpowered to definitively rule out the contribution of p53 mutational status (42). It should be noted that although immunohistochemical alterations in p53 expression have been associated with p53 mutation, a full genetic analysis was not performed for the current study.
In conclusion, pre-operative autophagy inhibition with hydroxychloroquine in PDA represents a novel treatment strategy that is safe with encouraging clinical outcomes compared to historical controls. Peripheral blood measurement of autophagy inhibition correlated with survival. P53 status did not appear to be important in defining responses. Based on these results we are actively accruing to an NCI supported (R01CA181450) randomized trial of neoadjuvant gemcitabine/nab-paclitaxel with and without hydroxychloroquine (NCT01978184) to better assess the true contribution of autophagy inhibition in the setting of effective chemotherapy
Supplementary Material
Synopsis.
This phase I/II clinical trial evaluated pre-operative autophagy inhibition in pancreatic adenocarcinoma. Treatment was found to be safe with encouraging responses in surrogate markers that were associated with the degree of autophagy inhibition, even in patients with p53 alteration.
Acknowledgments
This work was supported by T32CA113263 (BB) and an R01CA181450 (HJZ and MTL). Correlative studies were supported by philanthropic donors to the UPMC Pancreatic Cancer Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH. This project used the UPCI Animal Research Facility and Biostatistics Shared Resource Facility that are supported in part by award P30CA047904.
Financial Support: This work was supported by training grant number T32CA113263 (BB) from the National Cancer Institute and an R01CA181450 (HJZ and MTL). This project used the UPCI Animal Research Facility and Biostatistics Shared Resource Facility that are supported in part by award P30CA047904.
Footnotes
Disclosures: None
The original publication is available at the Annals website at http://link.springer.com/article/10.1245/s10434-015-4566-4.
References
- 1.Mathew R, White E. Autophagy, stress, and cancer metabolism: what doesn't kill you makes you stronger. Cold Spring Harb Symp Quant Biol. 2011;76:389–96. doi: 10.1101/sqb.2012.76.011015. [DOI] [PubMed] [Google Scholar]
- 2.Maycotte P, Thorburn A. Autophagy and cancer therapy. Cancer Biol Ther. 2011;11(2):127–37. doi: 10.4161/cbt.11.2.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchser WJ, Laskow TC, Pavlik PJ, Lin HM, Lotze MT. Cell-mediated autophagy promotes cancer cell survival. Cancer Res. 2012;72(12):2970–9. doi: 10.1158/0008-5472.CAN-11-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang R, Tang D. Autophagy in pancreatic cancer pathogenesis and treatment. Am J Cancer Res. 2012;2(4):383–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Kang R, Tang D, Lotze MT, Zeh HJ., 3rd AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 2012;8(6):989–91. doi: 10.4161/auto.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109(18):7031–6. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17(4):666–76. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wang XD, Lapi E, Sullivan A, Jia W, He YW, et al. Autophagic activity dictates the cellular response to oncogenic RAS. Proc Natl Acad Sci U S A. 2012;109(33):13325–30. doi: 10.1073/pnas.1120193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang R, Tang D, Lotze MT, Zeh HJ., 3rd RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy. 7(4):442–4. doi: 10.4161/auto.7.4.14681. [DOI] [PubMed] [Google Scholar]
- 11.Kang R, Tang D, Loze MT, Zeh HJ. Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE) Autophagy. 7(1):91–3. doi: 10.1038/cdd.2009.149. [DOI] [PubMed] [Google Scholar]
- 12.Fujii S, Mitsunaga S, Yamazaki M, Hasebe T, Ishii G, Kojima M, et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99(9):1813–9. doi: 10.1111/j.1349-7006.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, et al. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med (Berl) 2011;89(9):877–89. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 14.Livesey KM, Tang D, Zeh HJ, Lotze MT. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs. 2009;10(12):1269–79. [PubMed] [Google Scholar]
- 15.Munster T, Gibbs JP, Shen D, Baethge BA, Botstein GR, Caldwell J, et al. Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46(6):1460–9. doi: 10.1002/art.10307. [DOI] [PubMed] [Google Scholar]
- 16.Momose Y, Arai S, Eto H, Kishimoto M, Okada M. Experience with the use of hydroxychloroquine for the treatment of lupus erythematosus. J Dermatol. 2012 doi: 10.1111/1346-8138.12005. [DOI] [PubMed] [Google Scholar]
- 17.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao P, Potter D, Eisenber DP, Lenzner D, Zeh HJ, Lee KK, et al. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB. 2009;11(7):606–11. doi: 10.1111/j.1477-2574.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 20.Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127(11):1335–9. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 21.Bao P, Potter D, Eisenberg DP, Lenzner D, Zeh HJ, Lee KK, Iii, et al. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB (Oxford) 2009;11(7):606–11. doi: 10.1111/j.1477-2574.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima M, Okano K, Muraki S, Haba R, Maeba T, Suzuki Y, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013;258(2):336–46. doi: 10.1097/SLA.0b013e3182827a65. [DOI] [PubMed] [Google Scholar]
- 23.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 24.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammad N, Heilbrun LK, Philip PA, Shields AF, Zalupski MM, Venkatramanamoorthy R, et al. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia Pac J Clin Oncol. 2010;6(2):98–105. doi: 10.1111/j.1743-7563.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148(1-2):362–75. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balic A, Sorensen MD, Trabulo SM, Sainz B, Jr, Cioffi M, Vieira CR, et al. Chloroquine Targets Pancreatic Cancer Stem Cells via Inhibition of CXCR4 and Hedgehog Signaling. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0948. [DOI] [PubMed] [Google Scholar]
- 28.Bauer TM, El-Rayes BF, Li X, Hammad N, Philip PA, Shields AF, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119(2):285–92. doi: 10.1002/cncr.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topkan E, Parlak C, Kotek A, Yapar AF, Pehlivan B. Predictive value of metabolic 18FDG-PET response on outcomes in patients with locally advanced pancreatic carcinoma treated with definitive concurrent chemoradiotherapy. BMC Gastroenterol. 2011;11:123. doi: 10.1186/1471-230X-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147(8):753–60. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 31.Bao P, Potter D, Eisenberg DP, Lenzner D, Zeh HJ, Lee KK, Iii, et al. Validation of a prediction rule to maximize curative (R0) resection of early-stage pancreatic adenocarcinoma. HPB (Oxford) 2009;11(7):606–11. doi: 10.1111/j.1477-2574.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, et al. Phase II and Pharmacodynamic Study of Autophagy Inhibition Using Hydroxychloroquine in Patients With Metastatic Pancreatic Adenocarcinoma. The oncologist. 2014;19(6):637–8. doi: 10.1634/theoncologist.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenfeldt MT, O'Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504(7479):296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 35.Iacobuzio-Donahue CA, Herman JM. Autophagy, p53, and pancreatic cancer. N Engl J Med. 2014;370(14):1352–3. doi: 10.1056/NEJMcibr1400189. [DOI] [PubMed] [Google Scholar]
- 36.Starobinets H, Debnath J. Cancer: A suppression switch. Nature. 2013;504(7479):225–6. doi: 10.1038/nature12841. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto D, Blauer M, Hirota M, Ikonen NH, Sand J, Laukkarinen J. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. European journal of cancer. 2014;50(7):1382–90. doi: 10.1016/j.ejca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Donohue E, Thomas A, Maurer N, Manisali I, Zeisser-Labouebe M, Zisman N, et al. The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model. J Cancer. 2013;4(7):585–96. doi: 10.7150/jca.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papademetrio DL, Cavaliere V, Simunovich T, Costantino S, Campos MD, Lombardo T, et al. Interplay between autophagy and apoptosis in pancreatic tumors in response to gemcitabine. Targeted oncology. 2013 doi: 10.1007/s11523-013-0278-5. [DOI] [PubMed] [Google Scholar]
- 40.Yang A, Rajeshkumar NV, Wang X, Yabuuchi S, Alexander BM, Chu GC, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang S, Kimmelman AC. A critical role for autophagy in pancreatic cancer. Autophagy. 7(8):912–3. doi: 10.4161/auto.7.8.15762. [DOI] [PubMed] [Google Scholar]
- 42.Kim H, Saka B, Knight S, Borges M, Childs E, Klein A, et al. Having pancreatic cancer with tumoral loss of ATM and normal TP53 protein expression is associated with a poorer prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(7):1865–72. doi: 10.1158/1078-0432.CCR-13-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangwala R, Leone R, Chang YC, Fecher L, Schuchter L, Kramer A, et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8) doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S, et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8) doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangwala R, Chang YC, Hu J, Algazy K, Evans T, Fecher L, et al. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8) doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10(8) doi: 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi R, Davis LE, et al. Combined autophagy and HDAC inhibition: A phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10(8) doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, et al. Combined autophagy and proteasome inhibition: A phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10(8) doi: 10.4161/auto.29264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of clinical investigation. 2014;124(3):1406–17. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou W, Zhang Q, Yan Z, Chen R, Zeh HJ, Iii, Kang R, et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell death & disease. 2013;4:e966. doi: 10.1038/cddis.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner LM, Lotze MT. Tumor-cell death, autophagy, and immunity. The New England journal of medicine. 2012;366(12):1156–8. doi: 10.1056/NEJMcibr1114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925–37. [PubMed] [Google Scholar]
- 53.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


