Abstract
The potential anti-tumor agent wentilactones were produced by a newly isolated marine fungus Aspergillus dimorphicus. This fungus was derived from deep-sea sediment and identified by polyphasic approach, combining phenotypic, molecular, and extrolite profiles. However, wentilactone production was detected only under static cultures with very low yields. In order to improve wentilactone production, culture conditions were optimized using the response surface methodology. Under the optimal static fermentation conditions, the experimental values were closely consistent with the prediction model. The yields of wentilactone A and B were increased about 11-fold to 13.4 and 6.5 mg/L, respectively. The result was further verified by fermentation scale-up for wentilactone production. Moreover, some small-molecule elicitors were found to have capacity of stimulating wentilactone production. To our knowledge, this is first report of optimized production of tetranorlabdane diterpenoids by a deep-sea derived marine fungus. The present study might be valuable for efficient production of wentilactones and fundamental investigation of the anti-tumor mechanism of norditerpenoids.
Keywords: Aspergillus dimorphicus, anti-tumor agent, wentilactones, optimization
1. Introduction
According to the International Agency for Research on Cancer, lung cancer remains the most common cancer, with estimated 1.8 million new cases (12.9% of total) and 1.6 million deaths (19.4%) globally in 2012 [1]. Non-small-cell lung cancer, with a five-year survival rate of only about 15%, accounts for approximately 80%~85% of all lung cancer cases [2]. The most common liver cancer hepatocellular carcinoma accounts for 70%~85% of the total liver cancers [3,4]. It is estimated nearly 782,000 cases and 745,000 deaths in 2012 worldwide, among which 83% were in developing regions and 50% in China alone [1]. Nowadays, chemotherapy remains an effective and powerful option for cancer therapy [5]. For example, the anti-cancer drug Taxol, a taxane diterpenoid which was originally isolated from the Pacific yew tree, was later found in endophytic fungi and some precursor was also overproduced in E. coli [6,7]. However, there are still some limitations and side-effects of conventional chemotherapy drugs. Therefore, more novel effective anti-cancer agents are urgently needed to minimize the cancer incidence and severity [2,4,8].
Diterpenoids are a group of compounds with many pharmaceutical and industrial applications [9,10]. The tetranorditerpenoids wentilactones, which showed plant growth inhibition activity, were originally isolated from Aspergillus wentii [11,12]. Our group has been working on the isolation, identification, and bioactivity evaluation of marine-derived natural products from algae and endophytic fungi for many years [13,14]. Recently, we obtained several new tetranorlabdane diterpenoids with anti-cancer activities from a marine alga-derived endophytic fungus A. wentii [14]. Among these tetranorditerpenoids, we have proved that wentilactone A (WA) could induce apoptosis and G2/M arrest of human lung carcinoma cells [2]. Wentilactone B (WB) could inhibit proliferation and migration of human hepatoma SMMC-7721 cells [15]. These findings provide potential effectiveness and a theoretical basis for the therapeutic use of wentilactones in the treatment of malignancies [8]. However, the yields of wentilactones in A. wentii were relatively low under static cultures, which limited further investigations [14]. There are few reports of enhanced production of norditerpenoids from marine fungus so far [9,16].
In order to obtain fungal strains that could produce more wentilactones and other diterpenoids with similar structures, several marine fungi have been isolated and screened. Recently, we isolated and identified an Aspergillus dimorphicus from deep-sea sediment, which could produce slightly higher yields of wentilactones with more structural diversity. There was rare report of A. dimorphicus isolates from the marine environment, and only a few natural products have been identified [17]. To get the natural products from marine fungi, culture conditions might be optimized trying to simulate its original habitats. As for these anti-cancer wentilactones in A. dimorphicus, they could be detected only under static cultures. How these silent natural products are activated by environmental factors might be interesting for further investigation [16].
For the static fermentation of A. dimorphicus, we have tested different media, salinity, initial pH, culture time, temperature, and light conditions. According to the results of single-factor experiments, effects of three factors (salinity, initial pH, and culture time) were chosen for further optimization. The wentilactones production was evaluated by employing response surface methodology (RSM) based on Box-Behnken design [18,19]. The generated conditions were applied to wentilactone production and the results were reevaluated. Furthermore, some small-molecule elicitors were found to have capacity of stimulating the wentilactones production, especially with addition of 3% methanol. We hope that these data presented here could provide new methods for wentilactone production and therefore promote the investigation of the anti-tumor mechanism of tetranorditerpenoids.
2. Results and Discussion
2.1. Identification of Fungal Strain by Polyphasic Approach
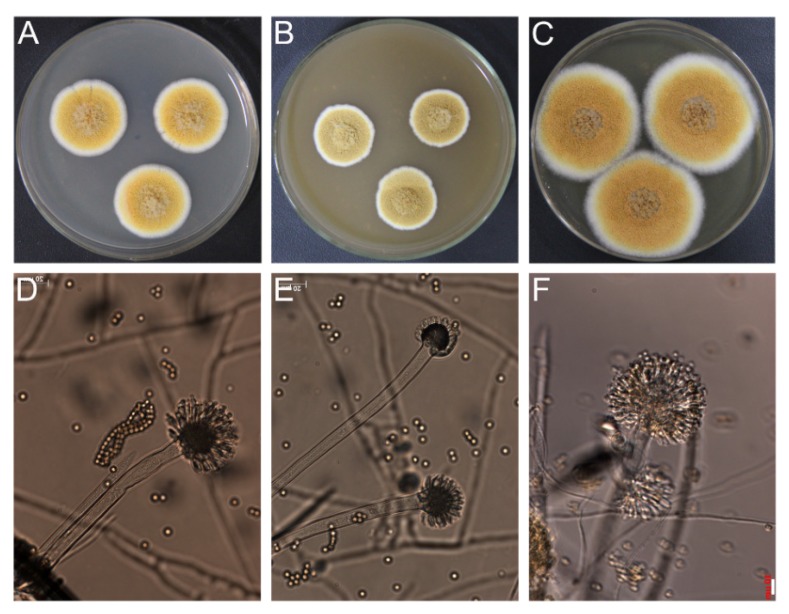
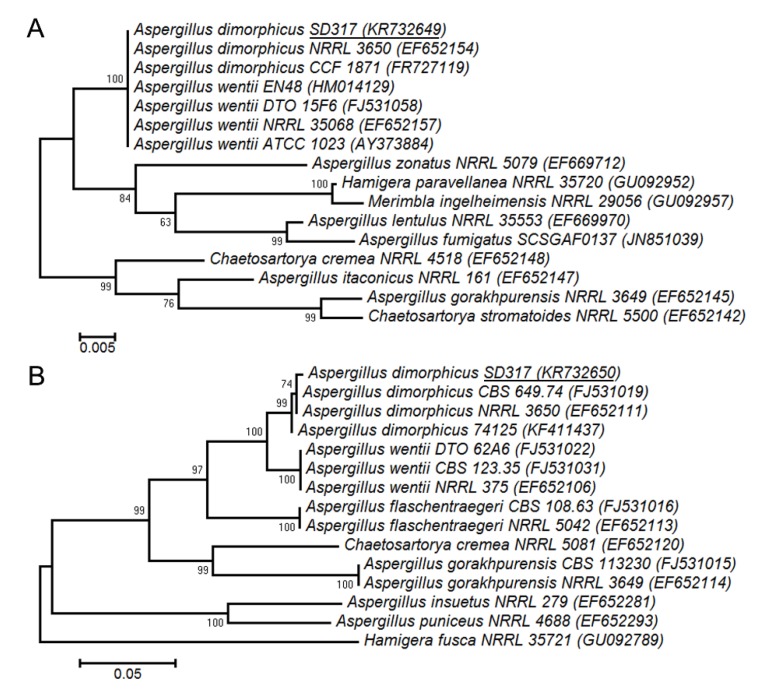
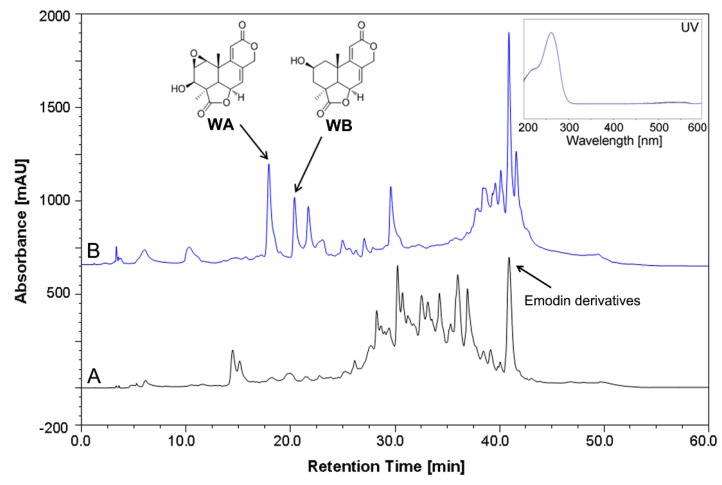
The fungal strain SD317 was recently isolated from deep-sea sediment of the South China Sea. The taxonomic status of this fungus was determined using the polyphasic approach, combining morphological, molecular, and extrolite characteristics. Morphological analysis of the strain indicated that the colonies grown on CYA, MEA, and YES plates have the main characteristics of Aspergillus (Figure 1A–F). The colonies reached 2~4 cm diameter in seven days, at first yellowish but later brownish. The mycelia and spores were closely similar to the morphological descriptions of Aspergillus section Cremei. Genotypic identification of the strain was authenticated by the fungal ITS and beta-tubulin gene partial sequences. After BLAST search in the GenBank database and some of the closely related species selected, the phylogenetic trees were constructed using the MEGA6.0 software [20]. The neighbor-joining tree based on fungal ITS sequence (Figure 2A) showed the clustering of strain SD317 within the A. dimorphicus and A. wentii, which could not be distinguished separately [21]. However, the phylogenetic tree based on beta-tubulin gene sequence (Figure 2B) showed that strain SD317 was assigned to the A. dimorphicus, which is a distinct species [22]. The beta-tubulin gene sequence of strain SD317 has the highest identity value (99%) with several A. dimorphicus strains. Strains of A. wentii have been reported to produce important metabolites, like aflatoxin, emodin, and wentilactone [11,23]. Extrolite analysis showed that strain SD317 could also produce wentilactones and emodin related derivatives (Figure 3). Based on these combined morphological, molecular, and extrolite porfiles, this marine fungus from deep-sea sediment was identified as Aspergillus dimorphicus.
Figure 1.
Morphological characteristics of the fungal strain SD317. Colonies on agar media (A) CYA; (B) MEA; (C) YES; (D–F) conidiophores. Bar, 10 μm.
Figure 2.
Phylogenetic trees of the fungal strain SD317 and related Aspergillus spp. (A) Neighbor-joining tree based on the fungal ITS rDNA sequence; (B) Neighbor-joining tree based on the beta-tubulin gene sequence. The Kimura two-parameter method was used, with the evolutionary distances bar illustrated and the bootstrap values above the branches.
Figure 3.
HPLC profiles of the crude extracts of A. dimorphicus cultures. (A) Extracts under shaking cultures; (B) Extracts under static cultures; the structures and peaks of WA and WB are shown with the UV spectrum.
Aspergillus dimorphicus was originally reported to be isolated from Indian soils [17]. But after that there were only a few descriptions, some strains even deposited in the GenBank with the name Aspergillus aff. dimorphicus [24]. This might due to its difficulty in identification without molecular methods. Some early studies suggested that A. dimorphicus might be a synonym of A. wentii, which have similar morphological and extrolite characteristics [21]. However, phylogenetic analysis of Aspergillus species using DNA sequences from four loci indicated that A. dimorphicus was a distinct species [22]. As to genotypic identification of A. dimorphicus, it could be easily separated from the closely related species A. wentii by more than one gene loci. To our knowledge, this is the first characterization of A. dimorphicus from the deep-sea marine environment.
2.2. Quantitative Analysis of Wentilactone Production by HPLC
In order to detect these wentilactones’ production in this fungus, an HPLC analysis method was developed for the identification and quantification. The standard calibration curves method was used since we have obtained pure wentilactones [14]. Separations were achieved using conventional C18 column with UV detection at 200~400 nm (254 nm for quantification). The mobile phase consists of water and methanol, with methanol varying from 10% to 100% over 40 min (Figure 3). The current method was specific and suitable for routine analysis due to the simplicity, accuracy, and reproducibility. These wentilactones in the crude extracts were determined by this HPLC method for quantitative analysis. There were few reports of HPLC method for norditerpenoid quantification in fungus [11,25]. This method developed in our lab might be useful for quantitative detection of other related tetranorlabdane diterpenoids with similar chromophores.
2.3. Effects of Environmental Factors on Wentilactone Production
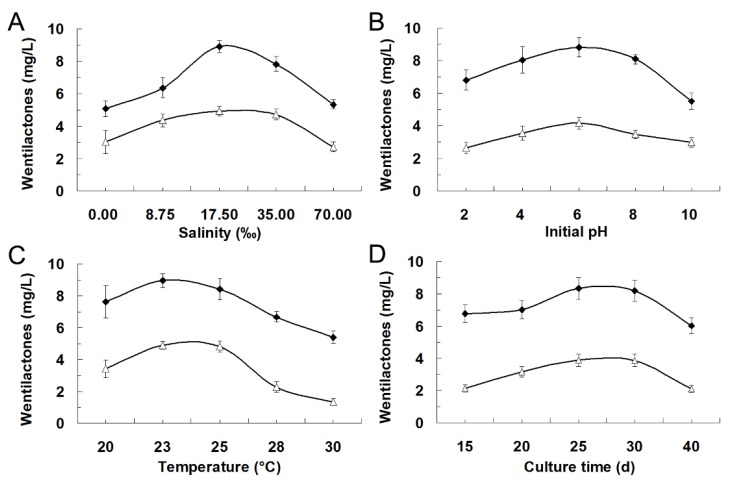
Previously, we found that these wentilactones could only be detected in static cultures (Figure 3). Almost no detectable wentilactones were produced in shaking cultures, which might be silent under these culture conditions. Therefore, different environmental factors were tested to get the optimal culture conditions. During our previous study, this fungus could grow and produce spores well in the modified PDB medium. It could also yield the largest biomass and total extracts. So the modified PDB medium was used for following static fermentation [14]. Then, a series of experiments were carried out with various salinity, initial pH, temperature, and culture time to determine their effects on wentilactone production [26]. As shown in Figure 4A–D, the salinity (17.5‰~35.0‰), initial pH (6~8), temperature (23~25 °C), and culture time (25~30 days), were better for wentilactone production. Salinity might be the main factor that affects the secondary metabolism of marine fungi, which is different from the terrestrial environment [27]. The pH value also affects the biosynthesis and accumulation of fungal products during fermentation [28]. The culture temperature might affect the biosynthesis of wentilactones by fungal growth [17]. Wentilactone production accumulated to high level at later stages, which is consistent with other fungal secondary metabolites.
Figure 4.
Effects of environmental factors on the wentilactones production. WA (♦); WB (△). (A) Salinity; (B) initial pH; (C) temperature; and (D) culture time.
2.4. Optimization of Wentilactone Production by RSM
Based on the results of single-factor experiments, three fermentation factors (initial pH, salinity and culture time) have significant influence on wentilactone production. The effects of initial pH, salinity, and culture time on wentilactone production were further optimized by using RSM. The actual values and the corresponding coded levels of factors and results for each independent variable are given in Table 1. The statistical software Design Expert (Version 8.0.6) was applied to carry out quadratic polynomial regression fitting.
Table 1.
Box-Behnken design of three factors and three levels and the experimental results.
| Runing Order | Variables and Their Coded Values | Yields (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | Salinity (‰) | Cluture Time (d) | Ya (WA) | Yb (WB) | ||||
| Code X1 | X1 | Code X2 | X2 | Code X3 | X3 | |||
| 1 | −1 | 4.0 | −1 | 0.0 | 0 | 25 | 5.16 | 1.26 |
| 2 | 1 | 8.0 | 1 | 35.0 | 0 | 25 | 12.28 | 6.21 |
| 3 | −1 | 4.0 | 1 | 35.0 | 0 | 25 | 6.28 | 3.49 |
| 4 | 0 | 6.0 | 1 | 35.0 | 1 | 30 | 10.74 | 5.64 |
| 5 | −1 | 4.0 | 0 | 17.5 | −1 | 20 | 5.66 | 2.80 |
| 6 | 0 | 6.0 | 0 | 17.5 | 0 | 25 | 12.17 | 5.77 |
| 7 | 0 | 6.0 | −1 | 0.0 | 1 | 30 | 5.96 | 2.97 |
| 8 | 1 | 8.0 | 0 | 17.5 | 1 | 30 | 11.92 | 6.53 |
| 9 | 0 | 6.0 | −1 | 0.0 | −1 | 20 | 3.43 | 1.50 |
| 10 | 1 | 8.0 | −1 | 0.0 | 0 | 25 | 7.00 | 3.16 |
| 11 | 0 | 6.0 | 0 | 17.5 | 0 | 25 | 13.15 | 6.55 |
| 12 | 0 | 6.0 | 0 | 17.5 | 0 | 25 | 12.93 | 5.83 |
| 13 | −1 | 4.0 | 0 | 17.5 | 1 | 30 | 9.09 | 4.81 |
| 14 | 1 | 8.0 | 0 | 17.5 | −1 | 20 | 8.14 | 3.62 |
| 15 | 0 | 6.0 | 1 | 35.0 | −1 | 20 | 6.44 | 3.16 |
2.4.1. Response Surface Analysis of WA
The regression equation describing the relationship between the WA production and the test variables in coded units is:
| Ya = 12.75 + 1.64X1 + 1.77X2 + 1.75X3 + 1.04X1X2 + 0.44X2X3 − 1.50X12 − 3.56X22 − 2.54X32 | (1) |
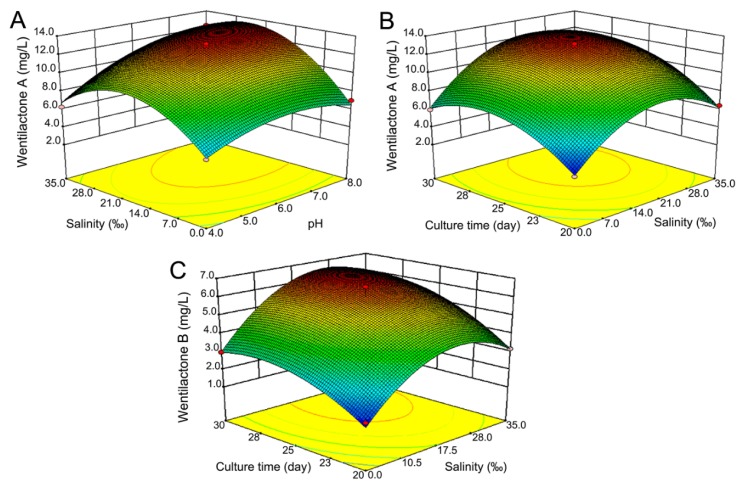
where Ya is the predicted response of WA production; X1, X2, and X3 are the coded values of pH, salinity, and culture time, respectively. The AVONA analysis results of WA were shown in Table 2. The model F-value of 67.53 and p-value of 0.0012 indicate that model terms are significant. The lack of fit, with the F-value of 1.02, is insignificant. The coefficient of determination (R2) was calculated as 0.9890 for the WA production, indicating that the statistical model can explain 98.90% of variability in the response (Table 2). Adequate precision measures the signal-to-noise ratio. An adequate precision of 22.764 for the WA production was recorded. The predicted R2 value is in reasonable agreement with the value of adjusted R2. This indicates good agreement between the experimental and predicted values for WA production. Thus it could be applied to predict the theoretical WA production. The results of the regression parameter estimate indicated that linear and square terms of pH (X1), salinity (X2), and culture time (X3) values, as well as the interaction term of pH (X1) and salinity (X2) were highly significant (p < 0.05). The relation between factors and WA production was indicated by the 3D surface (Figure 5A,B).
Table 2.
ANOVA for response surface reduced quadratic model analysis of variance table for WA.
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 146.79 | 8 | 18.35 | 67.53 | <0.0001 |
| X1 | 21.64 | 1 | 21.64 | 79.63 | 0.0001 |
| X2 | 25.15 | 1 | 25.15 | 92.57 | <0.0001 |
| X3 | 24.61 | 1 | 24.61 | 90.58 | <0.0001 |
| X1X2 | 4.32 | 1 | 4.32 | 15.90 | 0.0072 |
| X2X3 | 0.78 | 1 | 0.78 | 2.89 | 0.1403 |
| X12 | 25.93 | 1 | 8.36 | 30.76 | 0.0015 |
| X22 | 86.86 | 1 | 46.92 | 172.69 | <0.0001 |
| X32 | 23.86 | 1 | 23.68 | 87.82 | <0.0001 |
| Residual | 1.63 | 6 | 0.27 | ||
| Lack of fit | 1.30 | 4 | 0.27 | 1.02 | 0.5484 |
| Pure error | 0.53 | 2 | 0.27 | ||
| Cor. total | 148.42 | 14 |
Figure 5.
Response surface plots for maximal production of wentilactones. WA: (A) pH and salinity; (B) salinity and culture time; WB: (C) salinity and culture time. The Box-Behnken design of three factors and three levels and experimental results are shown in Table 1.
2.4.2. Response Surface Analysis of WB
The regression equation describing the relationship between the WB production and the test variables in coded units is:
| Yb = 6.05 + 0.90X1 + 1.2X2 + 1.11X3 + 0.25X2X3 − 0.70X12 − 1.82X22 − 0.91X32 | (2) |
where Yb is the predicted response of WB production; X1, X2, and X3 are the coded values of pH, salinity, and culture time, respectively. The AVONA analysis results for WB were shown in Table 3. The model F-value (28.64) and p-value (0.0001) indicate that model terms are significant. The lack of fit (F-value 1.23) is insignificant. The R2 was calculated as 0.9663 for the WB production, indicating that the statistical model can explain 96.63% of variability in the response. An adequate precision of 15.248 for WB production was recorded. The results of the regression parameter estimate indicated that linear and square terms of pH (X1), salinity (X2), and culture time (X3) values were highly significant (p < 0.05), but the interaction terms of the three factors showed less significant effects (p > 0.05) on the WB production. The relation between factors and WB production was indicated by the 3D surface (Figure 5C).
Table 3.
ANOVA for response surface reduced quadratic model analysis of variance table for WB.
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 43.54 | 7 | 6.22 | 28.64 | 0.0001 |
| X1 | 6.42 | 1 | 6.42 | 29.57 | 0.0010 |
| X2 | 11.57 | 1 | 11.57 | 53.27 | 0.0002 |
| X3 | 9.87 | 1 | 9.87 | 45.44 | 0.0003 |
| X2X3 | 0.26 | 1 | 0.26 | 1.19 | 0.3121 |
| X12 | 1.80 | 1 | 1.80 | 8.30 | 0.0236 |
| X22 | 12.26 | 1 | 12.26 | 56.46 | 0.0001 |
| X32 | 3.07 | 1 | 3.07 | 14.11 | 0.0071 |
| Residual | 1.52 | 7 | 0.22 | ||
| Lack of fit | 1.15 | 5 | 0.23 | 1.23 | 0.5047 |
| Pure error | 0.37 | 2 | 0.19 | ||
| Cor. total | 45.06 | 14 |
By solving the equations, the optimum conditions of cultivation could be initial pH 7.3, salinity 24.5‰, and culture time 27 days. The theoretical highest production of WA and WB could be obtained at 13.9 and 6.9 mg/L. The verification test was done after repeated experiments employing the optimized cultivation conditions. The average actual production of WA and WB reached to 13.4 and 6.5 mg/L, respectively. The results were further verified three times by fermentation scale-up for wentilactone production, each time with 400 flasks totally about 100 L. The purified compounds WA and WB were used for further anti-tumor mechanism studies [2,15]. Therefore, the RSM optimized production of wentilactones was feasible and effective. These might be valuable for fermentation conditions for other norditerpenoid production.
2.5. Elicitation of Wentilactone Production by Small Molecules
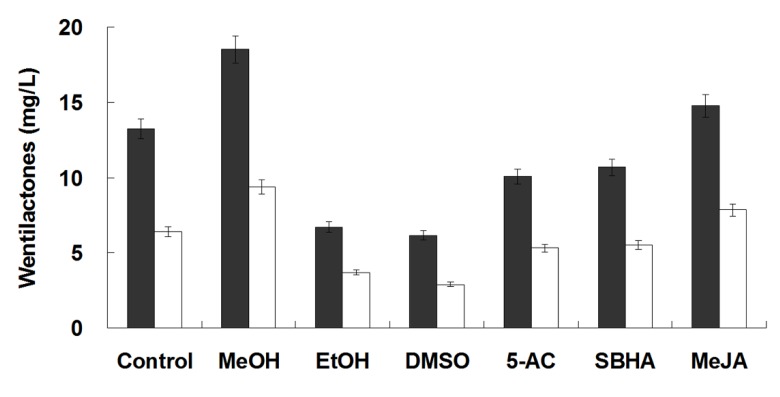
Three organic solvents (methanol, ethanol, and DMSO) with 3% final concentration, DNA methyltransferase inhibitor 5-AC, histone deacetylase inhibitor SBHA, and plant hormone MeJA with 100 μM were added to the culture for their effects on wentilactone production. With the treatment of 3% methanol on day 15, the production of WA and WB increased to 18.5 and 9.3 mg/L, respectively (Figure 6). Wentilactone yield increased slightly by feeding with MeJA, while a slight decrease was observed with the addition of ethanol, DMSO, 5-AC, and SBHA. Chemical elicitation using small molecules is proving to be an effective way for enhanced production and activation of silent microbial secondary metabolites. Some organic solvents may induce stress response and increases the synthesis of antibiotics [29]. The DNA methyltransferase and histone deacetylase inhibitors might activate unknown cryptic metabolic pathways by epigenetic modifications [16]. Rational design of the optimal time and concentration for the induction of secondary metabolites by addition of small-molecule elicitors still needs detailed investigation. The specific elicitation mechanisms and signal transduction pathways are very interesting for further study.
Figure 6.
Effects of small-molecule elicitors on the wentilactones production. WA (■); WB (□). Elicitors: MeOH (methanol, 3%), EtOH (ethanol, 3%), DMSO (dimethylsulfoxide, 3%), 5-AC (5-azacytidine, 100 μM), SBHA (suberohydroxamic acid, 100 μM), MeJA (methyl jasmonate, 100 μM).
3. Experimental Section
3.1. Fungal Strain and Chemicals
The fungus Aspergillus dimorphicus SD317 was isolated from deep-sea sediment (South China Sea 2038 m). The fungal ITS (KR732649) and beta-tubulin gene (KR732650) partial sequences of strain SD317 were deposited at the GenBank. The modified Potato Dextrose Broth (PDB) medium was used for liquid static fermentation as described previously [14]. All chemicals used were of analytical grade and commercially available.
3.2. Identification of Fungal Strain by Polyphasic Approach
For macro-morphological observations, Czapek Yeast Autolysate (CYA), Malt Extract Autolysate (MEA) agar, and Yeast Extract Sucrose (YES) agar media were used. The isolates were inoculated at three points on each plate of each medium and incubated at 25 °C in the dark for seven days [30]. For micro-morphological observations, microscopic mounts were made in lactic acid from MEA colonies and a drop of alcohol was added to remove air bubbles. Fungal materials were examined using light microscopy (Zeiss Axio Imager 2, Jena, Germany). For genotypic analysis, fungal DNA was extracted using Genomic DNA purification kit (TIANGEN, Beijing, China) according to the instructions. The fungal ITS and beta-tubulin genes were amplified by PCR and sequenced as described previously [30]. After BLAST search of the GenBank database (blast.ncbi.nlm.nih.gov), selected sequences alignment was performed using the CLUSTAL W. Phylogenetic analyses were conducted in MEGA6.0 with the Neighbor-joining method and the Kimura two-parameter model [20]. The bootstrap test was performed with 1000 replications and indicated as percentages. For extrolite analysis, the isolates were grown on CYA and YES at 25 °C for seven days. The extracts were filtered and analyzed by HPLC using UV-VIS detection as described previously [30].
3.3. Fungal Fermentation and Metabolite Extraction
Spore suspension was inoculated on Potato Dextrose Agar (PDA) plate and cultured at 28 °C for seven days. The fresh mycelia were inoculated into 1 L flasks containing modified PDB liquid medium. Then the incubated flasks were cultured under different conditions for wentilactone production. After static fermentation, the mycelia and culture broth of were separated and exhaustively extracted with acetone and ethyl acetate, respectively. The combined extracts were subjected to ultrasonic disruption. The metabolites extraction procedure was carried out as described previously [14].
3.4. Quantification of Wentilactone Production by HPLC Analysis
The fungal metabolites were analyzed by HPLC (Dionex, Sunnyvale, USA) with C18 column (4.6 × 250 mm, 5 μm) and UV-DAD detector (200–600 nm) at 254 nm. A mixture of water and methanol (0~5 min 10% methanol, 5~40 min 10%~100% methanol, 40~50 min 100% methanol) was the mobile phase at a flow rate of 1.0 mL/min. For wentilactone quantification, purified samples (1.0 mg) were dissolved in 1.0 mL of methanol as standard stock solutions, diluted, and analyzed by HPLC using standard calibration curves [14].
3.5. Effects of Environmental Factors on Wentilactone Production
The effects of various environmental factors on wentilactone production were studied to determine the optimal culture conditions [26]. A series of experiments were carried out with different salinity (0‰, 8.75‰, 17.5‰, 35.0‰, and 70.0‰), initial pH (2, 4, 6, 8, and 10), temperature (20, 23, 25, 28, and 30 °C), and culture time (15, 20, 25, 30, and 40 days). The extraction procedure, metabolite analyses, and production of wentilactones were performed and determined using the methods described above [14]. All the experiments were performed in triplicate, and the mean values ± standard deviations (SD) were used.
3.6. Optimization of Wentilactone Production by RSM
The RSM and Box-Behnken design were employed in further fermentation optimization [19]. According to the results of single-factor experiments, three fermentation factors (initial pH, salinity, and culture time) have significant influence on wentilactone production. Considering the feasible conditions for large scale static fermentation in the lab, a three factor and three level experiment was designed. The actual values and the corresponding coded levels of factors and results for each independent variable are given in Table 1. The experiment consisted of 15 trials including three center points, and the value of dependent response was the mean of three independent experiments. The response variable obtained using RSM was fitted to the second order polynomial equation:
| Yi = β0+ ∑βixi + ∑βiixi2+ ∑βijxixj | (3) |
where Yi is the predicted response; xi and xj are input variables which affect the response variable Y; β0 is the offset term; βi is the ith linear coefficient; βii is the ith quadratic coefficient; βij is the ijth interaction coefficient. The second order polynomial coefficients were calculated and analyzed using the statistical software package Design Expert (Version 8.0.6, Stat-Ease Inc., Minneapolis, MN, USA). Statistical analysis of the model was performed by the analysis of variance (ANOVA). All the experiments were performed in triplicates, and the mean values ± SD were used.
3.7. Effects of Small-Molecule Elicitors on Wentilactone Production
The effects of different small-molecule elicitors on wentilactone production were also detected during static fermentation under optimized culture conditions [26,29]. Various small-molecule elicitors, including methanol (MeOH 3%), ethanol (EtOH 3%), dimethylsulfoxide (DMSO, 3%), 5-azacytidine (5-AC, 100 μM), suberohydroxamic acid (SBHA, 100 μM), methyl jasmonate (MeJA, 100 μM), were introduced on day 0 or 15 to stimulate wentilactone production. The extraction and metabolite analyses were performed using the methods described above, and wentilactone production was determined by HPLC for quantification.
4. Conclusions
In summary, the newly isolated marine fungus Aspergillus dimorphicus was identified using a polyphasic approach, combining phenotypic, molecular, and extrolite profiles. Production of the potential anti-tumor agent wentilactones was determined by HPLC quantitatively. The optimal static fermentation conditions were: modified PDB 250 mL/1 L flasks, initial pH 7.3, salinity 24.5‰, culture time 27 days, temperature 23 °C, with the production of WA and WB increased to 13.4 and 6.5 mg/L, respectively. The result was also verified by fermentation scale-up for wentilactone production at room temperature (23 ± 1 °C) and natural light conditions. Furthermore, some small-molecule elicitors could also stimulate wentilactone production, especially with the addition of 3% methanol. To the best of our knowledge, this is the first report of optimized production of tetranorditerpenoids by a deep-sea derived marine fungus. These are valuable for druggability assessment and anti-tumor mechanism studies of these tetranorditerpenoids. Further research would be performed by directed mutations and metabolic engineering to investigate the molecular regulation mechanism of how silent diterpenoids biosynthesis might be activated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31200042 and 31570038) and the Ministry of Science and Technology of China (2012AA092104). We would like to thank Yanchun Yan and Ruth Nahurir of the Chinese Academy of Agricultural Sciences for their kind help in the revision of our manuscript.
Author Contributions
R.X. and G.-M.X. conceived and designed the experiments; G.-M.X. performed the marine fungi isolation and characterization; R.X. and G.-M.X. performed the fermentation and data analyses; X.-M.L. and B.-G.W. contributed reagents, materials, and analysis tools; C.-S.L. and B.-G.W. made a substantial contribution to the design of the experiments and gave suggestions in drafting the manuscript. B.-G.W. revised it critically and gave final approval of the version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lv C., Hong Y., Miao L., Li C., Xu G., Wei S., Wang B., Huang C., Jiao B. Wentilactone A as a novel potential antitumor agent induces apoptosis and G2/M arrest of human lung carcinoma cells, and is mediated by HRas-GTP accumulation to excessively activate the Ras/Raf/ERK/p53-p21 pathway. Cell Death Dis. 2013;4:e952. doi: 10.1038/cddis.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J.D., Roberts L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Miao L., Sun W., Jiao B., Wang B., Yao L., Huang C. Wentilactone B from Aspergillus wentii induces apoptosis and inhibits proliferation and migration of human hepatoma SMMC-7721 cells. Biol. Pharm. Bull. 2012;35:1964–1971. doi: 10.1248/bpb.b12-00368. [DOI] [PubMed] [Google Scholar]
- 5.Chabner B.A., Roberts T.G.J. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 6.Ajikumar P.K., Xiao W.H., Tyo K.E., Wang Y., Simeon F., Leonard E., Mucha O., Phon T.H., Pfeifer B., Stephanopoulos G. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong Z.Q., Yang Y.Y., Zhao N., Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 2013;13:71. doi: 10.1186/1471-2180-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes N., Lefranc F., Kijjoa A., Kiss R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar. Drugs. 2015;13:3950–3991. doi: 10.3390/md13063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromann K., Toivari M., Viljanen K., Vuoristo A., Ruohonen L., Nakari-Setala T. Identification and characterization of a novel diterpene gene cluster in Aspergillus nidulans. PLoS ONE. 2012;7:e35450. doi: 10.1371/journal.pone.0035450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007;3:387–395. doi: 10.1038/nchembio.2007.8. [DOI] [PubMed] [Google Scholar]
- 11.Dorner J.W., Cole R.J., Springer J.P., Cox R.H., Cutler H., Wicklow D.T. Isolation and identification of two new biologically-active norditerpene dilactones from Aspergillus wentii. Phytochemistry. 1980;19:1157–1161. doi: 10.1016/0031-9422(80)83075-5. [DOI] [Google Scholar]
- 12.Barrero A.F., Herrador M.M., Quilez del Moral J.F., Valdivia M.V. A convenient synthesis of a-ring-functionalized podolactones. Revision of the structure of wentilactone B. Org. Lett. 2002;4:1379–1382. doi: 10.1021/ol0257070. [DOI] [PubMed] [Google Scholar]
- 13.Wang B.G., Gloer J.B., Ji N.Y., Zhao J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013;113:3632–3685. doi: 10.1021/cr9002215. [DOI] [PubMed] [Google Scholar]
- 14.Sun H.F., Li X.M., Meng L., Cui C.M., Gao S.S., Li C.S., Huang C.G., Wang B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012;75:148–152. doi: 10.1021/np2006742. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z., Miao L., Lv C., Sun H., Wei S., Wang B., Huang C., Jiao B. Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells. Cell Death Dis. 2013;4:e657. doi: 10.1038/cddis.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao F.-P., Liang X.-R., Liu X.-H., Ji N.-Y. Aspewentins A–C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod. 2014;77:429–432. doi: 10.1021/np401047w. [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra B.S., Prasad R. Aspergillus dimorphicus and Emericella cleisto-minuta spp. nov. from Indian soils. Trans. Br. Mycol. Soc. 1969;52:331–336. doi: 10.1016/S0007-1536(69)80047-1. [DOI] [Google Scholar]
- 18.Box G.E.P., Hunter W.G., Hunter J.S.B. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. Wiley; New York, NY, USA: 1978. [Google Scholar]
- 19.Zhu Y.X., Yao L.Y., Jiao R.H., Lu Y.H., Tan R.X. Enhanced production of Fumigaclavine C in liquid culture of Aspergillus fumigatus under a two-stage process. Bioresour. Technol. 2014;152:162–168. doi: 10.1016/j.biortech.2013.10.089. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson S.W. Phylogenetic analysis of Aspergillus sections Cremei and Wentii, based on ribosomal DNA-sequences. Mycol. Res. 1995;99:1349–1355. doi: 10.1016/S0953-7562(09)81220-3. [DOI] [Google Scholar]
- 22.Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100:205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- 23.Wells J.M., Cole R.J., Kirksey J.W. Emodin, a toxic metabolite of Aspergillus wentii isolated from weevil-damaged chestnuts. Appl. Microbiol. 1975;30:26–28. doi: 10.1128/am.30.1.26-28.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daynes C.N., Zhang N., Saleeba J.A., McGee P.A. Soil aggregates formed in vitro by saprotrophic Trichocomaceae have transient water-stability. Soil Biol. Biochem. 2012;48:151–161. doi: 10.1016/j.soilbio.2012.01.010. [DOI] [Google Scholar]
- 25.Li W.K., Fitzloff J.F. HPLC-PDA determination of bioactive diterpenoids from plant materials and commercial products of Andrographis paniculata. J. Liq. Chromatogr. Relat. Technol. 2004;27:2407–2420. doi: 10.1081/JLC-200028162. [DOI] [Google Scholar]
- 26.Pu X., Qu X., Chen F., Bao J., Zhang G., Luo Y. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 2013;97:9365–9375. doi: 10.1007/s00253-013-5163-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Zheng J., Liu P., Wang W., Zhu W. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar. Drugs. 2011;9:1368–1378. doi: 10.3390/md9081368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen M.R., Lehmann L., Nielsen J. Systemic analysis of the response of Aspergillus niger to ambient pH. Genome Biol. 2009;10:R47. doi: 10.1186/gb-2009-10-5-r47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettit R.K. Small-molecule elicitation of microbial secondary metabolites. Microb. Biotechnol. 2011;4:471–478. doi: 10.1111/j.1751-7915.2010.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samson R.A., Peterson S.W., Frisvad J.C., Varga J. New species in Aspergillus section Terrei. Stud. Mycol. 2011;69:39–55. doi: 10.3114/sim.2011.69.04. [DOI] [PMC free article] [PubMed] [Google Scholar]