Abstract
The results from epidemiological studies between dietary vitamin A intake and glioma risk is not consistent. Thus, a meta-analysis was conducted to confirm the exact relationship between them. PubMed and Web of Knowledge were used to search the relevant articles up to May 2015. Pooled relative risk (RR) with 95% confidence interval (CI)was calculated using random-effect model. Egger’s test was used to assess the small-study effect. At the end, seven articles with eight case-control studies involving 1841 glioma cases and 4123 participants were included. Our study indicated that highest category of dietary vitamin A intake was significantly associated with reduced risk of glioma (RR = 0.80, 95% CI = 0.62–0.98, p = 0.014, I2 = 54.9%). Egger’s test did not find any publication bias. In conclusion, our study indicated that higher category of dietary vitamin A intake could reduce the glioma risk. However, we could not do a dose-response analysis for vitamin A intake with glioma risk due to the limited data in each reported individual article. Due to this limitation, further studies with detailed dose, cases and person-years for each category is wanted to assess this dose-response association.
Keywords: vitamin A, glioma, meta-analysis
1. Introduction
Approximately 70% of adult brain tumors are glioma, and it is the most common primary brain tumor that occurs most frequently in brain among adults [1,2]. Two recent meta-analyses had been published to confirm the associations between vitamin C [3] and vitamin E [4] and glioma risk. The results concluded that higher category of dietary vitamin C intake could reduce the glioma risk and dietary vitamin E intake could not affect the glioma risk. Dietary antioxidants, including vitamin A and vitamin C intake, have been shown in laboratory studies to enhance growth restriction of cancer cells in general [5] and glioma cells in particular [6,7]. Vitamin A, vitamin C, and vitamin E are all antioxidant nutrients. And they may influence the process since their being free radical scavengers, indicating that they could play an important role in glioma prevention. To date, many epidemiological studies were conducted to explore the relationship between vitamin A intake and glioma risk. Two studies obtain an inverse association between dietary vitamin A intake and glioma risk [8,9], while five studies found a negative association between them [10,11,12,13,14]. Furthermore, Giles et al. found an increased risk of glioma in males with higher category of dietary vitamin A intake [11]. Considering the results are not consistent, we therefore conduct this comprehensive meta-analysis to assess the association between them.
2. Methods
2.1. Search Strategy
The relevant articles were searched using the databases of PubMed [15] and Web of Knowledge [16] up to May 2015. We also reviewed the computer retrieved studies for reference lists by hand-searching. We used the following search terms: “glioma” or “brain cancer” combined with “vitamin A” or “antioxidants” or “lifestyle” or “diet”. Two investigators (Wen Lv and Xian Zhong) searched the relevant articles and independently reviewed all retrieved studies.
2.2. Inclusion Criteria
The following inclusion criteria were used: (1) the studies were using a prospective design or a case-control design; (2) the exposure of interest was dietary vitamin A intake; (3) the ending outcome was glioma; (4) odds ratio (OR) or relative risks (RR) and their 95% confidence intervals (CI) were available for highest category of dietary vitamin A vs. lowest category of dietary vitamin A intake.
2.3. Data Extraction
Two researchers (Wen Lv and Xian Zhong) extracted the following information from the included study independently: the last name of the first author’s, publication years, geographic locations of the study, study design, sample source, the age for cases and participants, the number of cases and person-years, and RR (95% CI) for the highest category of dietary vitamin A intake versus lowest category of vitamin A intake. The multivariable adjustment RR (95% CI) was used from each reported study if possible.
2.4. Statistical Analysis
The multivariate adjusted RR with 95% CI for highest category of vitamin A vs. lowest category for the glioma risk was pooled using a random-effects model [17]. The I2 of Higgins and Thompson [18] was used to assess the between-study heterogeneity. I2 described the proportion of total variation attributable to between-study heterogeneity, and I2 values of 0, 25%, 50% and 75% represent no, low, moderate, and high heterogeneity, respectively [19]. Meta-regression and subgroup analyses (geographic locations and study design) were performed to explore the potential between-study heterogeneity [20]. Egger’s test was used to evaluate the small-study effect [21]. A sensitivity analysis was conducted to describe if the pooled RR lies outside the 95% CI when removal of one individual studies at a time [22]. If so, then the one study was considered to be an impact on the overall result. We used STATA software, version 10.0 to analyze all the data (StataCorp LP, College Station, TX, USA). Two-tailed p ≤ 0.05 was accepted as statistically significant.
3. Results
3.1. Characteristics of Included Studies
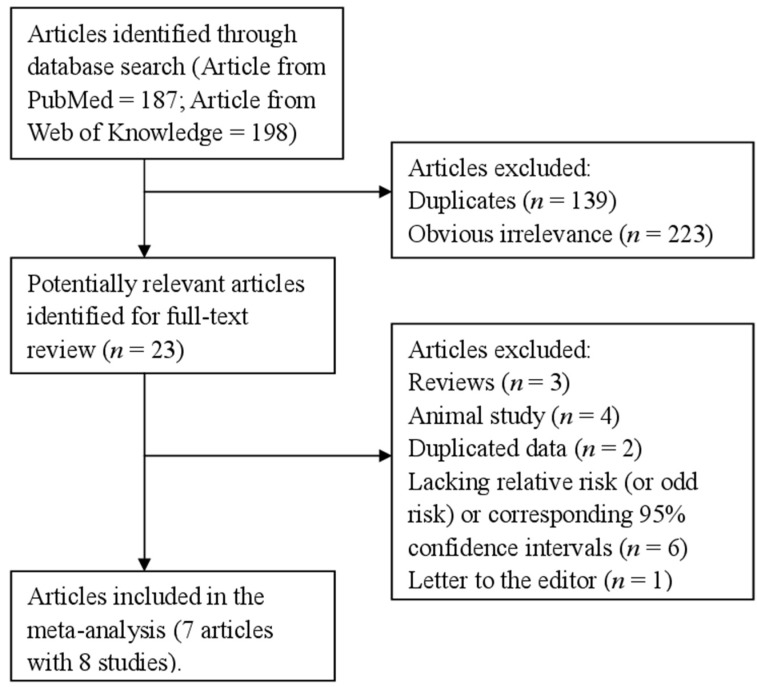
We identified 385 articles from all the databases, and twenty-three articles were reviewed in full after reviewing the title/abstract. Sixteen studies were further excluded from this analysis for various reasons showed in Figure 1. Therefore, seven articles [8,9,10,11,12,13,14] with 8 case-control studies comprising 1841 glioma cases and 4123 participants were used in this study. Table 1 showed the characteristics of the included studies. Five studies were conducted in United States, two studies in Australia, and one study in China.
Figure 1.
The detailed steps of our literature search.
Table 1.
Characteristics of studies on vitamin A intake and glioma risk.
| First Author, Year | Country | Study Design | Cases, Age (year) | Comparison Groups | Dietary Assessment | RR (95% CI) for Highest versus Lowest Category | Adjustment or Matched for |
|---|---|---|---|---|---|---|---|
| Bunin et al. 1994 [10] | United States | Case-control (PCC) | 155, <6 | Quartile 4 vs. Quartile 1 | FFQ | 0.70 (0.30–1.40) | Adjusted for income level. |
| Giles et al. 1994 [11] | Australia | Case-control (PCC) | 416, 20–70 | Tertile 3 vs. Tertile1 | FFQ | Females: 0.79 (0.42–1.48); Males: 1.67 (1.04–2.68) | Adjusted for alcohol and tobacco. |
| Blowers et al. 1997 [12] | United States | Case-control (PCC) | 94, 25–74 | Quartile 4 vs. Quartile 1 | FFQ | 0.70 (0.30–1.90) | Matched the patient on age (within five years) and race (Black or White). |
| Hu et al. 1999 [13] | China | Case-control (HCC) | 73, 20–74 | Quartile 4 vs. Quartile 1 | FFQ | 0.38 (0.10–1.60) | Matched to each case by sex, age within five-year intervals and area of residence (same or adjacent city and country). |
| Chen et al. 2002 [8] | United States | Case-control (PCC) | 236, ≥21 | Quartile 4 (mean = 5078) vs. Quartile 1 (mean = 1269.5) | FFQ | 0.50 (0.30–0.80) | Adjusting for age, age squared, gender, total energy intake, respondent type, education level, family history, and farming experience. |
| Tedeschi-Blok et al. 2006 [9] | United States | Case-control (PCC) | 802, ≥20 | Quartile 4 (mean = 5512) vs. Quartile 1 (mean = 1378) | FFQ | 0.72 (0.54–0.98) | Adjusted for age, gender, ethnicity, SES, total calories, and supplement use |
| DeLorenze et al. 2010 [14] | United States | Case-control (PCC) | 72, ≥20 | Tertile 3 (mean = 3757.6) vs. Tertile 1 (mean = 257.7) | FFQ | 0.97 (0.66–1.41) | Adjusted for reporting status, age at diagnosis, treatment, education, marital status, total calories, pack years, and age at alcoholic drink. |
Abbreviations: RR: relative risk; CI: confidence intervals; PCC: population-based case–control study; HCC: hospital-based case–control study; FFQ: food-frequency questionnaire; SES: socio-economic status.
3.2. Vitamin A and Glioma
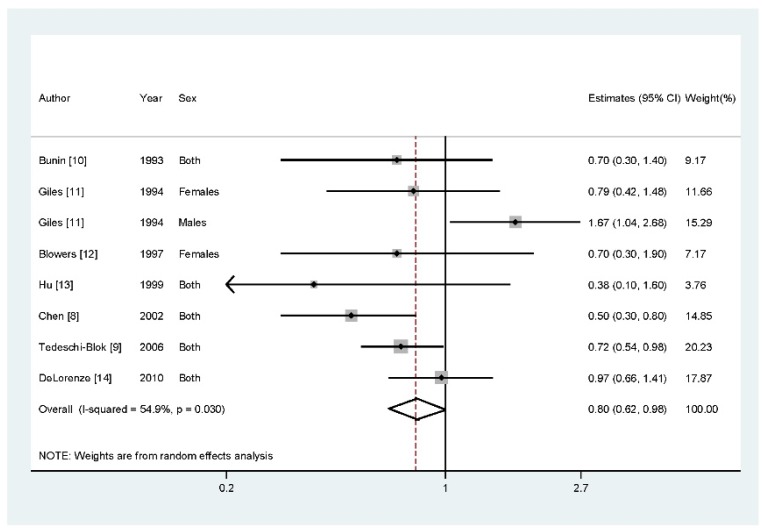
Data from seven articles with eight studies involving 1841 glioma cases were included in this study. Two studies reported that higher vitamin A intake could reduce the risk of glioma, while five studies found a negative association between them. However, there is one study found an increased risk of glioma with higher category of vitamin A intake. Results from our study suggested that highest category of dietary vitamin A intake versus lowest category could reduce the glioma risk (RR = 0.80, 95% CI = 0.62–0.98, p = 0.014, I2 = 54.9%) (Figure 2).
Figure 2.
The forest plot between highest versus lowest categories of vitamin A intake and glioma risk.
3.3. Meta-Regression
Moderate of heterogeneity (I2 = 54.9%, pheterogeneity = 0.030) was detected in our results. We then used meta-regression with the covariates of publication years, geographic locations where the study conducted, cases and source of controls to explore the potential heterogeneity. However, no significant finding was found in the above-mentioned analysis (the detailed results were shown in Supplement Table A1).
3.4. Influence Analysis and Publication Bias
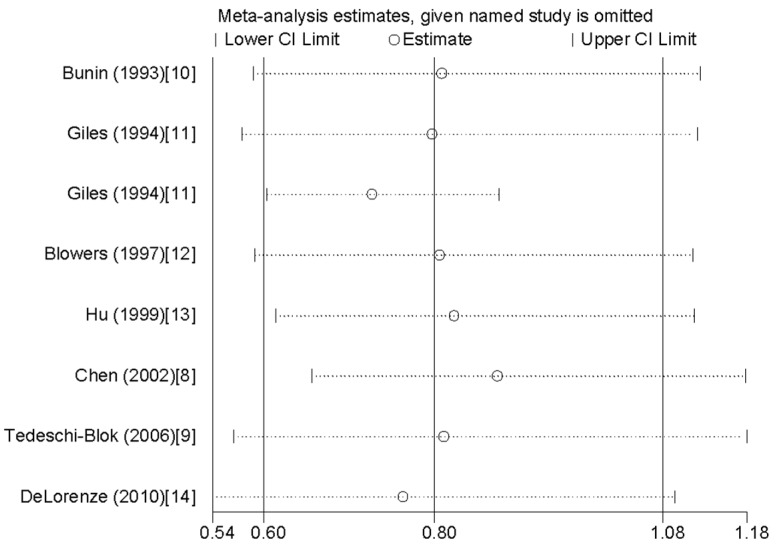
Influence analysis showed that the pooled RR did not lie out of the 95% CI when we removed one individual study at a time (Figure 3). There is no publication bias found by Egger’s regression asymmetry test (p = 0.564).
Figure 3.
Analysis of influence of individual study on the association between vitamin A intake and glioma risk. Open circle indicates the pooled relative risk, given named study is omitted. Horizontal lines represent the 95% CI.
In stratified analysis by geographic locations, highest category of dietary vitamin A intake could reduce glioma risk among American populations (RR = 0.73, 95% CI = 0.59–0.91, p < 0.001), but not in the other countries (summary RR = 0.95, 95% CI = 0.45–2.01). Seven of the included studies were population-based case-control (PBCC) studies, and only one study was hospital-based case-control (HBCC) study. We then combined the data for PBCC studies, but not for HBCC studies. And the result was only significant in PBCC studies (summary RR = 0.83, 95% CI = 0.68–0.98, p = 0.011).
4. Discussion
Findings from this study indicated that higher category of vitamin A intake may prevent against of glioma risk. The association was also significant among American populations for vitamin A intake and glioma risk.
The mechanisms of vitamin A for preventing glioma risk have not been thoroughly investigated. Previous studies had suggested that antioxidants, such as vitamin A, vitamin C and vitamin E might prevent the risk of glioma. A recent meta-analysis had reported that higher category of dietary vegetables and fruits intake could prevent against the glioma [23]. Vegetables and fruit included antioxidants vitamins, beta-carotene, fiber, and folate, this may be a protective effects of glioma prevention [24]. Vitamin A is involved in cell growth, normal synthesis of DNA methylation, and protection against oxidative stress and DNA damage.
One paper had reported that heterogeneity in the meta-analysis is common [25], and exploring the potential sources is therefore an essential component of meta-analysis. In our pooled results, moderate of heterogeneity (I2 = 54.9%, pheterogeneity = 0.030) was found. As we all know, the publication years, geographic location where the study conducted, and source of controls might arise the heterogeneity. We therefore performed meta-regression and subgroup analyses to explore the potential heterogeneity. As a result, we did not find the potential heterogeneity from the above analyses. Considering other genetic and environment variables may affect the heterogeneity, further studies with multivariate adjustment RR should assess this association.
Our meta-analysis had some advantages. To the best of our knowledge, we conducted the first comprehensive meta-analysis between dietary vitamin A intake and glioma risk based on highest level versus lowest level. Second, our meta-analysis included much more cases and participants; this may obtain a more reasonable result. Third, there is no significant publication bias tested by the Egger’ test. However, some limitations should be concerned in this study. First, the included studies were all case-control design. Case-control studies may suffer from recall bias and selection bias, but they are important methods in etiology research. Further studies original with prospective design and other design are wanted to confirm this result. Second, different measurement could affect the dietary intake. And the range of intake may be changed during the different measurement [26]. Third, we did not do the dose-response analysis due to the limited data reported in each individual study. Fourth, one study (Giles et al. 1994) [11] strongly suggested an association in the opposite way for a male study. However, we could not conduct the subgroup analysis by sex due to the limited data from each individual study, since only one study (Giles et al. 1994) [11] reported the association for males and females respectively and one study (Blowers et al. 1997) [12] reported the association for females only. Further studies should assess these associations. Fifth, different genetic aberrations are associated with susceptibility and onset of glioma. Evidences for genetic aberration leading to defects in the regulation of expression of enzymes that are involved in the biosynthesis of retinoic acid and its association with susceptibility to glioma exist [27,28]. But we could not conduct the subgroup analysis by gene and subtypes due to the limited data in each individual study. Further studies with detailed information should explore these associations. Finally, moderate between-study heterogeneity was found in our study, but the heterogeneity was not explained by subgroup analyses and meta-regression. However, other environment variables may affect this disease-effect unconformity.
5. Conclusions
In conclusions, findings from this study indicated that higher category of dietary vitamin A intake could reduce glioma risk. However, dose-response analysis was not performed between vitamin A intake and glioma risk due to the limited data reported in each individual article. Due to this limitation, further studies with detailed dose, cases, and participants (or person-years) for each category is wanted to assess this dose-response association.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number 81372621) to Weidong Han.
Appendix
Table A1.
The detailed results for the covariates by univariate meta-regression.
| Covariates | p-Value |
|---|---|
| Publication year | 0.185 |
| Geographic locations | 0.294 |
| Number of cases | 0.386 |
| Source of controls | 0.815 |
Author Contributions
Wen Lv and Xian Zhong conceived and designed the experiments; Wen Lv performed the experiments; Xian Zhong and Lingmin Xu analyzed the data; Weidong Han contributed reagents/materials/analysis tools; Wen Lv wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ricard D., Idbaih A., Ducray F., Lahutte M., Hoang-Xuan K., Delattre J.Y. Primary brain tumours in adults. Lancet. 2012;379:1984–1996. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 2.Wen P.Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S., Wang X., Tan Y., Qiu L., Fang H., Li W. Association between vitamin C intake and glioma risk: Evidence from a meta-analysis. Neuroepidemiology. 2015;44:39–44. doi: 10.1159/000369814. [DOI] [PubMed] [Google Scholar]
- 4.Qin S., Wang M., Zhang T., Zhang S. Vitamin E intake is not associated with glioma risk: Evidence from a meta-analysis. Neuroepidemiology. 2014;43:253–258. doi: 10.1159/000369345. [DOI] [PubMed] [Google Scholar]
- 5.D’Archivio M., Santangelo C., Scazzocchio B., Vari R., Filesi C., Masella R., Giovannini C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008;9:213–228. doi: 10.3390/ijms9030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoshyomn S., Nathan D., Manske G.C., Osler T.M., Penar P.L. Synergistic effect of genistein and BCNU on growth inhibition and cytotoxicity of glioblastoma cells. J. Neurooncol. 2002;57:193–200. doi: 10.1023/A:1015765616484. [DOI] [PubMed] [Google Scholar]
- 7.Pouliquen D., Olivier C., Hervouet E., Pedelaborde F., Debien E., Le Cabellec M.T., Gratas C., Homma T., Meflah K., Vallette F.M., et al. Dietary prevention of malignant glioma aggressiveness, implications in oxidant stress and apoptosis. Int. J. Cancer. 2008;123:288–295. doi: 10.1002/ijc.23513. [DOI] [PubMed] [Google Scholar]
- 8.Chen H., Ward M.H., Tucker K.L., Graubard B.I., McComb R.D., Potischman N.A., Weisenburger D.D., Heineman E.F. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes Control. 2002;13:647–655. doi: 10.1023/A:1019527225197. [DOI] [PubMed] [Google Scholar]
- 9.Tedeschi-Blok N., Lee M., Sison J.D., Miike R., Wrensch M. Inverse association of antioxidant and phytoestrogen nutrient intake with adult glioma in the San Francisco Bay Area: A case-control study. BMC Cancer. 2006;6:148. doi: 10.1186/1471-2407-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunin G.R., Kuijten R.R., Boesel C.P., Buckley J.D., Meadows A.T. Maternal diet and risk of astrocytic glioma in children: A report from the Childrens Cancer Group (United States and Canada) Cancer Causes Control. 1994;5:177–187. doi: 10.1007/BF01830264. [DOI] [PubMed] [Google Scholar]
- 11.Giles G.G., McNeil J.J., Donnan G., Webley C., Staples M.P., Ireland P.D., Hurley S.F., Salzberg M. Dietary factors and the risk of glioma in adults: Results of a case-control study in Melbourne, Australia. Int. J. Cancer. 1994;59:357–362. doi: 10.1002/ijc.2910590311. [DOI] [PubMed] [Google Scholar]
- 12.Blowers L., Preston-Martin S., Mack W.J. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA) Cancer Causes Control. 1997;8:5–12. doi: 10.1023/A:1018437031987. [DOI] [PubMed] [Google Scholar]
- 13.Hu J., La Vecchia C., Negri E., Chatenoud L., Bosetti C., Jia X., Liu R., Huang G., Bi D., Wang C. Diet and brain cancer in adults: A case-control study in northeast China. Int. J. Cancer. 1999;81:20–23. doi: 10.1002/(SICI)1097-0215(19990331)81:1<20::AID-IJC4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.DeLorenze G.N., McCoy L., Tsai A.L., Quesenberry C.P., Jr., Rice T., Il’yasova D., Wrensch M. Daily intake of antioxidants in relation to survival among adult patients diagnosed with malignant glioma. BMC Cancer. 2010;10:215. doi: 10.1186/1471-2407-10-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Databases of PubMed. [(accessed on 1 April 2015)]; Available online: http://www.ncbi.nlm.nih.gov/pubmed.
- 16.Web of Knowledge. [(accessed on 1 April 2015)]. Available online: http://apps.webofknowledge.com.
- 17.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 21.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobias A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999;8:1–48. [Google Scholar]
- 23.Li Y. Association between fruit and vegetable intake and risk for glioma: A meta-analysis. Nutrition. 2014;30:1272–1278. doi: 10.1016/j.nut.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Chang E.T., Adami H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 25.Munafo M.R., Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Prentice R.L. Dietary assessment and the reliability of nutritional epidemiology reports. Lancet. 2003;362:182–183. doi: 10.1016/S0140-6736(03)13950-5. [DOI] [PubMed] [Google Scholar]
- 27.Campos B., Weisang S., Osswald F., Ali R., Sedlmeier G., Bageritz J., Mallm J.P., Hartmann C., von Deimling A., Popanda O., et al. Retinoid resistance and multifaceted impairment of retinoic acid synthesis in glioblastoma. Glia. 2015;63:1850–1859. doi: 10.1002/glia.22849. [DOI] [PubMed] [Google Scholar]
- 28.Xu S.L., Liu S., Cui W., Shi Y., Liu Q., Duan J.J., Yu S.C., Zhang X., Cui Y.H., Kung H.F., et al. Aldehyde dehydrogenase 1A1 circumscribes high invasive glioma cells and predicts poor prognosis. Am. J. Cancer Res. 2015;5:1471–1483. [PMC free article] [PubMed] [Google Scholar]