Abstract
Mesenchymal stem cells (MSCs) are known to have a protective effect on islet cells. Cell sheets developed using tissue engineering help maintain the function of the cells themselves. This study describes a tissue engineering approach using islets with MSC sheets to improve the therapeutic effect of islet transplantation. MSCs were obtained from Fischer 344 rats and engineered into cell sheets using temperature-responsive culture dishes. The islets obtained from Fischer 344 rats were seeded onto MSC sheets, and the islets with MSC sheets were harvested by low-temperature treatment after coculture. The functional activity of the islets with MSC sheets was confirmed by a histological examination, insulin secretion assay, and quantification of the levels of cytokines. The therapeutic effects of the islets with MSC sheets were investigated by transplanting the sheets at subcutaneous sites in severe combined immunodeficiency (SCID) mice with streptozotocin-induced diabetes. Improvement of islet function and viability was shown in situ on the MSC sheet, and the histological examination showed that the MSC sheet maintained adhesion factor on the surface. In the recipient mice, normoglycemia was maintained for at least 84 days after transplantation, and neovascularization was observed. These results demonstrated that islet transplantation in a subcutaneous site would be possible by using the MSC sheet as a scaffold for islets.
Introduction
In the field of cell transplantation therapy, mesenchymal stem cells (MSCs) have been shown to have a protective effect on islet cells.1,2 Cell sheets developed using tissue engineering help maintain the function of the cells through a trophic effect.3,4 The protective effect of MSCs engineered into a cell sheet is thus thought to be improved. In the present study, we attempted to create MSC sheets cocultured with islets as an approach to islet transplantation.
In islet transplantation recipients who have undergone an intraportal injection, the ability to achieve long-term glycemic control remains insufficient.5 During intraportal transplantation, 60–80% of the islets are lost within 1 h after transplantation due to immediate blood-mediated inflammatory reactions, activation by direct exposure to foreign immunological cells, and the toxic effects of the immunosuppressive compounds on the transplanted islets.6 Additionally, an insufficient blood supply and immunoreactions associated with intraportal islet transplantation are the primary causes of islet loss.6,7
Several studies described the transplantation of islets at extrahepatic sites, including the omentum,8 spleen,9 testis,10 and renal subcapsular space.11 However, a sufficient long-term control of blood glucose levels has not been shown after implantation at these sites. In 2009, Shimizu et al.12 reported the creation of islet cell sheets using a tissue engineering method involving subcutaneous transplantation. Saito et al.13 reported that subcutaneously transplanted islet cell sheets maintain their function over the long term. Tissue engineering methods used for islet transplantation could generate islets to be transplanted at subcutaneous sites and serve as the foundation for generating a new therapeutic modality.
MSCs are known to differentiate into endothelial cells14,15 and improve the engraftment of islets by secreting antiapoptotic and angiogenic cytokines, such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and transforming growth factor beta 1 (TGFβ1).16–18 Rehman et al.16 reported that the secretion of VEGF increases in hypoxia, and that ischemic hindlimbs were improved by MSC therapy. Boumaza et al.18 reported that MSCs spontaneously secrete HGF and TGFβ1 and establish a tissue microenvironment that supports β cells. Chan et al.19 proposed the feasibility of MSC therapy for chronic stroke.
These earlier studies suggested that MSCs promote tissue repair by secreting these soluble factors that inhibit inflammation and improve angiogenesis. This ability of MSCs could solve some of the problems associated with graft loss subsequent to various forms of transplantation. However, the major issue of MSC therapy is that MSCs could not engraft sufficiently in vivo.20 It is thus necessary to modify the MSC transplantation procedure to improve MSCs' engraftment in vivo.
In the field of tissue engineering, cell sheets have been fabricated by tissue engineering methods that use temperature-responsive dishes.3,4 A temperature-responsive dish is coated with a temperature-responsive polymer. This polymer changes from hydrophobic to hydrophilic at temperatures under 32°C, and the cells can then be recovered as a sheet without using trypsin. The cell sheet thus preserves cellular communication junctions and the endogenous extracellular matrix (ECM), such as integrative adhesive agents,3 and the cell sheet further maintain the functions of the cells themselves.4 The ECM provides the essential structural and adhesive properties that maintain the integrity of the cell sheet during transplantation, and the ECM has the capability to cause the cells to differentiate in a particular direction.21 We, therefore, hypothesized that (1) the abundant ECM obtained by modifying MSCs into a cell sheet could promote the engraftment, and (2) the trophic effect of an MSC sheet on islets would be improved compared to MSCs alone. Islet transplantation at a subcutaneous site would be possible by using the MSC sheet as a scaffold for islets. Our group recently reported that human hepatic cell sheets could be made rapidly and efficiently by using fibroblast cells.22 We propose that the protective and engraftment effects of MSCs for transplanted islets could be improved by using MSCs engineered into cell sheets.
In the present study, we applied a tissue engineering approach using islets with MSC sheets (islets+MSC sheet) for transplantation at subcutaneous sites. The purpose of the study was to confirm the protective and therapeutic effects of using MSCs engineered into a cell sheet by tissue engineering as a scaffold for islet cell transplantation at an extrahepatic site.
Materials and Methods
Animals
Eight-week-old male Fischer 344 rats, 200–300 g male Fischer 344 rats, and 6-week-old male severe combined immunodeficiency (SCID) mice (Charles River Laboratories Japan, Yokohama, Japan) were used.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Nagasaki University.
Isolation and culture of MSCs derived from bone marrow
We isolated bone marrow cells from the femurs of 8-week-old rats by flushing the femurs with alpha minimum essential medium (αMEM) (Invitrogen GIBCO, Carlsbad, CA) containing 10% fetal bovine saline (FBS) (Invitrogen), 100 IU/mL penicillin (Invitrogen), and 100 μg/mL streptomycin (Invitrogen). These MSCs were used for the experiments at passage 2 or 3.
Characterization of the MSCs
Flow cytometry (FACS) analysis
The MSCs were prepared for use with the following markers: CD29, CD31, CD34, and CD90. First, MSCs were incubated with fluorescence-conjugated primary antibodies for 1 h. Then the cells were washed three times in phosphate-buffered saline and incubated with secondary antibodies for 1 h. After three washing steps, the MSCs were acquired using a FACSCanto II (Becton Dickinson, Lincoln Park, NY) flowcytometer and analyzed with the FACSDiva software program (Becton Dickinson). The following antibodies were employed: PE mouse anti-rat CD29 (Miltenyi Biotec, Auburn, CA), FITC mouse anti-rat CD31 (LSBio, Seattle, WA), PE mouse anti-CD34 (Santa Cruz Biotechnology, Santa Cruz, CA), and FITC mouse anti-rat Thy-1/CD90 (LSBio).
Differentiation of MSCs in vitro
We induced the differentiation of isolated MSCs into mesenchymal osteogenic and adipogenic lineages according to published protocols.23 Adipocytes were detected by standard Oil Red O staining. Osteocytes were detected by Alizarin Red staining.
Isolation of islets
Fischer 344 rats (200–300 g) were used as donors for islet transplantation. The islets were isolated using collagenase digestion according to published methods.24 The islets were stained with dithizone (140 mM) and counted under a microscope, and the number was converted into standard islet equivalents (IEs).
Preparation of islets cocultured with the MSC sheets (islets+MSC sheets)
We seeded MSCs at a density of 5×105 cells/dish onto 35-mm diameter temperature-responsive culture dishes (CellSeed, Tokyo, Japan). For the cells' culture, αMEM supplemented with 10% FBS was used. Overconfluent MSCs on the temperature-responsive dishes were transferred to another incubator set at 20°C for ∼30 min, causing the MSC sheet to detach spontaneously. To create islets+MSC sheets, we seeded 400–500 islets onto the MSCs after the MSCs reached 90% confluence. Following an additional 48–72 h in culture, confluent MSCs topped with islets were harvested as an islet+MSC sheet. MSCs were seeded at the density of 5×105 cells/dish onto 35-mm diameter dishes. After 7 days' cultivation, the MSCs were overconfluent in the culture dish, and the number of MSCs was 1×106 cells/dish. We found that 96–120-h cultivation was required for the cells to reach 90% confluence. Islets+MSC sheets were detached by the same procedure as that used for the MSC sheets. As a control, 400–500 islets were cultivated alone under the same conditions in sterilized, low-attachment culture dishes.
Histological and immunohistochemical analyses
The islets+MSC sheets were fixed in 10% formalin and sectioned. Serial sections were then cut from the paraffin-embedded blocks and stained with Hematoxylin and eosin (H&E). The presence of cytoplasmic insulin and glucagon in the islets on the MSC sheets was confirmed through immunostaining using pig polyclonal anti-insulin antibodies (LSBio) and mouse polyclonal anti-glucagon antibodies (Sigma Chemical, St. Louis, MO).
Electron microscopy
We used electron microscopy to confirm the presence of ECM on the surface of the MSC sheet and to observe the adhesion between islets and the MSC sheet. The islets+MSC sheets were fixed with 2.5% glutaraldehyde in 0.1 M phosphate-buffered 1% osmium tetroxide. The dehydrated samples were cut into ultrathin sections and then examined using an electron microscope (JEM-1200EX; JEOL, Tokyo, Japan).
Islet recovery after incubation
Islets were cocultured with MSCs or an MSC sheet, and we counted the number of islets and calculated the IEs after 24 and 72 h of incubation. The IE of the islets present following the incubation/IE of the seeded islets was considered to indicate the recovery rate.
Islet viability after incubation
We assessed the viability of the islets cocultured with MSCs or an MSC sheet after 72 h of incubation using calcein-AM and propidium iodide (PI) (Cellstain-Double Staining Kit; Dojindo, Kumamoto, Japan) staining. The samples were placed on a fluorescent microscope (Eclipse Ti-U; Nikon, Tokyo, Japan). Viable cells were stained green and dead cells were stained red. The degree of cell viability was assessed according to published protocols.2 We also evaluated the viability by cell size (<150, 151–250, and 251–500 μm). Ten or more islets were evaluated for each size category.
Insulin secretion assay
For the insulin secretion assay, islets cocultured with MSCs or an MSC sheet after 72 h of incubation were preincubated for 1 h at 37°C with RPMI-1640 medium containing 3.3 mM glucose. After the preincubation step, the culture medium was changed to fresh RPMI-1640 containing 3.3 mM glucose for an additional 1 h. The media were replaced with 20 mM glucose for 1 h. For the final step, the medium was changed to 3.3 mM glucose for 1 h. The culture medium was collected and frozen at −20°C until the analysis. The amount of secreted insulin was measured using an Ultra Sensitive Rat Insulin ELISA Kit (Morinaga Institute of Biological Science, Kanagawa, Japan). The stimulation index (SI) was calculated as follows: SI=(insulin content in the 20 mM glucose media)/(insulin content in the initial 3.3 mM glucose media).
Cytokine quantification
We measured the secretion of cytokines in the supernatants using the VEGF Rat ELISA Kit (Abcam, Cambridge, MA), Rat HGF EIA (Institute of Immunology Co., Tokyo, Japan), and the Rat TGFβ1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
Induction of diabetes mellitus and transplantation of the islet+MSC sheets
SCID mice were rendered diabetic through an intraperitoneal injection of 200 mg/kg of streptozotocin (Sigma). SCID mice were defined as diabetic mice that exhibited a nonfasting blood glucose (NFBG) level of more than 350 mg/dL for 2 consecutive days. SCID mice were divided into five groups: those transplanted with 2000 islets alone (n=5), 2000 islets + 4×106 MSCs (n=5), MSC sheet alone (n=5), 1000 islets+MSC sheets (n=5), and 2000 islets+MSC sheets (n=6). In the first two groups, isolated islets were cocultured without or with MSCs in a floating condition using 35-mm low-attachment culture dishes for 24 h, and we injected the islets together into a subcutaneous site of the abdomen of SCID mice. In the latter three groups, the MSC sheets or islets+MSC sheets were fabricated using 35-mm temperature-responsive culture dishes as well as in vitro, and harvested through low temperature treatment. Glass plates (GPs) were used for subcutaneous transplantation into the abdomen. To transplant the islets+MSC sheets (500 islets were riding on each MSC sheet), we created an arc-shaped incision in the abdominal skin of the mouse. After the attachment of the islets+MSC sheet to the subcutaneous site, the GP was immediately and carefully removed. Another islets+MSC sheet was transplanted on the initial sheet. Two or four islets+MSC sheets were transplanted in the subcutaneous site. As controls, diabetic sham-operated (DM-sham) mice (n=5) were investigated. In addition, another six mice were transplanted with four islets+MSC sheets (2000 islets) to evaluate the long-term efficacy.
Validation of the therapeutic effects of the islets+MSC sheets
The NFBG levels of the mice were measured twice weekly. On day 28, we obtained serum samples to measure the rat nonspecific insulin levels using the ELISA kits (Morinaga Institute of Biological Science). In other experiments, to confirm the long-term therapeutic effects (at day 84), five diabetic SCID mice underwent transplantation of four islets+MSC sheets, and the transplanted islets+MSC sheets were removed by abdominal wall resection on day 84.
Intraperitoneal glucose tolerance test
We evaluated the functionality of the islets+MSC sheets in vivo by conducting intraperitoneal glucose tolerance tests (IPGTTs) on day 56 in the mice transplanted with four islets+MSC sheets. The mice received an intraperitoneal inoculation of glucose solution (2 g/kg body weight) after 18 h of fasting.
Immunohistochemical and immunofluorescence examinations
On day 28, specimens of subcutaneous tissue were fixed in 10% buffered formalin, sectioned (5-μm-thick sections), and stained with H&E and Azan-Mallory. For the evaluation of the degree of vascularization, the specimens were immunostained using anti-von Willebrand factor (vWF) polyclonal antibodies (1:50; Chemicon–Millipore, Billerica, MA), anti-insulin polyclonal antibodies (1:50; Santa Cruz Biotechnology), and anti-pancreas duodenum homeobox Pdx1 antibodies (1:100; Upstate, Charlottesville, VA). The number of vessels was determined by counting the vessels randomly in five different subcutaneous areas.
Statistical analyses
Data are presented as the mean±standard error (SEM). Statistical analyses were performed using GraphPad Prism software (version 6:00; GraphPad, San Diego, CA) for numerical variables, using a repeated-measures analysis of variance (ANOVA) when comparing more than two groups, Student's t-test when comparing two groups, and the Mann–Whitney U-test. p-Values<0.05 were considered significant.
Results
Characteristics of MSCs isolated from rat bone marrow
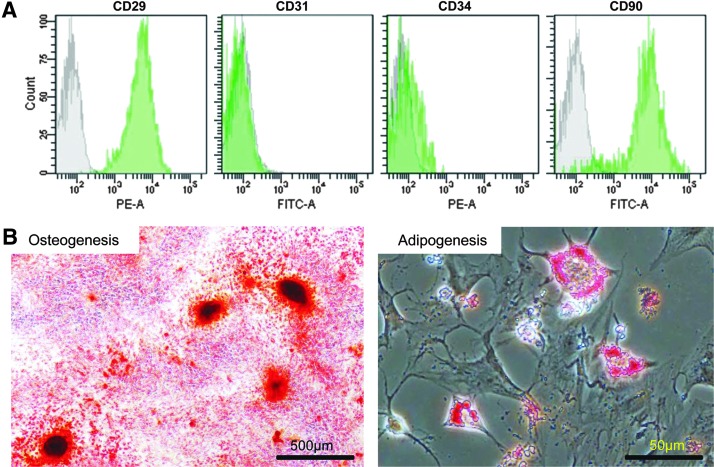
The isolated cells were positive for the mesenchymal markers CD29 and CD90, and negative for the hematopoietic markers CD31 and CD34 (Fig. 1A). These findings indicated that the characteristic immunophenotype of rat bone marrow-derived MSCs was exhibited in the isolated cells.25–27 MSCs have the ability to differentiate into osteogenic and adipogenic lineages.25,26 The cells described herein are able to differentiate into osteocytes and adipocytes (Fig. 1B).
FIG. 1.
(A) A flow cytometric analysis. CD29 and CD90 such as mesenchymal markers were positive, and CD31 and CD34 such as hematopoietic markers were negative. (B) To confirm a capability of MSC differentiation into osteogenesis and adipogenesis, Alizarin Red S staining and Oil Red O staining were performed. MSCs, mesenchymal stem cells.
Observation of the islet+MSC sheet
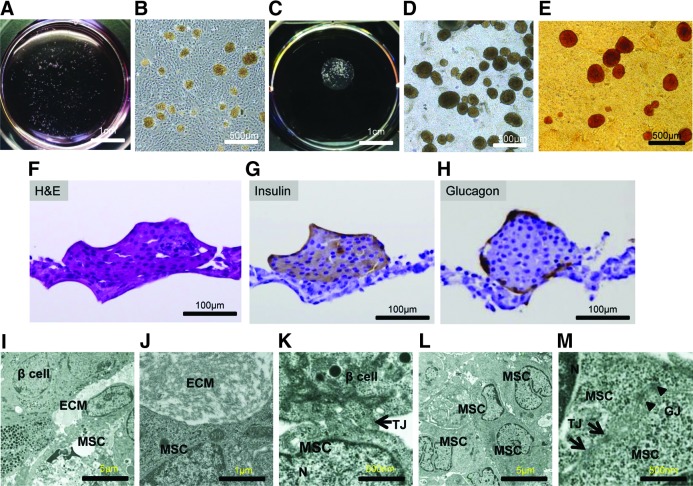
The islets seeded on the MSC sheet immediately sank to the bottom of the culture dishes and came into contact with the MSCs (Fig. 2A, B). After a 72-h culture, the islets adhered to the MSCs. The MSCs detached from the culture dish while shrinking slowly as a cell sheet following low temperature treatment (Fig. 2C, D). The presence of islets on the sheet was confirmed by dithizone staining (Fig. 2E). H&E staining showed that the islets adhered to the sheet while maintaining a spherical shape in their physiological form. In addition, the MSC sheets firmly adhered to the islets (Fig. 2F), which demonstrated cytoplasmic immunostaining for both insulin and glucagon (Fig. 2G, H). The transmission electron microscopy examination revealed that the islets adhered firmly to the MSC sheets and partially to the ECM (Fig. 2I, J), forming tight junctions (Fig. 2K). The MSC sheets contained multiple cell layers (Fig. 2L) that established cell-to-cell connections through the formation of tight junctions and gap junctions (Fig. 2M).
FIG. 2.
(A, B) The islets were seeded at a density of 50 islets/cm2 in 35-mm diameter temperature-responsive dish. The islets were riding on the confluent MSCs in the temperature-responsive dish. (C) The islets+MSC sheets were harvested by low-temperature treatment after 72 h coculture. (D) The islet+MSC sheets were harvested while shrinking during low-temperature treatment. (E) The islets were stained with dithizone. (F) H&E staining showed that the sheets adhered to the islets in the shape of spheres. (G, H) Rat insulin and glucagon immunostaining of islets cocultured with MSC sheets. (I–M) Ultrastructures of the islets+MSC sheets were observed by electron microscopy. (I, J) ECM was partially detected between the islets and MSC sheets. (K) The islets and MSC sheets were connected through the formation of tight junctions. (L) The MSC sheets consisted of multiple layers. (M) Cell-to-cell connections were observed in the MSC sheets due to the formation of tight and gap junctions. ECM, extracellular matrix; H&E, hematoxylin and eosin; N, nucleus; TJ, tight junctions (arrow); GJ, gap junctions (arrowhead).
Efficacy of MSC sheet for islets
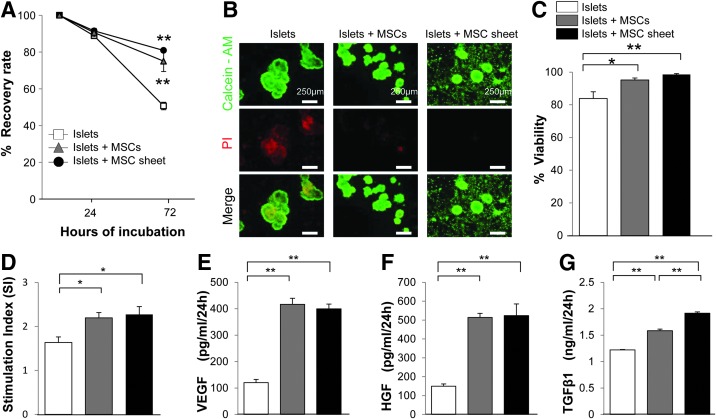
After 24 h, there were no differences in the recovery rate among the islets cocultured with the MSC sheet and the groups of MSCs and islets cultured alone. However, the recovery rate significantly improved in the cocultured groups compared to the group of islets cultured alone. There were no significant differences between the MSCs group and the MSC sheet group at 72-h culture (Fig. 3A). We assessed the viability of the islets using calcein-AM/PI staining. Viable cells were stained in green, and dead cells were stained in red (Fig. 3B). The MSCs were almost 100% viable in the range observed. The 72-h viability of the cocultured islets group was significantly improved compared to the islets cultured-alone group (Fig. 3C). There were no significant differences between the MSCs group and the MSC sheet group.
FIG. 3.
(A) The recovery rate was calculated after 24 and 72 h of incubation. (B) The viability of the islets was assessed using calcein-AM and PI. Viable cells were stained green and dead cells were stained red. Almost all MSCs and MSC sheets were viable. (C) Viability of the islets cultured alone and cocultured with MSCs and MSC sheets. (D) The insulin levels changed along with the change in the glucose concentration. The SI was calculated in the cultured-alone group and cocultured with MSCs and MSC sheet groups. (E–G) The secretions of VEGF, HGF, and TGFβ1 in the supernatants obtained from the islets alone, islets cocultured with MSCs, and MSC sheet groups. n=5 each. *p<0.05, **p<0.01 compared to the group of islets cultured alone. HGF, hepatocyte growth factor; PI, propidium iodide; SI, stimulation index; TGFβ1, transforming growth factor beta 1; VEGF, vascular endothelial growth factor.
In the coculture with MSCs and MSC sheet groups, the SI values were significantly higher than in the cultured-alone group. There were no significant differences between the MSCs group and the MSC sheet group (Fig. 3D). The VEGF, HGF, and TGFβ1 levels were significantly higher in the coculture groups compared to the cultured-alone islets group. The TGFβ1 level was significantly higher in the coculture with MSC sheet group than in the coculture with MSCs group (Fig. 3E–G).
Therapeutic effects of the engrafted islets+MSC sheets
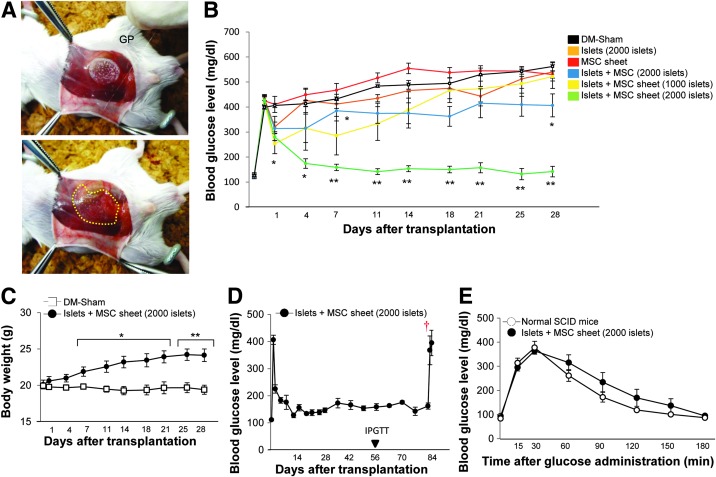
Islets+MSC sheet transplantation was performed as described in the Materials and Methods section (Fig. 4A). In the recipient SCID mice treated with two islet+MSC sheets transplantation, three of the five recipient mice had maintained normoglycemia at 2 weeks after transplantation, two of the five recipient mice had maintained normoglycemia at 3 weeks after transplantation, and all mice became hyperglycemic more than 3 weeks after transplantation. Therefore, four islets+MSC sheet transplantation was performed. All recipient SCID mice returned to a normoglycemic state within 1 week, whereas all sham-operated mice remained hyperglycemic.
FIG. 4.
(A) The islets+MSC sheets adhered to GP. The islets+MSC sheets were attached to the surrounding tissue. The implanted islets+MSC sheets are indicated by a dashed line. (B) The blood glucose levels of diabetic sham-operated (DM-sham) mice (n=5) and those of the recipient mice: 2000 islets alone (n=5), MSC sheet alone (n=5), 2000 islets with MSCs (n=5), two islets+MSC sheets (1000 islets) (n=5), and four islets+MSC sheets (2000 islets) (n=6). *p<0.05, **p<0.01 compared to the DM-sham group. (C) Body weight changes in the recipient mice treated with four islets+MSC sheets (2000 islets; black circles, n=6) and the DM-sham mice (white squares, n=5). *p<0.05, **p<0.01 compared to the DM-sham group. (D) The recipient mice were transplanted with four islets+MSC sheets (2000 islets; black circles, n=6). The graft tissue was surgically removed on day 84. †Graft removal. (E) The IPGTT was performed in the normal SCID mice (white circles, n=9) and recipient mice treated with four islets+MSC sheets (2000 islets; black circles, n=6) on day 56. DM, diabetes mellitus; GP, glass plates; IPGTT, intraperitoneal glucose tolerance tests; SCID, severe combined immunodeficiency.
The transplantation of 2000 islets alone and that of the same numbers of islets with MSCs was performed within 24 h after islet isolation. All recipient mice exhibited a minimal decrease in the NFBG level and remained hyperglycemic. In the diabetic SCID mice transplanted with MSC sheets only, all mice remained hyperglycemic (Fig. 4B). The body weight, as a clinical condition of recipient mice in the four islet+MSC sheets transplantation group, improved (Fig. 4C). All recipient SCID mice treated with a four islet+MSC sheets remained normoglycemic for 84 days, and the NFBG levels rose rapidly after graft removal (Fig. 4D). An IPGTT was performed in the recipient mice treated with four islet+MSC sheets and control (nondiabetic naive) SCID mice. The blood glucose levels returned to normal levels after elevations at 15 and 30 min (Fig. 4E).
Assessment of the engrafted islets and serum insulin levels
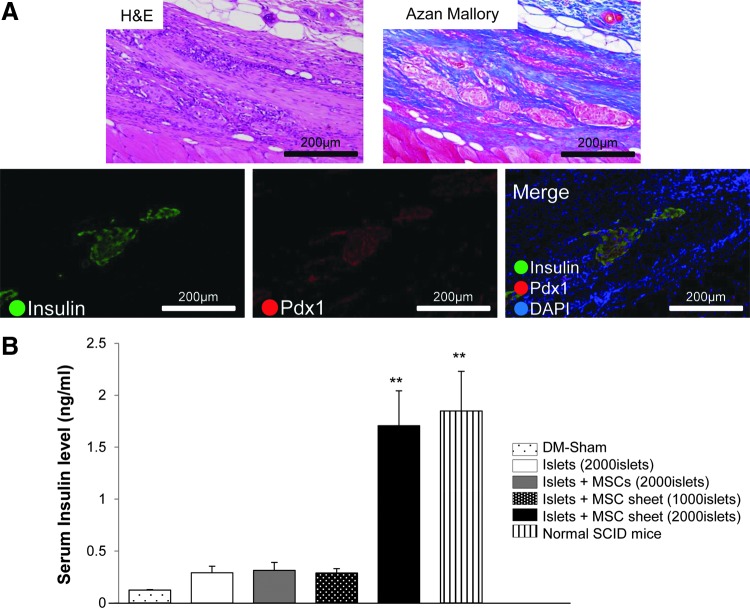
The appearance of connective tissue was observed at the subcutaneous sites of transplantation, and collagen-rich connective tissue was noted on Azan-Mallory staining. In addition, on immunofluorescence, islets with the expression of insulin and Pdx1 were observed in the new tissue. Islets maintaining their physiological shape were also detected (Fig. 5A), although no islets were apparent at the subcutaneous sites in the specimens in the islet transplantation-alone group (data not shown). However, the existence of transplanted MSC sheets could not be confirmed. A significant amount of insulin was seen in the four islet+MSC sheets transplantation group compared to that observed in the DM-sham mice group. There was no significant difference among the 2000 islets, 2000 islets with MSCs, two islet+MSC sheets transplantation, and DM-sham-operated groups (Fig. 5B).
FIG. 5.
(A) Histological, immunohistochemical, and immunofluorescence analyses on day 28 after the subcutaneous transplantation of the islets+MSC sheets. The expression of insulin and Pdx1 on islets was observed in the connective tissue. (B) Serum insulin level was investigated in the DM-sham (n=5), recipient SCID mice [2000 islets (n=5), 2000 islets with MSCs (n=5), two islets+MSC sheets (1000 islets) (n=5), four islets+MSC sheets (2000 islets) (n=6)], and normal SCID mice (n=7). **p<0.01 compared to the DM-sham group.
Angiogenesis associated with the transplantation of islets+MSC sheets
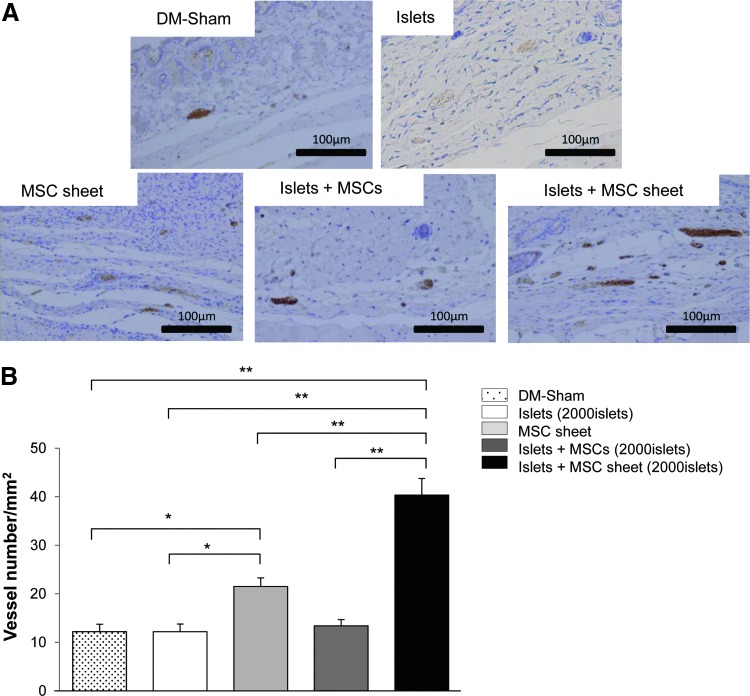
The numbers of detectable vessels in the MSC sheet and islet+MSC sheet groups were significantly higher than in the sham-operated and islets alone groups. There was no significant difference in the vessel numbers among the DM-sham-operated islets alone, and islets with MSCs groups (Fig. 6A, B).
FIG. 6.
(A) The expression of vWF was observed in the subcutaneous site. (B) The degree of vascularization was evaluated according to the number of vessels for anti-vWF immunostaining. In the implanted subcutaneous tissue, the number of vessels per square millimeter was counted. At 28 days after treatment, specimens were obtained from DM-sham mice (n=5), recipient SCID mice treated with islets alone (n=5), 4 MSC sheets alone (n=5), 2000 islets with MSCs (n=5), and 4 islets+MSC sheet (n=6). *p<0.05, **p<0.01. vWF, von Willebrand factor.
Discussion
Previous studies have examined MSCs' abilities to preserve the inflammatory response,28,29 modulate the immune reaction,30,31 inhibit apoptosis,32 and promote vascularization.33,34 Such effects may solve problems associated with graft loss following various forms of transplantation. In islet transplantation, MSCs secrete a variety of cytokines that modulate intracellular signaling related to the cell function and increased survival of islets both in vitro and in vivo.1,2 The results of the present study suggest that the use of the islet+MSC sheets improves graft survival compared to islets with MSCs in transplantation into diabetic mice.
VEGF, HGF, and TGFβ1 are the major cytokines secreted by MSCs that exhibit effects in graft protection. TGFβ1 stimulates the production of heat shock protein HSP32 and X-linked inhibitor of apoptosis protein (XIAP).35 HSP32 has a protective effect on islets and suppresses inflammatory reactions and oxidative stress.36,37 XIAP is known to have an antiapoptotic effect on β cells.38,39 Previous studies indicated that MSCs promote vascularization not only by secreting angiogenic growth factors such as VEGF and HGF,16,40 but also by the differentiation into endothelial cells.41,42 In the present study, we did not clarify particular elements that improve the function and survival of islets; however, we observed improvement in the function and survival of islets in situ on the MSCs and MSC sheets.
Several studies have described the usefulness of islet transplantation using MSCs,1,2 but islet transplantation using MSC sheets is thought to be more useful regarding the therapeutic effect. Although the MSCs were sheeted using tissue engineering techniques, the MSCs maintained their function, including the production of cytokines and their protective effects on islets. Our present findings verified that the secretion of angiogenic factors is not deteriorated even when MSCs are modified into a cell sheet.
Electron microscopy showed the attachment between the islet cells and MSCs to be sufficient for harvesting sheets as islets+MSC sheets, and the attachment withstood the transplantation procedures. ECM components such as adhesion factor were also observed on the surface of the MSC sheets. Previous studies revealed that the use of tissue-engineered cell sheets resulted in a greater degree of engraftment at the transplantation sites compared to cell transplantation43,44 because the cell sheets preserve adhesion factors when harvested without trypsin. We also found that although the protective effect of MSCs was comparable to that of MSC sheets in vitro, the therapeutic effects of the islets+MSC sheet transplantation were significantly higher than those of islet transplantation with MSCs.
These findings suggest that MSC sheets improve the engraftment rate of islets at subcutaneous sites depending on the presence of adhesion factors in the MSC sheets. The MSC sheet improved the efficiency of islet transplantation more than the same number of MSCs did. Moreover, the creation of the MSC sheets does not require a scaffold, such as a laminin coating or Matrigel, and only donor cells will be used. The main advances shown by our study were the fabrication of the islet+MSC sheets and the evaluation of the efficacy of islet+MSC sheet transplantation in a subcutaneous site. We used an immunodeficiency animal model in the experiments, and the immune activity of the MSC sheets was not clarified in this study. The immunomodulatory ability of the MSCs should be evaluated in a future study using an immunocompetent animal model.
The successful improvement of the blood glucose levels in diabetic mice was reported in a study using ∼400–500 islets for portal vein islet transplantation.45 A marginal amount of islets was detected following the transplantation of 1000–2000 islets using the islet+MSC sheet procedure. The major reasons underlying these findings are thought to be: (1) the engrafted islets were damaged following culture for over 48 h to establish islet-MSC cocultured sheets, and (2) the effects of vascularization in the MSC sheets were insufficient in the early phase of islet engraftment at the subcutaneous sites, although the MSC sheets exerted a stimulating effect on angiogenesis. Fumimoto et al.46 reported that in their study, the engraftment and function of islets were remarkably increased following transplantation at subcutaneous sites treated prevascularization with MSCs.
When performing transplantation into subcutaneous tissue lacking an adequate blood flow, vascularization is an important factor enabling the therapeutic effects of islet+MSC sheet transplantation. To improve the efficiency of transplantation, obtaining efficient early angiogenesis and/or using other sites with a sufficient blood flow for engraftment is required. In addition, in the present study angiogenesis was significantly increased in the islet+MSC sheet group compared to the MSC sheet alone group. We suspect that the islets exposed to hypoxia induced some signals to the MSC sheet for the angiogenesis effect.
Another possible candidate cell that produces angiogenic factors is fibroblast cells. As we mentioned above, our group recently reported the usefulness of a fibroblast cell sheet as the functional scaffold for hepatocytes.22 Fibroblasts are also known to secrete cytokines such as VEGF, and it is possible that fibroblast cells have protective effects on islets.47,48 At this time, it is not clear which type of cell is more feasible as a functional scaffold/cell sheet for islets. A comparison study is required to identify the most effective cells for islets.
Hasegawa et al.49 reported that MSCs can differentiate into β cells, but another study reported low levels of regeneration.50 In the present study, no insulin-positive or Pdx1-positive cells were detected by immunofluorescence examinations around the islets in the specimens of the recipient mice treated with islet+MSC sheet transplantation. These results indicate that MSC sheets could not differentiate into β cells around islets at subcutaneous sites under hyperglycemic conditions. Although the differentiation or the existence of transplanted MSC sheets was not confirmed absolutely in the present study, connective tissues containing a rich vascular bed were observed at the transplantation sites in the islet transplantation group using an MSC sheet, whereas the outgrowth of connective tissues was not detected in the specimens of the recipient mice transplanted with MSCs. These results suggest that the MSC sheets promote islets' engraftment in the early phase of transplantation more effectively than islets transplanted with MSCs, not only by the secretion of cytokines, but also by providing abundant ECM. Moreover, no abnormal overgrowth of MSC sheets was observed within at least 84 days following transplantation.
Conclusions
We successfully fabricated islet+MSC sheets by using tissue engineering. Our findings indicate that the MSC sheets exerted protective effects on the viability and function of islets and improved the engraftment of islets at subcutaneous sites. The islets engrafted with MSC sheets at subcutaneous sites had a therapeutic effect on hyperglycemia compared to that observed following islet transplantation without MSC sheets. In the future, a more detailed elucidation of the mechanisms of MSCs activity and cell sheet potentiality would help to expand the clinical applications of islet transplantation therapy.
Acknowledgment
The authors thank Dr. Tatsuya Kin (University of Alberta) for providing technical advice regarding the isolation of islets.
Author Contributions
M.H. and T.K. designed the research; M.H., T.K., T.A., A.K., S.O., T.T., H.M., Y.S., M.T., T.O., and S.E. performed and analyzed the research; M.H. and T.K. wrote the article.
Disclosure Statement
T.O. is an investor in CellSeed, Inc., and is an inventor/developer designated on the patent for temperature-responsive culture surfaces. The other authors declare that they have no duality of interests associated with this article.
References
- 1.Ito T., Itakura S., Todorov I., Rawson J., Asari S., Shintaku J., Nair I., Ferreri K., Kandeel F., and Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89, 1438, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Karaoz E., Genc Z.S., Demircan P.C., Aksoy A., and Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis 1, e36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu T., Yamato M., Kikuchi A., and Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 24, 2309, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Memon I.A., Sawa Y., Fukushima N., Matsumiya G., Miyagawa S., Taketani S., Sakakida S.K., Kondoh H., Aleshin A.N., Shimizu T., Okano T., and Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg 130, 1333, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ryan E.A., Paty B.W., Senior P.A., Bigam D., Alfadhli E., Kneteman N.M., Lakey J.R., and Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes 54, 2060, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Korsgren O., Lundgren T., Felldin M., Foss A., Isaksson B., Permert J., Persson N.H., Rafael E., Ryden M., Salmela K., Tibell A., Tufveson G., and Nilsson B. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 51, 227, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Ryan E.A., Lakey J.R., Rajotte R.V., Korbutt G.S., Kin T., Imes S., Rabinovitch A., Elliott J.F., Bigam D., Kneteman N.M., Warnock G.L., Larsen I., and Shapiro A.M. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 50, 710, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kin T., Korbutt G.S., and Rajotte R.V. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant 3, 281, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y., Zhang J.L., Liu Y.F., Li T.M., and Zhao N. Islet transplantation for diabetic rats through the spleen. Hepatobilliary Pancreat Dis Int 4, 203, 2005 [PubMed] [Google Scholar]
- 10.Nasr I.W., Wang Y., Gao G., Deng S., Diggs L., Rothstein D.M., Tellides G., Lakkis F.G., and Dai Z. Testicular immune privilege promotes transplantation tolerance by altering the balance between memory and regulatory T cells. J Immunol 174, 6161, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Leow C.K., Gray D.W., and Morris P.J. The long-term metabolic function of intraportal and renal subcapsular islet isografts and the effect on glomerular basement membrane thickness in rats. Diabetologia 38, 1014, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Shimizu H., Ohashi K., Utoh R., Ise K., Gotoh M., Yamato M., and Okano T. Bioengineering of a functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials 30, 5943, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Saito T., Ohashi K., Utoh R., Shimizu H., Ise K., Suzuki H., Yamato M., Okano T., and Gotoh M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation 92, 1231, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Oswald J., Boxberger S., Jorgensen B., Feldmann S., Ehninger G., Bornhauser M., and Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22, 377, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Silva G.V., Litovsky S., Assad J.A., Sousa A.L., Martin B.J., Vela D., Coulter S.C., Lin J., Ober J., Vaughn W.K., Branco R.V., Oliveira E.M., He R., Geng Y.J., Willerson J.T., and Perin E.C. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111, 150, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., and March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Aksu A.E., Horibe E., Sacks J., Ikeguchi R., Breitinger J., Scozio M., Unadkat J., and Feili-Hariri M. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol 127, 348, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Boumaza I., Srinivasan S., Witt W.T., Feghali-Bostwick C., Dai Y., Garcia-Ocana A., and Feili-Hariri M. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 32, 33, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Chan T.M., Harn H.J., Lin H.P., Chiu S.C., Lin P.C., Wang H.I., Ho L.I., Chuu C.P., Chiou T.W., Hsieh A.C., Chen Y.W., Ho W.Y., and Lin S.Z. The use of ADSCs as a treatment for chronic stroke. Cell Transplant 23, 541, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Lin H.P., Chan T.M., Fu R.H., Chuu C.P., Chiu S.C., Tseng Y.H., Liu S.P., Lai K.C., Shih M.C., Lin Z.S., Chen H.S., Yeh D.C., and Lin S.Z. Applicability of adipose-derived stem cells in Type 1 diabetes mellitus. Cell Transplant 24, 521, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Chan T.M., Lin H.P., and Lin S.Z. In situ altering of the extracellular matrix to direct the programming of endogenous stem cells. Stem Cells (Dayton, Ohio) 32, 1989, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Sakai Y., Koike M., Hasegawa H., Yamanouchi K., Soyama A., Takatsuki M., Kuroki T., Ohashi K., Okano T., and Eguchi S. Rapid fabricating technique for multi-layered human hepatic cell sheets by forceful contraction of the fibroblast monolayer. PLoS One 8, e70970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., and Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto T., Kuroki T., Adachi T., Ono S., Hayashi T., Tajima Y., Eguchi S., and Kanematsu T. Effect of zinc on early graft failure following intraportal islet transplantation in rat recipients. Ann Transplant 16, 114, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa H., Fujimoto Y., Teratani T., Iwasaki J., Kasahara N., Negishi K., Tsuruyama T., Uemoto S., and Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One 6, e19195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forte A., Finicelli M., Mattia M., Berrino L., Rossi F., De Feo M., Cotrufo M., Cipollaro M., Cascino A., and Galderisi U. Mesenchymal stem cells effectively reduce surgically induced stenosis in rat carotids. J Cell Physiol 217, 789, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Sun Z., Qiu X., Li Y., Qin J., and Han X. Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 390, 1309, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K., and Phinney D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104, 11002, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi H., Soto-Gutierrez A., Navarro-Alvarez N., Nahmias Y., Goldwasser Y., Kitagawa Y., Tilles A.W., Tompkins R.G., Parekkadan B., and Yarmush M.L. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 18, 1857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., and Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Gieseke F., Bohringer J., Bussolari R., Dominici M., Handgretinger R., and Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 116, 3770, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Block G.J., Ohkouchi S., Fung F., Frenkel J., Gregory C., Pochampally R., DiMattia G., Sullivan D.E., and Prockop D.J. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells 27, 670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milovanova T.N., Bhopale V.M., Sorokina E.M., Moore J.S., Hunt T.K., Hauer-Jensen M., Velazquez O.C., and Thom S.R. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol (1985) 106, 711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang M., Wang B., Wang C., He B., Fan H., Guo T.B., Shao Q., Gao L., and Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem 103, 321, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kutty R.K., Nagineni C.N., Kutty G., Hooks J.J., Chader G.J., and Wiggert B. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J Cell Physiol 159, 371, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Pileggi A., Molano R.D., Berney T., Cattan P., Vizzardelli C., Oliver R., Fraker C., Ricordi C., Pastori R.L., Bach F.H., and Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 50, 1983, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Lee D.Y., Lee S., Nam J.H., and Byun Y. Minimization of immunosuppressive therapy after islet transplantation: combined action of heme oxygenase-1 and PEGylation to islet. Am J Transplant 6, 1820, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Emamaullee J.A., Rajotte R.V., Liston P., Korneluk R.G., Lakey J.R., Shapiro A.M., and Elliott J.F. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes 54, 2541, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Plesner A., Liston P., Tan R., Korneluk R.G., and Verchere C.B. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes 54, 2533, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y., Kikuchi Y., Saito Y., Tamai K., Ogihara T., and Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol 25, 2542, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Cao Y., Sun Z., Liao L., Meng Y., Han Q., and Zhao R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332, 370, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Planat-Benard V., Silvestre J.S., Cousin B., Andre M., Nibbelink M., Tamarat R., Clergue M., Manneville C., Saillan-Barreau C., Duriez M., Tedgui A., Levy B., Penicaud L., and Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109, 656, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Kushida A., Yamato M., Konno C., Kikuchi A., Sakurai Y., and Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res 45, 355, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., Kikuchi A., Umezu M., and Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90, e40, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Kim J.S., Lim J.H., Nam H.Y., Lim H.J., Shin J.S., Shin J.Y., Ryu J.H., Kim K., Kwon I.C., Jin S.M., Kim H.R., Kim S.J., and Park C.G. In situ application of hydrogel-type fibrin-islet composite optimized for rapid glycemic control by subcutaneous xenogeneic porcine islet transplantation. J Control Release 162, 382, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Fumimoto Y., Matsuyama A., Komoda H., Okura H., Lee C.M., Nagao A., Nishida T., Ito T., and Sawa Y. Creation of a rich subcutaneous vascular network with implanted adipose tissue-derived stromal cells and adipose tissue enhances subcutaneous grafting of islets in diabetic mice. Tissue Eng Part C Methods 15, 437, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Jalili R.B., Moeen Rezakhanlou A., Hosseini-Tabatabaei A., Ao Z., Warnock G.L., and Ghahary A. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol 226, 1813, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Liu D., Xiao H., Du C., Luo S., Li D., and Pan L. The effect of fibroblast activation on vascularization in transplanted pancreatic islets. J Surg Res 183, 450, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa Y., Ogihara T., Yamada T., Ishigaki Y., Imai J., Uno K., Gao J., Kaneko K., Ishihara H., Sasano H., Nakauchi H., Oka Y., and Katagiri H. Bone marrow (BM) transplantation promotes beta-cell regeneration after acute injury through BM cell mobilization. Endocrinology 148, 2006, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Choi J.B., Uchino H., Azuma K., Iwashita N., Tanaka Y., Mochizuki H., Migita M., Shimada T., Kawamori R., and Watada H. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia 46, 1366, 2003 [DOI] [PubMed] [Google Scholar]