Abstract
Background: Our study sought to identify independent risk factors predisposing patients with necrotizing soft tissue infections (NSTIs) to mortality from among laboratory values, demographic data, and microbiologic findings in a small population. To this end, a retrospective review was conducted of the medical records of all patients with NSTI who had been treated at our institution from 2003 to 2012 (n=134).
Methods: Baseline demographics and comorbidities, clinical and laboratory values, hospital course, and the microbiologic characteristics of surgical incision cultures were recorded. Each variable was tested for association with survival status and all associated variables with p<0.15 were included in a logistic regression model to seek factors associated independently with mortality.
Results: Surprisingly, no demographic or pre-existing condition proved to be a predictor of mortality. Two laboratory values had an inverse correlation to mortality: High C-reactive protein (CRP) and highest recorded CRP. Of surgical incisions that grew bacteria in culture, 33.6% were polymicrobial. Mortality rates were highest with Enterococcus-containing polymicrobial infections (50%), followed by those containing Pseudomonas (40%), and Streptococcus spp. (27%). Understanding why so many studies across the literature, now including our own, find such disparate results for correlation of NSTI mortality with patient data may lie in the fundamentally dynamic nature of the organisms involved.
Conclusions: This study suggests that no single factor present on admission is a robust predictor of outcome; it is likely that survival in NSTI is predicated upon a complex interaction of multiple host and microbial factors that do not lend themselves to reduction into a simple formula. It is also abundantly clear that the well-established principles of NSTI surgery should continue to be followed in all cases, with an emphasis on early debridement, irrespective of apparent severity of initial presentation.
Necrotizing soft tissue infections (NSTIs) are complicated skin and soft tissue infections associated with necrotizing changes in the dermis, subcutaneous tissue, superficial fascia, deep fascia, and muscle. These infections are characterized by the potential for rapid progression, loss of limbs, and mortality rates as high as 76% [1], although most modern series report mortality between 10% and 25% [2–4]. Patients with NSTIs are often referred to critical care and burn centers because of their need for complex care, multiple operations, and reconstruction of large tissue and skin defects.
Although uncommon compared with other skin infections such as cellulitis or abscesses, the incidence of NSTI appears to be increasing, believed to be attributable to increasing immunosuppression and increases in obesity and type 2 diabetes mellitus, all of which may predispose an individual to NSTI infection [2,5]. The progression of the infection is often fulminant, and prognosis depends on quick diagnosis and appropriate treatment. The presenting signs of NSTI can be quite similar to those of other common soft tissue infections, such as cellulitis and skin abscesses, making early diagnoses of the disease challenging [6]. It has been shown that the only signs presenting in more than 50% of patients with NSTI were erythema, tenderness, or edema beyond the confines of apparent infection [7]. The necessity for physicians to grant this cluster of signs and symptoms a high index of suspicion for NSTI is crucial to early recognition and to instituting life-saving intervention without delay [8,9].
The current standard of care for treatment of NSTI includes prompt identification, early surgical debridement, antibiotic therapy, and supportive care. It is widely believed that early surgical debridement improves outcomes, although other research has shown that mortality rates are dependent on patient characteristics and the etiologic microbial pathogens [10]. Various studies have examined specific sets of parameters to differentiate between necrotizing and non-necrotizing infections. Wong et al. [11] found six independent variables to be associated with soft tissue infections and used these variables to develop a diagnostic tool for scoring (Laboratory Risk Indicator for Necrotizing Fasciitis, or LRINEC). The purpose of this work, however, was limited to diagnosis and did not extend to prediction of patient mortality, with the latter certainly a desideratum for the practicing surgeon.
Our study sought to identify independent risk factors predisposing patients with NSTI to mortality from among laboratory values, demographic data, and microbiologic findings in a small population. Certainly, there have been excellent, larger scale investigations conducted [12–14], but the advantage of smaller studies such as ours is a level of detail unavailable in larger databases. Despite efforts toward standardization, larger databases such as the American College of Surgeons National Surgical Quality Improvement Program® (ACS NSQIP®, American College of Surgeons, Chicago, IL) inherently suffer from internal lack of consistency among contributions from various reporting institutions. Smaller studies such as ours have the advantage of revealing some interesting and surprising correlations.
Patients and Methods
An Institutional Review Board-approved retrospective review was conducted of the medical records of all patients who had been treated at University Medical Center, Lubbock, Texas, from 2003 to 2012 with discharge diagnosis of NSTI identified by International Classification of Diseases, Ninth Revision (ICD-9) codes 785 (disorders of muscle, ligament, and fascia) and 728 (including diagnosis of shock). Necrotizing soft tissue infection was defined by the presence of necrosis of the subcutaneous tissue and fascia, with variable involvement of the skin and muscle. A list of patients was also obtained independently from the surgery billing offices to cross-check records and reduce the possibility of missed documents. The diagnosis was then confirmed by screening each patient's medical record for documentation of NSTI or related diagnosis (to include Fournier gangrene, clostridial myonecrosis, or necrotizing fasciitis). Patients with diagnoses of cellulitis only were excluded, as well as patients transferred from other hospitals. Baseline demographics and comorbidities including cardiovascular disease, diabetes mellitus, smoking, and alcohol and illicit drug use upon admission were recorded. Clinical and laboratory variables on admission were also collected, as well as hospital course including time to diagnosis, time to operation, number of operations, and overall length of stay (LOS). The microbiologic characteristics of surgical incision cultures were also recorded. The primary outcome measured was patient mortality.
Each demographic variable was tested for association with survival status using the Wald statistic, with the null hypothesis that there was no association between a variable and survival status. Associated variables where p<0.15 were included in a logistic regression model to determine factors associated independently with patient mortality. Forward selection, backward elimination, and stepwise selection methods were used for the variable selection, and for our final parameters, all three of these methods suggested the same model. Statistical Analysis Software (SAS; SAS Institute, Cary, NC) was used for these analyses, and “complete-case” analysis was used for both univariable and multivariable modeling. For each demographic and laboratory variable, we specify in Tables 1 and 2 the percentage of values missing from the dataset and consider less than 5% missing data to be negligible. The univariable microbiologic data (Table 3) were also analyzed using multivariable modeling techniques. Because of the retrospective nature of data collection, absence of a positive indication of each microbiologic was considered a negative result. As such, there were no missing data in the forward, backward, and stepwise selection methods that all yielded a single, concordant model (Table 4), which found non-methicillin–resistant/oxacillin–resistant Staphylococcus aureus (MRSA/ORSA) Staphylococcus spp. colonization to be a protective factor (odds ratio [OR]=0.27) and colonization with Pseudomonas spp. to be a risk factor (OR=3.51) for mortality. A similar method was attempted with the admission laboratory values of Table 2, but the selection methods disagreed on the appropriate model, so no meaningful results emerged.
Table 1.
Descriptive Statistics of Clinical Data
| Variable | Mean/percent | Standard deviation | 95% CI | Non-survivor | Survivor | p | Missing values [%] |
|---|---|---|---|---|---|---|---|
| Time to diagnosis [days] | 1.1 | 1.1 | (0.9, 1.3) | 1.0 | 1.2 | 0.62 | 0.75 |
| Time to first surgery [days] | 2.0 | 3.8 | (1.3, 2.6) | 3.3 | 1.5 | 0.10 | 0.75 |
| LOS [days] | 17.6 | 14.3 | (15.2, 20.1) | 22.8 | 16.0 | 0.09 | 0.75 |
| Number of operations | 3.3 | 2.9 | (2.8, 3.8) | 3.5 | 3.2 | 0.09 | 0.00 |
| Location, % | |||||||
| No data | 0.8 | 0.7 | (0.0, 2.2) | 0 | 1.0 | ||
| Extremity | 55.2 | 4.3 | (46.8, 63.6) | 43.8 | 58.9 | ||
| Body | 35.1 | 4.1 | (27.0, 43.2) | 56.3 | 28.4 | ||
| Body+extremity | 9.0 | 2.5 | (4.1, 13.8) | 0 | 11.8 | 0.03 | 0.00 |
| Number of comorbidities, % | |||||||
| 0 | 33.6 | 4.1 | (25.6, 41.6) | 31.3 | 34.1 | ||
| 1 | 25.4 | 3.8 | (18.0, 32.7) | 18.8 | 27.5 | ||
| 2 | 20.9 | 3.5 | (14.0, 27.8) | 18.8 | 21.6 | 0.16 | 14.93 |
| 3 | 14.9 | 3.1 | (8.9, 21.0) | 21.9 | 12.8 | ||
| 4 | 5.2 | 1.9 | (1.5, 9.0) | 9.4 | 3.9 | ||
| Alcohol, tobacco, drug use, % | |||||||
| Denied | 64.2 | 4.1 | (56.1, 72.3) | 68.8 | 62.8 | ||
| 1 substance | 19.4 | 3.4 | (12.7, 26.1) | 9.4 | 22.6 | 0.80 | 16.42 |
| 2 substances | 12.7 | 2.9 | (7.1, 18.3) | 15.6 | 11.8 | ||
| 3 substances | 3.7 | 1.6 | (0.5, 6.9) | 6.3 | 2.9 | ||
For each variable in column 1, column 2 shows percentages for categorical variables and means for numerical variables. Columns 3 and 4 contain standard deviations and 95% confidence intervals (CI), respectively. Columns 5 and 6 have the percentages/means conditioned on survival status. Column 7 has p values of the Wald statistic, and missing data values are noted in column 8, and when greater than 5% these present a limitation of the data set because of small sample size.
LOS=length of stay.
Table 2.
Descriptive Statistics of Admission Laboratory Data
| Variable | Mean/percent | Standard deviation | 95% CI | Non- survivor | Survivor | p | Missing values [%] |
|---|---|---|---|---|---|---|---|
| WBC [k/mcL] | 17.3 | 9.7 | (15.6, 18.9) | 17.8 | 17.1 | 0.72 | 0.00 |
| Hb [g/dL] | 11.3 | 2.3 | (10.9, 11.7) | 11.1 | 11.4 | 0.51 | 0.00 |
| Platelets [k/mcL] | 275.6 | 169.8 | (246.6, 304.6) | 289.0 | 271.4 | 0.61 | 0.00 |
| Hematocrit [%] | 33.6 | 6.7 | (32.5, 34.8) | 32.6 | 33.9 | 0.33 | 0.00 |
| Na [mmol/L] | 133.8 | 5.9 | (132.7, 134.8) | 134.6 | 133.5 | 0.35 | 0.00 |
| Lowest Na [mmol/L] | 131.5 | 5.2 | (130.6, 132.4) | 131.7 | 131.4 | 0.81 | 5.97 |
| CI [mmol/L] | 100.9 | 9.0 | (99.4, 102.5) | 101.6 | 100.7 | 0.62 | 1.49 |
| BUN [mg/dL] | 25.6 | 19.5 | (22.3, 29.0) | 23.5 | 26.3 | 0.47 | 2.99 |
| K [mmol/L] | 3.9 | 0.6 | (3.8, 4.0) | 4.0 | 3.9 | 0.68 | 0.00 |
| CO2 [mmol/L] | 20.8 | 5.6 | (19.9, 21.8) | 21.4 | 20.7 | 0.54 | 0.00 |
| CRP [mg/L] | 18.9 | 14.1 | (16.0, 21.8) | 13.2 | 20.7 | 0.03 | 32.09 |
| Highest CRPa [mg/L] | 21.5 | 15.0 | (18.2, 24.9) | 13.6 | 23.9 | 0.01 | 42.54 |
| Prealbumin [mg/dL] | 7.8 | 5.5 | (6.6, 8.9) | 7.8 | 7.7 | 0.93 | 35.07 |
| Cre [mg/dL] | 1.5 | 1.5 | (1.2, 1.7) | 1.3 | 1.5 | 0.52 | 0.00 |
| Highest Crea [mg/dL] | 1.9 | 1.7 | (1.6, 2.2) | 1.81 | 1.57 | 0.86 | 3.73 |
| Glu [mg/dL] | 181.4 | 128.1 | (159.5, 203.3) | 188.6 | 179.2 | 0.72 | 0.00 |
| Highest Glua [mg/dL] | 237.0 | 164.1 | (208.4, 265.6) | 264.8 | 228.1 | 0.29 | 3.73 |
| Ca [mg/dL] | 8.2 | 1.2 | (8.0, 8.4) | 8.1 | 8.2 | 0.52 | 0.00 |
| Ph [mg/dL] | 3.8 | 1.5 | (3.5, 4.1) | 4.0 | 3.8 | 0.60 | 17.91 |
| Mg [mg/dL] | 1.9 | 0.3 | (1.8, 1.9) | 1.9 | 1.9 | 0.53 | 18.66 |
| Temp [°F] | 98.7 | 1.8 | (98.4, 99.1) | 98.7 | 98.8 | 0.85 | 16.42 |
| Systolic BP [mm Hg] | 121.9 | 26.8 | (116.9, 126.8) | 125.1 | 120.8 | 0.46 | 14.93 |
| Diastolic BP [mm Hg] | 69.4 | 17.3 | (66.2, 72.6) | 71.4 | 68.7 | 0.49 | 15.67 |
| HR [beats/min] | 102.0 | 19.7 | (98.3, 105.6) | 103.8 | 101.4 | 0.57 | 14.93 |
| RR [breaths/min] | 20.2 | 9.3 | (18.5, 22.0) | 19.8 | 20.4 | 0.75 | 18.66 |
| O2 Saturation [%] | 96.4 | 3.8 | (95.6, 97.1) | 96.7 | 96.2 | 0.59 | 24.63 |
The means, standard deviations, and 95% confidence intervals (CI) are listed in columns 2–4. Columns 5 and 6 show the means conditioned on survival status, and column 7 has p values of the Wald statistic. Missing data values are reported in column 8, and when greater than 5% these present a limitation of the data set because of small sample size.
Highest values indicate the highest value recorded across patient's entire hospital stay while under treatment for NSTI.
WBC=white blood cell count; Hb=hemoglobin; Na=sodium; Cl=confidence interval; BUN=blood urea nitrogen; K=potassium; CO2= carbon dioxide; CRP=C-reactive protein; Cre=creatinine; Glu=serum glucose; Ca=calcium; Ph=phosphate; Mg=magnesium; BP=blood pressure; HR=heart rate; RR=respiratory rate; O2=oxygen; NSTI=necrotizing soft tissue infections.
Table 3.
Descriptive and Mortality Statistics for Microbiologic Data
| Variable | Percent of surgical infections infected | Standard deviation | 95% CI | Non -survivor | Survivor | p |
|---|---|---|---|---|---|---|
| Staphylococcus, % | 35.1 | 4.1 | (27.0, 43.2) | 15.6 | 41.2 | 0.01 |
| MRSA, % | 3.0 | 1.5 | (0.1, 5.9) | 3.1 | 2.9 | 0.96 |
| ORSA, % | 5.2 | 1.9 | (1.5, 9.0) | 3.1 | 5.9 | 0.55 |
| Pseudomonas, % | 9.0 | 2.5 | (4.1, 13.8) | 18.8 | 5.9 | 0.03 |
| Klebsiella, % | 4.5 | 1.8 | (1.0, 8.0) | 6.3 | 3.9 | 0.58 |
| Fungi, % | 9.7 | 2.6 | (4.7, 14.7) | 9.4 | 9.8 | 0.94 |
| Other gram-positive, % | 6.7 | 2.2 | (2.5, 11.0) | 6.3 | 6.9 | 0.90 |
| Other gram-negative, % | 9.0 | 2.5 | (4.1, 13.8) | 9.4 | 8.8 | 0.92 |
| Polymicrobial, % | 33.6 | 4.1 | (25.6, 41.6) | 28.1 | 34.3 | 0.45 |
The percentages, standard deviations and 95% confidence intervals are listed in columns 2–4. Columns 5 and 6 show the percentages conditioned on the survival status, and column 7 has p values of the Wald statistic.
CI=confidence interval; MRSA=methicillin-resistant Staphylococcus aureus; ORSA=oxacillin-resistant Staphylococcus aureus.
Table 4.
Model Estimation Results for Microbiologic Data
| Selection criteria | Intercept | Staphylococcus | Pseudomonas |
|---|---|---|---|
| Backward elimination Forward selection Stepwise selection | |||
| Parameter estimate | −0.9464 | −1.3046 | 1.2557 |
| Standard error | 0.25 | 0.53 | 0.64 |
| p | (0.0001) | (0.01) | (0.05) |
| OR | 0.27 | 3.51 | |
| 95% CI for OR | (0.10, 0.77) | (1.00, 12.31) | |
Note that the presence of Enterococcus spp. within a polymicrobial NSTI has a strong positive correlation with mortality whereas non-MRSA Staphylococcus spp. appear to exert a protective effect.
NSTI=necrotizing soft tissue infections; OR=odds ratio; CI=confidence interval.
Results
Population data and demographics
Of 134 patients with NSTI treated at our institution during the period under review, 31 did not survive. The mortality rate of the population is 23.8%. Nineteen patients required amputation (14.1%), of whom six were non-survivors.
Table 5 shows some descriptive measures of the demographic variables, including means/percentages, standard deviations, and 95% confidence intervals. The study population had an average age of 51 years (range, 10 to 92 years), 57% were male, and the average body mass index (BMI) was 33. Racial demographics were 50% Hispanic, 40% white, and 7.5% black.
Table 5.
Descriptive Statistics of Demographic Data
| Variable | Mean/percent | Standard deviation | 95% CI | Non-survivor | Survivor | p | Missing values (%) |
|---|---|---|---|---|---|---|---|
| Gender, % | |||||||
| Female | 43.3 | 4.3 | (34.9, 51.7) | 43.8 | 43.1 | ||
| Male | 56.7 | 4.3 | (48.3, 65.1) | 56.3 | 56.9 | 0.95 | 0.00 |
| Age [y] (range, 10–92) | 50.6 | 14.2 | (48.1, 53.0) | 48.4 | 51.3 | 0.32 | 0.00 |
| BMI | 33.3 | 10.6 | (31.1, 35.8) | 35.8 | 32.4 | 0.18 | 29.10 |
| Race, % | |||||||
| Hispanic | 50.0 | 4.3 | (41.5, 58.5) | 40.6 | 40.2 | ||
| White | 40.3 | 4.2 | (32.0, 48.6) | 53.1 | 49.0 | ||
| Black | 7.5 | 2.3 | (3.0, 11.9) | 6.3 | 10.8 | 0.70 | 2.24 |
For each variable in column 1, column 2 shows percentages for categorical variables and means for numerical variables. Columns 3 and 4 contain standard deviations and 95% confidence intervals, respectively. Columns 5 and 6 have the percentages/means conditioned on survival status. Column 7 has p values of the Wald statistic, and the percentage of missing data values is indicated in column 8.
CI=confidence interval; BMI=body mass index.
The corresponding measures used for comparison (means/percentages) and the p values of Wald tests are given in the last three columns of Table 5. Larger p values in Table 5 suggest that neither gender, age, BMI, nor race could be linked to patient mortality in the population under study.
Clinical course
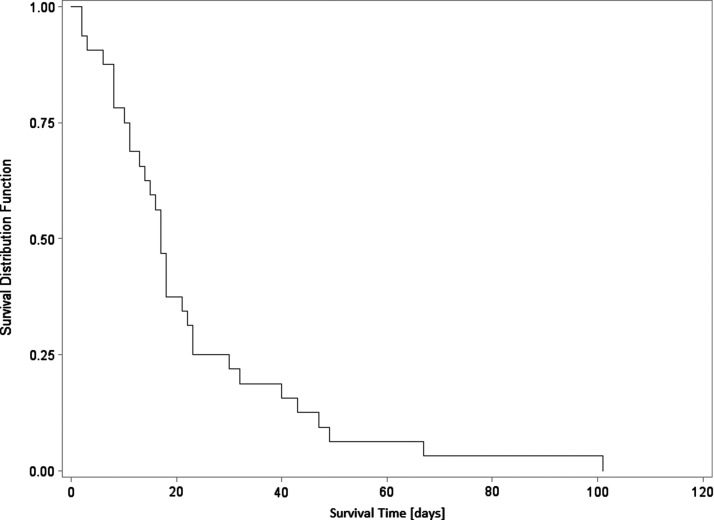
Table 1 shows clinical data associated with our patient population and association of these data with survival of NSTI. The only significant association with patient mortality in comparing anatomic sites of infection is with infection limited to an extremity predictive of greater survivability than infection found on the body/trunk or in both body/trunk plus extremity. Pre-existing conditions considered as comorbidities included diabetes mellitus, peripheral vascular disease, hypertension, heart disease, and hyperlipidemia; somewhat surprisingly, none of these proved to be a predictor of mortality. There were associations of mortality with LOS, number of operations, and time to first operation—with p values of 0.09, 0.09, and 0.10, respectively—which suggests these variables may have influenced patient outcomes in a clinically significant way although falling short of the threshold for statistical significance. Indeed, it is a biologically plausible supposition that the short time to debridement for all patients in this study might mask variations in outcome that might otherwise be apparent. Furthermore, in Figure 1, a Kaplan-Meier plot for non-survivors demonstrates an abrupt cutoff around the median survival time (at which 50% of the cohort is still alive) of approximately 17 days.
FIG. 1.
Kaplan-Meier plot for non-survivors. This plot demonstrates an abrupt cutoff around the median survival time (at which 50% of the cohort is still alive) of approximately 17 days, illustrative of the timing of death in the current study.
Laboratory values
Table 2 shows a host of laboratory values gathered on admission, only one of which has a significant association with mortality: High values for CRP and highest recorded CRP were both related to increased survival of NSTI. Interestingly, there were particularly high degrees of variability for serum sodium, blood urea nitrogen (BUN)/creatinine, glucose concentrations, temperature, and white blood cell (WBC) count. Additionally, we have considered WBC count as both a categorical and a continuous variable to seek correlation with mortality, dichotomizing based on values within versus outside normal limits. Even when abnormal WBC values (either less than 5,000 per microliter or greater than 25,000 per microliter) were compared with values within normal range, still no correlation could be drawn (as Fisher exact test p=0.82) for a variable predictive of mortality.
Microbiology
Table 3 shows the species of bacteria cultured from surgical incisions in monomicrobial NSTIs, with polymicrobial infections indicated separately. Of surgical incisions that grew bacteria in culture, 66.4% were monomicrobial NSTIs, with a large portion (65.2%) of these infections attributable to S. aureus in particular. The most common organism was S. aureus, with 18.9% of Staphylococcus infections being MRSA/ORSA. In 18 cases of 134 (13% of records), no indication in the medical record of infection was coded as no infection.
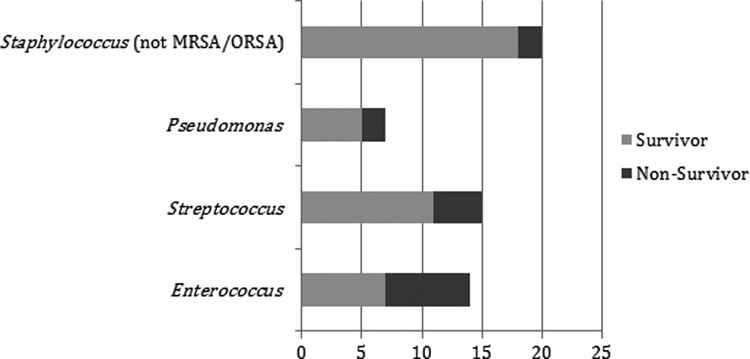
In this sample there were 45 polymicrobial infections with 32 different combinations of microbial species represented. There were 20 such infections containing Staphylococcus spp. (not MRSA/ORSA), seven containing Pseudomonas spp., 15 with Streptococcus spp., and 14 with Enterococcus spp. Mortality rates were highest with Enterococcus-containing polymicrobial infections (50%), followed by those containing Pseudomonas (40%), Streptococcus (27%), and non-MRSA/ORSA Staphylococcus (10%). Breakdown by specific species involvement is shown graphically in Figure 2. The effect of bacterial species on mortality is shown in Table 5.
FIG. 2.
Mortality in polymicrobial infections containing certain species. The x-axis indicates the percentage of all polymicrobial necrotizing soft tissue infections (NSTIs) in the current study in which certain species were found. Mortality rates varied as illustrated when these four species were detected, with Enteroroccus-containing polymicrobial NSTIs portending the worst (50% mortality), non-methicillin–resistant Staphylococcus areus (MRSA) Staphylococcus-containing NSTIs leading to highest survivability (10% mortality), and Pseudomonas spp. and Streptococcus spp. yielding intermediate values, with mortalities of 40% and 27%, respectively. Note that with respect to the overall mortality rate for this study (24%), the presence of non-MRSA Staphylococcus spp. appears to exert a protective effect in polymicrobial NSTI.
Discussion
Our study showed no significant correlation between mortality outcomes and either demographics, comorbidities, or most laboratory values. Although it is possible that this is simply the result of an underpowered study, the sample size we utilized is larger than many similar single-center studies and therefore was a surprising finding. This highlights the difficulty of using any of these parameters to predict outcome, and therefore the need for rigorous application of best practices including early surgical debridement in all these cases. In fact, the results suggested that the time to operation was shorter in survivors, although this did not reach statistical significance. This evidence corroborates the conclusions of numerous previous studies that have found that swift debridement in cases of NSTI is the single most influential determinant of patient survival. It is possible that our center's focus on early intervention may well have reduced the impact of other measures on patient mortality leading to the lack of correlation with parameters previously shown to impact NSTI outcome.
The conundrum of scoring systems
Historically, NSTIs have been a condition of special interest to clinicians because of the critical dependence of patient survival on early diagnosis and swift, aggressive intervention. Identification of consistent predictors of patient mortality focuses not only on guiding clinical decisions but also would enable us to communicate accurate prognoses more effectively with patients and their families [13–15].
Necrotizing soft tissue infections are fairly unique, not only in their emergent nature and serious mortality rate among other types of infections, but also in the protean character of the criteria by which they elude consistent characterization. In fact, it has been suggested there might not exist a single, simple pattern governing NSTI presentation as may be found for other types of infections [14,16–18]. Although the LRINEC system [11] has been utilized beyond its initial diagnostic scope to serve as a predictor of outcomes [18], even the LRINEC criteria have been known to fall short of accurate differentiation of NSTIs from other infections with sufficient sensitivity in more than one reported situation [19,20] and in at least one case it has been shown to fail completely [21]. Many data that arouse a high index of suspicion for NSTIs come in the form of physical findings such as crepitus or pain disproportionate to appearance [6,22], which are not only generally less quantifiable than laboratory values but also can have low predictive value when considered alone [11,23]. Many different subsets of laboratory values and other patient data have been put forth as the relevant cluster of criteria that should be universally predictive of mortality in patients with NSTI. One group claims that a combination of leukocytosis and hyponatremia is diagnostic [24], a statement thereafter directly refuted by a large-scale retrospective study [13]. Advanced patient age is often cited as predictive of mortality, whereas proponents of elevated WBC count [24,25] or serum procalcitonin [26] or lactate concentrations [27–29] as predictors stand in smaller company in their assertions. One researcher, citing multiple studies, admits the influence of NSTI comorbidities such as diabetes mellitus and hypertension to be a point of general disagreement [30]. Some note that no scoring system has been confirmed universally as a valid predictor of mortality in cases of NSTI [13], although internal validation of such paradigms can lead to high positive and negative predictive values [12]. Regardless, a single surgical solution does present itself clearly: It is generally agreed that quick and aggressive surgical intervention consistently correlates with better prognosis for patients with NSTIs [3,8,9].
Microbiologic considerations
The results of our study did not divide themselves naturally into the classic patterns of offending organisms by which Giuliano et al. [31], and later Morgan [32], classify NSTIs, although our analysis certainly sought possible synergistic polymicrobial relations. Rather, we believe the results to be reflective of our patient population and the relative virulence of various microbial species in our local surroundings.
It can be observed that there is a negative impact of Pseudomonas on survival compared with Staphylococcus. The odds ratio of mortality with Pseudomonas spp. present is 3.51 and that for Staphylococcus spp. is 0.27. The fact that the two species show opposing impacts on mortality can be explained using the parameter estimates as well. Positive impact of Pseudomonas spp. (1.2557) on mortality is controlled by the negative impact of Staphylococcus spp. (−1.3046) on patient mortality.
Results for polymicrobial infections are concordant intuitively with these findings as well, with Pseudomonas-containing infections more likely to result in mortality (40%) than those containing non-MRSA/ORSA Staphylococcus spp. (10%). Interestingly although not surprisingly, the presence of Enterococcus spp. in a polymicrobial infection has an even stronger association with mortality (50% rate), which is likely related to the enteral origin of such species. By nature, infections involving gut flora would most likely arise either from major trauma involving the intestines or from bowel perforation because of the NSTI itself, neither of which conditions especially portend a speedy recovery. The relative virulence of gut flora would be a difficult variable to isolate and consider independently, and we shall not attempt such analysis here.
Whether these results could translate into anything useful for other groups or regions, however, is questionable. Some studies have highlighted a possible dependence of NSTI mortality correlations with local factors—from population profiles of both the micro-organisms in question and the patients whose tissues they infiltrate—and thus these factors are believed to account for the high degree of variability in prognoses even within smaller geographic regions [10,33]. One researcher, noting mutually contradictory results among series of investigations similar in scale to our own, cites the diversity of populations and variables studied as well as small sample sizes as explanatory of such divergent results [16]. Globally, the datum with the least predictive power for mortality seems to be whether an infection is monomicrobial or polymicrobial, with particularly broad variability across the literature [34,35] and even reports of a shift in prevalence from one microbial type to the other within a single study of NSTIs [36]. Therefore, it may even be useful to reframe the term “locale” so as to distinguish not only separate geographical regions but also multiple investigations of a single region separated significantly in time.
Further considerations
What could explain such a divergence of results in our collective attempt to identify predictors of mortality in NSTI cases? Initially, it may be puzzling as to why certain laboratory findings appear in one study as distinctly correlative but in another study may not even merit a statistical sideways glance. Understanding microfloral populations as dynamic—key to explaining ailments with etiologies as straightforward as post-clindamycin Clostridium difficile colitis [37]—may underlie our quandary. The human microbiome is a subject of intense study, its scope by definition to include micro-organisms found within the human system in the state of health or disease [38,39]. However, we would propose that investigations of NSTI ultimately will characterize a greater “microbiosphere,” the scope of which would include micro-organisms resident in the immediate environment of a patient population in a certain locale. Such virulent species would play a key role in NSTIs whether such organisms should come into contact with patients through trauma or contact with an open surgical incision or mucosal membrane, or by other means.
It also may be useful explicitly to consider hybrid organisms brought about by the exchange of genetic material between microbiome and microbiosphere within a patient population as infections develop and progress. The confluence of these two “seas” of microflora in patients is brought about, in many cases, through incidents that typically necessitate surgical intervention: Traumatic introduction of external microbes into soft tissues in which they do not normally reside. Especially in cases of NSTI, for which the doubling time is exceedingly rapid, it is likely that microbiospheres (and even microbiomes) routinely undergo isolated pockets of evolution within themselves, and characterizing the organisms defining each locale may be the first step toward making sense of a cacophony of disparate results from multiple retrospective studies.
Making the distinction that may prove decisive in separating out organisms predictive of mortality, however, may extend well beyond the scope of simply culturing for genus and species. As databases from genomic sequencing platforms have only recently begun to take shape, the immense depth of microbiologic variability presents a relatively new frontier in medicine, much of the exploration of which has been confined so far to investigations of gut flora of the microbiome [40–42]. However, with increasing technologic ease and advances in throughput, it also may be possible to draw explicative correlations among genetically identifiable virulence factors within a single species, specifically those factors involving plasmid-dependent transfer of genetic material within each single species of invasive microflora.
However, this is not to imply that only local factors should inform the clinician's evaluation of NSTI, but rather that a standardized scoring system to predict NSTI mortality, should one be developed for universal use, ought not to be the first tool to which the physician should look in making treatment decisions. Continuing to use well-established principles of treatment for NSTI—early aggressive debridement, broad spectrum antibiotic coverage, and aggressive resuscitation—to treat each and every case and tailoring antibiotics to the particular microbiome of each case will provide each patient with the best possible chance of survival, regardless of initial laboratory values or presentation.
Study limitations
Limitations of the study in question must be acknowledged, especially with respect to the small sample size of a single institution study. Effective sample size was significantly reduced for some data points (secondary to greater than 5% missing data values), especially for laboratory studies not ordered routinely such as CRP, pre-albumin, phosphorus, and magnesium concentrations. Another important limitation is that we did not have information on antibiotic therapy and appropriateness of coverage for organisms involved in each case and therefore were unable to judge appropriateness of antibiotic therapy which, of course, is a significant factor for mortality in NSTI.
Conclusion
Despite efforts to build algorithms for rapid diagnosis of NSTI to increase survival rates [11,34], this study suggests that no single factor present on admission is a robust predictor of outcome. It is likely that survival in NSTI is predicated upon a complex interaction of multiple host and microbial factors that do not lend themselves to reduction into a simple formula. The best one can do in practice is to take a fairly inclusive superset of all significant factors reported from different populations to see which ones apply in one's own regional and temporal locales. In our investigations, we have found a correlation between mortality in patients with NSTI and two genus of bacteria, which we believe reflective of the local microbiome and microbiosphere and their effects on the patients we treat.
Regardless, it is clear that anything delaying early debridement and rapid surgical intervention, such as patient transfer [13,43], leads to increased mortality in NSTI [23,27,44,45] and that well-established principles of NSTI surgery should continue to be followed in all cases, irrespective of apparent severity of initial presentation.
Acknowledgments
The authors would like to thank Chance Witt, MD, and Jane A. Colmer-Hamood, PhD, of the Department of Immunology & Molecular Microbiology, as well as Phillip Watkins of the Clinical Research Institute, all of Texas Tech University Health Sciences Center, Lubbock Texas.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Martinschek A, Evers B, Lampl L, et al. . Prognostic aspects, survival rate, and predisposing risk factors in patients with fournier's gangrene and necrotizing soft tissue infections: Evaluation of clinical outcome of 55 patients. Urol Int 2012;89:173–179 [DOI] [PubMed] [Google Scholar]
- 2.Soltani AM, Best MJ, Francis CS, et al. . Trends in the incidence and treatment of necrotizing soft tissue infections: An analysis of the national hospital discharge survey. J Burn Care Res 2014;35:449–454 [DOI] [PubMed] [Google Scholar]
- 3.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: Current concepts and review of the literature. J Am Coll Surg 2009;208:279–288 [DOI] [PubMed] [Google Scholar]
- 4.Anaya DA, McMahon K, Nathens AB, et al. . Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005;140:151–157 [DOI] [PubMed] [Google Scholar]
- 5.Keung EZ, Liu X, Nuzhad A, et al. . Immunocompromised status in patients with necrotizing soft-tissue infection. JAMA Surg 2013;148:419–426 [DOI] [PubMed] [Google Scholar]
- 6.Wong CH, Chang HC, Pasupathy S, et al. . Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003;85-A:1454–1460 [PubMed] [Google Scholar]
- 7.Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin 2013;29:795–806 [DOI] [PubMed] [Google Scholar]
- 8.Howell GM, Rosengart MR. Necrotizing soft tissue infections. Surg Infect 2011;12:185–190 [DOI] [PubMed] [Google Scholar]
- 9.Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin 2013;29:795–806 [DOI] [PubMed] [Google Scholar]
- 10.Kao LS, Lew DF, Arab SN, et al. . Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: A multicenter study. Am J Surg 2011;202:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CH, Khin LW, Heng KS, et al. . The LRINEC (Laboratory risk indicator for necrotizing fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535–1541 [DOI] [PubMed] [Google Scholar]
- 12.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: Diagnosis and management. Clin Infect Dis 2007;44:705–710 [DOI] [PubMed] [Google Scholar]
- 13.Mills MK, Faraklas I, Davis C, et al. . Outcomes from treatment of necrotizing soft-tissue infections: Results from the national surgical quality improvement program database. Am J Surg 2010;200:790–796 [DOI] [PubMed] [Google Scholar]
- 14.Faraklas I, Stoddard GJ, Neumayer LA, Cochran A. Development and validation of a necrotizing soft-tissue infection mortality risk calculator using NSQIP. J Am Coll Surg 2013;217:153–160 [DOI] [PubMed] [Google Scholar]
- 15.Golger A, Ching S, Goldsmith CH, et al. . Mortality in patients with necrotizing fasciitis. Plast Reconstr Surg 2007;119:1803–1807 [DOI] [PubMed] [Google Scholar]
- 16.Anaya DA, McMahon K, Nathens AB, et al. . Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 2005;140:151–157 [DOI] [PubMed] [Google Scholar]
- 17.Yilmazlar T, Ozturk E, Alsoy A, Ozguc H. Necrotizing soft tissue infections: APACHE II score, dissemination, and survival. World J Surg 2007;31:1858–1862 [DOI] [PubMed] [Google Scholar]
- 18.Su YC, Chen HW, Hong YC, et al. . Laboratory risk indicator for necrotizing fasciitis score and the outcomes. ANZ J Surg 2008;78:968–972 [DOI] [PubMed] [Google Scholar]
- 19.Swain RA, Hatcher JC, Azadian BS, et al. . A five-year review of necrotising fasciitis in a tertiary referral unit. Ann R Coll Surg Engl 2013;95:57–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilkorn DJ, Citak M, Fehmer T, et al. . Characteristics and differences in necrotizing fasciitis and gas forming myonecrosis: A series of 36 patients. Scand J Surg 2012;101:51–55 [DOI] [PubMed] [Google Scholar]
- 21.Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: Clinical suspicion should trump scoring systems. J Emerg Med 2013;44:928–931 [DOI] [PubMed] [Google Scholar]
- 22.Anaya DA, Bulger EM, Kwon YS, et al. . Predicting death in necrotizing soft tissue infections: A clinical score. Surg Infect 2009;10:517–522 [DOI] [PubMed] [Google Scholar]
- 23.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995;221:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall DB, de Virgilio C, Black S, Klein SR. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg 2000;179:17–21 [DOI] [PubMed] [Google Scholar]
- 25.Tillou A, St. Hill CR, Brown C, Velmahos G. Necrotizing soft tissue infections: Improved outcomes with modern care. Am Surg 2004;70:841–844 [PubMed] [Google Scholar]
- 26.Friederichs J, Hutter M, Hierholzer C, et al. . Procalcitonin ratio as a predictor of successful surgical treatment of severe necrotizing soft tissue infections. Am J Surg 2013;206:368–373 [DOI] [PubMed] [Google Scholar]
- 27.Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg 2000;179:361–366 [DOI] [PubMed] [Google Scholar]
- 28.Yaghoubian A, de Virgilio C, Dauphine C, et al. . Use of admission serum lactate and sodium levels to predict mortality in necrotizing soft-tissue infections. Arch Surg 2007;142:840–846 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz S, Kightlinger E, de Virgilio C, et al. . Predictors of mortality and limb loss in necrotizing soft tissue infections. Am Surg 2013;79:1102–1105 [PubMed] [Google Scholar]
- 30.Kuncir EJ, Tillou A, St. Hill CR, et al. . Necrotizing soft-tissue infections. Emerg Med Clin North Am 2003;21:1075–1087 [DOI] [PubMed] [Google Scholar]
- 31.Giuliano A, Lewis F, Jr., Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg 1977;134:52–57 [DOI] [PubMed] [Google Scholar]
- 32.Morgan MS. Diagnosis and management of necrotising fasciitis: A multiparametric approach. J Hosp Infect 2010;75:249–257 [DOI] [PubMed] [Google Scholar]
- 33.Tunovic E, Gawaziuk J, Bzura T, et al. . Necrotizing fasciitis: A six-year experience. J Burn Care Res 2012;33:93–100 [DOI] [PubMed] [Google Scholar]
- 34.Taviloglu K, Cabioglu N, Cagatay A, et al. . Idiopathic necrotizing fasciitis: Risk factors and strategies for management. Am Surg 2005;71:315–320 [PubMed] [Google Scholar]
- 35.Tsitsilonis S, Druschel C, Wichlas F, et al. . Necrotizing fasciitis: Is the bacterial spectrum changing? Langenbecks Arch Surg 2013;398:153–159 [DOI] [PubMed] [Google Scholar]
- 36.Bernal NP, Latenser BA, Born JM, Liao J. Trends in 393 necrotizing acute soft tissue infection patients 2000–2008. Burns 2012;38:252–260 [DOI] [PubMed] [Google Scholar]
- 37.Deshpande A, Pasupuleti V, Thota P, et al. . Community-associated clostridium difficile infection and antibiotics: A meta-analysis. J Antimicrob Chemother 2013;68:1951–1961 [DOI] [PubMed] [Google Scholar]
- 38.NIH HMP Working Group, Peterson J, Garges S, et al. . The NIH human microbiome project. Genome Res 2009;19:2317–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turnbaugh PJ, Ley RE, Hamady M, et al. . The human microbiome project. Nature 2007;449:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu CM, Lin FM, Chang TH, et al. . Clinical detection of human probiotics and human pathogenic bacteria by using a novel high-throughput platform based on next generation sequencing. J Clin Bioinforma 2014;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paliy O, Kenche H, Abernathy F, Michail S. High-throughput quantitative analysis of the human intestinal microbiota with a phylogenetic microarray. Appl Environ Microbiol 2009;75:3572–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schloissnig S, Arumugam M, Sunagawa S, et al. . Genomic variation landscape of the human gut microbiome. Nature 2013;493:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holena DN, Mills AM, Carr BG, et al. . Transfer status: A risk factor for mortality in patients with necrotizing fasciitis. Surgery 2011;150:363–370 [DOI] [PubMed] [Google Scholar]
- 44.Boyer A, Vargas F, Coste F, et al. . Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 2009;35:847–853 [DOI] [PubMed] [Google Scholar]
- 45.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: Current concepts and review of the literature. J Am Coll Surg 2009;208:279–288 [DOI] [PubMed] [Google Scholar]