Abstract
Chronic inflammation marked by elevated interleukin (IL)-6, soluble tumor necrosis factor (TNF)-α receptor (sTNFR)-1, and sTNFR-2 levels may play a detrimental role in aging and HIV infection. This study aimed to evaluate the relationships of circulating IL-6 with sTNFR-1 and sTNFR-2 levels in an aging cohort of injection drug users (IDUs) with or at high risk for HIV infection. The AIDS Linked to the Intravenous Experience (ALIVE) study is a community-recruited, prospective observational study of former and current IDUs in Baltimore, Maryland. Serum IL-6, sTNFR-1, and sTNFR-2 levels were measured using standard ELISA. Multivariate linear regression analysis was employed, adjusting for age, sex, HIV status, injection drug use, comorbidities, as well as HIV viral load, CD4 T cell counts, and antiretroviral therapy where appropriate. The analysis included 1,178 participants (316 HIV positive and 862 HIV negative). In the adjusted model, sTNFR-1 and sTNFR-2 were individually associated with IL-6 (regression coefficient: 0.877 and 0.556, respectively, for all participants; 0.607 and 0.407 for HIV positives; and 0.999 and 0.628 for HIV negatives, all p < 0.0001). In the model combining sTNFR-1 and sTNFR-2, the associations for sTNFR-1 remained significant (0.693 for all participants, p < 0.0001; 0.417 for HIV positives, p < 0.05; and 0.840 for HIV negatives), while those for sTNFR-2 were no longer significant. sTNFR-1 and sTNFR-2 were positively associated with IL-6 in ALIVE participants. These findings provide initial insight into the in vivo relationship between TNF-α activation and IL-6 and a basis for further investigations into potential mechanisms underlying chronic inflammation in aging and HIV infection.
Introduction
Interleukin-6 (IL-6) is a proinflammatory cytokine with its elevated circulating level known as a hallmark of chronic inflammation observed in aging, termed “InflammAging” by some.1,2 This age-dependent chronic inflammation has been implicated in almost all pathophysiological processes and chronic conditions in older adults, such as atherosclerosis and cardiovascular diseases, anemia, frailty, disability, and mortality.3–8 Tumor necrosis factor (TNF)-α is considered a central player in the inflammatory cascade leading to systemic inflammation.9 Detections of soluble tumor necrosis factor-α receptors 1 (sTNFR-1) and 2 (sTNFR-2) are known to be reliable for measuring in vivo TNF-α activity. While TNF-α is shown to induce IL-6 production in vitro,10 in vivo relationships among sTNFR-1, sTNFR-2, and IL-6 have not been adequately investigated.
The number of older persons living with human immunodeficiency virus (HIV)-1 or acquired immune deficiency syndrome (AIDS) has risen dramatically over the past decade or so. Chronic conditions commonly encountered in the geriatric population have increasingly become major health concerns for this vulnerable aging population. Substantial evidence suggests chronic inflammation marked by elevated IL-6 levels in HIV infection and its associations with AIDS-defining as well as HIV-associated non-AIDS (HANA) conditions. For example, a number of studies have demonstrated that levels of IL-6 and C-reactive protein (CRP) are elevated with HIV infection and remain so in patients treated with highly active antiretroviral therapy (HAART) even after HIV-RNA levels are suppressed.11–14 Higher pretreatment levels of IL-6, sTNFR-1, or sTNFR-2 have been shown to be associated with HIV disease progression and mortality in patients treated with HAART.15,16 More recently, several studies have shown that elevated IL-6, sTNFR-1, or sTNFR-2 levels are associated with AIDS-defining events or HANA conditions including functional impairment.17–19 In addition, we have identified significant associations between chronic inflammation marked by elevated levels of IL-6 and CRP with HIV infection among injection drug users (IDUs) with or at high risk for HIV infection.20
The objective of this study was to evaluate the relationships between circulating IL-6 and soluble TNF-α receptors (sTNFR-1 and sTNFR-2) in an aging cohort of IDUs with or at high risk for HIV infection. We hypothesized that IL-6 would be associated with sTNFR-1 and sTNFR-2. Addressing this hypothesis will provide initial evidence for direct in vivo associations between IL-6 and TNF-α activation that may lead to improved understanding of inflammatory pathways of chronic inflammation in HIV infection and aging. To test this hypothesis, we conducted a cross-sectional analysis to evaluate the relationships between serum IL-6 and sTNFR-1 and sTNFR-2 levels, adjusting for age, sex, injection drug use, comorbidities, and HIV infection.
Materials and Methods
Study population
The AIDS Linked to the Intravenous Experience (ALIVE) study is a community-recruited, prospective observational cohort composed of former and current IDUs based in Baltimore, Maryland. Methodology has been previously described.21 During semiannual visits, ALIVE participants completed standardized questionnaires and submitted biospecimens for testing. Smoking, alcohol consumption, and illicit injection drug use were self-reported for a period of the past 6 months. Comorbid conditions were determined by self-report of a physician diagnosis of diabetes, hypertension, obesity, obstructive lung disease, anemia, hepatitis C infection, and liver or renal disease. HIV serology was determined using enzyme-linked immunosorbent assay (ELISA) with Western blot confirmation (Dupont, Wilmington, DE). Serum IL-6, sTNFR-1, and sTNFR-2 levels were measured using commercially available ELISA described below. A total of 1,190 participants had serum IL-6, sTNFR-1, and sTNFR-2 measurements at baseline. Residuals were examined and participants with high influence (determined by Cook's D value >1) for one or more of the above inflammatory markers were excluded (n = 11). Two participants were also removed due to incomplete data, leaving the sample size of 1,178 for this analysis. The Johns Hopkins University Institutional Review Board approved the study and each participant provided written informed consent.
Measurements of serum IL-6, sTNFR-1, and sTNFR-2
Serum samples were obtained from each participant according to the standard protocol, and stored in aliquots at −80°C until analysis. Serum IL-6, sTNFR-1, and sTNFR-2 were measured using commercially available ELISA according to the procedures provided by manufacturers. IL-6 was measured using the High-Sensitivity Quantikine kit (R&D Systems, Minneapolis, MN) with a detection range of 0.156–10.0 pg/ml and an interassay coefficient of variance (CV) of 5.7%. sTNFR-1 and sTNFR-2 were measured using DuoSet ELISA kits (R&D Systems, Minneapolis, MN) with a sensitivity of 12.5 pg/ml or 7.8 pg/ml and an interassay CV of 4.9% or 6.1%, respectively. Measurements were performed in duplicate and repeated if the measures differed by more than 15% or were out of the measurable range. The average of the two values in duplicate was used for analysis.
Statistical analysis
Frequency distributions were determined for baseline population characteristics. The Fisher's exact test and Student's t-test were used to determine differences between categorical and continuous data by HIV status, respectively. The median (interquartile range) was calculated for inflammatory markers and the nonparametric Wilcoxon–Mann–Whitney test was used to compare distributions between groups stratified by HIV status. Bivariate associations between sTNFR-1 and sTNFR-2 with IL-6 levels were evaluated using cross-tabulations of means and standard deviations of sTNFR-1 and sTNFR-2 levels by quartiles of IL-6; analysis of variance was used to compare group differences. Multiple linear regression was used to examine the relationship between inflammatory marker levels for IL-6, sTNFR-1, and sTNFR-2.
Separate analyses were performed for each inflammatory marker adjusting for age, sex, injection drug use over the past 6 months, number of injections within the past 30 days, HIV status, the number of comorbidities (0 or 1, 2, ≥3), HIV status, as well as HIV viral load, CD4 T cell counts, and antiretroviral therapy for the past 6 months (for the HIV-positive subset only). Two models were created using IL-6 as the dependent variable. Model A was adjusted for sTNFR-1 or sTNFR-2 individually, while model B was adjusted for both sTNFR-1 and sTNFR-2 together. To account for nonnormal distributions, levels of IL-6, sTNFR-1, and TNFR-2 were log transformed to approximate normality for linear regression analyses. Regression effects can be interpreted as a 1% change in the median covariate value for each unit change in the median outcome value. Multicolinearity was examined using variance inflation factors (VIFs) with 2.5 as a cutoff. Assumptions were checked for all models by examining error properties and residual plots. All analyses were performed using SAS statistical software (Version 9.2, Cary, NC).
Results
The majority of study participants were African American (87.5%) and male (64.9%) with a mean age of 46.8 years (range 21.2–78.1 years). HIV infection prevalence was 26.8% with 21.2% of the population reporting daily injection drug use. Table 1 summarizes basic demographic and clinical characteristics as well as medians (IQR) of IL-6, sTNFR-1, and sTNFR-2 levels of the total population and HIV-positive and HIV-negative groups. Compared to HIV-negative participants, those who were HIV positive were more likely to be African American, never married, unemployed, use injection drugs less often, less intravenous drug use over the past 6 months or inject times within the past 30 days, consume fewer alcoholic drinks/day, and have two or more comorbidities.
Table 1.
Characteristics of ALIVE Participants, Total Population and Stratified by HIV Status (N = 1178)
| Total population(n = 1178) | HIV positive(n = 316) | HIV negative(n = 862) | p-value | |
|---|---|---|---|---|
| Age, mean in years (95% CI) | 46.8 (46.3–47.2) | 46.9 (46.2–47.6) | 46.7 (46.1–47.3) | 0.762 |
| Sex | ||||
| Male | 765 (64.9) | 203 (64.2) | 562 (65.2) | 0.783 |
| Female | 413 (35.1) | 113 (35.8) | 300 (34.8) | |
| Race | ||||
| White/other | 147 (12.5) | 20 (6.3) | 127 (14.7) | 0.0001 |
| African American | 1031 (87.5) | 296 (93.7) | 735 (85.3) | |
| Marital status | ||||
| Never married | 776 (65.9) | 230 (73.3) | 546 (63.4) | 0.002 |
| Ever married | 399 (33.9) | 84 (26.8) | 315 (36.6) | |
| Employed | ||||
| No | 870 (73.9) | 253 (80.1) | 617 (71.7) | 0.004 |
| Yes | 306 (26.0) | 63 (19.9) | 243 (28.3) | |
| Cigarette smoker | ||||
| No | 181 (15.4) | 56 (17.8) | 125 (14.5) | 0.164 |
| Yes | 994 (84.4) | 258 (82.2) | 736 (85.5) | |
| IV drug use | ||||
| None | 637 (54.1) | 182 (57.6) | 455 (52.8) | 0.007 |
| < daily | 293 (24.9) | 87 (27.5) | 206 (23.9) | |
| ≥ daily | 248 (21.1) | 47 (14.9) | 201 (23.3) | |
| Number of injections past 30 days, median (IQR) | 25 (4–60) | 12 (4–35) | 30 (4–60) | 0.038 |
| Number of alcoholic drinks/day | ||||
| 0 | 549 (46.6) | 173 (54.8) | 376 (43.6) | 0.009 |
| 1–2 | 339 (28.8) | 78 (24.7) | 261 (30.3) | |
| 3–4 | 166 (14.1) | 38 (12.0) | 128 (14.9) | |
| ≥5 | 124 (10.5) | 27 (8.5) | 97 (11.3) | |
| Comorbidities | ||||
| 0 | 293 (24.9) | 43 (13.6) | 250 (29.0) | <0.0001 |
| 1 | 406 (34.5) | 87 (27.5) | 319 (37.0) | |
| 2 | 294 (25.0) | 105 (33.2) | 189 (21.9) | |
| ≥ 3 | 185 (15.7) | 81 (25.6) | 104 (12.1) | |
| BMI | ||||
| < 30 | 931 (79.0) | 259 (82.0) | 672 (78.0) | 0.135 |
| ≥ 30 | 247 (21.0) | 57 (18.0) | 190 (22.0) | |
| Hepatitis C infection | ||||
| No | 166 (14.0) | 0 (0.0) | 166 (19.3) | <0.0001 |
| Yes | 1012 (86.0) | 316 (100.0) | 696 (80.7) | |
| Antiretroviral therapy for past 6 months | ||||
| No | 148 (48.0) | |||
| Yes | 163 (52.0) | |||
| CD4 T cells, cells/mm3, median (IQR) | 304 (180–437) | |||
| HIV viral load, copies/ml, median (IQR) | 961 (400–28,000) | |||
| IL-6, pg/ml, median (IQR) | 1.61 (1.01–2.75) | 1.81 (1.21–3.12) | 1.50 (0.95–2.67) | 0.0001 |
| sTNFR-1, pg/ml, median (IQR) | 1491 (1260–1834) | 1484 (1257–1955) | 1492 (1261–1808) | 0.416 |
| sTNFR-2, pg/ml, median (IQR) | 4976 (3833–6900) | 9682 (5113–9213) | 4487 (3592–5964) | <0.0001 |
Values are number (%) unless otherwise noted.
ALIVE, AIDS linked to the intravenous experience; IQR, interquartile range; IL-6, interleukin-6; sTNFR, soluble tumor necrosis factor-α receptor.
The prevalence of hepatitis C infection was 86% for the total population included in this analysis with hepatitis C coinfection present in all HIV-positive participants and 80.7% of hepatitis C infection among HIV-negative participants (p = 0.0001). Antiretroviral therapy was used for the past 6 months by 52.0% of HIV-positive participants. There was no statistical difference with regard to age, sex, smoking, or body mass index. The median (IQR) CD4+ T cell count and HIV viral load for HIV-positive participants were 304 cells/mm3 (180–437) and 961 copies/ml (400–28,000), respectively. Median (IQR) inflammatory marker levels for the total study population were 1.61 pg/ml (1.01–2.75) for IL-6, 1,491 pg/ml (1,260–1,834) for sTNFR-1, and 4,976 pg/ml (3,833–6,900) for sTNFR-2 (Table 1). HIV-positive participants had significantly higher IL-6 and sTNFR-2 levels than HIV-negative participants (median 1.81 vs. 1.50 pg/ml, p = 0.0001 and 9,682 vs. 4,487 pg/ml, p < 0.0001, respectively), while there was no significant difference in sTNFR-1 levels between the two groups (1,484 vs. 1,492 pg/ml, p = 0.416).
The potential associations of age, sex, race, and HIV status in the total study population were assessed by bivariate regression analyses (Table 2). IL-6 levels were significantly associated with age, sex, and HIV status, but not race. Levels of sTNFR-1 were significantly associated with age and race but not sex or HIV status. Levels of sTNFR-2 were significantly associated with age and HIV status but not sex or race. Median IL-6 levels were increased by 19.8% and sTNFR-2 levels by 51.5% in HIV-positive persons compared to HIV-negative persons (p < 0.05). Females had median IL-6 levels that were 17.7% higher compared to males. Levels of sTNFR-1 were significantly lower in African American participants than in all other races.
Table 2.
Effects of Age, Race, and HIV Status on Interleukin-6, Soluble Tumor Necrosis Factor-α Receptor-1, and Soluble Tumor Necrosis Factor-α Receptor-2 Levels as Shown by Regression Coefficients (95% CI)
| Age (years) | Female vs. male | African-American vs. white/other | HIV positive vs. HIV negative | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| IL-6 (pg/ml) | 1.008* | (1.001, 1.015) | 1.177* | (1.053, 1.315) | 1.07 | (0.912, 1.257) | 1.198* | (1.063, 1.350) |
| sTNFR-1 (pg/ml) | 1.006* | (1.004, 1.008) | 1.006 | (0.969, 1.045) | 0.902* | (0.854, 0.953) | 1.017 | (0.977, 1.060) |
| sTNFR-2 (pg/ml) | 1.005* | (1.002, 1.008) | 1.018 | (0.964, 1.076) | 0.945 | (0.872, 1.023) | 1.515* | (1.435, 1.600) |
p < 0.05.
Since log-transformed scores were used in the regression analyses, values presented are exponentiated regression coefficients.
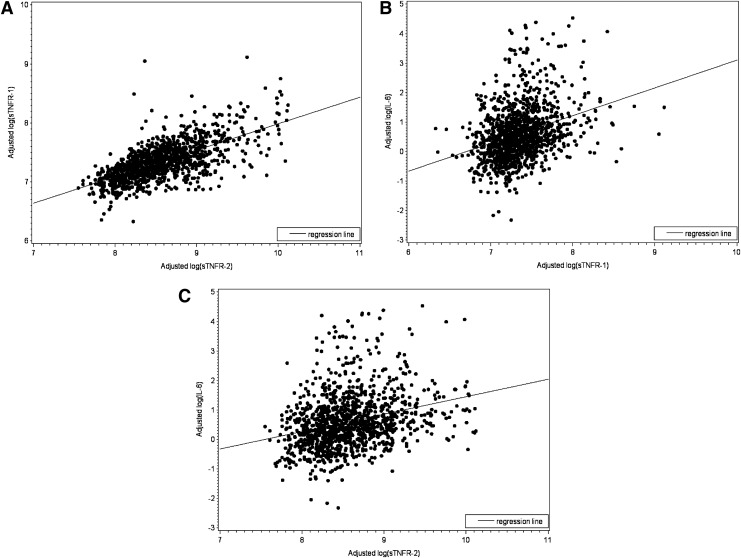
The relationships between levels of sTNFR-1 and sTNFR-2 and that of IL-6 in the total study population were evaluated next. First, the means and standard deviations of sTNFR-1 and sTNFR-2 levels were cross-tabulated with quartiles of the IL-6 level. This analysis was to evaluate the relationships using raw data stratified by IL-6 quartiles. We found stepwise increases in sTNFR-1 and sTNFR-2 levels across IL-6 quartiles (Table 3). We then assessed the associations between log-transformed sTNFR-1, sTNFR-2, and IL-6 levels, adjusting for age, sex, comorbidities, injection drug use over the past 6 months, number of injections in the past 30 days, and HIV status. This analysis was to evaluate bilateral associations between sTNFR-1, sTNFR-2, and IL-6 levels. Log(sTNFR-1) and log(sTNFR-2) were strongly associated with each other (partial correlation coefficient r = 0.681, p < 0.0001, Fig. 1A). Moreover, log(IL-6) was significantly associated with log(sTNFR-1) (r = 0.246, p < 0.0001, Fig. 1B) and log(sTNFR-2) (r = 0.250, p < 0.0001, Fig. 1C).
Table 3.
Mean (SD) of Soluble Tumor Necrosis Factor-α Receptor-1 and Soluble Tumor Necrosis Factor-α Receptor-2 Levels Across Interleukin-6 Quartiles
| IL-6 quartiles (range in pg/ml) | |||||
|---|---|---|---|---|---|
| 0–25% (0–1.1) | 26–50% (1.1–1.6) | 51–75% (1.6–2.7) | 76–100% (2.7–93.5) | p-value* | |
| sTNFR–1 (pg/ml) | 1395 (444) | 1555 (486) | 1719 (701) | 1885 (828) | <0.0001 |
| sTNFR–2 (pg/ml) | 4591 (2182) | 5696 (3219) | 6434 (3593) | 7073 (4064) | <0.0001 |
p value is from the trend test of the difference in sTNFR-1 and sTNFR-2 levels across IL-6 quartiles.
FIG. 1.
Scatterplots with regression line shows, respectively the fitted relationship of (A) log soluble tumor necrosis factor-α receptor (sTNFR)-2 and log sTNFR-1, (B) log sTNFR-1 and log interleukin (IL)-6, and (C) log sTNFR-2 and log IL-6 in all AIDS Linked to the Intravenous Experience (ALIVE) participants, adjusting for age, sex, number of comorbidities (0 or 1, 2, 3), injection drug use over the past 6 months and number of injections within the past 30 days, and HIV status.
Multiple linear regression analyses were performed using log(IL-6) as the outcome measure and log(sTNFR-1) or log(sTNFR-2) as predictors both in the total study population and in HIV-positive and HIV-negative subgroups, adjusting for age, sex, race, comorbidities, injection drug use in the past 6 months, number of injections in the past 30 days, and HIV status (for the total study population only). Analysis for HIV-positive subgroup was also adjusted for HIV viral load and CD4+ T cell count. In Model A, log(sTNFR-1) and log(sTNFR-2) individually had significant associations with log(IL-6) in the total study population [regression coefficients 0.877 (standard error, 0.084) and 0.556 (0.064), respectively, both p < 0.001] as well as in both HIV-positive [0.607 (0.145), p < 0.001 and 0.407 (0.105), p < 0.05, respectively] and HIV-negative [0.999 (0.103) and 0.628 (0.081), respectively, both p < 0.001] subgroups (Table 4). When both log(sTNFR-1) and log(sTNFR-2) were included in the same model (Model B), the association between log(IL-6) and log(sTNFR-1) remained statistically significant both in the total study population [0.693 (0.115), p < 0.001] and in HIV-positive [0.417 (0.193), p < 0.05] and HIV-negative [0.840 (0.143), p < 0.001] subgroups.
Table 4.
Adjusted Regression Coefficients of Inflammatory Markers (SE)
| Total population | HIV positive | HIV negative | |
|---|---|---|---|
| log(IL-6) Model A | |||
| log(sTNFR-1) | 0.877 (0.084)* | 0.607 (0.145)* | 0.999 (0.103)* |
| log(sTNFR-2) | 0.556 (0.064)* | 0.407 (0.105)** | 0.628 (0.081)* |
| log(IL-6) Model Ba | |||
| log(sTNFR-1) | 0.693 (0.115)* | 0.417 (0.193)** | 0.840 (0.143)* |
| log(sTNFR-2) | 0.204 (0.086)** | 0.207 (0.140) | 0.177 (0.110) |
p < 0.0001, **p < 0.05.
Model B adjusted for both sTNFR-1 and sTNFR-2.
All models adjusted for age, sex, race, number of comorbidities (0 or 1, 2, ≥3), drug use in the past 6 months (yes/no), number of injections in the past 30 days.
Total population group adjusted for HIV.
SE, standard error.
Discussion
In this study, we have identified significant in vivo associations of circulating sTNFR-1 and sTNFR-2 with IL-6 levels in an aging cohort of IDUs with and at risk for HIV infection, with adjustment for age, sex, race, comorbidities, injection drug use over the past 6 months and number of injections within the past 30 days, and HIV status. These positive associations remain valid in both HIV-positive and HIV-negative subgroups.
A large body of literature describes elevated levels of inflammatory cytokines in HIV infection and their associations with various clinical outcomes. In addition to the studies on IL-6 and CRP cited above,11–14,22 a significant number of studies have shown high levels of sTNFR-1 and/or sTNFR-2 levels and their associations with poor clinical outcomes or HANA conditions in HIV infection.15–19,23–27 Morlat and colleagues observed larger declines of TNF-α and sTNFR-2 levels during the early stages of HIV infection among nonprogressors than in progressors, suggesting the potential importance of chronic TNF-α activation in AIDS progression.28
In the IDU population, several studies have shown elevated levels of IL-6, TNF-α, or sTNFR-2.20,29–32 Other in vivo studies outside of HIV infection, either in a murine model or in patients with melanoma, have also shown that stimulation with LPS or TNF leads to elevated sTNFR-1 and sTNFR-2 levels.33,34 Our results of no significant difference in sTNFR-1 levels between HIV-positive and HIV-negative subgroups (Table 1) are somewhat surprising, but consistent with a number of previous studies in which only sTNFR-2 levels were shown to be elevated in HIV infection and associated with poor clinical outcomes.24,25,28,35 However, recent studies have also reported significant associations of elevated levels of sTNFR-1 with AIDS-defining events/progression or HANA conditions, suggesting that chronic TNF-α activation represented by sTNFR-1 may play a critical role in the pathogenesis of HIV infection and progression.15,17,19,27
Few studies have evaluated the relationships between inflammatory markers in HIV-infected or IDU populations. Such an evaluation is important as it may shed light on the underlying mechanisms of chronic inflammation observed in these two unique and yet related vulnerable populations. The significance of such evaluation is illustrated in rheumatoid arthritis, a common systemic inflammatory condition. While a number of inflammatory markers (TNF-α, IL-6, CRP, etc.) are known to be elevated in rheumatoid arthritis, it is the identification of TNF-α as the key cytokine mediator in relation to other inflammatory markers that has led to the highly effective anti-TNF-α therapy that has now become the standard care for this condition.36,37 To the best of our knowledge, this study is the first to report significant in vivo associations of circulating sTNFR-1 and sTNFR-2 with IL-6 levels in IDUs with or at high risk for HIV infection.
While the etiology of chronic inflammation in IDUs is largely unknown at the present time, hepatitis C, which is a common chronic viral infection in IDUs and coinfection in those with HIV infection, could be an important factor. Although the observed associations could simply be explained by correlations among parameters reflecting the overall inflammatory milieu, TNF-α, once activated, may trigger downstream biological activities including activation of the inflammatory cascade through two receptors, TNFR-1 and TNFR-2. Numerous cell types including immune cells, endothelial cells, and fibroblasts are known to express TNFR-1, whereas TNFR-2 is expressed only by immune cells (T and B lymphocytes, monocytes, and macrophages). In vitro studies showed that TNFR-1 mediated stimulation of HIV replication at a postintegration level while TNFR-2 inhibited HIV replication at viral entry in primary human macrophages.38 Douni and Kollias observed that production of TNFR-2 in transgenic mice resulted in severe systemic inflammatory syndrome independent of TNFR-1.39 Taken together, while sTNFR-1 and sTNFR-2 levels are both indicators of TNF-α activation in vivo, they appear to have different biological characteristics and play distinct roles in systemic inflammation and HIV biology.
Consistent with this, our results demonstrated significant associations between sTNFR-1 and sTNFR-2 levels (r = 0.681), but the r2 value was far from 1.0 (Fig. 1A). The significant associations of sTNFR-1 and sTNFR-2 with IL-6 levels individually in the total study population as well as in HIV-positive and HIV-negative subgroups with the adjustment of potential covariates suggest a role of TNF-α activation in vivo contributing to chronic and systemic inflammation in this population.
The finding that the association between sTNFR-1 and IL-6 levels remained significant when both sTNFR-1 and sTNFR-2 were included in the same model (Table 4, Model B) is intriguing. Lederman et al. described a model suggesting that HIV replication induces TNF-α and TNFR-2 production, which, in turn, induces multiple other indices of inflammation and immune activation.35 However, sTNFR-1 levels were not included in their analysis. One possible explanation for our finding is that as TNFR-1 is produced by many other cell types in addition to the immune cells, its association with systemic inflammation is likely stronger than that of sTNFR-2, which is primarily expressed by the immune cells only. Further investigations into the role and regulation of TNFR-1 and TNFR-2 in the development of chronic systemic inflammation and HIV infection are indicated.
This study has several limitations. First, although we vigorously excluded participants with outlier values and adjusted for possible confounding factors in our analysis, potential contributions from chronic infections or other factors that were not in the dataset or unknown to the participants could not be completely eliminated. In addition, we could not determine causal directionality of the identified associations in this cross-sectional analysis. Another interpretation of our findings is that sTNFR-1, sTNFR-2, and IL-6 are all produced by the immune cells. However, Barcellini and colleagues have reported no increase in IL-6 production by the peripheral blood mononuclear cells (PBMCs) from IDUs,40 suggesting that sources other than the immune cells are likely the main contributors to elevated IL-6 levels in IDUs. Further studies, including longitudinal analyses, are needed to determine the causal directionality of these associations.
We did not include CRP or other inflammatory markers in the analysis as we intended to focus on IL-6, the most consistent and widely measured cytokine mediator in the aging and HIV literature that is not known to be affected by individual organ function (e.g., CRP is primarily produced by the liver and its levels may be influenced by liver disease common among IDUs).20 Finally, we could not address the sources of circulating sTNFR-1, sTNFR-2, and IL-6 in this epidemiological study. This may be important as TNFR-1 and TNFR-2 are known to be produced by different cell types and injection drug behavior and HIV infection may preferentially impact different tissue or organ systems and their function.
Despite these limitations, findings from this study do support our original hypothesis and suggest a potential role of TNF-α activation marked by elevated sTNFR-1 and sTNFR-2 levels contributing to chronic inflammation in IDUs with or at high risk for HIV infection. This initial in vivo evidence, if confirmed and further expanded, will provide a basis for future investigations into TNF-α activation as a potential target for the development of interventional strategies for chronic inflammation and its associated chronic conditions in the vulnerable aging IDU population with or at high risk for HIV infection.
Acknowledgments
The authors would like to thank the ALIVE study participants and staff for their contributions to this research.
This work is supported in part by NIH Grants R01-DA-004334, U01-DA-036297, and RC1-AI-086053 (PI: Dr. Gregory D. Kirk), R01-DA-12568 (PI: Dr. Shruti Mehta), R21-AG-043874 (PI: Dr. Sean X. Leng), and funding from the Irma and Paul Milstein Program for Senior Health of the Milstein Medical Asian and American Partnership (MMAAP) Foundation (www.mmaapf.org) (to Dr. Sean X. Leng).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Maggio M, Guralnik JM, Longo DL, and Ferrucci L: Interleukin-6 in aging and chronic disease: A magnificent pathway. J Gerontol A Biol Sci Med Sci 2006;61(6):575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franceschi C. and Campisi J: Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014;69(Suppl 1):S4–S9 [DOI] [PubMed] [Google Scholar]
- 3.Rodondi N, Marques-Vidal P, Butler J, et al. : Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol 2010;171(5):540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrucci L, Semba RD, Guralnik JM, et al. : Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010;115(18):3810–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen HJ, Harris T, and Pieper CF: Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med 2003;114(3):180–187 [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Penninx BW, Volpato S, et al. : Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002;50(12):1947–1954 [DOI] [PubMed] [Google Scholar]
- 7.Leng SX, Xue QL, Tian J, et al. : Inflammation and frailty in older women. J Am Geriatr Soc 2007;55(6):864–871 [DOI] [PubMed] [Google Scholar]
- 8.Yao X, Li H, and Leng SX: Inflammation and immune system alterations in frailty. Clin Geriatr Med 2011;27(1):79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann M: The cytokine network in rheumatoid arthritis: Definition of TNF alpha as a therapeutic target. J R Coll Physicians Lond 1996;30(6):560–570 [PMC free article] [PubMed] [Google Scholar]
- 10.Van DJ, Opdenakker G, Simpson RJ, et al. : Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med 1987;165(3):914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regidor DL, Detels R, Breen EC, et al. : Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS 2011;25(3):303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. : Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201(12):1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armah KA, McGinnis K, Baker J, et al. : HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012;55(1):126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware DR, Hullsiek KH, Puronen CE, et al. : Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011;203(11):1637–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalayjian RC, Machekano RN, Rizk N, et al. : Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis 2010;201(12):1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saves M, Morlat P, Chene G, et al. : Prognostic value of plasma markers of immune activation in patients with advanced HIV disease treated by combination antiretroviral therapy. Clin Immunol 2001;99(3):347–352 [DOI] [PubMed] [Google Scholar]
- 17.McComsey GA, Kitch D, Sax PE, et al. : Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr 2014;65(2):167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlandson KM, Allshouse AA, Jankowski CM, et al. : Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013;208(2):249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenorio AR, Zheng Y, Bosch RJ, et al. : Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014;210:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter ML, Lau B, Mehta SH, et al. : Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr 2013;64(5):488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlahov D, Anthony JC, Munoz A, et al. : The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: Description of methods and characteristics of participants. NIDA Res Monogr 1991;109:75–100 [PubMed] [Google Scholar]
- 22.Bastard JP, Soulie C, Fellahi S, et al. : Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir Ther 2012;17(5):915–919 [DOI] [PubMed] [Google Scholar]
- 23.Zangerle R, Steinhuber S, Sarcletti M, et al. : Serum HIV-1 RNA levels compared to soluble markers of immune activation to predict disease progression in HIV-1-infected individuals. Int Arch Allergy Immunol 1998;116(3):228–239 [DOI] [PubMed] [Google Scholar]
- 24.Godfried MH, van der Poll T, Weverling GJ, et al. : Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis 1994;169(4):739–745 [DOI] [PubMed] [Google Scholar]
- 25.Stein DS, Lyles RH, Graham NM, et al. : Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: A comparative analysis of quantification of HIV RNA, soluble tumor necrosis factor type II receptors, neopterin, and beta2-microglobulin. Multicenter AIDS Cohort Study. J Infect Dis 1997;176(5):1161–1167 [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen PH, Dodt KK, Meyer CN, et al. : Increased levels of soluble tumour necrosis factor receptor-I (P55) and decreased IgG1 reactivities in HIV-1 patients with cytomegalovirus disease. Scand J Immunol 1998;47(6):591–595 [DOI] [PubMed] [Google Scholar]
- 27.Brown TT, Tassiopoulos K, Bosch RJ, et al. : Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010;33(10):2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morlat P, Pereira E, Clayette P, et al. : Early evolution of plasma soluble TNF-alpha p75 receptor as a marker of progression in treated HIV-infected patients. AIDS Res Hum Retroviruses 2008;24(11):1383–1389 [DOI] [PubMed] [Google Scholar]
- 29.Ryan LA, Brester M, Bohac D, et al. : Up-regulation of soluble tumor necrosis factor receptor two in plasma of HIV-seropositive individuals who use opiates. AIDS Res Hum Retroviruses 2004;20(1):41–45 [DOI] [PubMed] [Google Scholar]
- 30.Li JR, Gong RY, Li YP, et al. : Research on HIV/Toxoplasma gondii co-infection and cytokine levels among intravenous drug users. Parasite Immunol 2010;32(2):161–164 [DOI] [PubMed] [Google Scholar]
- 31.Warshow UM, Riva A, Hegazy D, et al. : Cytokine profiles in high risk injection drug users suggests innate as opposed to adaptive immunity in apparent resistance to hepatitis C virus infection. J Viral Hepat 2012;19(7):501–508 [DOI] [PubMed] [Google Scholar]
- 32.Ajello F, La LR, Lodato M, et al. : Soluble tumor necrosis factor alpha receptors (sTNF-Rs) in HIV-1-infected intravenous drug users: Change in circulating sTNF-R type II level and survival for AIDS patients. Eur J Epidemiol 2000;16(3):209–216 [DOI] [PubMed] [Google Scholar]
- 33.Bemelmans MH, Gouma DJ, and Buurman WA: LPS-induced sTNF-receptor release in vivo in a murine model. Investigation of the role of tumor necrosis factor, IL-1, leukemia inhibiting factor, and IFN-gamma. J Immunol 1993;151(10):5554–5562 [PubMed] [Google Scholar]
- 34.Gerain J, Lienard D, Pampallona S, et al. : Systemic release of soluble TNF receptors after high-dose TNF in isolated limb perfusion. Cytokine 1997;9(12):1034–1042 [DOI] [PubMed] [Google Scholar]
- 35.Lederman MM, Kalish LA, Asmuth D, et al. : ‘Modeling’ relationships among HIV-1 replication, immune activation and CD4+ T-cell losses using adjusted correlative analyses. AIDS 2000;14(8):951–958 [DOI] [PubMed] [Google Scholar]
- 36.Wiens A, Venson R, Correr CJ, et al. : Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy 2010;30(4):339–353 [DOI] [PubMed] [Google Scholar]
- 37.Jin J, Chang Y, and Wei W: Clinical application and evaluation of anti-TNF-alpha agents for the treatment of rheumatoid arthritis. Acta Pharmacol Sin 2010;31(9):1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbein G. and Gordon S: 55- and 75-kilodalton tumor necrosis factor receptors mediate distinct actions in regard to human immunodeficiency virus type 1 replication in primary human macrophages. J Virol 1997;71(5):4150–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douni E. and Kollias G: A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J Exp Med 1998;188(7):1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barcellini W, Rizzardi GP, Velati C, et al. : In vitro production of type 1 and type 2 cytokines by peripheral blood mononuclear cells from high-risk HIV-negative intravenous drug users. AIDS 1995;9(7):691–694 [DOI] [PubMed] [Google Scholar]