Abstract
Background: Our previous work has shown that there are sex differences in subjective responses to acute caffeine administration in adolescents. The purpose of this study was to determine if these sex differences are dependent on pubertal development.
Materials and Methods: We examined subjective responses before and after administration of 0, 1, and 2 mg/kg of caffeine in pre- and postpubertal boys and girls (n = 112). In addition, we examined differences in subjective responses to acute caffeine in both the luteal and follicular phases of the menstrual cycle in postpubertal girls.
Results: Caffeine at both doses resulted in greater changes in responses on the Addiction Research Center Inventory and the Brief Assessment of Mood States compared with placebo. Girls reported greater increases from baseline to peak in feeling different and liking the feeling than boys after 2 mg/kg of caffeine regardless of pubertal stage. Postpubertal girls also had a greater decrease from baseline in reports of feeling high and greater increases from baseline in reports of wanting more than postpubertal males. Finally, girls had greater changes (both increases and decreases) in responses on the Brief Mood Questionnaire when in the follicular phase compared with the luteal phase. This was also true for reports of feeling high and feeling different on the Drug Effects Questionnaire. None of these effects varied as a function of usual caffeine use, suggesting that differences are not the result of tolerance or sensitization.
Conclusions: These results suggest that subjective responses to caffeine emerge before puberty, but sex differences may be strengthened after pubertal development.
Introduction
Caffeine intake among US children continues to be high.1 Indeed, caffeine from soda is being replaced by intake from sources such as coffee and energy drinks, which are readily available and high in caffeine content.1–3 Recent high-profile deaths associated with energy drink consumption have prompted the American Academy of Pediatrics to officially discourage caffeine use among children and adolescents and led the Food and Drug Administration (FDA) to review the empirical research concerning the effects of caffeine consumption in children and adolescents. Caffeine is known to disrupt sleep patterns4–6 and to increase blood pressure and decrease heart rate7–9 in children and adults. In addition, children and adolescents tend to consume caffeine delivered in sweetened soda and energy drinks,1,2 which promotes consumption of sugar-sweetened beverages1,2,10–12 and therefore may indirectly contributes to weight gain and dental caries.13,14
Acute doses of caffeine produce mild, subjective psychostimulant effects, which may promote its use.15 For example, doses ranging from 30 to 200 mg increase reports of positive mood,16–19 alertness,17,20 and feelings of euphoria.16 These effects are comparable with low doses of amphetamine21,22 and appear to be moderated by sex and menstrual cycle phase.23,24 We showed that caffeine also produced subjective effects in postpubertal adolescents (aged 15–17 years), including feeling high and feeling different,24 and that higher estradiol levels predicted reduced responses in females, but were associated with the opposite effect in males. Although it is not clear if this is the direct effect of estradiol levels, it suggests that steroid hormones may play a role in the sex differences in response to caffeine. The subjective effects of caffeine in prepubertal boys and girls have not been examined.
Men and women differ in the use of many licit and illicit substances.25–27 The mechanism for this may be physiological, psychosocial, or a combination of the two. Cytochrome p450 is the enzyme that metabolizes both estrogens and caffeine,28,29 thus levels of this enzyme availability may vary with menstrual cycle phase or as a function of gender and may alter the rate of caffeine metabolism.30 This difference in metabolism may relate to how the downstream effects of caffeine are experienced. As mentioned above, we have shown that boys are more likely to report using caffeine to experience drug-like effects,24 but when drug effects are directly assessed after acute caffeine administration, we find that girls often report stronger drug effects than do boys and that these effects differ as a function of menstrual cycle phase.24 Alternatively, sex differences in caffeine use may be related to psychosocial factors. For example, energy drinks are heavily marketed toward adolescent and young adult males.31 This may selectively promote energy drink use among males and create sex differences in use patterns. It is important to understand the development of sex differences in substance use, but it is not ethical or advisable to give illicit drugs to naive children and adolescents. Caffeine, however, is commonly used among this population and can be administered in a dose-dependent manner. We have sought to determine the developmental trajectory of sex differences in caffeine response with the idea that these differences will inform our understanding of sex differences in substance use more broadly.
The purpose of this study was to examine the subjective effects of acute caffeine administration in pre- and postpubertal boys and girls. In addition, we investigated whether the effects of caffeine varied across the menstrual cycle in postpubertal females. The results from this study build upon our previous work on subjective responses by examining a broader range of drug effects and extend our findings to prepubertal children.
Materials and Methods
Participants and recruitment
Participants were prepubertal boys (n = 27) and girls (n = 28) between the ages of 8 and 9 years and postpubertal boys (n = 30) and girls (n = 27) between the ages of 15 and 17 years. Pubertal status was confirmed using self- and parental reports of Tanner stage and responses on a Pubertal Development Questionnaire (described below). Eligible participants were not cigarette smokers, had previous experience with caffeine and had no adverse reactions, were not using hormone-based contraceptives and/or not pregnant, were not on any medications affecting caffeine metabolism, and were willing to abstain from regular caffeine use. Only 13 participants reported medication use and, of these, one participant was taking an inhaled steroid with potential effects on heart rate and blood pressure.
Experimental procedures
Participants visited the laboratory on six occasions, three visits 1 week with the remaining three visits occurring 2 weeks later. For 15- to 17-year-old girls, three of the sessions were scheduled during the mid-follicular phase of the menstrual cycle and three were scheduled during the mid-luteal phase (described below). All subjects participated in six sessions (each dose condition twice) to normalize the number of visits to the postpubertal girls. Participants were randomized to an order of caffeine administration using a random number table. Participants were asked to abstain from all soda and other caffeine-containing products for 24 hours before their appointment times, as well as from all food and drink other than water for 2 hours before their appointments.
Upon arrival at the laboratory, participants and parents read and signed consent and assent forms and parents completed a demographic questionnaire. The participants had their height and weight measured and parents and children completed the Tanner stage and Pubertal Development Questionnaires (described later). Participants then completed a Caffeine Use Questionnaire to determine usual caffeine intake (described later).
At each visit, participants completed 24-hour food and physical activity recalls and completed baseline questionnaires (described below). Then, participants consumed a 300 mL portion of a beverage (lemon–lime-flavored soda, orange juice, or lemonade) containing either placebo or caffeine (1.0 or 2.0 mg/kg; order counterbalanced). These beverages were chosen because they contain a similar amount of sugar (22–26 g) and energy (105–110 kcals) per serving and none of them contain caffeine, which minimizes expectancy about potential caffeine effects. They completed the Drug Effects Questionnaire (DEQ) every 10 minutes for an hour, the Addiction Research Center Inventory (ARCI) and Brief Assessment of Mood Questionnaire at baseline, and again after 30 and 60 minutes (questionnaire described later). To reduce potential effects of expectancy, participants and parents were told that the levels of soda ingredients may be manipulated and caffeine was listed as one of the possible ingredients. At the end of the final visit, participants were debriefed and compensated for their time. These data were collected between August of 2013 and October of 2014. All procedures were approved by the University at Buffalo Social and Behavioral Sciences Institutional Review Board.
Caffeine preparation
Caffeine solutions were created by dissolving caffeine in flattened lemon–lime-flavored soda at two doses: 1 mg/kg (10 mg/mL caffeine/soda) and 2 mg/kg (20 mg/mL caffeine/soda) and freezing aliquots. These solutions were then thawed and added to the drinks mentioned above according to the participants' body weight. All solutions were prepared and coded by a researcher not involved in the data collection or entry. The experimental procedures were double blind.
Measurements
Anthropometrics
Body weight was assessed by the use of a digital scale (SECA, Hanover, MD). Height was assessed using an SECA digital stadiometer.
Menstrual cycle determination
Postpubertal girls visited the laboratory during both the mid-luteal and mid-follicular phases of the menstrual cycle. For the mid-follicular phase, girls telephoned the laboratory on the first day of their period and were scheduled for visits 3–5 days later. For the mid-luteal phase, the girls were given ovulation predictor kits and told to check for ovulation beginning 9 days after the first day of their last period. They telephoned the laboratory when they received a positive test for ovulation and were scheduled for laboratory visits 3–5 days later.
Questionnaires
Tanner stage evaluation
Boys and girls were given line drawings of each of the five Tanner stages of pubertal development and asked to circle the one that looks most like them. Girls were given drawings of breast development and boys were given drawings of genital and public hair development. Parents were independently given the same drawings and asked to circle the one that they felt best resembled their child. The average of the self- and parental reports was taken to determine the Tanner stage (I–V). These drawings were given to the children in an envelope and they were given verbal instructions to circle the picture that looked most like how they look and to return the questionnaire to the envelope. The experimenter left the room and returned 2 minutes later. Self-assessment of pubertal stage has been shown to be accurate and an acceptable substitute when physical examinations are not feasible.32,33
Pubertal Development Questionnaire
This questionnaire asks questions about secondary sex characteristics for boys (growth of body hair, changes in voice, acne, and height) and girls (breast development, menstruation, and acne). This was given along with the Tanner stage drawings and used as a secondary measure of pubertal development. This questionnaire was also given to both the child and the parent, and the scores averaged together.
Addiction Research Center Inventory
Participants completed the short form of the ARCI, which is a 48-item questionnaire, adapted by Martin et al. for use in developing children,34 and consists of five subscales: amphetamine (A), a scale that provides an assessment of amphetamine-like effects, including “I have a weird feeling” or “I feel more excited than dreamy”; benzedrine group (BG), a scale that provides a measure of benzedrine-like effects, intellectual efficiency, and energy, including “My movements seem slower today” and “People might say I am a little too dull today”; lysergic acid diethylamide (LSD), a scale that provides a measure of dysphoria and somatic complaints, including “I would be happy all the time if I felt as I do now” and “My movements seem faster than usual”; morphine–benzedrine group (MBG), a scale that provides a measure of euphoria, including “Things around me seem more pleasing than usual” and “I feel so good that I know other people can tell it”; and pentobarbital–chlorpromazine–alcohol group (PCAG), a scale that provides a measure of sedation, including “I feel drowsy” and “It seems harder to move around today”.35 Subjects completed the ARCI once before and ∼30 and 60 minutes after beverage consumption.
Brief Assessment of Mood States
This questionnaire was adapted from the profile of mood states and measures six dimensions of affect or mood, including tension–anxiety, depression–dejection, anger–hostility, vigor–activity, fatigue–inertia, and confusion–bewilderment. The questionnaire consists of 19 adjectives and participants were asked to circle the number that best described how they were feeling at that moment, ranging from none (1) to extremely (2). This questionnaire has been adapted for use in elementary school-aged children and was administered at 0, 30, and 60 minutes after beverage consumption.
Drug Effects Questionnaire
Participants were asked to respond to the following statements: “Do you feel high right now?,” “Do you feel hungry right now?,” “Do you feel different now than you did at the beginning of this session?,” “Do you like the way you are feeling?,” and “Do you want more of what you were given to drink?” by drawing a vertical line along a 100 mm continuum anchored by “not at all” and “very much”.36 This questionnaire was administered every 10 minutes from baseline to 60 minutes after beverage consumption.
Caffeine Use Questionnaire
The Caffeine Use Questionnaire was used to determine participants' usual caffeine intake. This questionnaire assessed sources, amounts, and frequency of caffeinated food and beverage intake in our study population. It also assesses reasons why children or adolescents use and/or do not use caffeine.37,38 For example, participants were asked whether or not they consume soda. If they said yes, they were asked what type of soda, how often and when they consume soda, and how much was typically consumed. This information was used to calculate average daily caffeine consumption, using caffeine content estimates provided by the US Department of Nutritional Services, with the following average estimates for different caffeine sources used: tea (40 mg/5 oz), soda (40 mg/12 oz), coffee (100 mg/5 oz), energy drinks (∼150 mg/12 oz), chocolate (10 mg/oz), and caffeine-containing pills (Excedrin or No-Doze: 130 mg–200 mg/pill). This questionnaire was administered on the first visit only.
Demographic and medical history
Parents completed a demographic questionnaire providing information about household income, education, profession, and race/ethnicity. In addition, during the screening, parents were asked to report any medical condition or medications that their child had that could have influenced their responses to caffeine, such as stimulant medications for attention deficit hyperactivity disorder (ADHD). Parents were asked if their child had ever had any adverse reaction to caffeine. Finally, potential participants were asked if they were taking oral contraceptives (girls) or smoke cigarettes. Any child for whom there was a positive response for any of the above questions was excluded from the study.
Analytic plan
Sex and pubertal group differences in participant characteristics were analyzed using either an analysis of variance (ANOVA; body–mass index [BMI], age, caffeine consumption, and Tanner stage) or chi-squared analyses for categorical variables (race, household income, and parental education). To analyze the effects of caffeine dose, sex, and pubertal stage on questionnaire responses, we conducted a peak analysis. First, the data from each session were analyzed and the peak response over the course of the 60 minutes was identified for each individual at each caffeine dose. We then conducted a mixed analysis of covariance with sex and pubertal stage as the between-subjects factors, caffeine dose (0, 1, and 2 mg/kg) as the within-subjects factor, baseline to peak as repeated measure, and usual caffeine intake as the covariate. Post hoc analyses were conducted using linear contrasts to determine group differences. A Bonferroni correction was applied to adjust for multiple comparisons such that data where there were two comparisons made would be significant if p < 0.025 and data where there were three comparisons made would be significant if p < 0.017. Data without multiple comparisons were considered significantly different if p < 0.05. All analyses were conducted using SYSTAT (SYSTAT version 11.0, 2005; Systat Software, Inc., San Jose, CA).
Results
Participant characteristics
One hundred twenty-nine participants began the study, but 17 did not complete the study or were removed from the analyses for having scheduling conflicts (n = 2), for not meeting Tanner stage requirements (n = 2), for disclosing medication use after they began the study (n = 4), for failing to return for follow-up visits (n = 8), and for being unable to generate saliva (n = 1). This left 112 participants. Male (n = 27) and female (n = 28) participants aged 8–9 years and males (n = 30) and females (n = 27) aged 15–17 years were included in the analyses. Table 1 shows the mean ± SEM for age (years), BMI (mg/kg), and average daily caffeine consumption (mg/day) of participants in each group along with demographic data. As expected, there was a main effect of pubertal status on age, BMI, and average daily caffeine consumption where the postpubertal group was significantly older, had higher BMI, and consumed more daily caffeine than the prepubertal group (all p < 0.03; Table 1). There were no main effects of sex, and no interactions between sex and pubertal status, on any characteristics of the study population.
Table 1.
Characteristics of the Study Population
| Males | Females | ME | |||||
|---|---|---|---|---|---|---|---|
| Prepubertal (n = 27) | Postpubertal (n = 30) | Prepubertal (n = 28) | Postpubertal (n = 27) | Sex | Pubertal phase | Sex × pubertal phase | |
| Category | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | p | p | p |
| Age (years) | 8.6 ± 0.14a | 15.8 ± 0.13 | 8.5 ± 0.13a | 15.8 ± 0.14 | 0.89 | <0.0001 | 0.99 |
| BMI (kg/m2) | 19.2 ± 0.98a | 24.4 ± 0.93 | 19.1 ± 0.96a | 23.0 ± 0.98 | 0.71 | <0.0001 | 0.81 |
| Average daily caffeine consumption (mg/day) | 47.1 ± 15.6a | 98.9 ± 14.8 | 47.4 ± 15.3a | 63.3 ± 15.6 | 0.25 | 0.029 | 0.24 |
| Child race | 0.27 | 0.79 | 0.98 | ||||
| Asian, n (%) | 1 (4) | 1 (3) | 1 (4) | 1 (4) | |||
| Black or African American, n (%) | 2 (7) | 6 (21) | 5 (17) | 6 (22) | |||
| White, n (%) | 23 (85) | 22 (73) | 18 (64) | 19 (70) | |||
| Other or mixed race, n (%) | 1 (4) | 1 (3) | 1 (4) | 1 (4) | |||
| Parental education: | 0.86 | 0.63 | 0.48 | ||||
| High school, n (%) | 3 (11) | 3 (10) | 4 (15) | 4 (15) | |||
| Some college, n (%) | 6 (22) | 9 (30) | 6 (23) | 4 (15) | |||
| Completed college, n (%) | 13 (48) | 14 (47) | 11 (43) | 14 (52) | |||
| Graduate school, n (%) | 5 (19) | 4 (13) | 5 (19) | 5 (18) | |||
| Household income: | 0.78 | 0.43 | 0.99 | ||||
| <$30,000, n (%) | 0 (0) | 4 (16) | 3 (13) | 7 (25) | |||
| $30,000–$50000, n (%) | 4 (18) | 4 (16) | 5 (22) | 3 (11) | |||
| $50,000–$70,000, n (%) | 7 (30) | 5 (20) | 3 (13) | 5 (19) | |||
| $70–$110,000 | 6 (26) | 8 (32) | 8 (35) | 5 (19) | |||
| >$110,000, n (%) | 6 (26) | 4 (16) | 4 (17) | 7 (26) | |||
Participant characteristics are shown as mean (SEM) or n (%). For mean (SEM), ANOVA analyses were conducted, and for n (%), chi-squared analyses were conducted to determine ME and interactions of sex and pubertal phase. The p-values for ME and interactions are shown in the columns to the right.
Significantly different from postpubertal participants of the same sex (p < 0.05).
ANOVA, analysis of variance; BMI, body–mass index; ME, main effect.
Caffeine effects on subjective responses
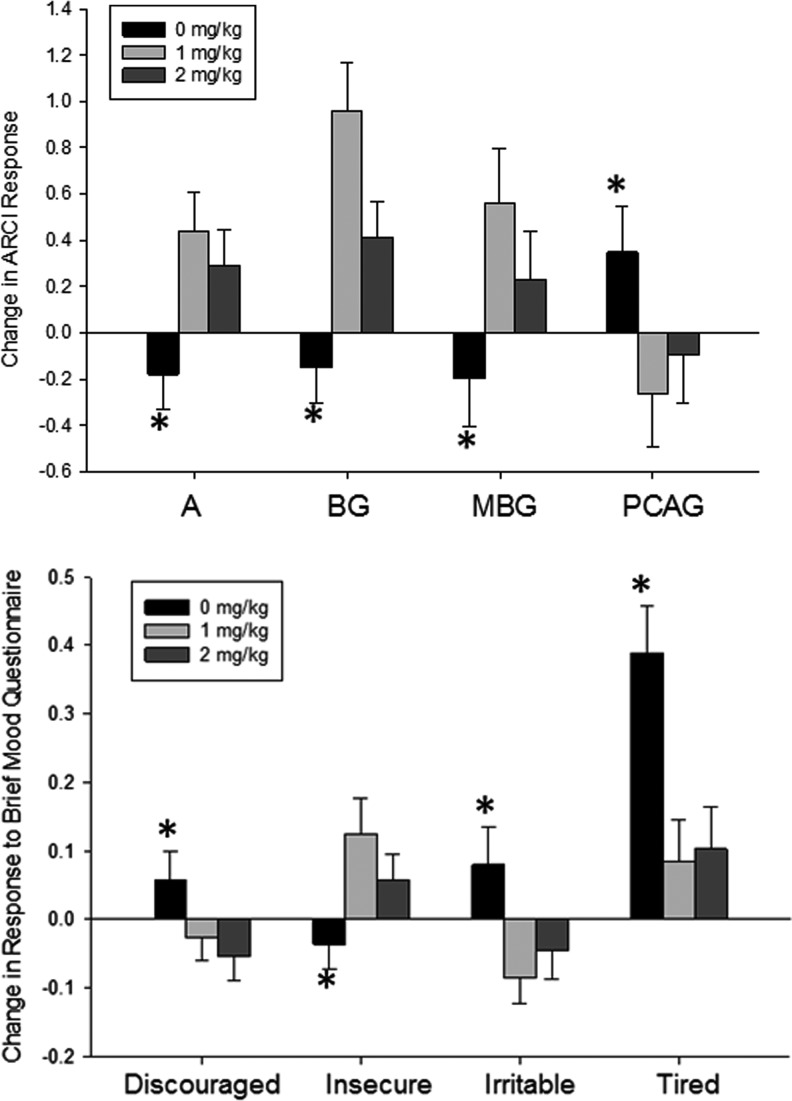
Caffeine (both doses) significantly increased responding on the amphetamine [A; F(2, 216) = 7.2; p = 0.001], energy [BG; F(2, 216) = 12.0; p < 0.0001], and euphoria [MBG; F(2, 216) = 5.2; p = 0.006] subscales of the ARCI from pre- to postsessions and decreased responding on the sedation subscale [PCAG; F(2, 216) = 74.7; p = 0.01] relative to the placebo condition (Fig. 1). We also found that caffeine (both doses) decreased from pre- to postsession ratings of feeling irritable [F(2, 216) = 4.4; p = 0.014] and tired [F(2, 216) = 9.6; p < 0.0001] and increased ratings of feeling insecure [F(2, 216) = 4.4; p = 0.019] compared with the placebo condition (Fig. 1). Ratings of feeling discouraged were decreased after caffeine administration, but this finding did not reach statistical significance after the correction for multiple comparisons [F(2, 216) = 3.6; p = 0.029].
FIG. 1.
Effects of acute caffeine on subjective responses. Mean ± SEM change in Addiction Research Center Inventory (ARCI) subscale response (top) and responses on Brief Mood Questionnaire (bottom) from baseline to peak after placebo (black bars), 1 mg/kg (light gray bars), or 2 mg/kg (dark gray bars) of caffeine. For all ARCI subscales and Brief Mood Questionnaire choices shown here, both doses of caffeine resulted in significantly greater changes in responses than did the placebo. * = significantly different from caffeine (1 mg/kg and 2 mg/kg); p < 0.05.
Caffeine dose and gender interactions on subjective responses
There were trends for caffeine having different effects on boys and girls for responses on the energy subscale of the ARCI [BG; F(2, 216) = 3.6; p = 0.028], with girls reporting greater responses than boys at the 1 mg/kg dose. We found similar interactions on the Brief Assessment of Mood States, with reports of feeling discouraged [F(2, 216) = 3.2; p = 0.044], insecure [F(2, 216) = 3.7; p = 0.026], and tired [F(2, 216) = 3.5; p = 0.03] greater in females than in males after both the 1 and 2 mg/kg doses of caffeine. None of these interactions remained significant after corrections for multiple comparisons.
Caffeine dose, gender, pubertal phase interactions on subjective responses
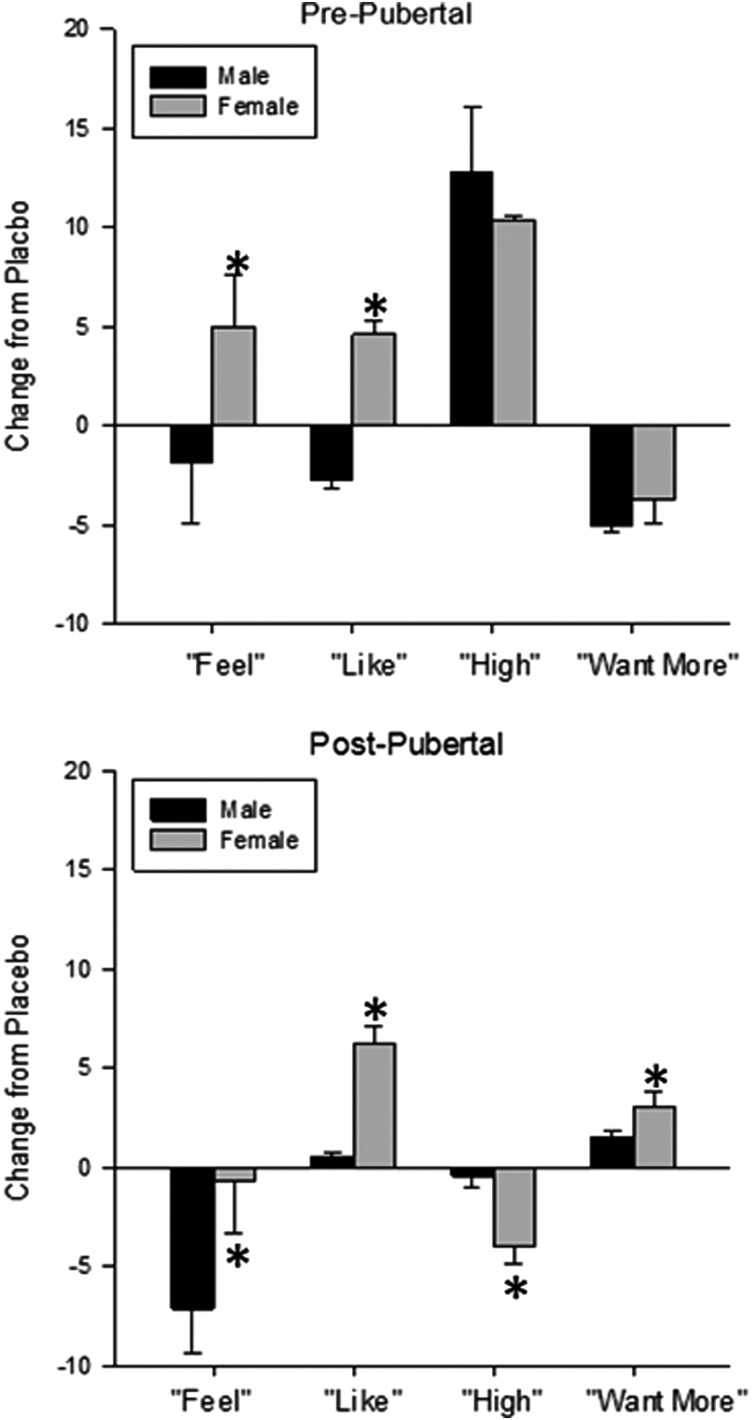
Two milligrams per kilogram of caffeine had different effects on responses on the DEQ in boys and girls depending on pubertal stage. There were main effects of sex on reports of feeling different [F(1, 107) = 6.3; p = 0.014] and a trend for liking the feeling [F(1, 107) = 3.9; p = 0.049]. When we examined sex and pubertal phase interactions, we found that prepubertal girls reported increases in liking the feeing, whereas prepubertal boys reported decreases. Postpubertal girls reported greater increases in liking the feeling compared with boys, but postpubertal girls showed no change in feeling different after 2 mg/kg of caffeine compared with placebo, while boys reported decreases in feeling different. For reports of feeling high [F(1, 54) = 8.1; p = 0.006] and wanting more [F(1, 55) = 2.6; p = 0.004], there were only sex differences in the postpubertal participants, with girls showing greater changes from placebo than boys for both (Fig. 2).
FIG. 2.
Responses on the Drug Effects Questionnaire (DEQ). Mean ± SEM change in DEQ responses from placebo after 2 mg/kg of caffeine in prepubertal (top) and postpubertal (bottom) males (black bars) and females (gray bars). In both pre- and postpubertal participants, there were sex differences in reporting of feeling different and liking the caffeine, but sex differences in feeling high and wanting more were only observed in postpubertal participants. * = significantly different from males; p < 0.05.
Caffeine dose and menstrual cycle phase effects on subjective responses in postpubertal girls
When postpubertal girls were examined alone, we found that caffeine (2 mg/kg) had differential effects depending on the phase of the menstrual cycle for ratings of feeling depressed [F(1, 25) = 18.6; p < 0.0001], happy [F(1, 25) = 10.3; p = 0.005], interested [F(1, 25) = 10.3; p = 0.004], tired [F(1, 25) = 11.4; p = 0.002], uneasy [F(1, 25) = 16.4; p < 0.0001], worried [F(1, 25) = 24.6; p < 0.0001], and bored [F(1, 25) = 5.8; p = 0.024] on the Brief Assessment of Mood States, with greater decreases in reports of feeling depressed, interested, uneasy, and worried and greater increases in feeling happy, tired, and bored when girls were in the follicular compared with the luteal phases (Fig. 3). There were menstrual cycle differences for all responses on the DEQ, except for feeling hungry (all p < 0.05; Fig. 3). In this case, girls reported greater increases in feeling high and feeling different, but lower reports of liking the feeling and wanting more, when in the follicular compared with the luteal phase. We found no menstrual cycle phase differences for responses to 0 or 1 mg/kg of caffeine.
FIG. 3.
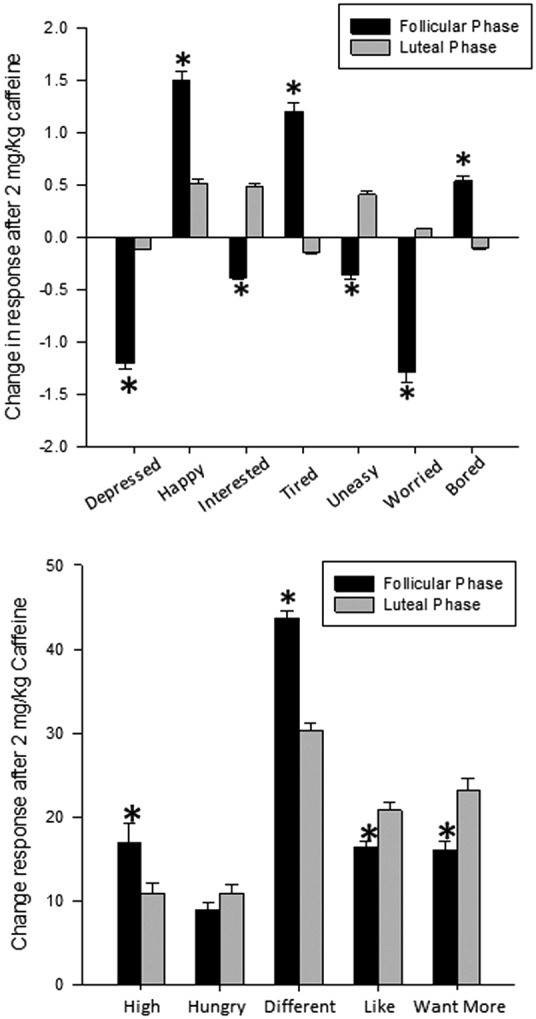
Menstrual cycle differences in responses to acute caffeine. Mean ± SEM change in peak responses from placebo after 2 mg/kg of caffeine in postpubertal girls on the Brief Mood Questionnaire (top) and the DEQ (bottom) when girls were in the follicular (black bars) or luteal (gray bars) phases of the menstrual cycle. In general, girls had greater changes in responses on the Brief Mood Questionnaire when in the follicular phase compared with the luteal phase. This was also true for reports of feeling high and feeling different on the DEQ, with reports of liking the feeling and wanting more being significantly lower in the follicular phase compared with the luteal phase. * = significantly different from luteal phase; p < 0.05.
Discussion
Acute caffeine administration produces subjective effects. These effects are dependent on caffeine dose and may vary as a function of gender, age, and/or menstrual cycle phase. To date, the majority of this work has been conducted in adults. The purpose of this study was to replicate and extend our previous findings on the effects of acute caffeine on subjective responses in young children and older adolescents. We tested the hypothesis that the effects of caffeine on subjective responses vary as a function of dose and differ as a function of pubertal stage. We found that caffeine had dose-dependent effects on many of the responses that we examined. We also found that subjective responses varied as a function of pubertal stage, sex, and menstrual cycle phase.
Acute caffeine administration has been shown to induce changes in subjective responses in adults. For example, studies by Childs et al. have shown that caffeine induces reports of feeing high and feeling different, as well as changes in mood, such as feeling more energetic and feeling happier.16,39,40 In a previous study in our laboratory, we examined these responses in 12- to 17-year-old adolescents and found that acute caffeine administration induced similar subjective responses.9,24 The purpose of the current study was to replicate and extend these findings to younger children and to determine if sex differences in subjective responses to caffeine are present in prepubertal children or emerge after puberty. We found that acute caffeine produced an increased reporting of positive subjective responses in all participants regardless of age. Specifically, both pre- and postpubertal children had increased scores on the euphoria (MBG) scale of the ARCI and lower scores on the sedation (PCAG) scale of the ARCI after caffeine administration compared with placebo. In addition, acute caffeine appeared to improve mood by decreasing ratings of feeling discouraged, irritable, and tired compared with placebo.
We also found sex differences in subjective responses to caffeine with girls in general, showing greater changes in subjective responses after caffeine administration compared with boys, with the direction of change dependent on the question of set of questions being asked. These results are inconsistent with a previous study that reported greater subjective responses to caffeine in men than women23 and a study showing that males had subjective responses to caffeine at lower doses than females.23 Another study showed that greater caffeine use was related to greater impulsivity in men, but there was no relationship in women.41 Finally, a study in adolescents showed that males found caffeinated beverages more reinforcing than females.11 It is unclear why our data suggest that females had greater subjective responses than males in our study when the majority of the literature supports the opposite relationship. One possibility is that we stratified by caffeine use in our study such that there was no difference in caffeine use between males and females in our study. This may have meant that we recruited females who were, in general, higher caffeine users than what might be found in the population. Another difference between this study and the others cited above is that our study was conducted in children and adolescents and the others were conducted in adults. It may be that as caffeine use is escalating during adolescents, females experience more subjective effects, but after caffeine use stabilizes in adulthood, a different pattern emerges. Additional, longitudinal studies are required to determine the developmental changes in subjective response to drugs that contribute to sex differences.
In addition to sex differences, we observed differences in subjective responses to caffeine in postpubertal girls as a function of menstrual cycle phase. On the Brief Assessment of Mood States, girls seemed to be not only more comfortable (decreased feelings of depressed, uneasy, and worried) but also more tired and bored after caffeine administration when in the follicular phase compared with the luteal phase. In addition, on the DEQ, the 2 mg/kg dose of caffeine produced greater reports of feeling high and feeling different, but lower ratings of liking the feeling and wanting more compared with placebo when girls were in the follicular phase compared with the luteal phase. This suggests that the drug-like effects were perceived as stronger, but may have been more aversive effects. This is consistent with the literature in adult females showing that subjective responses to drugs, such as amphetamine, differ as a function of menstrual cycle phase.42,43 We do not understand the implications of these menstrual cycle differences other than, when using postpubertal females to assess drug effects, it is important to take into account the phase of the menstrual cycle.
This study had several strengths. First, we used a double-blind, placebo-controlled dose–response design. This allowed us to examine within-subjects changes in responses to different doses of caffeine. Second, we examined postpubertal girls in both menstrual cycle phases. This allowed us to look at potential changes in responses to caffeine across the menstrual cycle. Finally, we had a fairly large sample size for this type of study, which allowed us to examine sex and pubertal phase interactions. This study was not without limitations. First, our study sample was primarily Caucasian and middle class. This limits our ability to generalize findings to other ethnic and socioeconomic groups. Second, we relied on self-reports for caffeine abstinence verification, thus some of our participants may not have disclosed recent consumption of caffeine. While troublesome, if anything, recent caffeine use would reduce the impact of acute caffeine, thus limiting our ability to observe changes in responding. Third, we relied on self-reports of menstrual cycle phase and ovulation in postpubertal girls. Although we provided ovulation predictor kits to assist girls in identification of their luteal phase, it would have strengthened the study to have an empirical verification of menstrual cycle phase, such as steroid hormone concentrations.
When taken together, this study suggests that acute caffeine elicits drug-like effects on children and adolescents. There were some differences as a function of pubertal stage and/or sex, as well as differences in responses across the menstrual cycle. These differences tended to be in magnitude of responses and not in direction, suggesting that the main effects of caffeine are similar. Future studies should examine the potential impact of acute responses to caffeine on initiation and use of other substances to determine if these drug-like effects generalize to other licit and illicit substances. In addition, this type of work may provide clues to identifying drugs with abuse potential and perhaps individuals at risk.
Acknowledgments
This research was supported by an NIDA grant, RO1 DA030386, to J.L.T. The authors acknowledge the assistance of Adam Graczyk, Karina Vattana, and Teresa Sion in the recruitment of participants and data collection and entry. The authors also thank Thomas Kelly for help with the data analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahluwalia N, Herrick K. Caffeine intake from food and beverage sources and trends among children and adolescents in the United States: review of national quantitative studies from 1999 to 2011. Adv Nutr. 2015;6:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahluwalia N, Herrick K, Moshfegh A, et al. . Caffeine intake in children in the United States and 10-y trends: 2001–2010. Am J Clin Nutr. 2014;100:1124–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branum AM, Rossen LM, Schoendorf KC. Trends in caffeine intake among U.S. children and adolescents. Pediatrics. 2014;133:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calamaro CJ, Yang K, Ratcliffe S, et al. . Wired at a young age: the effect of caffeine and technology on sleep duration and body mass index in school-aged children. J Pediatr Health Care. 2012;26:276–282 [DOI] [PubMed] [Google Scholar]
- 5.Lodato F, Araujo J, Barros H, et al. . Caffeine intake reduces sleep duration in adolescents. Nutr Res. 2013;33:726–732 [DOI] [PubMed] [Google Scholar]
- 6.Orbeta RL, Overpeck MD, Ramcharran D, et al. . High caffeine intake in adolescents: associations with difficulty sleeping and feeling tired in the morning. J Adolesc Health. 2006;38:451–453 [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama T, Xie D, Graham-Maar RC, et al. . Dietary and lifestyle factors associated with blood pressure among U.S. adolescents. J Adolesc Health. 2007;40:166–172 [DOI] [PubMed] [Google Scholar]
- 8.Temple JL, Ziegler AM, Graczyk A, Bendlin A, Sion T, Vattana K. Sex and pubertal stage differences in cardiovascular responses to caffeine in children. Pediatrics. 2014;134:e112–e119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temple JL, Dewey AM, Briatico LN. Effects of acute caffeine administration on adolescents. Exp Clin Psychopharmacol. 2010;18:510–520 [DOI] [PubMed] [Google Scholar]
- 10.Panek LM, Swoboda C, Bendlin A, et al. . Caffeine increases liking and consumption of novel-flavored yogurt. Psychopharmacology (Berl). 2013;227:425–436 [DOI] [PubMed] [Google Scholar]
- 11.Temple JL, Bulkley AM, Briatico L, et al. . Sex differences in reinforcing value of caffeinated beverages in adolescents. Behav Pharmacol. 2009;20:731–741 [DOI] [PubMed] [Google Scholar]
- 12.Temple JL, Ziegler AM, Graczyk A, et al. . Influence of caffeine on the liking of novel-flavored soda in adolescents. Psychopharmacology (Berl). 2012;223:37–45 [DOI] [PubMed] [Google Scholar]
- 13.Broffitt B, Levy SM, Warren J, et al. . Factors associated with surface-level caries incidence in children aged 9 to 13: the Iowa Fluoride Study. J Public Health Dent. 2013;73:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temple JL. Factors that influence the reinforcing value of foods and beverages. Physiol Behav. 2014;136:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferre S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;20 Suppl 1:S35–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childs E, de Wit H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology (Berl). 2006;185:514–523 [DOI] [PubMed] [Google Scholar]
- 17.Haskell CF, Kennedy DO, Wesnes KA, et al. . Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology (Berl). 2005;179:813–825 [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Sutherland D, Christopher G. Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J Psychopharmacol. 2005;19:620–626 [DOI] [PubMed] [Google Scholar]
- 19.Smith AP. Caffeine, cognitive failures and health in a non-working community sample. Hum Psychopharmacol. 2009;24:29–34 [DOI] [PubMed] [Google Scholar]
- 20.McHill AW, Smith BJ, Wright KP., Jr Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. 2014;29:131–143 [DOI] [PubMed] [Google Scholar]
- 21.Garrett BE, Griffiths RR. The role of dopamine in the behavioral effects of caffeine in animals and humans. Pharmacol Biochem Behav. 1997;57:533–541 [DOI] [PubMed] [Google Scholar]
- 22.Heishman SJ, Henningfield JE. Stimulus functions of caffeine in humans: relation to dependence potential. Neurosci Biobehav Rev. 1992;16:273–287 [DOI] [PubMed] [Google Scholar]
- 23.Adan A, Prat G, Fabbri M, et al. . Early effects of caffeinated and decaffeinated coffee on subjective state and gender differences. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1698–1703 [DOI] [PubMed] [Google Scholar]
- 24.Temple JL, Ziegler AM. Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J Caffeine Res. 2011;1:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penolazzi B, Natale V, Leone L, et al. . Individual differences affecting caffeine intake. Analysis of consumption behaviours for different times of day and caffeine sources. Appetite. 2012;58:971–977 [DOI] [PubMed] [Google Scholar]
- 26.Pettit ML, DeBarr KA. Perceived stress, energy drink consumption, and academic performance among college students. J Am Coll Health. 2011;59:335–341 [DOI] [PubMed] [Google Scholar]
- 27.Rodenburg EM, Eijgelsheim M, Geleijnse JM, et al. . CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking. Am J Clin Nutr. 2012;96:182–187 [DOI] [PubMed] [Google Scholar]
- 28.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514 [DOI] [PubMed] [Google Scholar]
- 29.Tantcheva-Poor I, Zaigler M, Rietbrock S, et al. . Estimation of cytochrome P-450 CYP1A2 activity in 863 healthy Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9:131–144 [PubMed] [Google Scholar]
- 30.Hong CC, Tang BK, Hammond GL, et al. . Cytochrome P450 1A2 (CYP1A2) activity and risk factors for breast cancer: a cross-sectional study. Breast Cancer Res. 2004;6:R352–R365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar G, Onufrak S, Zytnick D, et al. . Self-reported advertising exposure to sugar-sweetened beverages among US youth. Public Health Nutr. 2015;18:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonat S, Pathomvanich A, Keil MF, et al. . Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110:743–747 [DOI] [PubMed] [Google Scholar]
- 33.Schlossberger NM, Turner RA, Irwin CE., Jr Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13:109–113 [DOI] [PubMed] [Google Scholar]
- 34.Martin CA, Guenthner G, Bingcang C, et al. . Measurement of the subjective effects of methylphenidate in 11- to 15-year-old children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin WR, Sloan JW, Sapira JD, et al. . Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258 [DOI] [PubMed] [Google Scholar]
- 36.Leddy JJ, Epstein LH, Jaroni JL, et al. . Influence of methylphenidate on eating in obese men. Obes Res. 2004;12:224–232 [DOI] [PubMed] [Google Scholar]
- 37.Miller KE. Energy drinks, race, and problem behaviors among college students. J Adolesc Health. 2008;43:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller KE. Wired: energy drinks, jock identity, masculine norms, and risk taking. J Am Coll Health. 2008;56:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Childs E. Influence of energy drink ingredients on mood and cognitive performance. Nutr Rev. 2014;72 Suppl 1:48–59 [DOI] [PubMed] [Google Scholar]
- 40.Childs E, de Wit H. Enhanced mood and psychomotor performance by a caffeine-containing energy capsule in fatigued individuals. Exp Clin Psychopharmacol. 2008;16:13–21 [DOI] [PubMed] [Google Scholar]
- 41.Waldeck TL, Miller LS. Gender and impulsivity differences in licit substance use. J Subst Abuse. 1997;9:269–275 [DOI] [PubMed] [Google Scholar]
- 42.Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Exp Clin Psychopharmacol. 2010;18:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13 [DOI] [PubMed] [Google Scholar]