Abstract
There is a great need for living valve replacements for patients of all ages. Such constructs could be built by tissue engineering, with perspective of the unique structure and biology of the aortic root. The aortic valve root is composed of several different tissues, and careful structural and functional consideration has to be given to each segment and component. Previous work has shown that immersion techniques are inadequate for whole-root decellularization, with the aortic wall segment being particularly resistant to decellularization. The aim of this study was to develop a differential pressure gradient perfusion system capable of being rigorous enough to decellularize the aortic root wall while gentle enough to preserve the integrity of the cusps. Fresh porcine aortic roots have been subjected to various regimens of perfusion decellularization using detergents and enzymes and results compared to immersion decellularized roots. Success criteria for evaluation of each root segment (cusp, muscle, sinus, wall) for decellularization completeness, tissue integrity, and valve functionality were defined using complementary methods of cell analysis (histology with nuclear and matrix stains and DNA analysis), biomechanics (biaxial and bending tests), and physiologic heart valve bioreactor testing (with advanced image analysis of open–close cycles and geometric orifice area measurement). Fully acellular porcine roots treated with the optimized method exhibited preserved macroscopic structures and microscopic matrix components, which translated into conserved anisotropic mechanical properties, including bending and excellent valve functionality when tested in aortic flow and pressure conditions. This study highlighted the importance of (1) adapting decellularization methods to specific target tissues, (2) combining several methods of cell analysis compared to relying solely on histology, (3) developing relevant valve-specific mechanical tests, and (4) in vitro testing of valve functionality.
Introduction
Aortic valve pathology is an important chapter of cardiovascular diseases in adults and may also be the result of congenital malformations in children. Extensive research has uncovered important aspects related to the pathogenesis of valve disease.1–7 However, the knowledge is not advanced enough to offer drug therapy or prevention. Therefore, the only therapy for aortic valve disease is their surgical repair or replacement with artificial devices that mimic the function, but not the biology of the native valve. To date, it is estimated that about 1,000,000 aortic heart valves are replaced every year around the world,8 therefore, strategies to address these issues are bound to have a global impact. The most common devices are mechanical valves and valves made from glutaraldehyde-treated devitalized animal tissues. These provide significant improvement in cardiac function and life expectancy, but have functional limitations such as the need for lifelong anticoagulation (mechanical valves) and propensity to degenerate and calcify (tissue valves). Tissue valves fail within 15–20 years after implantation, and repeated open-heart surgery to replace defective implants is undesirable. These risks are particularly augmented in pediatric patients, where tissue valves degenerate and calcify at an accelerated rate and none of the implants grow with the somatic growth of the patients. For these reasons, tissue-based valve prostheses are implanted mostly in patients >65 years of age and mechanical valves in patients who accept lifelong anticoagulation medication.
Since no current valve prosthesis can withstand aortic conditions for a lifetime, “valve sparing” surgical techniques have been invented to preserve the aortic valve three-dimensional architecture; alternatively, when the valve cusps have been compromised by calcification, full aortic root replacement is performed.9 Other valve replacement options are the pulmonary autograft valves (i.e., the Ross procedure, whereby the patient's own living pulmonary valve is transplanted into the aortic position) and the human allograft valves (sterilized, cryopreserved cadaveric aortic roots obtained from humans). The latter exhibits good durability, but is not readily available, represents only a small proportion of total valve replacements, and eventually fails in the long term due to lack of living cells.9 Therefore, one can conclude that there is a great need for living valve replacements for patients of all ages. As has been eloquently said, “future advances with tissue-engineered heart valves […] may change the landscape for valve repair in the pediatric population”.10 Such constructs could be built by tissue engineering approaches using scaffolds and cells, with perspective of the unique biology of the aortic root.
The aortic root is a functional unit and complex three-dimensional extracellular matrix structure comprising the aortic valve cusps (leaflets), the sinuses of Valsalva, the transition area between the sinus and the aorta (“sinotubular junction”), and the crown-shaped basal ring (ventriculoaortic junction or “annulus”).11 Each anatomical component of the aortic root has unique features, and all elements work in perfect harmony to minimize stress on the cusps, thus being highly efficient in a variety of hemodynamic conditions.12 The aortic valve cusps are exposed to a unique hemodynamic environment, characterized by area-specific shear stress, bending forces and strains in circumferential and radial directions that vary in intensity and direction during each cardiac cycle.9 To respond to these challenges, cusps have a trilayered (fibrosa, spongiosa, ventricularis) and anisotropic structure (stiffer in the circumferential axis vs. radial axis), which is essential for mechanical durability and stress distribution.13,14
Currently, the most physiologic valve replacements are considered the homograft valves because of their outstanding valve function and hemodynamics. However, their durability is limited, showing 60% survival at 10 years and only 30% at 20 years,15 probably because most cells are lost during the freeze–thaw cycle or shortly after implantation.16,17 In this respect, we consider homografts as “unintentionally created” human acellular roots. In 2001, the first immersion-decellularized porcine aortic roots prepared by the proprietary SynerGraft CryoLife, Inc. procedures were implanted in pediatric patients. More recently, acellular porcine aortic roots prepared by immersion decellularization with trypsin treatments (Matrix P procedure) were implanted in patients in Europe and South America. Despite excellent hemodynamics, the SynerGraft prostheses failed dramatically (leading to three deaths) due to strong immune reactions caused by incomplete decellularization of the sinus and aortic wall components.18 Matrix P valves were infiltrated by “valve-like fibroblasts” and covered by “neoendothelium,” which increased the enthusiasm for the approach. However, in the long term, the infiltrating cells were identified as activated myofibroblasts, which induced chronic cusp contraction and fibrosis, the valves eventually becoming incompetent.19 The surface cell coverage was identified as transanastomotic tissue overgrowth, which is a typical host reaction to cardiovascular implants.20 Reviewing all data collected on implantation of acellular roots, Ingham and collaborators concluded recently that incomplete valve decellularization is associated with severe graft failure.21
Other experimental approaches to building tissue-engineered valves include use of cell-seeded biodegradable polymers (synthetic or fibrin-based, molded in the shape of an aortic valve root) to generate a homogeneous, isotropic collagenous structure lacking elastic fibers, before implantation,22,23 implantation of stent-mounted acellular valves seeded with vascular fibroblasts or stem cells,24 as well as use of polymers22,25 and layered composites.13,14,26 Creating structures as heterogeneous as that of the aortic roots using a bottom-up approach (such as three-dimensional printing27) is quite difficult and requires major technological advances. Taken together, none of the above referenced approaches satisfied the design criteria.22,28–30
Learning from these data, we hypothesized that decellularized xenogeneic aortic valve roots could serve as excellent scaffolds for heart valve tissue engineering. However, careful structural and functional consideration has to be given to each segment of the root. The main bottleneck remains decellularization of the aortic wall and the clear demonstration of complete removal of all foreign cells from all segments of the root. This study reveals that immersion techniques are inadequate for whole-root decellularization and that specialized perfusion systems and well-defined success criteria for evaluation of decellularization completeness, tissue integrity, biomechanics, and valve functionality are needed for generating functional acellular aortic valve roots.
Materials and Methods
Tissue collection and cleaning
Fresh porcine aortic roots with intact ascending aorta, coronaries, mitral valve, and thin endocardial wall at least 1 inch below the valve were collected from adult swine at a local abattoir (Snow Creek Meat Processing Center) and stored in ddH2O on ice during transportation to the laboratory. Aortic roots were further dissected to include 6–8 cm of intact ascending aorta (cut just below the branching of the brachiocephalic artery), 5–10 mm long coronaries, about 3 cm of mitral valve leaflet, and ventricular endocardium tissue with an intact mitral myocardial junction. Roots were macroscopically cleaned over ice by removing fat and other extraneous tissues as follows. For immersion decellularization, we completely removed the subvalvular fat, muscle, and mitral valve remnants; for perfusion decellularization, we thinned the subvalvular fat and muscle with intact endocardium all around the valve 360° to about 1–3 mm to match the thickness of the mitral valve leaflet. This latter step facilitated mounting of roots in the perfusion device (see perfusion decellularization system section).
Immersion decellularization
Cleaned aortic roots were decellularized by immersion in glass containers on an orbital shaker at 22°C, as described previously,31 using a ratio of 500 mL/5 roots. Decellularization steps consisted of hypotonic shock (ddH2O, 24 h, 4°C), loosening of extracellular matrix and initialization of cell removal (0.1 M NaOH, 2 h, 22°C), and detergents (1% sodium dodecyl sulfate, 1% Triton X-100, 1% Na-deoxycholate, and 0.2% ethylenediaminetetraacetic acid [EDTA] in 50 mM TRIS, pH 7.5) for 16 days, with fresh solution changed every 2 days. After rinsing, removal of nucleic acids was performed by incubation in 720 mU/mL deoxyribonuclease and 720 mU/mL ribonuclease in 5 mM magnesium chloride in 1× Dulbecco's phosphate-buffered saline (DPBS) for 4 days with one change at 2 days, 37°C. To reduce bioburden and quantitatively extract residual detergents, each step was preceded by a 15-min 70% ethanol treatment and rinsing. The roots were finally incubated in 70% ethanol (24 h, 22°C) for final bioburden reduction and sterilized in 0.1% peracetic acid in PBS, pH 7.5 (2 h, 22°C).
Perfusion decellularization system
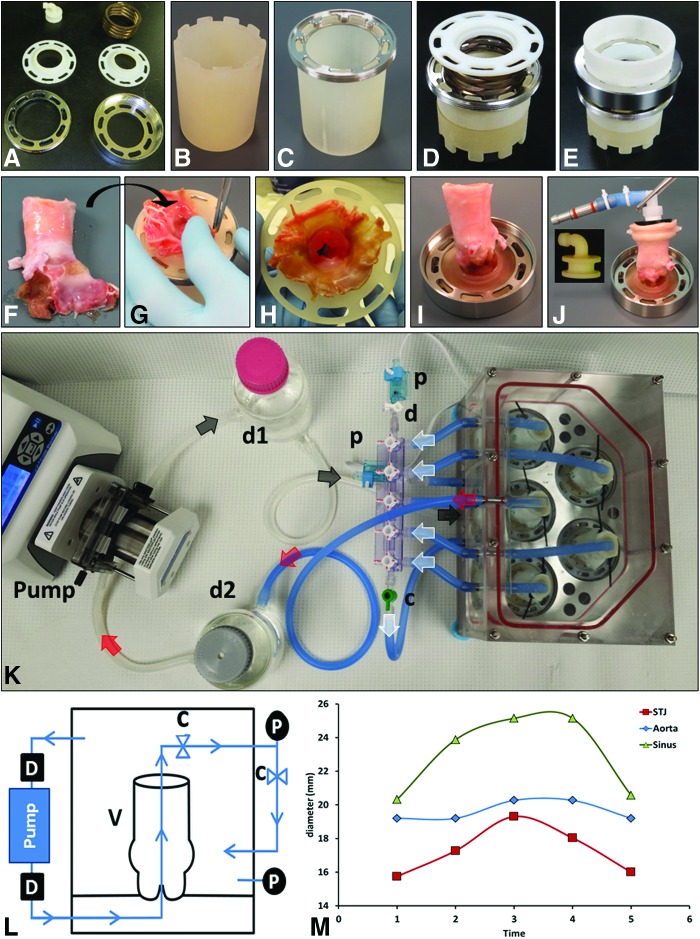
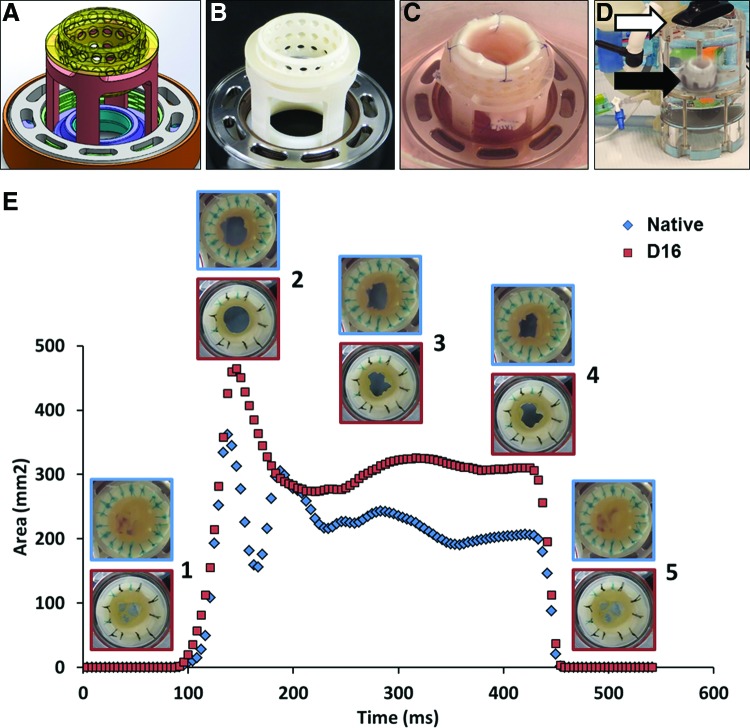
Roots were mounted (Fig. 1A–J) into the self-adjusting, no-touch valve-mounting devices (MechAnnulus™ Rings, patent pending; Aptus, LLC) at the level below the base of the cusps. After mounting, the coronaries were ligated shut, the distal ascending aorta was trimmed flat, and an outflow plug (Fig. 1J) was inserted into the aorta and secured with cable ties. By closing the coronaries and controlling the outflow through this plug, a pressure differential can be created between the interior and exterior of the valve root during processing. A perfusion system (designed in SolidWorks and manufactured from stainless steel, acrylic, and polycarbonate) was developed to pressurize the interior of up to five roots with a circulating fluid (PDCell System, patent pending; Aptus, LLC). The system (Fig. 1K) utilizes a peristaltic pump to create flow, incorporates a pulse dampener, reservoir, constrictor valve to build pressure, and a series of plates and chambers to appropriately direct flow through and around an aortic root (Fig. 1L). This system cyclically expands the root (Fig. 1M) and sinuses, driving fluids transmurally while maintaining low pressure differentials on the valve cusps. Pressure was measured in line communicating with the outside as well as the inside of the aortic roots by transducers (DTXPlus; Argon Medical) controlled by custom LabVIEW software, which displays inner, outer, and differential pressures. Digital video imaging was also used to evaluate root distention.
FIG. 1.
Valve mounting system as follows. (A) Ring system for aortic root mounting composed of two-threaded metal rings, a spring, and two internal plastic rings. (B) Support “tower” used during assembly. (C) Inside metal ring placed on support tower. (D) Plastic ring placed on top of spring and metal ring. (E) A second support tower on top aids with tightening the external and internal metal rings. (F–I) A fresh aortic root is cleaned, coronaries are ligated, and then the root is flipped, mounted inside the support system, and secured in place by the threaded outside ring. (J) The valve root is then adapted with an “elbow” fitting connector to the aorta (insert) and placed in the five-valve decellularization system (K). Direction of fluid flow indicated by arrows is as follows: from the pump through the dampener (d1) enters the bottom of the system (black arrows) and pushes fluid through the valves in an anterograde manner. Fluid from each root then collects through a manifold (white arrows) into a single line, which feeds the system again, bathing the valves from the outside. Fluid pooled from the top of the device (red arrows) is then pushed into the second dampener (d2) and back to the pump. The flow diagram (L) illustrates the main system components of the perfusion system comprising a valve (v), peristaltic pump, pulse dampener (d), check valves (c), and pressure transducers (p). (M) Distension of aortic roots during cyclical perfusion decellularization. Digital measurements of external diameters of the aorta, sinotubular junction (STJ), and sinus at the level of coronaries are shown for a representative inflation–deflation cycle. Color images available online at www.liebertpub.com/tec
Perfusion decellularization procedure
Aortic roots were assembled into the PDCell System as described above. All steps were performed with a cyclic transmural pressure gradient of about 52 ± 2 mmHg and mechanical stretching for 3 min on and 30 s off. Sequential steps consisted of hypotonic shock (ddH2O, 24 h, 22°C), loosening of the extracellular matrix and initialization of cell removal (0.1 M NaOH, 2 h, 22°C), detergent decellularization (1% sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate, and 0.2% EDTA in 50 mM TRIS, pH 7.5, for 8 and 16 days with fresh solution change every 4 days, 22°C), enzymatic removal of nucleic acids (720 mU/mL deoxyribonuclease and 720 mU/mL ribonuclease in 5 mM magnesium chloride in 1× DPBS, 4 days with fresh solution every 2 days at 37°C), and sterilization (0.1% peracetic acid in PBS, pH 7.5, 1 h, 22°C). Each step was followed by rinsing with water, ethanol, or 1× DPBS as appropriate. To reduce bioburden and quantitatively extract residual detergents, each step was preceded by a 15-min 70% ethanol treatment and rinsing. Immediately following, the distal root outflow constrictors were removed with sterile tools and the mounted valve roots were transferred to sterile individual 300-mL wide mouth glass containers with silicone membrane sealing lids on an orbital shaker for final sterilization (0.1% peracetic acid in PBS, pH 7.5, 1 h, 22°C). In addition, one group of roots (n = 4) underwent 16 days of detergent decellularization, but was stopped immediately before enzymatic removal of nucleic acids.
DNA analysis
DNA were extracted from aorta, sinus, cusp, and muscle tissue samples (n = 4) and purified with the DNeasy Blood & Tissue Kit (Qiagen) before analysis by ethidium bromide agarose gel electrophoresis. Samples were also quantified by reading absorbances at 260 nm on a NanoDrop 2000c (Thermo Scientific). Quantities of DNA were normalized to dry tissue weight and expressed as ng/mg dry tissue.
Histology and immunohistochemistry
For histology studies, samples collected from the aortic wall, sinus, cusp, and muscle were fixed in 10% formalin, embedded in paraffin, sectioned at 5 μm (3 μm for the aorta), and stained with hematoxylin and eosin (H&E) and 4′,6-diamidino-2-phenylindole (DAPI) for nuclei and Movat's pentachrome (Cat.# K042; Poly Scientific R&D Corp.) for overall tissue analysis. For quantification of matrix components, slides were also stained with Voerhoff van Gieson's stain for elastic fibers (Cat.# K059; Poly Scientific R&D Corp.), Masson's trichrome stain for collagen fibers (Cat.# K037; Poly Scientific R&D Corp.), and alcian blue stain for glycosaminoglycans/proteoglycans (Cat.# K066; Poly Scientific R&D Corp.), according to manufacturers' procedures. Stained slides were then scanned on an iSCAN HT Ventana whole slide scanner (University of Nebraska Medical Center, Tissue Science Facility, Omaha, NE). Three representative images were taken from each tissue section and stain, and digital image processing was performed using ImageJ, using the Color threshold plugin to select the desired color and then the resulting image was converted to binary. Percent areas corresponding to each component were then evaluated, and values were represented as mean ± standard deviation (SD).
Immunohistochemistry (IHC) was performed for type IV collagen as described previously32 in the Leica Bond Max automated IHC system (Leica Microsystems, Inc.) using a rabbit anti-human type IV collagen antibody (Abcam ab6586, cross reacts with pig antigen) at 1:250 dilution (4 μg/mL). Slides were incubated with primary antibody for 15 min. The system employs a proprietary epitope retrieval method (involving heat) and secondary detection methods. Primary antibody was omitted for staining negative controls.
Biaxial mechanical testing
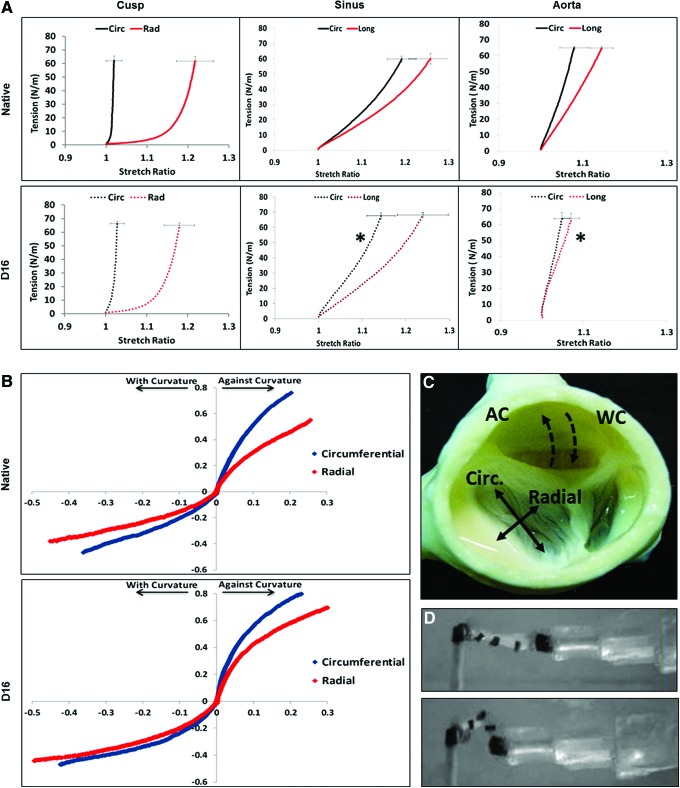
Biaxial mechanical testing (n = 5 for each group) was carried out for native and 16-day perfusion decellularized tissue specimens using the methodology previously published.32,33 Square-shaped samples of cca. 8 × 8 mm were dissected from the aortic valve cusps, sinuses, and aorta tissues of each group. For the aortic valve cusps, one edge of the sample was aligned with the circumferential direction and the other edge aligned with the radial direction; for the sinuses and aorta tissues, one edge of the sample was aligned with the circumferential direction and the other edge aligned with the longitudinal direction (Fig. 6C). Thickness of each sample was measured in triplicate using a digital caliper (Mitutoyo America Corporation). Four dark markers were placed in the center region of the square sample. Samples were mounted onto the biaxial testing system through stainless steel hooks attached to eight loops of 000 polyester suture of equal length (two suture loops per sample edge). A preload of 1 g was used during the biaxial mechanical testing. After 10 cycles of preconditioning, the sample was loaded to an equibiaxial tension (force/unit length) of 60 N/m. The sample extensibility was characterized by the maximum stretch ratio along the circumferential direction (λcirc) and radial direction (λrad). Biaxial testing was performed with the samples submerged in PBS bath at 37°C.
FIG. 6.
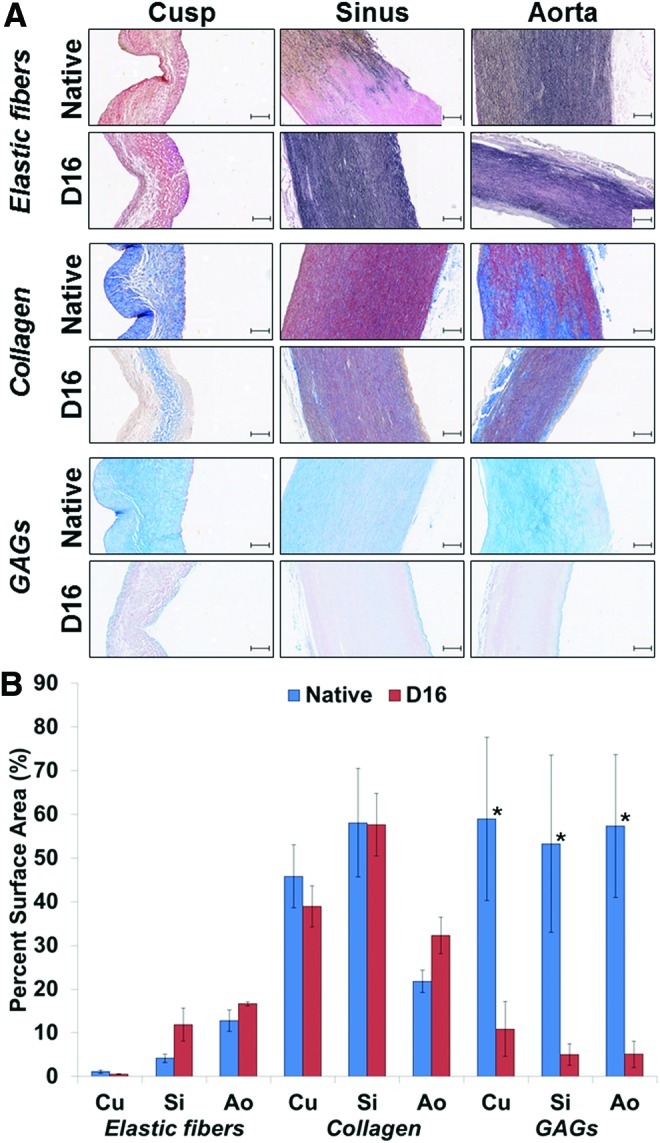
Representative histology sections (A) stained to reveal elastic fibers (dark brown), collagen (dark blue), and GAGs (light blue-turquoise) in aortic root tissues (cusp, sinus, aorta) before (native) and after 16 days (D16) of perfusion decellularization. All scale bars are 100 μm. (B) Graph depicting semiquantitative analysis of elastic fibers, collagen, and GAGs on sections from cusps (Cu), sinus (Si), and aorta (Ao) by digital image analysis. Values represent mean percent area occupied by each component relative to the total tissue section area analyzed. *Statistically significant differences. GAG, glycosaminoglycan. Color images available online at www.liebertpub.com/tec
Bending mechanical testing
Bending tests of aortic valve cusps were performed by following a previously reported protocol.34 Aortic valve cusp samples (native and decellularized) were dissected out of the belly region of the valve cusp. These samples were further trimmed to tissue strips (∼8 mm long by ∼4 mm wide) in both the circumferential (n = 5 each group) and radial directions (n = 5 each group). Thickness of each sample was measured in triplicate using a digital caliper. Two hollow posts (∼4 mm) were attached to each end of the tissue strip for mounting purposes. One end of the tissue strips was attached to a post that was fixed on the inside wall of the bath chamber, and the other end was mounted onto a 0.38 mm diameter bending bar (length of 14 cm). Each strip was mounted with the ventricularis side up and subjected to a simple bending test driven by a linear positioner and a stepper motor (Velmex, Inc.). Five dark contrast markers were used to track the cusp strip curvature, that is, marker 1 was pasted on the fixed post, marker 2–4 pasted along the edge of the tissue strip, and marker 5 pasted on the end of the bending bar (Fig. 6D). The bending movement was captured using a FireWire camera (DMK21AF04 model; The Imaging Source). Both the Velmex motor and FireWire camera were controlled by a custom written LabVIEW program (version 2000; National Instruments). In addition to circumferential and radial directions, each cusp strip was tested by flexing the strip with the natural curvature (where the ventricularis layer is in tension and the fibrosa layer in compression) and against the natural curvature (ventricularis in compression and the fibrosa layer in tension).
Hemodynamics evaluation
Fresh and 16-day perfusion decellularized aortic roots were placed in the purpose-designed holders (Fig. 7) and then mounted in upgraded versions (patent pending; Aptus, LLC) of the previously described heart valve bioreactor31,35 for functionality testing. The bioreactor was set at 70.58 bpm (350 ms systole and 500 ms diastole) and valves tested in aortic conditions of 120/80 mmHg pressures and 70 ± 1 mL stroke volume. For each valve, videos were obtained at 240 frames per second at a resolution of 320 × 240 pixels. Video converter software (Free Studio v. 6.4) was used to convert every video frame to its own numbered jpg file. ImageJ (NIH freeware) was then used for measurements of geometric orifice areas (GOAs) for each frame of three cycles of open and close per valve. Since the valves were of varying diameters, GOA data were normalized to diameter at maximum opening for each valve. Averages were then calculated for each point to generate data sets, which were plotted as GOAs as a function of time using 1/240 s intervals for each frame.
FIG. 7.
Mechanical properties of decellularized tissues. (A) Biaxial stress–strain analysis of aortic root tissue components before (native, full lines) and after complete decellularization by 16 days of perfusion (D16, dashed lines). Cusp tissues were tested in circumferential (Circ, black lines) and radial axis (Rad, red lines) and sinus and wall tissues in circumferential (Circ, black lines) and longitudinal (Long, red lines) axis. *Designates statistically significant differences in mean values of decellularized tissues compared to native tissues for each testing axis. (B) Bending properties of fresh and 16-day perfusion decellularized cusps (D16) tested with their natural curvature (WC) and against curvature (AC). (C) Top image of an acellular root depicting directions of the biaxial and bending analysis of aortic cusps shown in A and B. (D) Macroscopic aspects of a cusp strip during a bending test. Top, at rest; bottom, after full bending. Black contrast markers were glued onto the strip for tracking purposes. Color images available online at www.liebertpub.com/tec
Statistical analysis
Results are represented as mean ± SD. Statistical analysis was performed with one-way analysis of variances (ANOVA), and results were considered significantly different at p < 0.05.
Results
Novel system for perfusion decellularization
It is now evident that implantation of an incompletely decellularized xenogeneic scaffold is not realistic, as the cell remnants can induce severe immune reactions, inflammation, and calcification.18,21,36 While some tissues are readily decellularized by simple immersion, we and others have shown that immersion is not an effective means of cell removal from the aortic wall components of the root21 and also from extended lengths of small and medium diameter arteries.37 Therefore, we developed a unique perfusion decellularization setup (PDCell system; Aptus, LLC), which has been used extensively (∼50 roots have been decellularized using this system in two research laboratories) and was found to be reproducible and repeatable. The system was purposefully designed so that the aortic root is pressurized, expanded, and allowed to recoil cyclically to ensure flow of fluids through the thickness of the aortic wall. Simultaneously, the cusps are exposed to low-pressure gradients ensuring preservation of tissue integrity. To evaluate the efficacy of this system, we compared perfusion decellularized aortic roots with roots decellularized by immersion, specifically focusing on properties of the cusps, muscle component, sinus walls, and the aorta. In addition, we optimized perfusion time and conditions and established the need for nucleases as part of the procedure.
Decellularization effectiveness and quality control
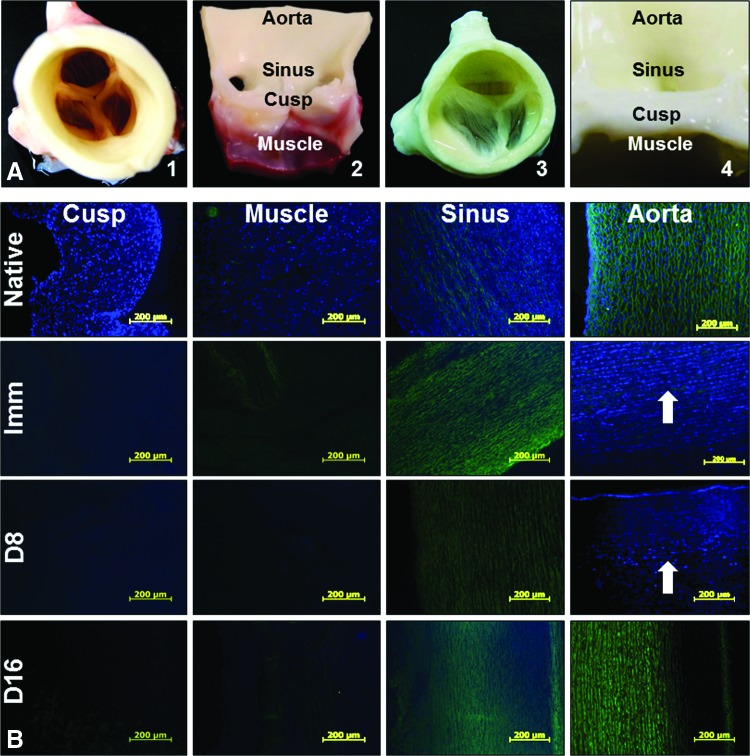
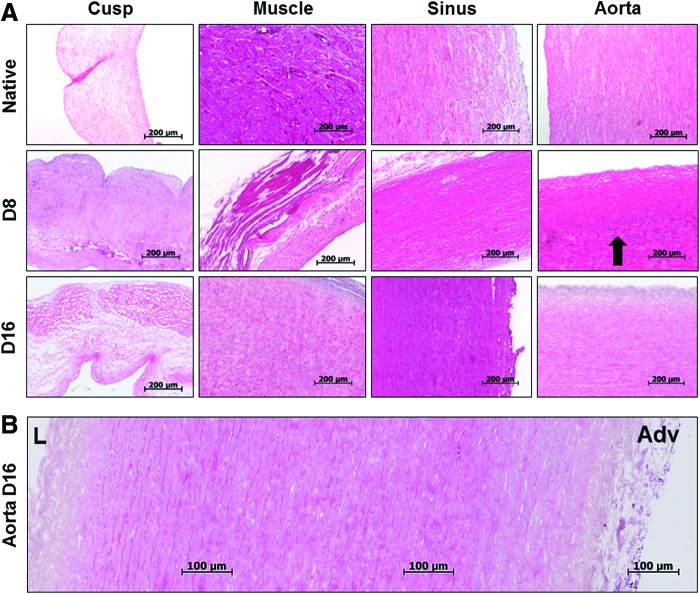
Macroscopically, the decellularized aortic roots appeared intact compared to native tissues (Fig. 2). Notably, acellular roots acquired a visible discoloration. Cusp, muscle, sinus, and wall tissue samples, collected at 8 and 16 days of perfusion decellularization (with and without nucleases) and from immersion decellularized roots, were analyzed for presence of cells by histology and DNA analysis and compared to fresh (native) tissues. Histology using DAPI nuclear staining (Fig. 2) showed that cusps, muscles, and sinus tissues were readily decellularized by immersion or 8 days of perfusion, as noted by disappearance of DAPI-stained nuclei from tissue sections. However, the wall component of the root contained large numbers of cell nuclei in the middle 1/3 of the media in both immersion and 8-day perfusion-treated roots, indicating restricted diffusion through the thick, dense, and elastic fiber-rich tissue. The aortic wall treated for 16 days in the PDCell system was completely devoid of DAPI-stained nuclei (Fig. 2B). These results were confirmed by H&E staining (Fig. 3), which was adequate for assessment of overall tissue morphology, presence of cell nuclei, and visualization of the “pores” created by cell removal. DNA analysis by EthBr agarose gel electrophoresis (Fig. 4) validated the histology data and showed that 16 days of perfusion decellularization followed by nuclease treatments were needed for complete DNA removal from the aortic wall. These results were also confirmed by NanoDrop quantification, showing a 10-fold reduction in DNA content after 16 days of perfusion decellularization (data not shown).
FIG. 2.
Representative macroscopic images (A) showing a native, fresh intact porcine aortic root in top view (1) and a fresh root cut open with one cusp removed to reveal the main internal anatomical coordinates (2), a fully decellularized aortic root (16-day perfusion) in top view (3) and cut open (4). (B) Representative histology images of fresh aortic root sections stained for nuclei with DAPI (blue) and overlaid with elastin autofluorescence (green) before (native) and after immersion decellularization (Imm) and after 8 days (D8) and 16 days (D16) of perfusion decellularization. White arrows point to DAPI-stained cell remnants. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tec
FIG. 3.
Hematoxylin and eosin-stained sections (A) showing representative aortic root tissues before (native) and after 8 days (D) and 16 days (D16) of perfusion decellularization. Arrows point to stained cell nuclei remnants. H&E stain shows nuclei in blue and cytoplasm and matrix in pink. (B) Representative panoramic composite of three images spanning the entire thickness of the acellular aortic wall (16-day perfusion). L, lumen; Adv, adventitia; H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tec
FIG. 4.
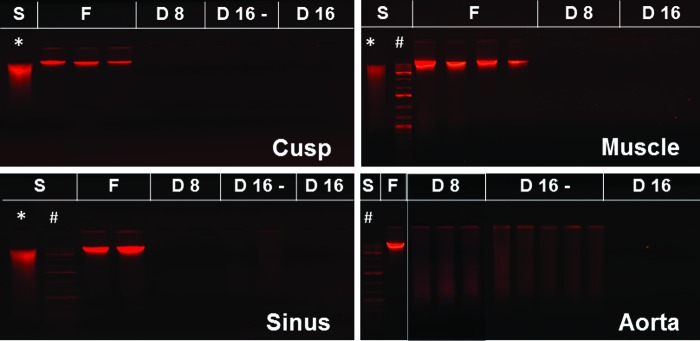
Ethidium bromide agarose gel electrophoresis of DNA extracted from fresh aortic root tissues (F) and tissues collected after 8 days (D8) and 16 days (D16) of perfusion decellularization. Samples were also collected before the nuclease treatment step from the 16-day perfusion groups (D16). S, DNA standards consisting of whole genomic DNA (*) and DNA ladder (#). Red fluorescent bands represent double-stranded DNA. Color images available online at www.liebertpub.com/tec
Extracellular matrix components
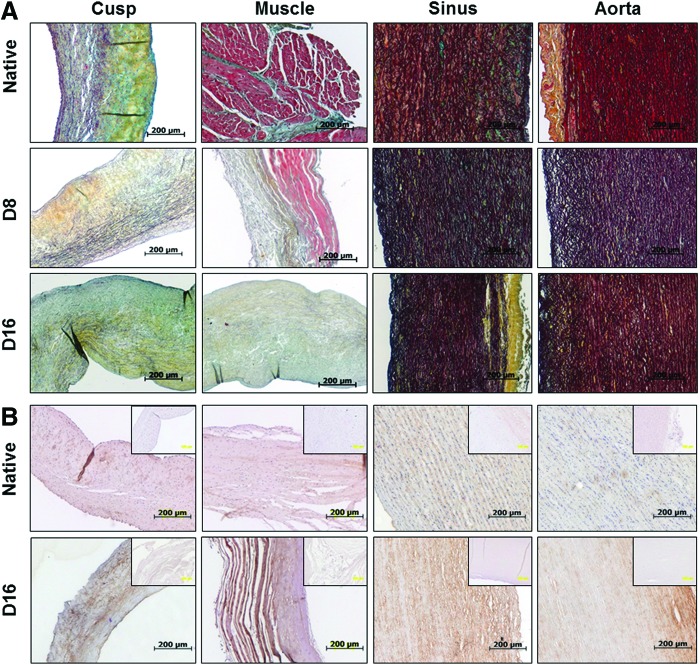
Initial Movat's pentachrome staining (Fig. 5) revealed apparent preservation of intact extracellular matrix primarily comprising collagen and elastic fibers, without any visible changes in tissue structure after complete decellularization. Glycosaminoglycans (light blue on Movat's stain) were present in all native tissues, but could not be detected in any tissues after decellularization. These observations were confirmed by digital image analysis of sections stained separately for elastic fibers, collagen, and glycosaminoglycans/proteoglycans (Fig. 6). Quantitative results showed excellent preservation of elastic fibers and collagen (p > 0.05) and statistically significant reduction in glycosaminoglycan (GAG) content in all tissues after complete decellularization (p < 0.05). IHC staining revealed preservation of type IV collagen component of the basement membranes in all fully decellularized tissues.
FIG. 5.
Representative Movat's pentachrome-stained sections (A) reveal collagen (yellow), elastin (dark brown), and muscle cells (red) in native aortic root tissues before (native) and after 8 days (D8) and 16 days (D16) of perfusion decellularization. (B) Representative images depicting IHC staining for type IV collagen (brown, positive IHC reaction; blue, nuclei) in native aortic root tissues before (native) and after 16 days (D16) of perfusion decellularization. Inserts, negative IHC controls. Color images available online at www.liebertpub.com/tec
Mechanical testing
Overall results (Fig. 7) showed that complete decellularization did not alter the natural anisotropy of root tissues. When comparing acellular cusps to native cusps, no statistically significant differences were found in biaxial mechanical properties (Fig. 7A) in either direction (p = 0.623 for circumferential, p = 0.330 for radial). When tested for bending characteristics (Fig. 7B), the moment–curvature curves for both native and acellular cusps showed that against curvature bending was stiffer than the with curvature bending and the circumferential direction bending was stiffer than the radial direction, as observed earlier in our previous study.34 Overall, the decellularized cusps showed an almost identical trend in moment–curvature relationship to that of the native aortic valve cusps. The biaxial properties of the acellular sinus were also not statistically different from native sinus (p = 0.7800 in the radial direction and marginally different in the circumferential direction, p = 0.040). Conversely, the acellular aortic wall portion of the root was stiffer in the longitudinal direction (p = 0.002) and not statistically different in the circumferential direction (p = 0.220).
Hemodynamics
To evaluate functionality of acellular roots, we designed a novel MechAnnulus Ring mounting system (Fig. 8A–C), which allowed for testing of whole roots under a variety of conditions in the updated heart valve bioreactor (Fig. 8D). The valves functioned well at aortic parameters of flow and pressures without regurgitation and their functionality did not change with time over multiple cycles. Maximum GOAs reached about 400–470 mm2 for the 22–24 mm diameter roots. During each cycle, both the native and acellular roots opened quickly (in cca. 50 ms) with well-coordinated cusp motions. While in the fully open segment, we recorded slight movement of the sinuses and apparent fluttering of the cusps for several hundreds of milliseconds. The valves remained open for about 300 ms after which they closed rapidly, within <20 ms, without regurgitation.
FIG. 8.
Hemodynamics of acellular aortic roots. For proper mounting, a four-legged support with a perforated top insert was designed in SolidWorks, a computer-aided design software (A) and three dimensionally printed (B). The support served mounting of the distal portion of the aortic wall using 12–14 individual sutures (C). The root was then placed (black arrow) into the heart valve bioreactor (D), adapted with a camera (white arrow) capable of acquiring top view images. (E) Average geometric orifice areas (in mm2), normalized to valve diameter as a function of time (means for n = 3 cycles per valve), are shown for fresh (native, blue line) and 16-day perfusion decellularized (D16, red line) aortic roots. Representative images (blue border, fresh roots; red border, decellularized roots) are shown as inserts for the closed position (1), fully open (2), midway through the open phase (3), just before starting to close (4), and fully closed again (5). Color images available online at www.liebertpub.com/tec
Discussion
Novel system for perfusion decellularization
Although widely used because of their simplicity, immersion decellularization techniques are inadequate for decellularizing whole aortic roots. This study and others26,38 showed that immersion is effective for tissues that are thin or relatively more porous than the aorta, which contains numerous layers of elastic fibers that restrict penetration of fluids. In some applications, immersion decellularization techniques are replaced with perfusion techniques for tissues that are difficult to decellularize.15,28 In the case of porcine aortic roots, it can be inferred that procedures, which are more rigorous, will be necessary to decellularize aortic portions than the procedures used to decellularize the cusps. However, the balance must be made to retain the structural integrity of the cusps while providing conditions harsh enough to decellularize the aortic wall. A device (PDCell system) and method of use were created for decellularizing the thick portions of the aortic root as well as the more fragile cusp portions. The cusps were subjected only to immersion-like conditions, and the aorta and sinus were subjected to a cyclical transmural pressure gradient and resultant stretch. This combination of isolated conditions resulted in an aortic root that was overall decellularized, yet maintained structural integrity in the most fragile and vital areas. A critically needed component of valve perfusion systems is the mounting mechanism, which secures the aortic root in place to provide a very tight seal without damaging tissues through the entire process of valve preparation and testing. Moreover, during this study, we learned that decellularization leads to loss of tissue thickness, thus potentially compromising the seal. For these reasons, we designed a self-adjusting mounting system (MechAnnulus Rings), which also allowed for no-touch handling of the aortic root during processing.
Efficiency of root decellularization
The root is a complex three-dimensional structure composed of functional elements that differ in composition, structure, and thickness. Therefore, our approach was to undertake a separate comprehensive analysis of the cusps, sinuses, and aortic wall components. Despite large utilization, simple H&E histology staining was not sufficient to demonstrate decellularization.21 In the current study, it was at times difficult to classify an object as a cell because the affinity of certain tissue components for traditional stains was altered after decellularization. In addition, incompletely extracted cell remnants appeared as dark blue smears upon H&E staining.39 Completeness of cell removal must be evaluated by several complementary methods, which focus on localizing cell nuclei, cell remnants, and DNA. Thus, we defined fully acellular tissues, as those that corroborated complete lack of nuclei staining on H&E sections, lack of DAPI nuclei staining (which detects intact double stranded DNA), and minimal content of DNA as evidenced by EthBr agarose gel electrophoresis of extracted and purified genomic DNA. A more than 95% reduction in DNA content (corresponding to <50 ng/mg dry tissue) was considered a satisfactory threshold by Badylak et al.40 Additional quantification by UV spectrophotometry (NanoDrop) has not always proven reliable, possibly because this method is not very sensitive at very low concentrations. To simplify data presentation, since immersion treatment was shown early on to be ineffective on the wall, the remainder of the results and analyses was focused only on the 8- or 16-day perfusion groups.
Extracellular matrix
Integrity of the extracellular matrix is the second criterion important in development of acellular tissue scaffolds. This was evaluated on histological sections using Movat's pentachrome as well as separate stains for elastic fibers, collagen, and GAGs. IHC was also employed to investigate type IV collagen, an essential component of the basement membrane. Overall, we noticed that the 16-day perfusion method preserved the main matrix components (collagen, elastic fibers, type IV collagen) in all segments of the root, with the exception of GAGs, which were lost from all tissues during decellularization. We have shown previously that GAGs are lost very easily during tissue preparation steps, which utilize aqueous solvents.41–43 It is not known what effects the paucity of GAGs would have on the durability of implanted acellular scaffolds; certainly this aspect requires more investigations.
Mechanical properties
It is well known that, for most published decellularization protocols, the aortic valve cusps experience an increase in overall tissue extensibility due to the microstructural disruptions of the collagen and elastic fiber networks.33 However, the current decellularization protocol preserved the biaxial behavior of cusps. This may be due to exposure of cusps to small differential pressures, low concentrations of detergents, and lack of protease treatments. When tested for bending, acellular cusps maintained moment–curvature trends comparable to that of native aortic valve cusps. The subtle variations of cusp flexure, when bended with and against curvature, in both circumferential and radial directions, were all preserved after the decellularization process. Taken together, these observations suggest that the decellularization process preserved the ultrastructural level subtleties of the aortic valve cusps. The sinus and the aortic wall have a different structure, and expectedly, effects of complete decellularization on the sinus and aortic wall were different compared to the cusps. The most evident alteration was stiffening of the aortic wall when tested in the longitudinal direction. The mechanism of this process is unknown, but we hypothesize that this may be due to removal of components involved with stabilizing interactions between collagen fibers. It remains to be determined whether these subtle changes in the mechanical properties of the acellular root wall components will influence the long-term durability of implanted roots. We did not show additional data on the muscle component since it is a nonfunctional element, appears in very small amounts, and will likely be dissected away during implantation.
Hemodynamics
Digital video imaging and analysis generated important data regarding functionality of acellular aortic roots. The roots were mounted onto the purpose-designed supports and tested under a variety of conditions in the updated heart valve bioreactor. Notably, both acellular and native valves opened and closed under very low pressures (not shown) indicating that the decellularization protocol did not change the cusp tissue response to minute changes in pressures and flow. Overall, the acellular valve roots functioned well and their functionality did not change with time over multiple cycles. The GOA for acellular valve roots was within acceptable limits for valves of 22–24 mm diameters.
Conclusions
The advancement of heart valve replacement and regeneration is clearly moving in the direction of tissue-engineered devices. One option for scaffold development is to employ decellularized xenogeneic aortic valve roots. The aortic root is composed of several different tissues, and careful structural and functional consideration has to be given to each segment and component. This study demonstrates that immersion techniques are inadequate for whole-root decellularization and that specialized tissue holding devices and pressurized perfusion systems are a vital part of the process of generating functional acellular aortic valve roots. The perfusion system designed for this research is the first of its kind for this specific application. Its capabilities to treat individual components of the valve root with independent conditions allow it to be rigorous enough to dynamically decellularize the aortic root while gentle enough to preserve the integrity of the cusps. Success criteria for evaluation of each root segment for decellularization completeness, tissue integrity, and valve functionality were defined using several complementary methods of cell analysis, biomechanics, and physiologic bioreactor testing. Fully acellular porcine roots exhibited preserved macroscopic structures and microscopic matrix components, which translated into conserved mechanical properties and excellent valve functionality when tested in aortic flow and pressure conditions. These results, together with the proof we have provided that shows excellent preservation of the root extracellular matrix and mechanical properties, strongly support the hypothesis that acellular roots generated with our method yield fully functional valves. Future studies call for more detailed characterization of scaffolds using transmission and scanning electronmicroscopy, proteomics and glycomics, as well as testing efficacy of such scaffolds, for heart valve tissue engineering and regeneration in vitro and in vivo.
Acknowledgments
The authors would like to acknowledge the Clemson University Machining and Technical Services for manufacturing the perfusion decellularization system and Snow Creek Meat Processing Center for generous donation of porcine valve roots. This project was funded, in part, by NHLBI of the National Institutes of Health under award number RO1HL093399, by NIGMS of the National Institutes of Health under award number 5P20GM103444-07, and by a grant from the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, project number PNII-ID-PCCE-2011-2-0036.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wirrig E.E., and Yutzey K.E. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol 34, 737, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieu P., and Boulanger M.C. Basic mechanisms of calcific aortic valve disease. Can J Cardiol 30, 982, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Weiss R.M., Miller J.D., and Heistad D.D. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res 113, 209, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towler D.A. Molecular and cellular aspects of calcific aortic valve disease. Circ Res 113, 198, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerstrom F., Barderas M.G., and Rodriguez-Padial L. Aortic stenosis: a general overview of clinical, pathophysiological and therapeutic aspects. Expert Rev Cardiovasc Ther 11, 239, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos A., Kaoukis A., Papadaki H., and Pyrgakis V. Pathophysiologic mechanisms of calcific aortic stenosis. Ther Adv Cardiovasc Dis 6, 71, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Ladich E., Nakano M., Carter-Monroe N., and Virmani R. Pathology of calcific aortic stenosis. Future Cardiol 7, 629, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Elder M. Global Surgical Procedure Volumes. Kalorama Information, New York, NY, 2014 [Google Scholar]
- 9.Chester A.H., El-Hamamsy I., Butcher J.T., Latif N., Bertazzo S., and Yacoub M.H. The living aortic valve: from molecules to function. Glob Cardiol Sci Pract 2014, 52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henaine R., Roubertie F., Vergnat M., and Ninet J. Valve replacement in children: a challenge for a whole life. Arch Cardiovasc Dis 105, 517, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Piazza N., de Jaegere P., Schultz C., Becker A.E., Serruys P.W., and Anderson R.H. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv 1, 74, 2008 [DOI] [PubMed] [Google Scholar]
- 12.David T.E., Gavra G., Feindel C.M., Regesta T., Armstrong S., and Maganti M.D. Surgical treatment of active infective endocarditis: a continued challenge. J Thorac Cardiovasc Surg 133, 144, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Simionescu D.T., Chen J., Jaeggli M., Wang B., and Liao J. Form follows function: advances in trilayered structure replication for aortic heart valve tissue engineering. J Healthc Eng 3, 179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tedder M.E., Simionescu A., Chen J., Liao J., and Simionescu D.T. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A 17, 25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grocott-Mason R.M., Lund O., Elwidaa H., Mazhar R., Chandrasakeran V., Mitchell A.G., Ilsley C., Khaghani A., Rees A., and Yacoub M. Long-term results after aortic valve replacement in patients with congestive heart failure. Homografts vs prosthetic valves. Eur Heart J 21, 1698, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Allen M.D., Shoji Y., Fujimura Y., Gordon D., Thomas R., Brockbank K.G., and Disteche C.M. Growth and cell viability of aortic versus pulmonic homografts in the systemic circulation. Circulation 84, III94, 1991 [PubMed] [Google Scholar]
- 17.McNally R.T., and Brockbank K.G. The correlation between improved cellular viability and clinical performance in 5,000 cryopreserved human heart valves. ASAIO Trans 37, M355, 1991 [PubMed] [Google Scholar]
- 18.Simon P., Kasimir M.T., Seebacher G., Weigel G., Ullrich R., Salzer-Muhar U., Rieder E., and Wolner E. Early failure of the tissue engineered porcine heart valve synergraft in pediatric patients. Eur J Cardiothorac Surg 23, 1002, 2003; discussion 1006 [DOI] [PubMed] [Google Scholar]
- 19.Voges I., Brasen J.H., Entenmann A., Scheid M., Scheewe J., Fischer G., Hart C., Andrade A., Pham H.M., Kramer H.H., and Rickers C. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg 44, e272, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Pennel T., Fercana G., Bezuidenhout D., Simionescu A., Chuang T.H., Zilla P., and Simionescu D. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials 35, 6311, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paniagua Gutierrez J.R., Berry H., Korossis S., Mirsadraee S., Lopes S.V., da Costa F., Kearney J., Watterson K., Fisher J., and Ingham E. Regenerative potential of low-concentration sds-decellularized porcine aortic valved conduits in vivo. Tissue Eng Part A 21, 332, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber B., Dijkman P.E., Scherman J., Sanders B., Emmert M.Y., Grunenfelder J., Verbeek R., Bracher M., Black M., Franz T., Kortsmit J., Modregger P., Peter S., Stampanoni M., Robert J., Kehl D., van Doeselaar M., Schweiger M., Brokopp C.E., Walchli T., Falk V., Zilla P., Driessen-Mol A., Baaijens F.P., and Hoerstrup S.P. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 34, 7269, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Syedain Z.H., Meier L.A., Reimer J.M., and Tranquillo R.T. Tubular heart valves from decellularized engineered tissue. Ann Biomed Eng 41, 2645, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock U.A., Degenkolbe I., Attmann T., Schenke-Layland K., Freitag S., and Lutter G. Prevention of device-related tissue damage during percutaneous deployment of tissue-engineered heart valves. J Thorac Cardiovasc Surg 131, 1323, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Mol A., Smits A.I., Bouten C.V., and Baaijens F.P. Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices 6, 259, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Tedder M.E., Liao J., Weed B., Stabler C., Zhang H., Simionescu A., and Simionescu D.T. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A 15, 1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butcher J.T., Mahler G.J., and Hockaday L.A. Aortic valve disease and treatment: the need for naturally engineered solutions. Adv Drug Deliv Rev 63, 242, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Tudorache I., Calistru A., Baraki H., Meyer T., Hoffler K., Sarikouch S., Bara C., Gorler A., Hartung D., Hilfiker A., Haverich A., and Cebotari S. Orthotopic replacement of aortic heart valves with tissue-engineered grafts. Tissue Eng Part A 19, 1686, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syedain Z.H., Bradee A.R., Kren S., Taylor D.A., and Tranquillo R.T. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A 19, 759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers P., De Somer F., Cornelissen M., Thierens H., and Van Nooten G. Decellularization of heart valve matrices: search for the ideal balance. Artif Cells Blood Substit Immobil Biotechnol 40, 151, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Sierad L.N., Simionescu A., Albers C., Chen J., Maivelett J., Tedder M.E., Liao J., and Simionescu D.T. Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc Eng Technol 1, 138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow J.P., Simionescu D.T., Warner H., Wang B., Patnaik S.S., Liao J., and Simionescu A. Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol. Biomaterials 34, 685, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao J., Joyce E.M., and Sacks M.S. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 29, 1065, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brazile B., Wang B., Wang G., Bertucci R., Prabhu R., Patnaik S., Butler J., Claude A., Brinkman-Ferguson E., Williams L., and Liao J. On the bending properties of porcine mitral, tricuspid, aortic, and pulmonary valve leaflets. J Long Term Eff Med Implants 25, 41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sierad L.N., Shaw E.L., Launius R., McBride S., Storholt C., Poole R., Spence D., Miller K., Sosdian L., Allen K., Burton L., Iari A., Nagatomi J., and Simionescu D.T. Toward an endothelial-cell covered mechanical valve; surface re-engineering and bioreactor testing of mechanical heart valves. Challenges Regener Med 1, 22, 2014 [Google Scholar]
- 36.Keane T.J., Londono R., Turner N.J., and Badylak S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Fercana G., Bowser D., Portilla M., Langan E.M., Carsten C.G., Cull D.L., Sierad L.N., and Simionescu D.T. Platform technologies for decellularization, tunic-specific cell seeding, and in vitro conditioning of extended length, small diameter vascular grafts. Tissue Eng Part C Methods 20, 1016, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercuri J.J., Patnaik S., Dion G., Gill S.S., Liao J., and Simionescu D.T. Regenerative potential of decellularized porcine nucleus pulposus hydrogel scaffolds: stem cell differentiation, matrix remodeling, and biocompatibility studies. Tissue Eng Part A 19, 952, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Schulte J.B., Simionescu A., and Simionescu D.T. The acellular myocardial flap: a novel extracellular matrix scaffold enriched with patent microvascular networks and biocompatible cell niches. Tissue Eng Part C Methods 19, 518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badylak S.F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 42, 1517, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Lovekamp J.J., Simionescu D.T., Mercuri J.J., Zubiate B., Sacks M.S., and Vyavahare N.R. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials 27, 1507, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simionescu D.T., Lovekamp J.J., and Vyavahare N.R. Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: an ultrastructural study. J Heart Valve Dis 12, 226, 2003 [PubMed] [Google Scholar]
- 43.Simionescu D.T., Lovekamp J.J., and Vyavahare N.R. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: implications for bioprosthetic heart valve degeneration. J Heart Valve Dis 12, 217, 2003 [PubMed] [Google Scholar]