Abstract
The presentation and controlled release of bioactive signals to direct cellular growth and differentiation represents a widely used strategy in tissue engineering. Historically, work in this field has primarily focused on the delivery of large cytokines and growth factors, which can be costly to manufacture and difficult to deliver in a sustained manner. There has been a marked increase over the past decade in the pursuit of lipid mediators due to their wide range of effects over multiple cell types, low cost, and ease of scale-up. Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are two bioactive lysophospholipids (LPLs) that have gained attention for use as pharmacological agents in tissue engineering applications. While these lipids can have similar effects on cellular response, they possess distinct chemical backbones, mechanisms of synthesis and degradation, and signaling pathways using a discrete set of G-protein-coupled receptors (GPCRs). LPA and S1P predominantly act extracellularly on their GPCRs and can directly regulate cell survival, differentiation, cytokine secretion, proliferation, and migration—each of the important functions that must be considered in regenerative medicine. In addition to these potent physiological functions, these LPLs play pivotal roles in a number of pathophysiological processes. To capitalize on the promise of these molecules in tissue engineering, these lipids have been incorporated into biomaterials for in vivo delivery. Here, we survey the effects of LPA and S1P on both cellular- and tissue-level phenotypes, with an eye toward regulating stem/progenitor cell growth and differentiation. In particular, we examine work that has translational applications for cell-based tissue engineering strategies in promoting cell survival, bone and cartilage engineering, and therapeutic angiogenesis.
Introduction
One of the fundamental tenets of tissue engineering is the presentation and controlled release of bioactive signals to direct cellular growth and differentiation. Historically, work in this field has centered on the delivery of large cytokines and growth factors.1 These biomacromolecules play critical roles in regulating endogenous tissue growth and maturation and are widely investigated for their therapeutic potential to drive stem cell proliferation and differentiation. However, recombinant proteins are costly to manufacture and difficult to deliver in a sustained manner over time,1 necessitating the use of supraphysiological doses in clinical applications that can lead to uncontrolled tissue growth. Although there has been less focus on the use of secondary metabolites for tissue engineering applications, there has been a marked increase over the past decade in the pursuit of lipid mediators due to their wide range of effects over multiple cell types,2,3 low cost, and ease of scale-up.
Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are two bioactive lysophospholipids (LPLs) that have gained attention for use as pharmacological agents in tissue engineering applications. While these lipids can have similar effects on cellular response, they possess distinct chemical backbones, mechanisms of synthesis and degradation, and signaling pathways mediated by a discrete set of G-protein-coupled receptors (GPCRs). LPA and S1P predominantly act extracellularly on their GPCRs and can directly regulate cell survival,2,4,5 differentiation,2,6–8 cytokine secretion,9,10 proliferation,11–13 and migration2,12,13—each of the important functions that must be considered in regenerative medicine. In addition to these potent physiological functions, these LPLs play pivotal roles in pathophysiological processes, including autoimmune disease, fibrotic disease, cancer, inflammation, and bone disease.2,14 These lipids have been incorporated into biomaterials for in vivo delivery4,9,11,15,16 and are several orders of magnitude cheaper than recombinant proteins.9 Here, the effects of LPA and S1P on both cellular- and tissue-level phenotypes are surveyed, with an eye toward regulating stem/progenitor cell growth and differentiation. In particular, this review examines work that has translational applications for cell-based tissue engineering strategies.
Metabolism and Cellular Signaling
The chemical structures of LPA and S1P have both a phosphate head group and a single fatty acid chain attached to a three-carbon backbone.14 Their levels in circulation are maintained via tightly regulated metabolic pathways, with plasma levels typically ranging from 500 to 1000 nM.3,17 While there are multiple routes toward LPA biosynthesis, leading to structural heterogeneity,18 S1P is solely created through sphingolipid turnover.10
The bulk of LPA found in the circulation is generated by the action of autotaxin (ATX), a circulating lysophospholipase D enzyme that cleaves the phosphodiester bonds of LPLs.2,19 In particular, activated platelets secrete large amounts of lysophosphatidylcholine, lysophosphatidylserine, and lysophosphatidyl-ethanolamine, which are subsequently converted to LPA by ATX.20 Sphingolipid turnover is initiated when sphingomyelinase converts sphingomyelin into ceramide.17 In sphingolipid metabolism, ceramide is first converted into sphingosine by enzyme ceramidase and S1P is then produced by the subsequent phosphorylation of sphingosine by sphingosine kinase (SK).10
G protein-coupled receptors
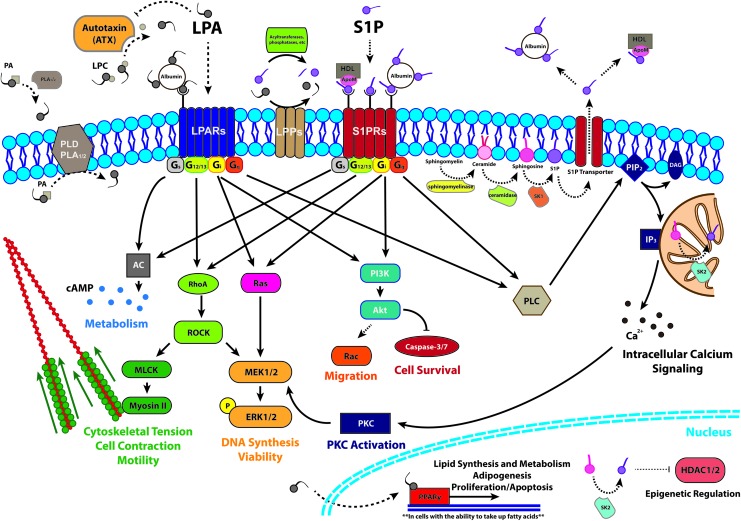
S1P and LPA elicit pleiotropic cellular effects by activating GPCRs on the cell surface. Both LPLs signal through the endothelial differentiation gene (EDG) family of receptors and are ligands for the P2Y10 receptor (Table 1).21,22 Extracellular LPA can affect cellular response through at least six GPCRs (LPA1–6), while S1P engages at least five (S1P1–5). These GPCRs are differentially expressed in various tissues, can change with cellular differentiation state, and have separate coupled subunits that trigger distinct intracellular signaling cascades (Fig. 1).23–27
Table 1.
G-Protein-Coupled Receptors
| Receptor | Synonyms | Receptor family | G-protein-coupled subunits | References |
|---|---|---|---|---|
| LPA1 | LPAR1, EDG2 | EDG | Gi, Gq, G12/13 | 23,28,32,122,125 |
| LPA2 | LPAR2, EDG4 | EDG | Gi, Gq, G12/13 | 23,28,32,122,125 |
| LPA3 | LPAR3, EDG7 | EDG | Gi, Gq | 23,28,32,122,125 |
| LPA4 | LPAR4, GPR23, P2Y9 | P2Y | Gq, G12/13, GS | 23 |
| LPA5 | LPAR5, GPR92 | P2Y | Gq, G12/13 | 23 |
| LPA6 | LPAR6, P2RY5 | P2Y | G12/13 | 23 |
| S1P1 | S1PR1, EDG1 | EDG | Gi | 122,125 |
| S1P2 | S1PR2, EDG5 | EDG | Gi, Gq, G12/13 | 122,125 |
| S1P3 | S1PR3, EDG3 | EDG | Gi, Gq, G12/13 | 122,125 |
| S1P4 | S1PR4, EDG6 | EDG | Gi, G12/13, GS | 122,125 |
| S1P5 | S1PR5, EDG8 | EDG | Gi, G12/13 | 122,125 |
LPA, lysophosphatidic acid; S1P, sphingosine-1-phosphate; EDG, endothelial differentiation gene.
FIG. 1.
Examples of the most widely understood mechanisms of LPA synthesis, degradation, and intracellular signaling. LPA, lysophosphatidic acid; PA, phosphatidic acid; PLA, phospholipase A; PLD, phospholipase D; LPP, lipid phosphate phosphatase; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacyl glycerol; AC, adenylyl cyclase; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PLC, phospholipase C; IP3, inositol 1,4,5-trisphosphate; ROCK, Rho-associated protein kinase; MLCK, myosin light chain kinase; MEK, mitogen-activated protein kinase kinase; ERK, extracellular-signal-regulated kinases; PKC, protein kinase C; PPARγ, peroxisome proliferator-activated receptor gamma; HDAC, histone deacetylase.3,15,18,21,23,24,27–29,32,71,73,112,116,118–124 Color images available online at www.liebertpub.com/teb
Indeed, the engagement of GPCRs is the most widely accepted primary mechanism by which LPA influences cell behavior.3,23,24,28,29 LPA1, LPA2, and LPA3 belong to the EDG family of receptors and were the first to be identified and characterized. LPA4, LPA5, and LPA6 were discovered later and classified as purinergic-like (P2Y) receptors, with distinct amino-acid identities and biological functions.23,24 Three other putative LPA receptors—GPR35,30 GPR87,31 and P2Y1021—may also be involved in LPA signaling, but their roles are not yet fully characterized.24,32
The most important biological role of S1P is to serve as a natural ligand for the EDG family of GPCRs.26 At least five distinct GPCRs, S1P1–5, are known to bind S1P with a high affinity (Kd of 2–30 nM) and each elicits distinct biological actions of which conflicting results have been reported.25,33,34 To demonstrate the pleiotropic nature of S1P receptors (S1PRs), S1P1 activation enhances barrier integrity and vessel maturation whereas S1P3 conversely promotes vessel permeability and remodeling.35,36 While S1P1–3 are ubiquitously expressed, S1P4 is solely expressed in lymphoid and lung tissues, and S1P5 expression is localized to the brain and spleen.37
Intracellular signaling
Intracellular LPA signals primarily through activation of peroxisome proliferator-activated receptor gamma (PPARγ) and may play a key role in regulation of fatty acid metabolism.29,32 The effects of PPARγ include adipogenic differentiation, lipid metabolism, arterial wall remodeling,38,39 and dendritic cell differentiation.40 However, LPA binds strongly at a 3:1 stoichiometric ratio to serum albumin, which serves as a stabilizer and carrier protein.3 With the exception of cells such as macrophages, vascular smooth muscle cells (SMCs), and platelets that can internalize oxidized low-density lipoprotein associated with LPA,41,42 the LPA-albumin complex is unable to enter cells in high quantities.32 This likely limits the effectiveness of strategies seeking to directly stimulate PPARγ signaling when applied to cells cultured in serum-supplemented media in vitro.
While capable of eliciting intracellular actions, the intracellular targets of S1P are only recently being discovered.10,27 S1P produced by SK 2, highly localized in the nucleus, has been reported to bind and inhibit histone deacetylase (HDAC) activity.27,43 HDAC inhibition is a growing target for cancer therapies via reversal of aberrant epigenetic changes associated with human disease. Independent of S1PRs, S1P can activate nuclear factor-κB (NF-κB), a key inflammatory transcription factor.44 Furthermore, S1P produced in the cell is exported by specific membrane transporters, including spinster 2, which has a role in establishing a vascular gradient of S1P in mice.17 This release of S1P might also contribute to inside-out signaling by stimulating S1PRs after being transported outside of the cell membrane.10,45
Activity of Bioactive Phospholipids in Homeostasis and Disease
LPA and S1P have a wide range of effects on many tissue types and on cells at varying stages of differentiation and development. Many tissue engineering strategies seek to recapitulate or modulate similar cellular responses. Therefore, establishing an understanding of endogenous LPL signaling is critical to the success of such approaches. Furthermore, given the pleiotropic nature of LPL signaling, control over spatiotemporal presentation becomes important for establishing the intended selectivity of receptor activation. We highlight some of these areas and address current studies of LPA and S1P next.
Vascular health
Both LPA and S1P play significant roles in vascular development and disease, as might be expected from the close relationship with platelet activation. ATX-knockout mice fail to develop a functional vasculature and do not survive embryonic development, at least in part due to the lack of LPA synthesis.46 LPA (1 μM) also stimulates angiogenesis of developing blood vessels in a chick chorioallantoic membrane (CAM) model,47 achieving a response quantitatively similar to 50 ng vascular endothelial growth factor (VEGF). LPA-induced vessels were larger in diameter than those induced by VEGF. In mature vessels, LPA can induce endothelial cell mitogenesis48 and increase the permeability of cell–cell junctions,49 the latter of which can facilitate metastasis. LPA also induces SMC dedifferentiation50 and may lower expression of CD36 by endothelial cells.51,52 Consistent with its role in Rho-ROCK signaling, LPA sensitizes murine aortic endothelial cells to oscillatory shear stresses by regulating Ca2+ transients.53 Micromolar levels of LPA may induce a vasoconstrictive response54 in medial SMCs under high shear stresses, but lower doses cause endothelium-dependent vasodilation via endothelial nitric oxide synthase and phospholipase activity.55
VEGF is perhaps the most widely studied proangiogenic molecule whose activity has been targeted as a therapeutic for initiating neovascularization and for blocking tumor growth. S1P has also been touted as a complete angiogenic molecule given its contributions at both early stages of angiogenesis and later stages of neovessel stabilization.8,56–61 S1P plays a pivotal role in regulating angiogenesis and vascular tone.36,62 S1P1 is essential for vascular development, as S1P1-null mice exhibit lethal embryonic hemorrhage.8 S1P promotes initial sprouting of capillary-like structures by endothelial cells in vitro13 and synergistically acts with basic fibroblast growth factor for induction of in vitro angiogenesis.63 In addition, S1P plays a crucial role in stabilizing neovessels in arteriogenesis via the recruitment of mural cells and regulation of endothelial cell–cell junctions.11 While S1PR1 activation has been shown to inhibit VEGF-induced hyperpermeability and aberrant sprouting,64,65 S1PR3 conversely enhances vessel permeability and remodeling.35,66 Thus, temporal regulation of S1P signaling is imperative for dictating the overall outcome. In light of its potent activities throughout the process of catalyzing the formation and stabilization of neovessels, S1P has emerged as a promising target for novel therapeutic approaches to treating ischemic diseases.67 Many studies have also examined the angiogenic activity of LPLs as compared with proangiogenic growth factors, including VEGF, further highlighting their therapeutic potential.47,68–70 For example, S1P and LPA each induced a similar angiogenic response to VEGF in an in vitro chicken CAM assay.47 S1P has also been shown to surpass VEGF and independently induce sprouting and directed migration of outgrowth endothelial cells to a similar degree as the combination of S1P and VEGF under hypoxia.70 Thus, given its “bimodal” angiogenic ability, one may speculate that it may be more therapeutically effective to deliver S1P with spatiotemporal control rather than combinations of multiple growth factors.
Skeletal biology
LPA signaling influences bone biology and the maintenance of skeletal homeostasis. Developmentally, LPA1 KO mice exhibit decreased bone density, shorter bone length, and craniofacial dysmorphism. In addition, bone marrow (BM) stem/stromal cells isolated from these animals have reduced osteogenic potential.71,72 On the contrary, LPA4 KO mice manifest higher bone mass, with greater trabecular number and thickness.73
Mesenchymal stem/stromal cells (MSCs), which participate directly in developmental bone formation by differentiating into osteoblasts,74 also respond to LPA. In addition to inducing migration,75 albumin-bound LPA upregulates alkaline phosphatase (ALP) activity, an early marker of osteogenic differentiation, suggesting that LPA directs osteoblastogenesis in MSCs,7,73,76 likely through LPA3. However, LPA4 activation can inhibit osteogenesis,73 possibly due to its ability to bind intracellular Gs subunits. Therefore, additional regulation of cAMP signaling may be required for optimal bone formation. In bovine endometrial stromal cells, LPA upregulates production of prostaglandin E2 (PGE2),77 which promotes bone formation in osteoblastic cells through suppression of sclerostin expression and a corresponding increase in Wnt signaling.78 LPA has also been reported to induce MSC differentiation into myofibroblast-like cells.79 In addition to direct contributions to bone formation, MSCs promote the necessary angiogenic contributions to bone repair and homeostasis by secreting trophic factors that recruit and stabilize endothelial cells.80 LPA potentiates this behavior by stimulating MSCs to increase secretion of proangiogenic and other cytokines, including VEGF and stromal cell derived factor-1.81 Furthermore, LPA protects MSCs against apoptosis induced by ischemic conditions, likely through a phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt-mediated pathway.4,5,82,83
In mature bone, LPA modulates cytoskeletal organization in osteoblasts and may stimulate extracellular matrix production and organization.84 Osteoblasts increase ALP production in response to LPA, especially when concomitantly exposed to calcitriol,7,76 indicating a role in osteoblast maturation. LPA modulates motility in osteoblasts and osteocytes, inducing chemotaxis in murine MC3T3 preosteoblasts85 and dendrite outgrowth in MLO-Y4 osteoblasts.86 These findings suggest that LPA may be involved in directing osteoblast migration and morphology during bone remodeling. Recent data provide evidence that osteoclasts produce LPA, which can then act as an additional autocrine signal as well as a paracrine cue to osteoclasts and potentially MSCs.87,88
Indeed, osteoclasts, which arise from a hematopoetic rather than mesenchymal lineage, are also responsive to LPA and demonstrate marked increases in Ca2+-mediated intracellular signaling on exposure.89 LPA participates in regulation of osteoclast number and activity by influencing osteoclastogenesis and enhancing survival of this population through suppression of apoptosis.88,89 This increase in survival may be due to activation of the extracellular-signal-regulated kinases (ERK)-PI3K/Akt pathway88 or activation of calmodulin and downstream pro-survival pathways due to increases in cytosolic Ca2+.89 Consistent with its effect on other cell types, LPA also has cytoskeletal effects on osteoclasts, resulting in retraction of lamellipodia and pseudopodia and a decrease in cell area, although resorptive capacity is largely unaffected.89
S1P also promotes osteoblast migration, survival by inhibition of apoptosis,90,91 and proliferation92,93 at concentrations similar to those that occur in systemic circulation. Osteoblasts express S1P1, S1P2, and S1P5.92 As a mitogen, S1P appears to differentially activate the MAPK pathway in both human and rat osteoblasts in a species-specific manner.94 Primary human osteoblasts also have been shown to increase ALP production after 3 and 5 days of stimulation with S1P in vitro.95 S1P also was reported as a potent serum-derived chemoattractant in inducing MSC mobilization in vitro.25 MSCs express S1P1–3, but S1P3 signaling appears to be the predominant regulator of MSC trafficking.
S1P stimulation increases osteoclastogenesis by increasing RANKL in osteoblasts through cyclooxygenase-2 and PGE2 regulation demonstrated in co-culture studies of BM-derived macrophages and osteoblasts.90 Furthermore, S1P can contribute to the dynamic control of bone mineral homeostasis, as it induces migration of osteoclast precursor cells along concentration gradients of S1P both in vitro and in vivo.96 The regulation of osteoclast precursor cell trafficking to and from the bone surface is a crucial process in the mediation of bone resorption.
Clinical Applications of Bioactive Lipids in Drug Discovery
Based on the importance of the S1P and LPA signaling axes for various pathologies, a number of drugs are in clinical trials that target these pathways, as reviewed in Kunkel et al.97 FTY720 (fingolimod) is an S1P-based therapeutic with potent immunomodulatory capacity.14 FTY720 was first clinically studied for its use in improving renal transplantation outcomes and preventing allograft rejection, but it did not reach its clinical end-points.14,98 However, FTY720 became the first FDA-approved orally bioavailable drug for treating relapsing forms of multiple sclerosis.14 In a phase I clinical trial (ClinicalTrials.gov Identifier: NCT00661414), sonepcizumab, an S1P-specific monoclonal antibody, was evaluated as an anti-S1P treatment to reduce tumor volume and metastatic potential by inhibiting blood vessel formation.97,99 Given the pleiotropic actions of S1P and LPA in full, other therapies have used specific receptor targets for therapeutic application. RPC1063 is an S1P1 modulator that has undergone Phase II clinical trials for both relapsing-remitting multiple sclerosis and ulcerative colitis. BMS-986020 (AM152), an antagonist of LPA1, is also in Phase II clinical trials for treating idiopathic pulmonary fibrosis.14 In addition, LPA has been studied as a diagnostic for early detection of ovarian cancer (ClinicalTrials.gov Identifier: NCT00986206). While there are several ongoing efforts to target S1P- and LPA-signaling pathways, clinical trials using S1P and/or LPA directly as a therapeutic have not been performed to date.
Applications in Tissue Engineering
There have been comparatively few efforts to directly use LPLs for clinical treatments or regenerative medicine. Systemic delivery is impractical due to the extremely short circulatory half-life of these lipids,100 which are eliminated through first-pass hepatic clearance, and LPL quantification can be labor- and cost-intensive (Table 2). Nonetheless, the pleiotropic nature of LPA and S1P, in addition to their ready availability and low cost compared with recombinant proteins, makes these LPLs attractive targets for investigation in many applications. Therefore, these lipids lend themselves particularly well to tissue engineering strategies that utilize material-based approaches to closely regulate the spatiotemporal presentation of signaling molecules. Some of the more promising directions for LPLs in tissue engineering are highlighted next.
Table 2.
Chemical, Physical, and Biological Characteristics of Lysophosphatidic Acid and Sphingosine-1-Phosphate
| Chemical and physical characteristics | Biological characteristics | |||||
|---|---|---|---|---|---|---|
| LPL | Chemical structure | MW (g/mol) | ½ life | Physiological levels | In vivo production | Detection methods |
| LPA | 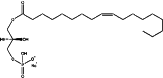 |
436.52 | <1 min; rapidly cleared by first-pass hepatic100 | ∼1–20 μM (serum)3,118 1–600 nm (plasma)32,118 |
Activated platelets, hair follicles, cancer cells | LC-MS/MS126,127; MALDI-TOF128,129 |
| S1P | 379.47 | ∼15 min (plasma)130 | ∼0.1–1.2 μM (plasma)13,17,130,131 0.5–75 pmol/mg wet weight (tissues)130 |
Activated platelets, red blood cells, mast cells, cancer cells, endothelial cells | ELISA70,132; LC-MS/MS133; Radiolabeling16; HPLC130,134 | |
LPA, lysophospholipid; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography; LC-MS/MS, liquid chromatography-mass spectrometry; MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight.
Pharmacological strategies for inhibiting apoptosis and promoting cell persistence
Maintaining cell persistence and survival postimplantation represents one of the biggest hurdles to successful in vivo translation of cell therapies. Serum-deprived and hypoxic (SD/H) culture conditions are catastrophic to MSC cultures,101 and more than 99% of cells delivered to ischemic heart tissue die after 72 h.102 Although recombinant growth factors such as angiopoietin-1 can confer apoptotic resistance,103 such proteins are prohibitively expensive, have short half-lives in vivo, and are difficult to deliver in a sustained manner. Thus, it is critically important to develop novel methods for enhancing cell survival.
The ability of LPA and LPA receptor agonists to protect multiple cell types against SD/H,104 combined with the relatively low cost, makes them ideal for many cell delivery applications. Micromolar concentrations of LPA protect neuronal precursors,105 osteoblasts, osteoclasts,88 rat and human MSCs,4,5 and other cell types against SD/H- and endoplasmic reticulum-stress induced apoptosis in vitro in a pertussis toxin- and PI3K-dependent manner.82 Furthermore, these protective effects extend to MSCs injected in vivo83 after preconditioning in medium containing LPA. Similarly, the development of nonlipid agonists of LPA2 is underway for protecting gastrointestinal tissue against apoptosis caused by high-dose γ-irradiation.106
However, most modern strategies for regenerative medicine utilize cell-instructive biomaterials such as hydrogels or polymer scaffolds to deliver cells to larger defects. Therefore, the ability to deliver signaling molecules such as LPA or LPA receptor agonists in a three-dimensional material is paramount. We have shown that MSCs in peptide-modified Arg-Gly-Asp (RGD)-alginate containing physically entrapped LPA exhibit improved persistence over 4 weeks in a subcutaneous implantation model (Fig. 2A). Furthermore, osteogenically induced MSCs responded differently to ischemic environments and varying doses of LPA compared with undifferentiated cells.4 Additional work is required to determine optimal concentrations and retention properties in a range of materials that are suited for distinct therapeutic applications.
FIG. 2.
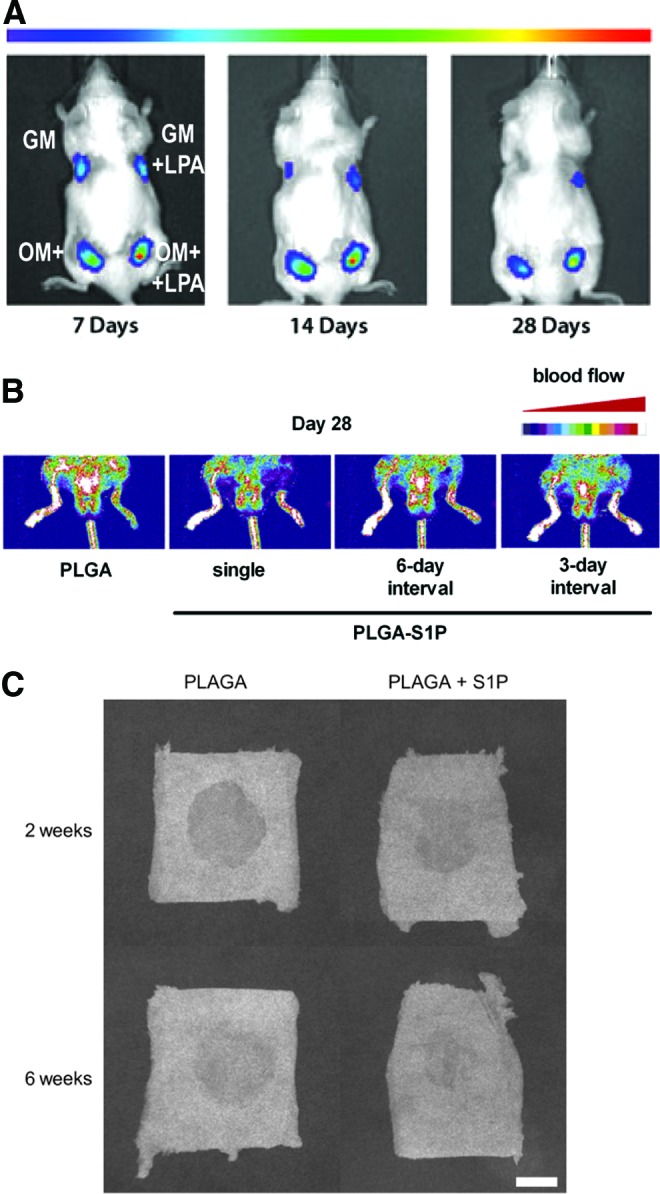
Delivery of lysophospholipids for tissue engineering applications. Delivery of LPA enhanced survival of transplanted human mesenchymal stem/stromal cells, preconditioned with either growth medium (GM) or osteogenic medium (OM), within alginate hydrogels after 28 days in vivo as assessed by bioluminescence imaging (A). Figure reproduced with permission from Mary Ann Liebert.4 Intermittently repeated, local injections of sphingosine-1-phosphate (S1P)-loaded poly(lactic-co-glycolic acid) (PLGA) microparticles (PLGA-S1P) resulted in enhanced blood flow recovery in murine ischemic hindlimbs after 28 days in vivo when examined by Laser Doppler blood flow analysis (B). Figure reprinted with permission from Elsevier.57 Sustained delivery of S1P from PLGA scaffolds within critical-sized, rat cranial defects led to greater cranial bone healing after 2 and 6 weeks in vivo when evaluated by X-ray imaging analysis (C). Figure reprinted with permission from Elsevier.11 Color images available online at www.liebertpub.com/teb
The sphingolipid rheostat (ratio of S1P to ceramide) can also dictate outcomes of cell survival (S1P-induced) versus cell death (ceramide-induced).107,108 S1P, generated by overexpression of SK1, protects against apoptosis by activating ERK1/2, Akt, and the NF-κB signaling pathways of cell survival.26,107 S1P further inhibits release of cytochrome c, activation of caspases, and activation of Jun amino-terminal kinase, a stress-activated protein kinase, in inhibition of apoptosis. S1P has been shown to suppress apoptosis by endothelial cells in SD in a dose-dependent manner.108 Interestingly, these anti-apoptotic actions appear to be independent of extracellular S1PR signaling.107
Therapeutic angiogenesis
Beyond short-term abrogation of SD/H-induced apoptosis, the reestablishment of a vascular supply is critical for successful cell therapies to treat ischemic defects, such as those arising from chronic and acute peripheral vascular disease or tissue loss due to trauma, surgery, or disease. Although surgical interventions for restoration of blood flow are possible, they are both costly and invasive. Growth factor-based approaches have been pursued as treatment options,1 but limitations related to regulating spatiotemporal release and uncontrolled vessel and tumor growth motivate the development of alternative strategies.
The direct mitogenic48 and proangiogenic47 effects of LPA on endothelial cells suggest that controlled release of this molecule may stimulate an angiogenic response in vivo. However, the challenges of accurately modeling release in in vitro systems, including artificial synthesis/degradation, make it difficult to effectively tailor material properties before in vivo implementation. An alternative strategy for using LPA in therapeutic angiogenesis is to take advantage of the ability of stromal cell populations to function as pericytes that promote and stabilize blood vessel formation.109 Such pericytes naturally secrete growth factors such as VEGF and have been investigated as a vehicle to continuously supply local angiogenic cues.110,111 Since LPA induces the secretion of proangiogenic and inflammatory cytokines from MSCs,75,81 entrapment of stromal cells in LPA-containing constructs could result in elevated secretion of angiogenic growth factors. Indeed, human adipose-derived stromal cells (ASCs) entrapped in fibrin gels containing LPA significantly improved recovery and functional outcome in a mouse model of critical limb ischemia.9 Two weeks after femoral artery ligation and resection, animals treated with both fibrin-entrapped ASCs and LPA showed significantly increased blood vessel formation compared with mice treated with only ASCs or LPA, while all groups receiving LPA exhibited reduced limb necrosis and loss. Improved tissue preservation without concomitant increases in vascularization in mice treated with LPA alone9 also supports mounting evidence that LPA can modulate local inflammation and the immune response.112 Given the close interplay between the angiogenic and inflammatory axes in wound healing, effective LPA presentation merits further investigation for applications involving ischemic defects or vascular injury.
The manipulation of local S1P gradients represents a novel and exciting approach to recruit endogenously or exogenously supplied stem/progenitor and effector immune cells for regenerative medicine in recent research.16 S1P has also been recognized as an attractive therapeutic agent for delivery based on its key involvement in both angiogenesis and arteriogenesis.11,58 S1P stimulates the proliferation and migration of endothelial cells and promotes vascular stabilization through recruitment of pericytes and SMCs to surround neovessels. S1P further supports luminal expansion of arterioles and venules by stimulating SMC proliferation, migration, and differentiation into a more contractile phenotype. S1P may also recruit BM-derived circulating endothelial precursor cells to ischemic tissues, potentially mediated through S1P3, to stimulate angiogenesis.25 Daily intramuscular injections of S1P increased capillary density and promoted blood flow recovery in murine ischemic hindlimbs.56 However, material-controlled sustained release would be a more clinically relevant approach toward therapeutic angiogenesis without the need for daily injections. The deployment of S1P-loaded poly(lactic-co-glycolic acid) (PLGA) microparticles improved blood flow recovery and reduced VEGF-induced edema (Fig. 2B).57 S1P encapsulated within PLGA thin films promoted short-term enlargement of arteriolar and venular diameters.11 Furthermore, temporally separated delivery of VEGF and S1P from porous hollow cellulose fibers has been shown to enhance cellular infiltration in a modified murine Matrigel plug assay.65 However, biomaterial-based gradients of S1P are short-lived in the tissue due to degradation by S1P lyase,16 thereby motivating the investigation of new methods to locally sustain these signals. One such method involving co-delivery of S1P and 4-deoxy-pyridoxine, an S1P lyase inhibitor, from PLGA films substantially increased local tissue S1P and sphingolipid concentrations over time.16
Orthopedic applications
LPA holds promise for successful use in bone repair applications, even though the LPA-directed signaling axis in osteoblast and osteoclast differentiation has not yet been fully elucidated. In addition to the pro-survival implications for cells delivered to ischemic defect sites, LPA may be targeted for use in directing differentiation or maturation of MSCs, preosteoblasts, or osteoblasts through the pathways previously described. Although the hydrogel-based systems that have been investigated for cell survival and therapeutic angiogenesis are only mildly osteoinductive, we have shown that the addition of mineralized PLGA microspheres to fibrin gels further enhances osteogenic differentiation113; such a system would allow for similar physical entrapment of LPA and presentation. Alternately, other groups are pursuing the covalent attachment of LPA to titanium constructs with the goal of stimulating osteoblast maturation and osseointegration114 on scaffolds better suited to load-bearing applications.
Given the pro-angiogenic and pro-arteriogenic nature of S1P, exogenous delivery has also been used in approaches for enhancing tissue-engineered bone regeneration.11,115 In a rat cranial defect model, defects filled with S1P-loaded PLGA macroporous scaffolds exhibited significantly increased bone volume after 2 and 6 weeks of healing versus empty scaffolds alone (Fig. 2C). The substantial bone healing correlated with an increased number of blood vessels. This functional involvement of S1P in the formation of new bone is hypothesized to be due to its ability to both remodel the microvasculature and stimulate recruitment and proliferation of osteoblast precursor cells.11 However, future studies are required to fully understand S1P signaling in bone formation.
Orthopedic applications of LPA are not limited to treatment of bony defects. LPA treatment of self-assembled fibrocartilage constructs synthesized by articular chondrocytes and meniscal cells resulted in tissue with improved tensile properties and superiorly aligned collagen structures.6 LPA is produced in situ by resting zone growth plate chondrocytes in response to 24R,25-dihydroxyvitamin D3 and inhibits apoptosis induced by inorganic phosphate.116 Similarly, LPA stimulates proliferation of rat chondrocytes in vitro.117 Since this cell type is notoriously difficult to expand in culture, successful induction of a proliferative response could have significant implications for scale-up of tissue engineered cartilages. These studies emphasize the feasibility of using bioactive lipids to enhance the physical and biochemical properties of tissue engineering strategies that call for ex vivo expansion or generation of tissue constructs for subsequent implantation into in vivo defect sites.
Conclusion
LPA and S1P are inexpensive lipid mediators that have pleiotropic effects in many different cell and tissue types. To date, these lipids have been primarily studied in biological and mechanistic contexts. However, if careful consideration is given to controlling presentation, release, and degradation in conjunction with established biomaterials-based delivery vehicles, these molecules hold great promise for enhancing the efficacy of tissue engineering solutions for a wide range of pathologies and defects. Given the diverse and pleiotropic nature of these LPLs, delivery of S1P or LPA may provide an attractive alternative to the delivery of multiple growth factors. Thus, further studies must be performed to compare the efficacy of these bioactive molecules for specific therapeutic applications.
Acknowledgments
The authors would like to acknowledge financial support from the National Institutes of Health (1R21AG036963 to J.K.L.), the California Institute for Regenerative Medicine UC Davis Stem Cell Training Program (CIRM T1-00006, CIRM TG2-01163) to B.Y.K.B., and the American Heart Association Western States Affiliate Predoctoral Fellowship (15PRE22930044) to P.A.W.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lee K., Silva E.A., and Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8, 153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pebay A., Bonder C.S., and Pitson S.M. Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat 84, 83, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Moolenaar W.H. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem 270, 12949, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Binder B.Y., Genetos D.C., and Leach J.K. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Part A 20, 1156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Baydoun A.R., Xu R., Deng L., Liu X., Zhu W., Shi L., Cong X., Hu S., and Chen X. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells 26, 135, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Hadidi P., and Athanasiou K.A. Enhancing the mechanical properties of engineered tissue through matrix remodeling via the signaling phospholipid lysophosphatidic acid. Biochem Biophys Res Commun 433, 133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansell J.P., and Blackburn J. Lysophosphatidic acid, human osteoblast formation, maturation and the role of 1alpha,25-dihydroxyvitamin D3 (calcitriol). Biochim Biophys Acta 1831, 105, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Wada R., Yamashita T., Mi Y., Deng C., Hobson J.P., Rosenfeldt H.M., Nava V.E., Chae S., Lee M., Liu C.H., Hla T., Spiegel S., and Proia R.L. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106, 951, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder B.Y., Sondergaard C.S., Nolta J.A., and Leach J.K. Lysophosphatidic acid enhances stromal cell-directed angiogenesis. PLoS One 8, e82134, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyne N.J., and Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10, 489, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Sefcik L.S., Petrie Aronin C.E., Wieghaus K.A., and Botchwey E.A. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials 29, 2869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snider A.J., Ali W.H., Sticca J.A., Coant N., Ghaleb A.M., Kawamori T., Yang V.W., Hannun Y.A., and Obeid L.M. Distinct roles for hematopoietic and extra-hematopoietic sphingosine kinase-1 in inflammatory bowel disease. PLoS One 9, e113998, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poitevin S., Cussac D., Leroyer A.S., Albinet V., Sarlon-Bartoli G., Guillet B., Hubert L., Andrieu-Abadie N., Couderc B., Parini A., Dignat-George F., and Sabatier F. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc Res 103, 121, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Kihara Y., Mizuno H., and Chun J. Lysophospholipid receptors in drug discovery. Exp Cell Res 333, 171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awojoodu A.O., Ogle M.E., Sefcik L.S., Bowers D.T., Martin K., Brayman K.L., Lynch K.R., Peirce-Cottler S.M., and Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A 110, 13785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogle M.E., Sefcik L.S., Awojoodu A.O., Chiappa N.F., Lynch K., Peirce-Cottler S., and Botchwey E.A. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater 10, 4704, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendelson K., Evans T., and Hla T. Sphingosine 1-phosphate signalling. Development 141, 5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki J., Inoue A., and Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781, 513, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Okudaira S., Yukiura H., and Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 92, 698, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T., Mizuno K., Saku K., Taguchi R., and Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277, 48737, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Murakami M., Shiraishi A., Tabata K., and Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun 371, 707, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Valentine W.J., Fells J.I., Perygin D.H., Mujahid S., Yokoyama K., Fujiwara Y., Tsukahara R., Van Brocklyn J.R., Parrill A.L., and Tigyi G. Subtype-specific residues involved in ligand activation of the endothelial differentiation gene family lysophosphatidic acid receptors. J Biol Chem 283, 12175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagida K., and Ishii S. Non-Edg family LPA receptors: the cutting edge of LPA research. J Biochem 150, 223, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Yanagida K., Kurikawa Y., Shimizu T., and Ishii S. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta 1831, 33, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Hsu A., Lee J.F., Cramer D.E., and Lee M.J. To stay or to leave: stem cells and progenitor cells navigating the S1P gradient. World J Biol Chem 2, 1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel S., and Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4, 397, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Spiegel S., and Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11, 403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi K., Herr D., Mutoh T., and Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol 9, 15, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Tigyi G., and Parrill A.L. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res 42, 498, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Oka S., Ota R., Shima M., Yamashita A., and Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun 395, 232, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Tabata K., Baba K., Shiraishi A., Ito M., and Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun 363, 861, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol 161, 241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch K.R., and Macdonald T.L. Sphingosine 1-phosphate chemical biology. Biochim Biophys Acta 1781, 508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi H., Kitayama J., Takuwa N., Arikawa K., Inoki I., Takehara K., Nagawa H., and Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J 374, 715, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerage D., Brindley D.N., and Hemmings D.G. Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 35 Suppl, S86, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Jung B., Obinata H., Galvani S., Mendelson K., Ding B.S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., and Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23, 600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takuwa Y., Okamoto Y., Yoshioka K., and Takuwa N. Sphingosine-1-phosphate signaling in physiology and diseases. BioFactors 38, 329, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y., Makarova N., Tsukahara R., Guo H., Shuyu E., Farrar P., Balazs L., Zhang C., and Tigyi G. Lysophosphatidic acid-induced arterial wall remodeling: requirement of PPARgamma but not LPA1 or LPA2 GPCR. Cell Signal 21, 1874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida K., Nishida W., Hayashi K., Ohkawa Y., Ogawa A., Aoki J., Arai H., and Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation 108, 1746, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Leslie D.S., Dascher C.C., Cembrola K., Townes M.A., Hava D.L., Hugendubler L.C., Mueller E., Fox L., Roura-Mir C., Moody D.B., Vincent M.S., Gumperz J.E., Illarionov P.A., Besra G.S., Reynolds C.G., and Brenner M.B. Serum lipids regulate dendritic cell CD1 expression and function. Immunology 125, 289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siess W., Zangl K.J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., and Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A 96, 6931, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francone O.L., Tu M., Royer L.J., Zhu J., Stevens K., Oleynek J.J., Lin Z., Shelley L., Sand T., Luo Y., and Kane C.D. The hydrophobic tunnel present in LOX-1 is essential for oxidized LDL recognition and binding. J Lipid Res 50, 546, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Hait N.C., Allegood J., Maceyka M., Strub G.M., Harikumar K.B., Singh S.K., Luo C., Marmorstein R., Kordula T., Milstien S., and Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siehler S., Wang Y., Fan X., Windh R.T., and Manning D.R. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem 276, 48733, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Aoyagi T., Nagahashi M., Yamada A., and Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat Res Biol 10, 97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., and Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281, 25822, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Rivera-Lopez C.M., Tucker A.L., and Lynch K.R. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 11, 301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panetti T.S., Chen H., Misenheimer T.M., Getzler S.B., and Mosher D.F. Endothelial cell mitogenesis induced by LPA: inhibition by thrombospondin-1 and thrombospondin-2. J Lab Clin Med 129, 208, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Schulze C., Smales C., Rubin L.L., and Staddon J.M. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem 68, 991, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Hayashi K., Takahashi M., Nishida W., Yoshida K., Ohkawa Y., Kitabatake A., Aoki J., Arai H., and Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res 89, 251, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Smyth S.S., Mueller P., Yang F., Brandon J.A., and Morris A.J. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler Thromb Vasc Biol 34, 479, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren B., Hale J., Srikanthan S., and Silverstein R.L. Lysophosphatidic acid suppresses endothelial cell CD36 expression and promotes angiogenesis via a PKD-1-dependent signaling pathway. Blood 117, 6036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohata H., Yamada H., and Momose K. Lysophosphatidic acid induces shear stress-dependent Ca2+ influx in mouse aortic endothelial cells in situ. Exp Physiol 96, 468, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Niioka T., Ohata H., Momose K., and Honda K. Lysophosphatidic acid induces shear stress-dependent contraction in mouse aortic strip in situ. J Cardiovasc Pharmacol 62, 530, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Ruisanchez E., Dancs P., Kerek M., Nemeth T., Farago B., Balogh A., Patil R., Jennings B.L., Liliom K., Malik K.U., Smrcka A.V., Tigyi G., and Benyo Z. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J 28, 880, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oyama O., Sugimoto N., Qi X., Takuwa N., Mizugishi K., Koizumi J., and Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res 78, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Qi X., Okamoto Y., Murakawa T., Wang F., Oyama O., Ohkawa R., Yoshioka K., Du W., Sugimoto N., Yatomi Y., Takuwa N., and Takuwa Y. Sustained delivery of sphingosine-1-phosphate using poly(lactic-co-glycolic acid)-based microparticles stimulates Akt/ERK-eNOS mediated angiogenesis and vascular maturation restoring blood flow in ischemic limbs of mice. Eur J Pharmacol 634, 121, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Wacker B.K., Scott E.A., Kaneda M.M., Alford S.K., and Elbert D.L. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules 7, 1335, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee M.J., Thangada S., Claffey K.P., Ancellin N., Liu C.H., Kluk M., Volpi M., Sha'afi R.I., and Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301, 1999 [DOI] [PubMed] [Google Scholar]
- 60.English D., Welch Z., Kovala A.T., Harvey K., Volpert O.V., Brindley D.N., and Garcia J.G. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J 14, 2255, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Paik J.H., Skoura A., Chae S.S., Cowan A.E., Han D.K., Proia R.L., and Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 18, 2392, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adamson R.H., Sarai R.K., Altangerel A., Thirkill T.L., Clark J.F., and Curry F.R. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res 88, 344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F., Van Brocklyn J.R., Hobson J.P., Movafagh S., Zukowska-Grojec Z., Milstien S., and Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem 274, 35343, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Gavrilovskaya I.N., Gorbunova E.E., Mackow N.A., and Mackow E.R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol 82, 5797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tengood J.E., Kovach K.M., Vescovi P.E., Russell A.J., and Little S.R. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials 31, 7805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaho V.A., and Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 55, 1596, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Visentin B., Vekich J.A., Sibbald B.J., Cavalli A.L., Moreno K.M., Matteo R.G., Garland W.A., Lu Y., Yu S., Hall H.S., Kundra V., Mills G.B., and Sabbadini R.A. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9, 225, 2006 [DOI] [PubMed] [Google Scholar]
- 68.English D., Kovala A.T., Welch Z., Harvey K.A., Siddiqui R.A., Brindley D.N., and Garcia J.G. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res 8, 627, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Kimura T., Watanabe T., Sato K., Kon J., Tomura H., Tamama K., Kuwabara A., Kanda T., Kobayashi I., Ohta H., Ui M., and Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J 348 Pt 1, 71, 2000 [PMC free article] [PubMed] [Google Scholar]
- 70.Williams P.A., Stilhano R.S., To V.P., Tran L., Wong K., and Silva E.A. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P). PLoS One 10, e0123437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blackburn J., and Mansell J.P. The emerging role of lysophosphatidic acid (LPA) in skeletal biology. Bone 50, 756, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Salles J.P., Laurencin-Dalicieux S., Conte-Auriol F., Briand-Mesange F., and Gennero I. Bone defects in LPA receptor genetically modified mice. Biochim Biophys Acta 1831, 93, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Liu Y.B., Kharode Y., Bodine P.V., Yaworsky P.J., Robinson J.A., and Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem 109, 794, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Park D., Spencer J.A., Koh B.I., Kobayashi T., Fujisaki J., Clemens T.L., Lin C.P., Kronenberg H.M., and Scadden D.T. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee M.J., Jeon E.S., Lee J.S., Cho M., Suh D.S., Chang C.L., and Kim J.H. Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells. J Cell Biochem 104, 499, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Mansell J.P., Nowghani M., Pabbruwe M., Paterson I.C., Smith A.J., and Blom A.W. Lysophosphatidic acid and calcitriol co-operate to promote human osteoblastogenesis: requirement of albumin-bound LPA. Prostaglandins Other Lipid Mediat 95, 45, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Woclawek-Potocka I., Kondraciuk K., and Skarzynski D.J. Lysophosphatidic acid stimulates prostaglandin E2 production in cultured stromal endometrial cells through LPA1 receptor. Exp Biol Med 234, 986, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Genetos D.C., Yellowley C.E., and Loots G.G. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One 6, e17772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeon E.S., Moon H.J., Lee M.J., Song H.Y., Kim Y.M., Cho M., Suh D.S., Yoon M.S., Chang C.L., Jung J.S., and Kim J.H. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells 26, 789, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Hoch A.I., Binder B.Y., Genetos D.C., and Leach J.K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One 7, e35579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeon E.S., Heo S.C., Lee I.H., Choi Y.J., Park J.H., Choi K.U., Park Do Y., Suh D.S., Yoon M.S., and Kim J.H. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Exp Mol Med 42, 280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Z., Wei H., Liu X., Hu S., Cong X., and Chen X. LPA rescues ER stress-associated apoptosis in hypoxia and serum deprivation-stimulated mesenchymal stem cells. J Cell Biochem 111, 811, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Hou J., Shi L., Chen J., Sang J., Hu S., Cong X., and Chen X. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev 18, 947, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Zhang Q., Magnusson M.K., and Mosher D.F. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 8, 1415, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masiello L.M., Fotos J.S., Galileo D.S., and Karin N.J. Lysophosphatidic acid induces chemotaxis in MC3T3-E1 osteoblastic cells. Bone 39, 72, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Waters K.M., Jacobs J.M., Gritsenko M.A., and Karin N.J. Regulation of gene expression and subcellular protein distribution in MLO-Y4 osteocytic cells by lysophosphatidic acid: relevance to dendrite outgrowth. Bone 48, 1328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panupinthu N., Rogers J.T., Zhao L., Solano-Flores L.P., Possmayer F., Sims S.M., and Dixon S.J. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol 181, 859, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sims S.M., Panupinthu N., Lapierre D.M., Pereverzev A., and Dixon S.J. Lysophosphatidic acid: a potential mediator of osteoblast-osteoclast signaling in bone. Biochim Biophys Acta 1831, 109, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Lapierre D.M., Tanabe N., Pereverzev A., Spencer M., Shugg R.P., Dixon S.J., and Sims S.M. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J Biol Chem 285, 25792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryu J., Kim H.J., Chang E.J., Huang H., Banno Y., and Kim H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J 25, 5840, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grey A., Chen Q., Callon K., Xu X., Reid I.R., and Cornish J. The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology 143, 4755, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Grey A., Xu X., Hill B., Watson M., Callon K., Reid I.R., and Cornish J. Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif Tissue Int 74, 542, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Pederson L., Ruan M., Westendorf J.J., Khosla S., and Oursler M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A 105, 20764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carpio L.C., Stephan E., Kamer A., and Dziak R. Sphingolipids stimulate cell growth via MAP kinase activation in osteoblastic cells. Prostaglandins Leukot Essent Fatty Acids 61, 267, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Dziak R., Yang B.M., Leung B.W., Li S., Marzec N., Margarone J., and Bobek L. Effects of sphingosine-1-phosphate and lysophosphatidic acid on human osteoblastic cells. Prostaglandins Leukot Essent Fatty Acids 68, 239, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J., Proia R.L., and Germain R.N. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kunkel G.T., Maceyka M., Milstien S., and Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12, 688, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salvadori M., Budde K., Charpentier B., Klempnauer J., Nashan B., Pallardo L.M., Eris J., Schena F.P., Eisenberger U., Rostaing L., Hmissi A., Aradhye S., and FTY720 0124 Study Group. FTY720 versus MMF with cyclosporine in de novo renal transplantation: a 1-year, randomized controlled trial in Europe and Australasia. Am J Transplant 6, 2912, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Ponnusamy S., Selvam S.P., Mehrotra S., Kawamori T., Snider A.J., Obeid L.M., Shao Y., Sabbadini R., and Ogretmen B. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med 4, 761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salous A.K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G.A., Smyth S.S., and Morris A.J. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J Lipid Res 54, 2775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Potier E., Ferreira E., Meunier A., Sedel L., Logeart-Avramoglou D., and Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng 13, 1325, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Das R., Jahr H., van Osch G.J., and Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16, 159, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Liu X.B., Jiang J., Gui C., Hu X.Y., Xiang M.X., and Wang J.A. Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta Pharmacol Sin 29, 815, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Kiss G.N., Fells J.I., Gupte R., Lee S.C., Liu J., Nusser N., Lim K.G., Ray R.M., Lin F.T., Parrill A.L., Sumegi B., Miller D.D., and Tigyi G. Virtual screening for LPA2-specific agonists identifies a nonlipid compound with antiapoptotic actions. Mol Pharmacol 82, 1162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun Y., Nam J.S., Han D.H., Kim N.H., Choi H.K., Lee J.K., Rhee H.J., and Huh S.O. Lysophosphatidic acid induces upregulation of Mcl-1 and protects apoptosis in a PTX-dependent manner in H19-7 cells. Cell Signal 22, 484, 2010 [DOI] [PubMed] [Google Scholar]
- 106.Patil R., Szabo E., Fells J.I., Balogh A., Lim K.G., Fujiwara Y., Norman D.D., Lee S.C., Balazs L., Thomas F., Patil S., Emmons-Thompson K., Boler A., Strobos J., McCool S.W., Yates C.R., Stabenow J., Byrne G.I., Miller D.D., and Tigyi G.J. Combined mitigation of the gastrointestinal and hematopoietic acute radiation syndromes by an LPA2 receptor-specific nonlipid agonist. Chem Biol 22, 206, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Le Stunff H., Milstien S., and Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem 92, 882, 2004 [DOI] [PubMed] [Google Scholar]
- 108.Hisano N., Yatomi Y., Satoh K., Akimoto S., Mitsumata M., Fujino M.A., and Ozaki Y. Induction and suppression of endothelial cell apoptosis by sphingolipids: a possible in vitro model for cell-cell interactions between platelets and endothelial cells. Blood 93, 4293, 1999 [PubMed] [Google Scholar]
- 109.Armulik A., Abramsson A., and Betsholtz C. Endothelial/pericyte interactions. Circ Res 97, 512, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9, 107, 2010 [DOI] [PubMed] [Google Scholar]
- 111.de Villiers J.A., Houreld N., and Abrahamse H. Adipose derived stem cells and smooth muscle cells: implications for regenerative medicine. Stem Cell Rev 5, 256, 2009 [DOI] [PubMed] [Google Scholar]
- 112.Knowlden S., and Georas S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol 192, 851, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davis H.E., Binder B.Y., Schaecher P., Yakoobinsky D.D., Bhat A., and Leach J.K. Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng Part A 19, 1773, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mansell J.P., Brown J., Knapp J.G., Faul C.F., and Blom A.W. Lysophosphatidic acid-functionalised titanium as a superior surface for supporting human osteoblast (MG63) maturation. Eur Cell Mater 23, 348, 2012 [DOI] [PubMed] [Google Scholar]
- 115.Das A., Tanner S., Barker D.A., Green D., and Botchwey E.A. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J Biomed Mater Res Part A 102, 1210, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hurst-Kennedy J., Zhong M., Gupta V., Boyan B.D., and Schwartz Z. 24R,25-Dihydroxyvitamin D3, lysophosphatidic acid, and p53: a signaling axis in the inhibition of phosphate-induced chondrocyte apoptosis. J Steroid Biochem Mol Biol 122, 264, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Hurst-Kennedy J., Boyan B.D., and Schwartz Z. Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes. Biochim Biophys Acta 1793, 836, 2009 [DOI] [PubMed] [Google Scholar]
- 118.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 15, 477, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Hama K., and Aoki J. LPA(3), a unique G protein-coupled receptor for lysophosphatidic acid. Prog Lipid Res 49, 335, 2010 [DOI] [PubMed] [Google Scholar]
- 120.Lin M.E., Herr D.R., and Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 91, 130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McIntyre T.M., Pontsler A.V., Silva A.R., St Hilaire A., Xu Y., Hinshaw J.C., Zimmerman G.A., Hama K., Aoki J., Arai H., and Prestwich G.D. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A 100, 131, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takuwa Y., Takuwa N., and Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem 131, 767, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Tania M., Khan A., Zhang H., Li J., and Song Y. Autotaxin: a protein with two faces. Biochem Biophys Res Commun 401, 493, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Poti F., Simoni M., and Nofer J.R. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P). Cardiovasc Res 103, 395, 2014 [DOI] [PubMed] [Google Scholar]
- 125.Anliker B., and Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem 279, 20555, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Baker D.L., Desiderio D.M., Miller D.D., Tolley B., and Tigyi G.J. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem 292, 287, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Scherer M., Schmitz G., and Liebisch G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin Chem 55, 1218, 2009 [DOI] [PubMed] [Google Scholar]
- 128.Morishige J., Urikura M., Takagi H., Hirano K., Koike T., Tanaka T., and Satouchi K. A clean-up technology for the simultaneous determination of lysophosphatidic acid and sphingosine-1-phosphate by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a phosphate-capture molecule, Phos-tag. Rapid Commun Mass Spectrom 24, 1075, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Tanaka T., Tsutsui H., Hirano K., Koike T., Tokumura A., and Satouchi K. Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate-capture molecule. J Lipid Res 45, 2145, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Venkataraman K., Lee Y.M., Michaud J., Thangada S., Ai Y., Bonkovsky H.L., Parikh N.S., Habrukowich C., and Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102, 669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sabbadini R.A. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer 95, 1131, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zeng Y., Adamson R.H., Curry F.R., and Tarbell J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol 306, H363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Merrill A.H., Jr., Sullards M.C., Allegood J.C., Kelly S., and Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 36, 207, 2005 [DOI] [PubMed] [Google Scholar]
- 134.Min J.K., Yoo H.S., Lee E.Y., Lee W.J., and Lee Y.M. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 303, 167, 2002 [DOI] [PubMed] [Google Scholar]